Abstract
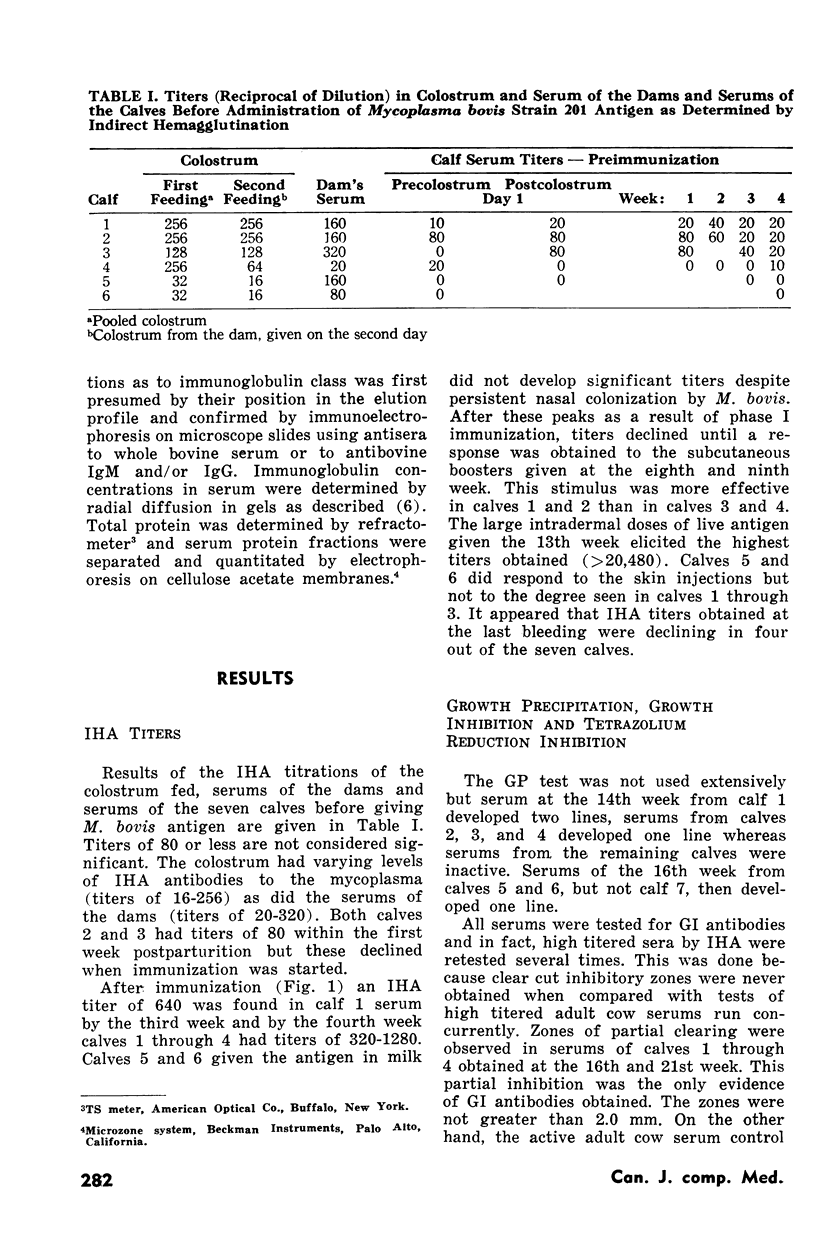
Seven calves seven to 30 days of age were given Mycoplasma bovis antigen by different routes. Immunization was in two phases. The first consisted of single or multiple SC, IV or oral doses of antigen for two to four weeks. The second phase consisted of multiple SC or ID injections given from the eighth to the 19th week. The experiment was terminated at 26 weeks. Antibody titers were followed by indirect hemagglutination, growth inhibition and tetrazolium reduction inhibition. Total serum protein, protein fractions and IgG and IgM concentrations were determined in serums of one calf and the distribution of indirect hemagglutination antibodies in IgG and IgM classes were determined in serums of two of the calves.
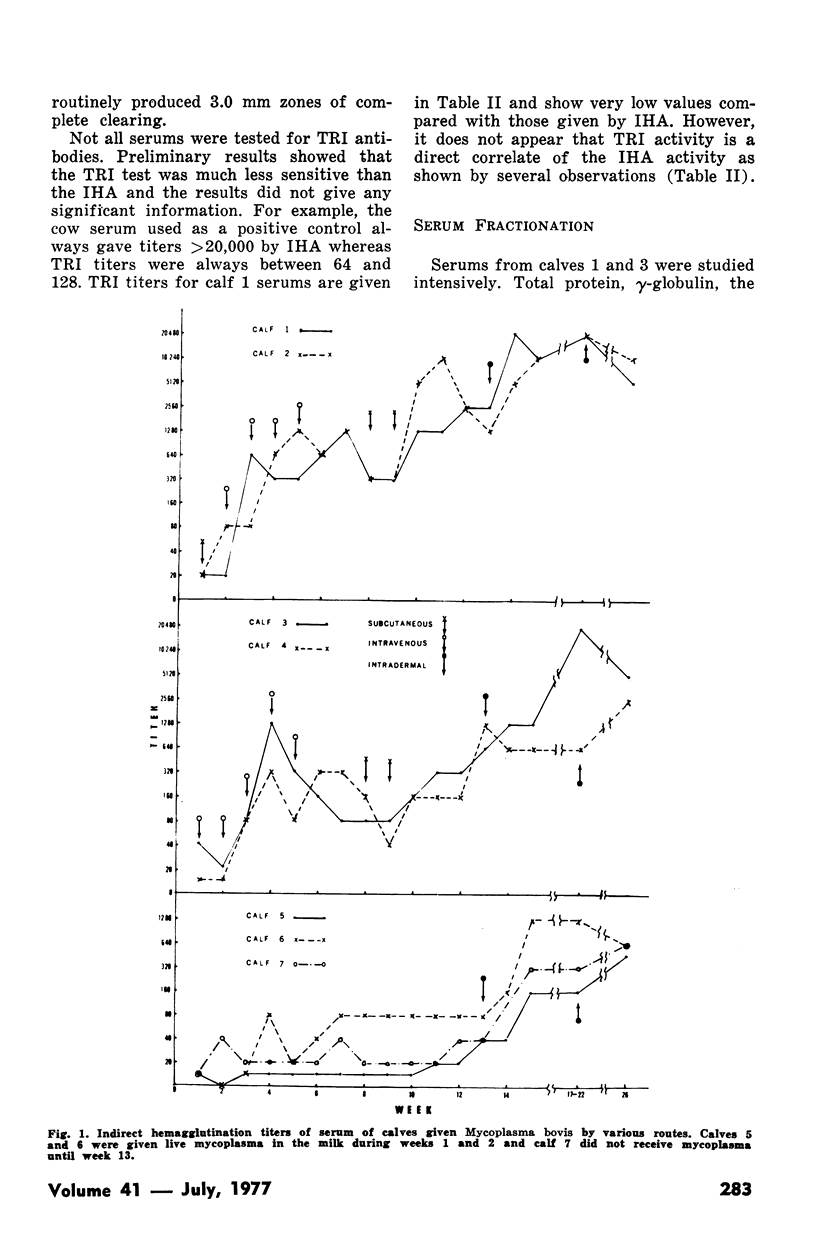
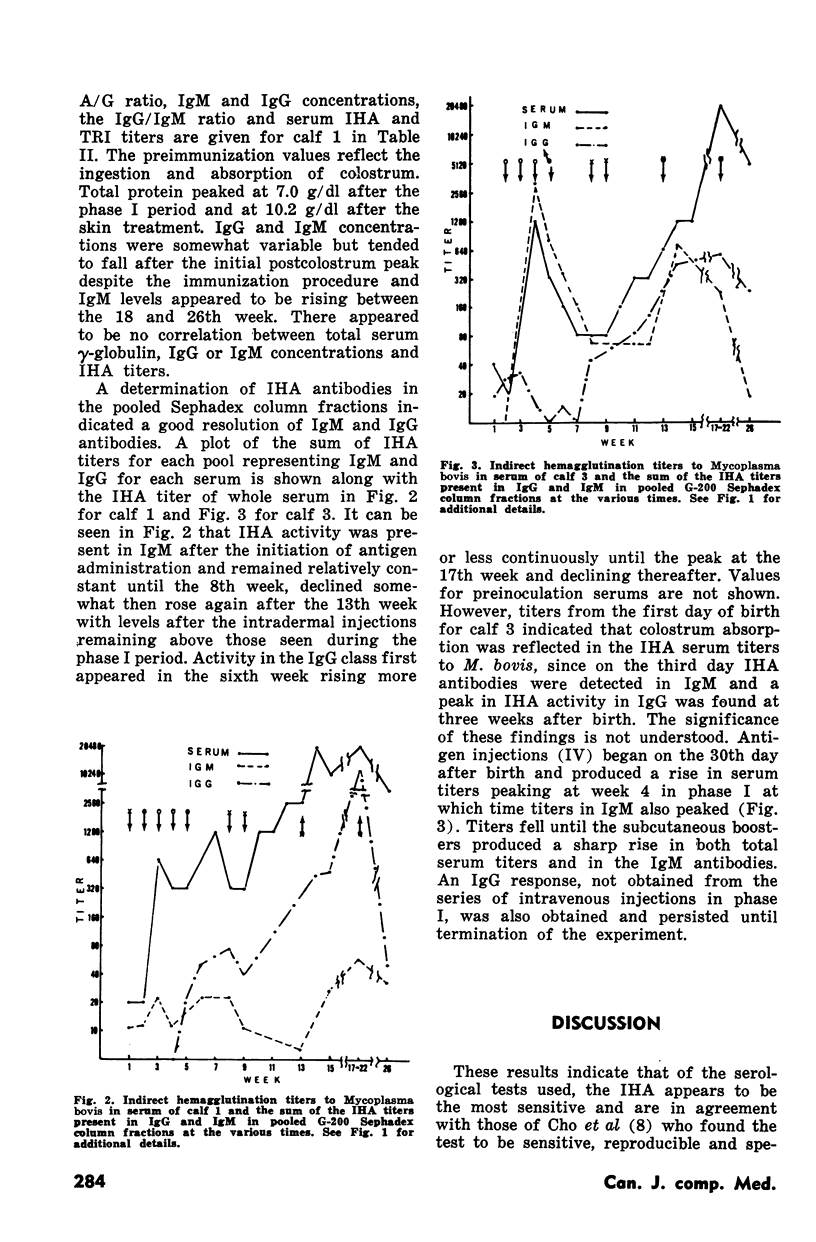
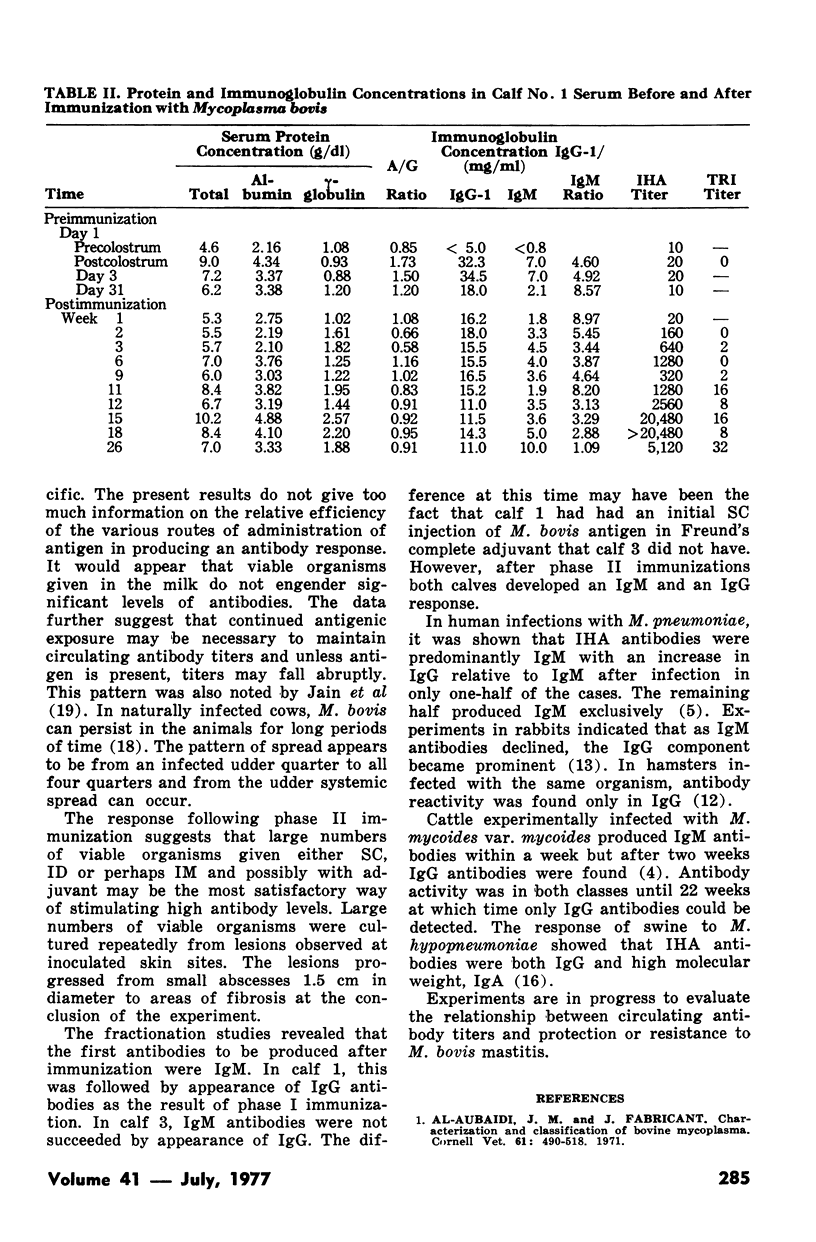
Indirect hemagglutination titers of 1280 and peak titers of >20,480 occurred after the first and second phases respectively. There was no relationship between total serum IgG or IgM concentrations and indirect hemagglutination titers. In one calf given M. bovis antigen in one dose SC and five weekly doses IV in phase I, indirect hemagglutination antibodies appeared in IgM within one week and IgG by four weeks, IgG antibody activity rose steadily until the 17th week but declined at the 26th week, whereas IgM activity after the initial rise dropped at the 13th week but rose even higher as a result of second phase ID injections. Another calf given six weekly IV doses of M. bovis antigen in phase I developed indirect hemagglutination antibodies in IgM peaking at four weeks then declining but with no IgG response. Activity in both IgM and IgG occurred after the second phase. Growth inhibition antibodies were found only on two occasions in one calf serum and tetrazolium reduction inhibition activity when tested never gave titres exceeding 1:32.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas E. J., Jasper D. E. Agar block technique for identification of mycoplasmas by use of fluorescent antibody. Appl Microbiol. 1972 Jun;23(6):1097–1100. doi: 10.1128/am.23.6.1097-1100.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber T. L., Stone S. S., Delay P. D. Antibody in cattle experimentally infected with contagious bovine pleuropneumonia. Infect Immun. 1970 Nov;2(5):617–622. doi: 10.1128/iai.2.5.617-622.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld G. Distribution of antibodies within 19 S and 7 S immunoglobulins following infection with Mycoplasma pneumoniae. J Immunol. 1968 Feb;100(2):338–347. [PubMed] [Google Scholar]
- CLYDE W. A., Jr MYCOPLASMA SPECIES IDENTIFICATION BASED UPON GROWTH INHIBITION BY SPECIFIC ANTISERA. J Immunol. 1964 Jun;92:958–965. [PubMed] [Google Scholar]
- Carroll E. J., Crenshaw G. L. Bactericidal activity of bovine neonatal serums for selected coliform bacteria in relation to total protein and immunoglobulin G1 and immunoglobulin M concentrations. Am J Vet Res. 1976 Apr;37(4):389–394. [PubMed] [Google Scholar]
- Carroll E. J., Rollins M., Jasper D. E. The immune response of rabbits to 3 strains of Mycoplasma agalactiae var. bovis isolated from mastitic bovine udders. Cornell Vet. 1976 Apr;66(2):143–151. [PubMed] [Google Scholar]
- Cho H. J., Ruhnke H. L., Langford E. V. The indirect hemagglutination test for the detection of antibodies in cattle naturally infected mycoplasmas. Can J Comp Med. 1976 Jan;40(1):20–29. [PMC free article] [PubMed] [Google Scholar]
- Erno H., Jurmanová K. Bovine mycoplasmas: serological studies by double immunodiffusion, growth precipitation and growth inhibition. Acta Vet Scand. 1973;14(4):524–537. [PubMed] [Google Scholar]
- Erno H., Jurmanová K., Leach R. H. Bovine mycoplasmas: a serological study by the metabolic inhibition test. Acta Vet Scand. 1973;14(4):511–523. [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A., Jr, Denny F. W. Nature of the immune response to Mycoplasma pneumoniae. J Immunol. 1967 May;98(5):1028–1038. [PubMed] [Google Scholar]
- Foggie A., Etheridge J. R., Erdag O., Arisoy F. Contagious agalactia of sheep and goats studies on live and dead vaccines in lactating sheep. J Comp Pathol. 1971 Jan;81(1):165–172. doi: 10.1016/0021-9975(71)90069-7. [DOI] [PubMed] [Google Scholar]
- Foggie A., Etheridge J. R., Erdag O., Arisoy F. Contagious agalactia of sheep and goats: preliminary studies on vaccines. J Comp Pathol. 1970 Jul;80(3):345–358. doi: 10.1016/0021-9975(70)90065-4. [DOI] [PubMed] [Google Scholar]
- Jain N. C., Jasper D. E., Dellinger J. D. Serologic response of cows to Mycoplasma under experimental and field conditions. Am J Vet Res. 1969 May;30(5):733–742. [PubMed] [Google Scholar]
- Jasper D. E., Al-Aubaidi J. M., Fabricant J. Epidemiologic observations on mycoplasma mastitis. Cornell Vet. 1974 Jul;64(3):407–415. [PubMed] [Google Scholar]
- Jasper D. E., Jain N. C., Brazil L. H. Clinical and laboratory observations on bovine mastitis due to Mycoplasma. J Am Vet Med Assoc. 1966 May 1;148(9):1017–1029. [PubMed] [Google Scholar]
- Kehoe J. M., Norcross N. L., Carmichael L. E. Bovine mycoplasma mastitis. Ann N Y Acad Sci. 1967 Jul 28;143(1):337–344. doi: 10.1111/j.1749-6632.1967.tb27673.x. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Jensen A. Mycoplasma: growth precipitation as a serodiagnostic method. Appl Microbiol. 1972 Mar;23(3):553–558. doi: 10.1128/am.23.3.553-558.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach R. H. Further studies on classification of bovine strains of mycoplasmatales, with proposals for new species, Acholeplasma modicum and Mycoplasma alkalescens. J Gen Microbiol. 1973 Mar;75(1):135–153. doi: 10.1099/00221287-75-1-135. [DOI] [PubMed] [Google Scholar]
- al-Aubaidi J. M., Fabricant J. Characterization and classification of bovine mycoplasma. Cornell Vet. 1971 Jul;61(3):490–518. [PubMed] [Google Scholar]


