Abstract
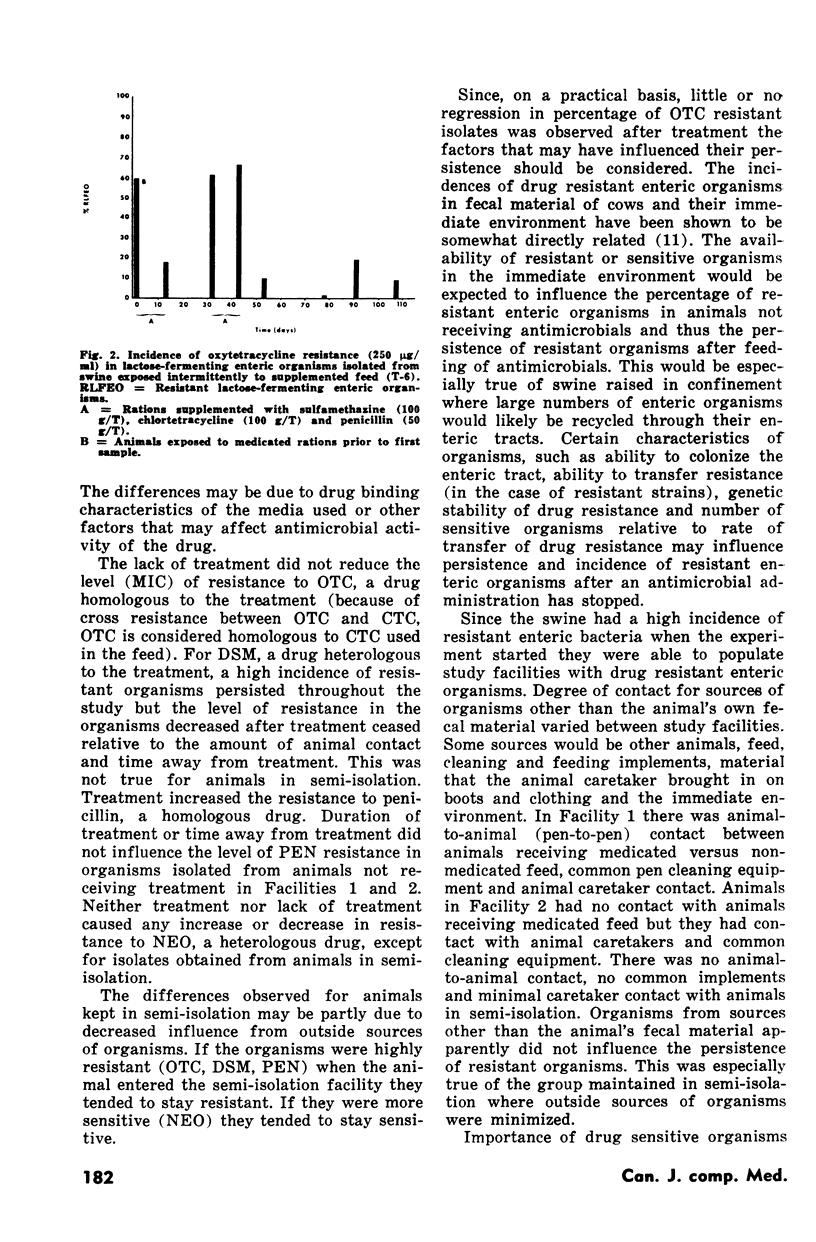
Six groups of swine (85 animals) were fed a combination of antimicrobial drugs (sulfamethazine 100 g/ton, chlortetracycline 100 g/ton and penicillin 50 g/ton). After two weeks the antimicronial drugs were removed from the diet of two groups (28 animals). These swine were compared to four groups fed the medicated diet to determine the effect of duration of treatment and degree of animal isolation on the persistence of resistance in lactose-fermenting enteric organisms. The degree of resistance to penicillin, oxytetracycline, dihydrostreptomycin and neomycin as determined by minimum inhibitory concentrations and the incidence of resistant organisms were examined during and after antibiotic feedings. Ninety-two percent or greater of all isolates tested during and after treatment had minimum inhibitory concentrations for oxytetracycline of greater than 100 mug/ml. Thirty-two weeks after cessation of dietary antibiotic, resistance to oxytetracycline and dihydrostreptomycin remained at 100% and 89% respectively. Variation in degree of contact between swine receiving medicated feed and those receiving nonmedicated feed was not sufficient to reduce the incidence of resistance to oxytetracycline or dihydrostreptomycin in all animals. Factors influencing persistence of resistant enteric organisms are discussed. Addition of the antimicrobials to the ration resulted in significantly greater weight gains for treated animals than for the controls but did not alter feed conversion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- EDWARDS S. J. Effect of streptomycin on the growth rate and intestinal flora (Escherichia coli) of piglets. J Comp Pathol. 1961 Jul;71:243–252. doi: 10.1016/s0368-1742(61)80030-1. [DOI] [PubMed] [Google Scholar]
- MacLowry J. D., Jaqua M. J., Selepak S. T. Detailed methodology and implementation of a semiautomated serial dilution microtechnique for antimicrobial susceptibility testing. Appl Microbiol. 1970 Jul;20(1):46–53. doi: 10.1128/am.20.1.46-53.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins L. D., Pocurull D. W., Mercer H. D., Natzke R. P., Postle D. S. Use of antibiotics in a dairy herd and their effect on resistance determinants in enteric and environmental Escherichia coli. J Dairy Sci. 1974 Aug;57(8):944–950. doi: 10.3168/jds.S0022-0302(74)84990-8. [DOI] [PubMed] [Google Scholar]
- Schroeder S. A., Terry P. M., Bennett J. V. Antibiotic resistance and transfer factor in Salmonella, United States 1967. JAMA. 1968 Sep 23;205(13):903–906. [PubMed] [Google Scholar]
- Smith H. W. Effect of prohibition of the use of tetracyclines in animal feeds on tetracycline resistance of faecal E. coli of pigs. Nature. 1973 May 25;243(5404):237–238. doi: 10.1038/243237a0. [DOI] [PubMed] [Google Scholar]
- Sokol A., Koppel Z., Mikula L. Dynamics of incidence of antibiotic-(chemotherapeutic-) resistant E. coli in pigs and types of polyresistance of the isolated strains depending on addition of aureovit12 C20 to food. J Hyg Epidemiol Microbiol Immunol. 1969;13(3):347–357. [PubMed] [Google Scholar]


