Abstract
Peptides derived from the whole sequence of mycobacterial GroES heat shock proteins were tested for the ability to induce the proliferation of blood mononuclear cells from tuberculosis patients and sensitized healthy subjects. The response to the N-terminal peptide (residues 1 to 16) was found to be more frequent and stronger in tuberculosis patients. This finding is exceptional, considering that recognition of all other GroES peptides by patients was either diminished or not different from that of controls.
Active tuberculosis (TB) is commonly associated with depressed delayed-type skin hypersensitivity and in vitro proliferation of blood lymphocytes (5). T-cell anergy in leprosy is closely linked to the pathology and clinical manifestations of the disease, but the implications for TB are less clear. One mechanism involved is the sequestration of CD4 T cells from the blood circulation to the site of the disease, e.g., pleural fluid (3, 12, 13), associated with a pronounced change from Th1 to Th0 cytokine secretion (4). There are no obvious grounds for expecting the depression in the blood T-cell response in TB to be antigen or epitope specific. Nevertheless, the proliferation of T cells in TB patients compared with that in sensitized healthy subjects showed at least certain peptide-specific differences (11). These could have been related to the extent of recovery of blood T-cell responses following chemotherapy (12), but the frequency of gamma interferon-secreting lymphocytes specific for ESAT-6 was shown actually to fall rather than rise during antituberculosis chemotherapy (9).
The GroES (cpn10) heat shock protein of Mycobacterium tuberculosis is strongly recognized by T cells from healthy tuberculin reactors and patients with pleural TB (1). GroES is conserved between species, and its homologues are present in both prokaryotic and eukaryotic cells. It has been used as an experimental vaccine both against bacterial, e.g., Helicobacter pylori (6), infection and against autoimmune diseases, e.g., adjuvant arthritis (10). As the repertoire of T-cell responses to GroES revealed a distinct peptide-related specificity in leprosy (2), it seemed of interest to investigate if patients with active TB have similar or different hierarchies of peptide recognition. We tested pairs of peptides of very similar GroES sequences from M. tuberculosis and M. leprae, the latter being relevant to potentially reflect environmental sensitization with organisms of the M. avium complex. Peptide 16-mers overlapping by eight residues covering the entire protein sequence were produced on the Milligen 9050 Peptide Synthesiser using 9-fluorenylmethoxy carbonyl technology and purified on Sephadex G-15. Sequence integrity was verified by mass spectrometry, and homogeneity was verified by reverse-phase high-performance liquid chromatography (2).
The patients (TBP) and healthy donors (HS) were diagnosed in the United Kingdom. Ethnic origins were not matched between the groups: Caucasian (4 TBP, 12 HS), Indian (12 TBP, 4 HS), or African (9 TBP, 1 HS). The diagnosis of TB was confirmed in 20 of 25 patients by culture or histology and in the remaining 5 by clinical criteria. Of the 20 patients with pulmonary TB, 3 had pleural effusions and of the 5 with extrapulmonary cases, 3 had lymphatic disease and 2 had peritoneal disease. There was no evidence of human immunodeficiency virus infection in any of the patients tested. Blood was drawn before the onset of chemotherapy from all except one patient. All 17 healthy donors (14 males and three females with an average age of 36 years) were BCG vaccinated and had no history of TB. Both TBP and HS reacted to 1 tuberculin unit with a skin induration of greater than 5 mm, and blood samples were obtained with informed consent.
Peripheral blood mononuclear cells (PBMC) and peptide (50 μg/ml) in quadruplicate wells were incubated for 7 days. [3H]thymidine incorporation in cultures with antigen, if greater than 2.5-fold that achieved with medium alone (stimulation index, ≥2.5) was considered positive. PBMC from all TBP and HS responded positively to at least one peptide and also to purified protein derivative (results not shown). The total number of peptides recognized showed a significant difference (Fisher exact test) between the groups tested. Considering mean values for M. tuberculosis- and M. leprae-derived peptides, subjects in the HS group recognized a larger number of peptides (71% recognized more than five, and 29% recognized five or fewer) whereas those in the TBP group recognized fewer peptides (28% recognized more than five, and 72% recognized five or fewer). Similar results have been reported with respect to acr/16-kDa peptides in TB (8) and GroES peptides in leprosy (2).
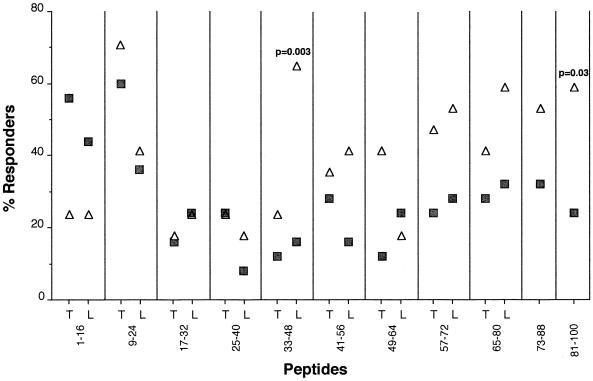
The frequencies of response to the M. tuberculosis (T)- and M. leprae (L)-derived peptides by PBMC from subjects in the HS and TBP groups are presented in Fig. 1. The responsiveness to both the 1-16T and 1-16L peptides was higher in the TBP group than in the HS group. In contrast, responses to the all of the other peptides tested were either similar between the HS and TBP groups (p9-24, p17-32, and p25-40) or diminished in the TBP group (all in the carboxy-terminal section from p41-56). Responsiveness to the T and L series of peptides was similar in most instances, with a few exceptions. The observed lower responsiveness to p33-48T, 49-64L, and p65-80T is difficult to explain, and the possibility that these peptide preparations were of poorer quality cannot be excluded.
FIG. 1.
Frequency of responders to GroES 16-mer peptides with sequences derived from M. tuberculosis (T) or M. leprae (L). PBMC (2 × 105) from the TBP (full squares) and HS (open triangles) groups were cultured with synthetic peptides (50 μg/ml). Responder status represents individuals with stimulation indexes of greater than 2.5. The significance of differences between the TB and HS groups was evaluated by Fisher's exact test.
As our interest in disease association was greater than our interest in fine specificity, we decided to evaluate the patterns of antipeptide responses with respect to the more stimulatory one from each T-L peptide pair. Stimulation indices of >2.5 to 6 were classified as moderately positive, and those of >6.0 were classified as strongly positive. We classified the stimulation with individual peptides into five categories (A to E) on the basis of TBP responsiveness in relation to HS responsiveness (Fig. 2). Category A represents a pronounced increase in the frequency and intensity of stimulation in the TBP group, which was observed only with respect to the N-terminal peptide p1-16. In contrast, no significant difference between the TBP and HS groups was found with either the strongly stimulatory peptide p9-24 (category B) or the weakly stimulatory peptides p17-32 and p25-40 (category C). By contrast, we observed a diminished response of PBMC from TBP to four peptides classified in category D and a marked decrease in responsiveness to the three peptides of category E (p33-48, p57-72, and p81-100). Combining categories D and E thus accounts for the overall narrowing of the peptide repertoire in TBP.
FIG. 2.
Different patterns of recognition of GroES peptides. Stack columns represent the frequency of negative and weakly and strongly positive PBMC proliferation in 17 HS group controls (C) and 25 untreated patients with active TB (T). Response patterns: A, p1-16; B,p9-24; C, p17-32 and p25-40; D, p41-56, p49-64, p65-80, and p73-88; E, p33-48, p57-72, and p81-100 (means for the individual peptides in categories C, D, and E). For each sequence, two peptides containing residues from M. tuberculosis and M. leprae were tested and the higher response from each pair was recorded.
The isolated case of an enhanced proliferative T-cell response of TBP to the amino-terminal peptide p1-16 is thus unusual and needs confirmation in a larger cohort of patients and controls. Notably, the responsiveness to p1-16 was most prominent in the 9 African patients tested, but the mean value was not statistically significantly different from that of the 12 Indian patients tested. We also searched for a possible association with HLA-DR alleles (comprehensively tested as described in reference 2), but considering the small size of the groups and that the patient and control populations were not ethnically matched, we could not draw any meaningful conclusions (results not shown). The enhanced response in active TB to only one peptide (p1-16) represents a special situation, considering that out of the 11 peptides of GroES tested, responses to 7 were diminished and responses to 3 were similar in patients and controls. The reasons for and mechanisms of this striking peptide specificity have not been investigated but could be related to the structural motif in residues 6 to 15, which have a pivotal role in the aggregation of GroES into tetramers or heptamers (7). It is a matter of speculation whether this structural feature plays a role in our empirical observation or whether the association is merely coincidental. The mechanisms may involve an abrogation of the sequestration of CD4 T cells from the blood to the site of disease. Therefore, comparison of T-cell responses between blood and pleural fluid, including their different cytokine profiles (4), would be of interest for further study. Considering the highly conserved structure and common occurrence of GroES, our finding could also be relevant to the immunogenicity and, by inference, to the vaccinal capacity of GroES in other infectious and autoimmune diseases.
Acknowledgments
We thank Adrian Hills for the synthesis of peptides and the clinical staff of Northwick Park Hospital for assistance in diagnosing TB.
REFERENCES
- 1.Barnes, P. F., V. Mehra, B. Rivoire, S. J. Fong, P. J. Brennan, M. S. Voegtline, P. Minden, R. A. Houghten, B. R. Bloom, and R. L. Modlin. 1992. Immunoreactivity of a 10-kDa antigen of Mycobacterium tuberculosis. J. Immunol. 148:1835-1840. [PubMed]
- 2.Chua-Intra, B., S. Peerapakorn, N. Davey, S. Jurcevic, M. Busson, H. M. Vordermeier, C. Pirayavaraporn, and J. Ivanyi. 1998. T-cell recognition of mycobacterial GroES peptides in Thai leprosy patients and contacts. Infect. Immun. 66:4903-4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieli, F., G. Friscia, C. Di Sano, J. Ivanyi, M. Singh, R. Spallek, G. Sireci, L. Titone, and A. Salerno. 1999. Sequestration of T lymphocytes to body fluids in tuberculosis: reversal of anergy following chemotherapy. J. Infect. Dis. 180:225-228. [DOI] [PubMed] [Google Scholar]
- 4.Dieli, F., M. Singh, R. Spallek, A. Romano, L. Titone, G. Sireci, G. Friscia, C. Di Sano, D. Santini, A. Salerno, and J. Ivanyi. 2000. Change of Th0 to Th1 cell-cytokine profile following tuberculosis chemotherapy. Scand. J. Immunol. 52:96-102. [DOI] [PubMed] [Google Scholar]
- 5.Ellner, J. J. 1978. Pleural fluid and peripheral blood lymphocyte function in tuberculosis. Ann. Intern. Med. 89:932-933. [DOI] [PubMed] [Google Scholar]
- 6.Ferrero, R. L., J. M. Thiberge, I. Kansau, N. Wuscher, M. Huerre, and A. Labigne. 1995. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc. Natl. Acad. Sci. USA 92:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fossati, G., P. Lucietto, P. Giuliani, A. R. Coates, S. Harding, H. Colfen, G. Legname, E. Chan, A. Zaliani, and P. Mascagni. 1995. Mycobacterium tuberculosis chaperonin 10 forms stable tetrameric and heptameric structures. Implications for its diverse biological activities. J. Biol. Chem. 270:26159-26167. [DOI] [PubMed] [Google Scholar]
- 8.Friscia, G., H. M. Vordermeier, G. Pasvol, D. P. Harris, C. Moreno, and J. Ivanyi. 1995. Human T cell responses to peptide epitopes of the 16-kD antigen in tuberculosis. Clin. Exp Immunol. 102:53-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalvani, A., P. Nagvenkar, Z. Udwadia, A. A. Pathan, K. A. Wilkinson, J. S. Shastri, K. Ewer, A. V. Hill, A. Mehta, and C. Rodrigues. 2001. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J. Infect. Dis. 183:469-477. [DOI] [PubMed] [Google Scholar]
- 10.Ragno, S., V. R. Winrow, P. Mascagni, P. Lucietto, F. Di Pierro, C. J. Morris, and D. R. Blake. 1996. A synthetic 10-kD heat shock protein (hsp10) from Mycobacterium tuberculosis modulates adjuvant arthritis. Clin. Exp. Immunol. 103:384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vordermeier, H. M., D. P. Harris, G. Friscia, E. Roman, H. M. Surcel, C. Moreno, G. Pasvol, and J. Ivanyi. 1992. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38-kDa antigen in patients with active disease. Eur. J. Immunol. 22:2631-2637. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson, R. J., H. M. Vordermeier, K. A. Wilkinson, A. Sjolund, C. Moreno, G. Pasvol, and J. Ivanyi. 1998. Peptide-specific T cell response to Mycobacterium tuberculosis: clinical spectrum, compartmentalization, and effect of chemotherapy. J. Infect. Dis. 178:760-768. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, M., M. K. Gately, E. Wang, J. Gong, S. F. Wolf, S. Lu, R. L. Modlin, and P. F. Barnes. 1994. Interleukin 12 at the site of disease in tuberculosis. J. Clin. Investig. 93:1733-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]