Abstract
We have employed a strategy utilizing differential fluorescence induction (DFI) in an effort to identify Staphylococcus aureus genes whose products can be targeted for antimicrobial drug development. DFI allows identification of promoters preferentially active under given growth conditions on the basis of their ability to drive expression of a promoterless green fluorescent protein gene (gfp). A plasmid-based promoter trap library was constructed of 200- to 1,000-bp fragments of S. aureus genomic DNA fused to gfp, and clones with active promoters were isolated under seven different in vitro growth conditions simulating infection. Six thousand two hundred sixty-seven clones with active promoters were screened to identify those that exhibited differential promoter activity. Bioinformatic analysis allowed the identification of 42 unique operons, containing a total of 61 genes, immediately downstream of the differentially active putative promoters. Replacement mutations were generated for most of these operons, and the abilities of the resulting mutants to cause infection were assessed in two different murine infection models. Approximately 40% of the mutants were attenuated in at least one infection model.
Infectious diseases remain the leading cause of death worldwide despite antibiotic therapy. Moreover, this situation is made worse by the emergence of antibiotic-resistant strains. Accordingly, efforts to identify novel drugs to treat them have intensified. In particular, infections caused by the bacterial pathogen Staphylococcus aureus are becoming increasingly difficult to treat due to the rising incidence of multidrug-resistant isolates. Selective pressure and the ease with which microorganisms can exchange genetic information have led to the clinical isolation of methicillin-resistant, vancomycin-resistant derivatives of this organism. S. aureus is a gram-positive bacterium that causes a broad range of severe infections in humans and is a major cause of nosocomial infection. To date, several S. aureus virulence determinants have been identified, including cell-bound and secreted toxins, hemolysins, proteases, and extracellular-matrix-binding proteins, but the mechanisms behind this organism's virulence and its broad repertoire of tissue tropisms remains obscure. Our objectives in the present study were to identify bacterial genes that may be induced during infection and determine whether they are essential for causing infection, with the intention of finding gene product targets for development of antimicrobial drugs.
Traditional methods for identifying microbial functions important for virulence have relied on the characterization of a defined mutant either in vivo or in surrogate tissue culture models of infection. This approach has yielded a great deal of information, but not all of it is relevant to disease. The unraveling of a pathogen's journey through its host requires the analysis of many genes and, consequently, the infection of large numbers of research animals. As a result, many groups have devised strategies that reduce the number of animals used by utilizing an in vivo selection approach.
Such methods include STM (signature-tagged mutagenesis; 9, 19), DNA microarray technology (8, 28, 29), IVET (in vivo expression technology; 14, 15), and DFI (differential fluorescence induction; 26, 27). STM is a negative selection in which a library of individually tagged, random mutants is used to establish an infection. Attenuated mutants that cannot be recovered after infection are identified by their tags. IVET allows identification of promoters active in vivo by selection for cloned promoters driving the expression of a gene whose function is essential for survival within the host or by marking strains carrying cloned promoters that are active. DNA microarray technology allows monitoring of gene expression by global analysis of mRNA levels.
In this study, we extended our previous application of DFI to gram-positive bacterial pathogens (4). By using DFI, the activity of large numbers of promoters can be measured easily and rapidly. DFI employs a library of random chromosomal DNA fragments, in this case from S. aureus, fused to a promoterless reporter gene encoding green fluorescent protein (GFP) and harbored in S. aureus. If a library fragment contains a promoter active under a given condition, i.e., in an in vivo infection environment or under a specific in vitro condition, gfp will be expressed and fluorescent cells can be isolated and analyzed by flow cytometry. Subsequently, the sequence of the promoter-containing fragment is determined and compared to the S. aureus genome sequence, thereby facilitating identification of operons downstream of the promoter. The contribution of genes in these operons to infection is assessed by directed mutagenesis and determination of the virulence of the mutants.
We have explored several in vitro conditions that may simulate an in vivo environment or an individual component of the in vivo environment to which an organism may respond. The conditions chosen were temperature shift of static and aerobic culture, low pH (LpH), divalent cation and iron starvation, and early logarithmic and stationary phase (SP) growth. With DFI, 46 unique inducible promoter clones were identified. Nearly 40% of the strains mutated in downstream operons had attenuated virulence in at least one animal infection model, thus validating this first application of DFI to S. aureus for the identification of potential antimicrobial targets.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains used in this study were S. aureus 8325 (20) and RN4220 (11). All S. aureus strains were grown in brain heart infusion medium (BHI) supplemented, when appropriate, with the following antibiotics at the indicated concentrations, unless otherwise noted: chloramphenicol (CM), 7 μg/ml; erythromycin (EM), 5 μg/ml. The Escherichia coli strains used were DH5α and DH12S (Life Technologies, Gaithersburg, Md.), grown in Luria broth supplemented with ampicillin at 100 μg/ml or EM at 100 μg/ml. Plasmids pSE1 (this work) and pTS1 (a gift from Tim Foster) are E. coli-S. aureus shuttle vectors conferring ampicillin resistance in E. coli and CM resistance in S. aureus. pTS1 is temperature sensitive for replication in S. aureus.
Transformation of S. aureus.
Plasmid DNA was introduced into S. aureus by electroporation. Electrocompetent cells were prepared as previously described (21). Cells were kept ice cold at all times, and plasmid DNA was added to 40 μl of electrocompetent cells in a 1-mm electroporation cuvette and pulsed with an Electrocell manipulator (BTX, San Diego, Calif.) set at 2.5 kV and 129 Ω for strain 8325 and at 1.5 kV and 186 Ω for strain RN4220. After the pulse, cells were resuspended in 500 μl of SMMP (21), incubated at 37°C (or 30°C for temperature-sensitive replication plasmids) for 30 to 60 min and then plated on selective agar medium.
Construction of plasmid pSE1gfpm2 and the S. aureus promoter trap library.
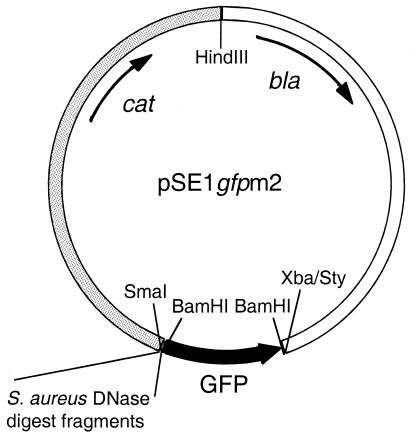
The S. aureus promoter trap vector pSE1 gfpm2 is shown in Fig. 1. Plasmid pSE1 is a shuttle plasmid generated by combining the β-lactamase gene-containing HindIII-StyI fragment of E. coli plasmid pBR322 with HindIII- and XbaI-digested plasmid RN5543 (a gift from R. Novick). RN5543 is a derivative of pC194 (13) with a pUC19 polylinker insert in the HindIII site. A BamHI fragment containing a promoterless gfpmut2 structural gene was generated by PCR with the oligonucleotide primers 5′-ATGCATGCGGATCCTTTAAGAAGGAGATATACATATG and 5′-ATGCATGCGGATCCTTATTTGTATAGTTCATCCATGCC with a template of the gfpmut2 gene (7) and inserted into the BamHI site of pSE1 to generate pSE1 gfpm2. To construct the library, S. aureus strain 8325 chromosomal DNA was prepared (23) and randomly cleaved with DNase in the presence of 10 mM MnCl2 to generate double-stranded breaks (3). The DNA was fractionated by agarose gel electrophoresis, and fragments of 200 to 1,000 bp were isolated. The ends of the fragments were blunted with the Klenow fragment of DNA polymerase, inserted into the SmaI site of pSE1 gfpm2, and introduced by electroporation into E. coli DH12S cells with selection for ampicillin-resistant colonies. Colonies were then pooled, and plasmid DNA was prepared with the QIAGEN (Valencia, Calif.) kit. This plasmid preparation was transformed by electroporation into S. aureus RN4220 while taking care to obtain enough transformants to maintain library complexity. Colonies were again pooled, and plasmid DNA was prepared with the QIAGEN kit and a protocol (provided by the supplier) adapted for S. aureus and subsequently transferred by electroporation into strain 8325. In both cases, S. aureus transformants were selected on BHI-CM agar. Strain 8325 transformant colonies were pooled, suspended in BHI broth, and stored at −80°C.
FIG. 1.
S. aureus promoter trap library. The promoter trap shuttle vector pSE1 gfpm2 is shown. The portion of the vector derived from pBR322 is white, the portion from RN5543 is shaded gray, and gfp is black. The SmaI site into which the chromosomal DNA fragments were inserted is indicated.
Fluorescence-activated cell sorting.
To minimize growth of the library so as to maintain equal representation of clones, aliquots of the library (∼106 CFU) were thawed and used to inoculate cultures (described for each condition below). Cells were grown under the inducing condition for 4 to 6 h to logarithmic phase (LP), collected by centrifugation, and resuspended in phosphate-buffered saline (PBS) with 0.5% bovine serum albumin. Cells were sorted on a FACStar Plus instrument (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) with a 488-nm emission argon laser. Cells were gated by size based on log scale forward and side scatter. Samples were run at low sample differential, resulting in a sample flow rate of 4,000 events/s. A minimum of 30,000 cells that exhibited higher fluorescence than the control strain (strain 8325 carrying vector pSE1 gfpm2) were collected. The sequence of induction and sorting was repeated, and cells were stored at −80°C. In preparation for screening, frozen stocks were diluted and plated on BHI-CM agar for single-colony isolation.
Screening of sorted promoter trap library clones.
For each condition, a protocol was established whereby S. aureus strain 8325 carrying the individual library clones (or the vector control) was subjected to the inducing condition and compared with the same strain grown under noninducing conditions in a high-throughput fashion. To control for any adverse effects a given condition may have on gfp activity, a positive control strain, 8325/p gfp+++, was used. This clone was identified by its high fluorescence in one screen and was found to constitutively express gfp at a high level. In most cases (except where noted), the noninducing condition was aerobic growth in BHI broth-CM, pH 8, at 37°C. To screen for differentially induced clones following sorting, unless described otherwise below, individual colonies from cell sorting were grown overnight under both the inducing and noninducing conditions in deep 96-well blocks (E & K Scientific, Saratoga, Calif.). Each culture was then diluted 1:50 in fresh medium and cultured for 3 h, again under both conditions. Cells were pelleted, washed in PBS, resuspended in PBS-0.5% bovine serum albumin, and analyzed with a FACScalibur instrument (Becton Dickinson) and the cell sorting parameters described above. Approximately 800 sorted clones were screened for each condition, and clones with differential GFP expression were screened again in quadruplicate. Data were analyzed with CellQuest software (Becton Dickinson).
Growth conditions. (i) STS.
For static temperature shift (STS), the inducing condition was growth at 37°C without aeration and the control condition was growth at 30°C without aeration, both in BHI broth-CM.
(ii) Aerobic temperature shift.
Aerobic temperature shift conditions were identical to STS conditions, with the exception that the aerobic cultures were shaken.
(iii) LpH.
For LpH, the inducing condition was static growth at 37°C in BHI-CM broth, pH 5.5 (prepared by adding 1/500 volume of concentrated HCl to BHI broth). The control condition was static growth at 37°C in BHI-CM broth, pH 8. Following the 1:50 dilution of the overnight cultures for screening, the control cultures were grown for 3 h while the induced cultures were grown for 4.5 h so that both reached approximately the same density.
(iv) DCS.
For divalent cation starvation (DCS), the inducing condition was aerobic growth at 37°C in BHI broth-CM depleted of divalent cations by addition of 6% (wt/vol) Chelex 100 (Sigma Chemical Co., St. Louis, Mo.), overnight incubation, and sterile filtration through a 0.2-μm-pore-size membrane prior to use. For screening, colonies were cultured overnight with aeration in BHI-CM broth in deep 96-well blocks at 37°C. Cells were washed twice with Chelex-treated BHI-CM broth and subcultured 1:50 into both BHI-CM broth and Chelex-treated BHI-CM broth and grown for 3 h at 37°C with aeration.
(v) LFe.
For low iron (LFe), the inducing condition was growth at 37°C in RPMI 1640 medium (BioWhittaker, Inc., Walkersville, Md.) in an atmosphere with 7.5% CO2 without aeration and the noninducing condition was growth at 37°C in RPMI 1640 medium supplemented with Fe2(SO4)3 to 20 μM in an atmosphere with 7.5% CO2 without aeration. Following sorting of the library after growth in inducing medium, colonies were inoculated in duplicate into wells containing 300 μl of inducing or noninducing medium and incubated at 37°C without aeration for 18 h in an atmosphere of 7.5% CO2.
LP.
For LP, the inducing condition was aerobic culture in BHI broth-CM at 37°C to mid-LP (optical density at 60 nm [OD600] of 1.2). The noninducing condition was aerobic culture in BHI broth-CM at 37°C to early LP (OD600 of 0.2).
SP.
For SP, the inducing condition was aerobic culture in BHI broth-CM at 37°C to early SP (OD600 of 1.8 to 2.0). The noninducing condition was aerobic culture in BHI broth-CM at 37°C to early LP (OD600 of 0.2).
Bioinformatic analysis of cloned differentially expressed promoters.
PCR products encompassing plasmid library inserts were generated from differentially expressed pSE1 gfpm2 promoter clones by using primers 5′-GTGAAAAGTTCTTCTCCTTTACTC-3′and 5′-GGCTCATATTTGCTTTTTAAG-3′, which anneal on each side of the insert-containing SmaI site. These DNAs were sequenced with an ABI Prism 377 automated DNA sequencer. Results from automated sequence base calls were visually verified (Seqman software; DNAStar Inc.). Verified sequences were compared to the preliminary S. aureus genomic sequence data obtained from The Institute for Genomic Research website at http://www.tigr.org with the basic local alignment search tool (BLASTn) algorithm (1). Nucleotide sequence identity of >90% over the full length of the promoter clones in the correct orientation was used as the confidence threshold for further open reading frame (ORF) analysis of genomic sequences. Genomic sequences homologous to the putative promoter clones and up to 10 kb downstream were used in ORF analysis with the OMIGA software ORF finder tool (Oxford Molecular, Oxford, United Kingdom). ORFs were annotated based on their homology to data available from GenBank as determined by the BLASTp algorithm.
Generation of replacement mutants of S. aureus.
Mutants with entire operons replaced were generated with pTS1, an E. coli-S. aureus shuttle plasmid that is temperature sensitive for replication in S. aureus. For each mutant, a mutagenic plasmid was made by ligating, in a single reaction, three fragments into BamHI- and SacI-digested pTS1. The first fragment is a PCR product of approximately 500 bp with BamHI and KpnI ends that is centered over the start of the first gene of the transcription unit. The second fragment is a PCR product (amplified with primers 5′-ATGCATGCCTCGAGCGCACCAGCGAAAACTGG-3′ and 5′-ATGCATGCGGTACCCAATAATCGCATCCGATTGCAG-3′) having KpnI and XhoI ends that contains the erm gene and upstream DNA from plasmid pE194 (10). The third fragment is a PCR product of approximately 500 bp with XhoI and SacI ends that is centered over the end of the last gene of the transcription unit. After construction, each mutagenic plasmid was shuttled through RN4220, selecting for resistance to EM at 5 μg/ml and 32°C and confirming resistance to CM at 5 μg/ml and then introduced into strain 8325. Strain 8325 with the mutagenic plasmid was cultured in BHI broth at 32°C with EM at 5 μg/ml to an OD600 of 0.5 and then plated to prewarmed BHI agar with EM at 1 μg/ml and incubated at 42°C. Emr Cms colonies marking a double crossover were identified by patching (usually 50 colonies) to BHI agar with appropriate antibiotic supplements and incubation at 42°C. Mutants were confirmed by PCR and/or Southern analyses.
Murine systemic infections.
Animals (female CD-1 mice 7 weeks old) were treated with a single 0.1-ml intraperitoneal (i.p.) dose of cyclophosphamide (250 mg/kg) 4 days prior to infection to generate immune cell depletion. Mice were returned to their cages and given food and water ad libitum. Bacterial inocula consisted of dilutions of overnight cultures of strains to be tested made in BHI and mixed 1:1 with BHI containing 4% debittered brewer's yeast (Red Star Yeast & Products, Milwaukee, Wis.). The approximate bacterial numbers were 101 to 107 CFU/mouse. The inoculum was confirmed by plating appropriate dilutions of bacteria on BHI agar. Mice were injected i.p. with 0.5 ml of the appropriate suspension, returned to their cages, and given food and water ad libitum. Six to 10 mice were infected per dose. Infected animals were checked twice daily to determine morbidity and mortality, and 50% lethal doses (LD50s) were calculated on the basis of the number of animals surviving at each dose at the 48-h time point. For all studies, a positive control (wild-type strain 8325) was included for comparison.
Murine kidney abscess infections.
Bacterial inocula were prepared from cultures grown overnight at 37°C with aeration in BHI broth. Bacteria were collected by centrifugation, washed once with PBS, and again collected by centrifugation. Bacteria were then resuspended in PBS to a concentration of approximately 108 CFU/ml. Mice (CD-1 females 8 weeks old) were injected with 0.2 ml of bacterial inoculum via the tail vein with a 30-gauge needle attached to a 1-ml tuberculin syringe. Five mice were injected per strain. Animals were allowed food and water ad libitum and examined twice daily for signs of distress, e.g., lethargy, rough hair coat, weight loss, and shortness of breath. To enhance infection, ferric ammonium citrate (Sigma Chemical Co.) was diluted in PBS and given via intramuscular injection twice daily at 5 mg/kg for 5 days, starting 1 day prior to infection (2). At 5 days following inoculation, animals were sacrificed by CO2 overdose and both kidneys were aseptically removed and homogenized in 1 ml of sterile PBS in a Stomacher (Labconco, Kansas City, Mo.). Tissue homogenates were serially diluted in PBS and plated on BHI agar plates for enumeration of bacterial colonization. Wild-type strain 8325 was used as a positive control in all experiments. All animal procedures were performed in accordance with the Protein Design Labs, Inc., Institutional Animal Care and Use Committee guidelines.
RESULTS
Promoter trap library construction.
Construction of the S. aureus pSE1 gfpm2 promoter trap library (Fig. 1) in E. coli strain DH12S yielded 380,000 colonies. PCR analysis of 96 individual clones showed that the insert frequency was 40%, resulting in approximately 150,000 recombinants, and insert sizes ranged from 200 to 1,000 bp, with an average of 500 bp (data not shown). Considering insert orientation and a genome size of 2.8 Mbp, the library in DH12S represents greater-than-13-fold coverage of the genome. When the plasmid library was transferred to S. aureus strain RN4220 and then to strain 8325, 500,000 and 2,000,000 colonies were obtained, respectively. Using Poisson's law, the library represents greater-than-10-fold coverage of the genome after transfer to strain 8325.
Choice and establishment of growth conditions.
The seven different growth conditions, referred to as inducing conditions, used in this study (see Materials and Methods) were chosen to mimic certain aspects of in vivo infection, as well as for amenability to the technical requirements of DFI. The parameters varied to generate the conditions were temperature, pH, cell density, gas exchange, and the availability of divalent cations and iron. The effect of each parameter on flow cytometry, growth kinetics, and fluorescence of GFP-expressing cells was determined. In general, the inducing condition was the maximum parameter variation from the uninduced condition (generally, aerobic growth in BHI broth at 37°C) that resulted in little or no adverse effect on the growth or fluorescence of the culture (data not shown).
Identification of differentially induced clones.
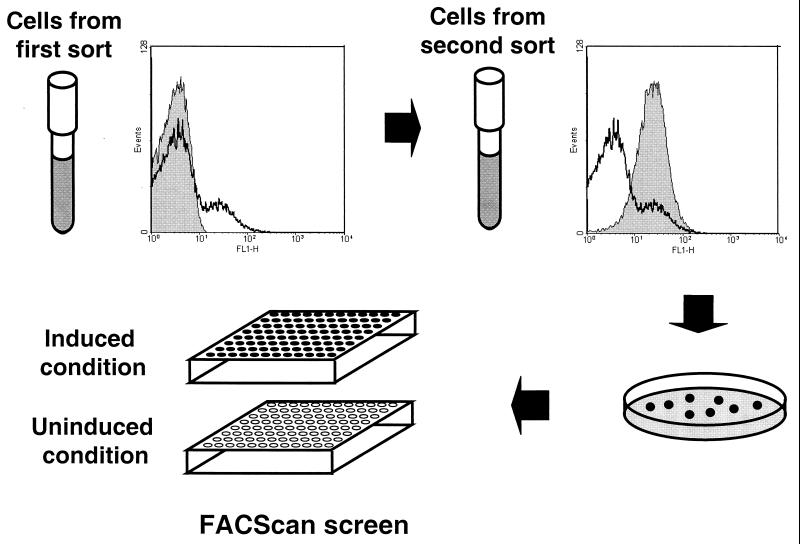
The strategy used to identify differentially induced clones is shown in Fig. 2. For each inducing condition, the promoter trap library in strain 8325 was cultured and subjected to flow cytometry with a gate encompassing the major bacterial population established with a negative control of promoterless, gfp-containing plasmid pSE1 gfpm2 in strain 8325. Cells more fluorescent than the negative control were collected and cultured again under the inducing condition for a second round of cell sorting. Two rounds of sorting generally yielded a completely fluorescent population of cells. The twice-sorted library sample was then plated to obtain single colonies for screening. A total of 6,267 individual fluorescent clones obtained by cell sorting under all seven inducing conditions were screened for differential induction. Of these, 164 showed reproducible GFP induction of twofold or greater. Two representative GFP induction profiles are shown in Fig. 3. Sequence determination of DNA inserts in clones showing reproducible induction yielded the identification of 46 unique sequences. The results of bioinformatic analysis of these clones are shown in Table 1. By sequence analysis, putative operons were identified that start either in, or immediately downstream of, 42 of the unique sequences. Of these, 33 were single-gene operons whereas 9 were polycistronic. Seven sequences were identified in more than one screen, indicating that these promoters respond to more than one environmental signal.
FIG. 2.
Scheme for identification and isolation of clones showing differential gfp expression. Cells carrying the promoter trap library and cultured under an inducing condition were sorted by fluorescence-activated cell sorter in two rounds. The left histogram shows a comparison of the negative control cells with the vector only (shaded gray) with a cell population after a first sorting (black line). The right histogram shows a comparison of cell populations following a first sorting (black line) and a second sorting (shaded gray). Following the second sorting, cells were plated for single-colony isolation. Single colonies were then used to inoculate individual cultures for screening.
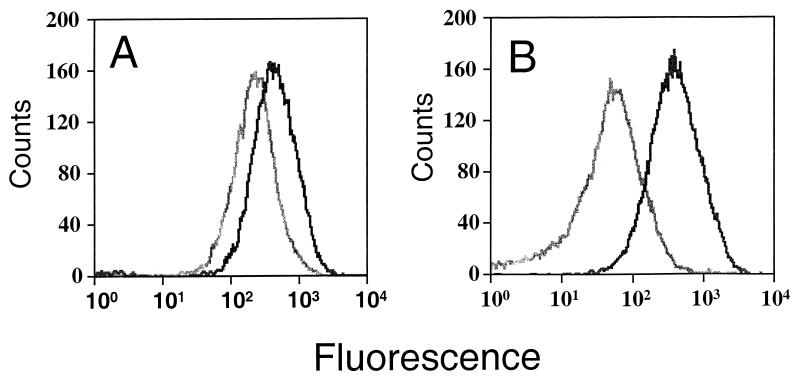
FIG. 3.
Fluorescence of selected library clones showing differential gfp expression. The histograms show the fluorescence of cells cultured under inducing (black) and noninducing (gray) conditions. Cells are strain 8325 carrying pSE1 gfpm2 with a fragment containing the DCS06/STS01 (A) or the LFe 06 (B) promoter fragment upstream of gfp.
TABLE 1.
Summary of promoter and downstream ORF analyses
| Promoter designationa | Induction ratio(s)b | No. of ORFs in operon | ORF description | GenBank accession no. | Mutant made | Systemic infection model LD50 | Attenuation in kidney abscess infection model | |
|---|---|---|---|---|---|---|---|---|
| LFe 01 | 3 | 1 | Probable MFS transporter | BAB41487 | Yes | WTg (1.5 × 102) | None | |
| LFe 02/DCS 05 | 3/2 | 1 | Penicillin amidase homolog | BAB41488 | Yes | WT | None | |
| LFe 03 | 2 | 1 | Fructose-1,6-bisphosphate aldolase homolog | BAB43704 | Yes | WT | 1.0-log attenuation | |
| LFe 04 | 3 | 4 | ORF1, membrane protein; sstA; ORF2, permease, sstB; ORF3, ATP-binding protein, sstC; ORF4, lipoprotein, sstD | BAB41921 | Yes | WT | None | |
| LFe 05/LpH 08 | 3/3 | 1 | Ferrichrome-binding protein-fhuD | BAB43376 | Yes | WT | None | |
| LFe 06 | 6 | 1 | Cell surface protein | BAB42226 | Noc | |||
| LpH 01 | 2 | 1 | Hypothetical protein | BAB42940 | Yes | 4.5 × 103 | None | |
| LpH 02 | 12 | 2 | ORF1, alpha-acetolactate synthase; ORF2, alpha-acetolactate decarboxylase | BAB43299 | Yes | WT | None | |
| LpH 03 | 7 | 7 | ORF1, urease gamma subunit; ORF2, urease beta subunit; ORF3, urease alpha subunit; ORF4, urease accessory protein, ureE; ORF5, urease accessory protein, ureF; ORF6, urease accessory protein, ureG; ORF7, urease accessory protein, ureD | BAB43380 | Yes | 1 × 103 | None | |
| LpH 04 | 4 | 1 | Fibrinogen-binding protein A | BAB41975 | Yes | WT | None | |
| LpH 05 | 6 | 1 | Glutamyl endopeptidase | BAB42146 | Yes | WT | 0.5-log attenuation | |
| LpH 06/DCS 09 | 4/3 | 1 | Peptidoglycan hydrolase | BAB41489 | Yes | 2.7 × 104 | 0.5-log attenuation | |
| LpH 07 | 3 | 1 | Flavohemoprotein homolog | BAB41454 | Yes | WT | None | |
| LpH 09 | 3 | 1 | mapW homolog | BAB42081 | Yes | WT | None | |
| LpH 10 | 8 | 1 | Copper-transporting ATPase, copA | BAB43648 | Yes | WT | None | |
| DCS 01 | 2 | 2 | ORF1, deoxyribose-phosphate aldolase; ORF2, phosphodeoxyribomutase, drm | BAB41353 | Yes | WT | 1.0-log attenuation | |
| DCS 02/ATS 05 | 5/2 | 1 | Coenzyme A disulfide reductase | BAB42070 | Yes | WT | 1.5-log attenuation | |
| DCS 03 | 2 | 1 | Molybdenum cofactor biosynthesis protein, moaC | BAB43366 | Yes | WT | 2.0-log attenuation | |
| DCS 04 | 2 | 1 | Esterase (Acinetobacter lwoffii) | BAB43626 | Yes | WT | None | |
| DCS 06/STS 01 | 2/2 | 1 | Elongation factor Tu | BAB41737 | Nod | |||
| DCS 07 | 2 | No ORF | ||||||
| DCS 08 | 2 | 4 | ORF1, CDP-ribitol pyrophosphorylase; ORF2, d-xylulose reductase; ORF3, hypothetical protein 3; ORF4, putative glycosyl transferase | BAB41469 | Nod | |||
| DCS 10/LP 01 | 2/3 | 1 | Glycerol ester hydrolase | BAB41533 | Yes | WT | None | |
| DCS 11 | 3 | 1 | Cell wall enzyme ebsB homolog | BAB42526 | Yes | WT | None | |
| DCS 12 | 6 | 2 | ORF1, formate dehydrogenase homolog, yigC; ORF2, conserved hypothetical protein, yjgD | BAB43401 | Nod | |||
| DCS 13 | 6 | 1 | Glycerophosphoryl diester phosphodiesterase homolog | BAB42217 | Yes | WT | None | |
| DCS 14 | 6 | 1 | Beta-hemolysin | BAB4309 | Yes | WT | None | |
| DCS 15 | 5 | 2 | ORF1, exotoxin 15; ORF2, hypothetical protein | BAB41622 | Yes | WT | 1.5-log attenuation | |
| DCS 16 | 5 | 1 | Teichoic acid translocation ATP-binding protein-tagH | BAB41825 | Nod | |||
| DCS 17 | 2 | 1 | l-Lactate dehydrogenase | BAB43700 | Yes | WT | None | |
| STS 02 | 3 | 2 | ORF1, superoxide-inducible protein 7; ORF2, amidotransferase | BAB41707 | Yes | WT | 2.0-log attenuation | |
| STS 03 | 3 | No ORF | ||||||
| STS 04 | 2 | 1 | Superoxide dismutase | BAB41348 | Yes | WT | None | |
| STS 05/ATS 04 | 2/2 | 2 | ORF1, hypothetical protein, yqeU; ORF2, hypothetical protein, yqeV | BAB42669 | Noe | |||
| STS 06 | 2 | 1 | Hypothetical protein, ypsC | BAB42537 | Yes | WT | None | |
| STS 07 | 3 | 1 | Methicillin resistance protein | BAB42845 | Nod | |||
| STS 08 | 2 | 1 | UDP-N-acetylglucosamine pyrophosphorylase homolog | BAB41687 | Nod | |||
| STS 09 | 2 | 1 | 50S ribosomal protein L33 | BAB42428 | Noe | |||
| ATS 01 | 2 | 1 | Asparaginyl-tRNA synthetase | BAB42547 | Yes | WT | 1.0-log attenuation | |
| ATS 02 | 2 | No ORF | ||||||
| ATS 03 | 3 | 1 | Unknown conserved protein | BAB42720 | Yes | WT | 0.5-log attenuation | |
| LP 02 | 3 | 1 | Putative integral membrane protein | BAB41396 | Yes | 9.2 × 102 | None | |
| LP 03 | 3 | 1 | Dihydroorotate dehydrogenase homolog | BAB43680 | Yes | WT | None | |
| SP 01 | 6 | 1 | NAD-specific glutamate dehydrogenase | BAB42058 | Yes | WT | None | |
| SP 02 | 5 | No ORF | ||||||
| SP 03 | 6 | 3 | ORF1, putative ribose transporter, rbsU homolog; ORF2, ribose permease, rbsD; ORF3, ribokinase, rbsK | BAB41484 | Yesf | WT | None |
The promoter designation is derived from the inducing condition in which the promoter was isolated: LFe for low iron, LpH for low pH, DCS for divalent cation starvation, STS for static temperature shift, ATS for aerobic temperature shift, LP for logarithmic phase, and SP for stationary phase. Promoters with two designations were isolated in both conditions.
Average of quadruplicate determinations. Where two promoters are listed, induction ratios for both are shown.
Not attempted.
Unable to make mutant, putative in vitro essential gene(s).
Unable to make mutagenic plasmid.
Mutation directed to ORF1 only.
WT, wild type.
Generation of replacement mutants.
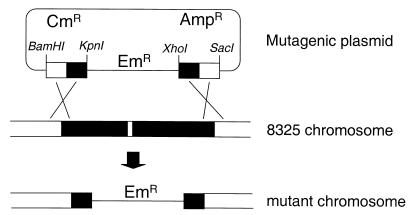
Of the 42 operons identified downstream of inducible promoters, we had sufficient genomic sequence data to attempt to make replacement mutations in 41 of them (Table 1). Of these, 33 mutants were successfully generated as shown schematically in Fig. 4. The eight mutants we were unable to make fell into two categories. For two, we were unable to construct the mutagenic plasmids, usually due to failure to obtain the correct PCR products. For the other six, mutagenic plasmids were constructed but we were unable to generate bona fide mutants despite extensive screening for the Emr Cms phenotype (see Materials and Methods). This suggests, but does not prove, that a gene(s) in each of these six operons is essential for growth in vitro. Genes in two of these operons include that encoding the essential protein elongation factor Tu and that encoding TagH, which has been suggested to be essential in B. subtilis (12).
FIG. 4.
Generation of S. aureus replacement mutants. In this example, mutants of a bicistronic operon are made by selecting for integration of a mutagenic plasmid into the chromosome. The double-crossover product replacement mutant is identified by screening for an Emr Cms phenotype (see Materials and Methods).
In vivo characterization of replacement mutants.
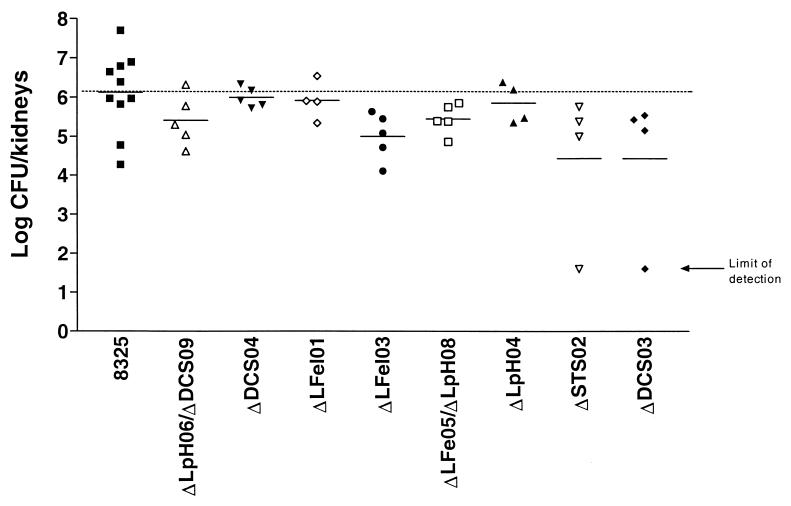
Replacement mutants were compared to wild-type strain 8325 in kidney abscess and systemic-infection models. The kidney abscess infection model measures bacterial colonization based on the number of bacteria recovered from kidney homogenates, while the LD50 is a measure of bacterial virulence in the systemic-infection model. Thirty-three mutants were individually used to infect mice, and the results are shown in Table 1. Wild-type strain 8325 consistently exhibited an LD50 of 150 organisms in the systemic-infection model, and four mutants showed at least sixfold attenuation. In the kidney abscess infection model, 10 of the mutants examined showed threefold or greater attenuation. A representative experiment demonstrating kidney colonization is shown in Fig. 5. Only one of the four mutants with an increased LD50 in the systemic-infection model was also attenuated in the kidney abscess infection model. To determine whether virulence attenuation is associated with the in vitro growth rate, the doubling times of the 13 mutants with attenuated virulence and strain 8325 in static BHI culture at 37°C were compared. All 14 strains had equivalent LP doubling times of approximately 55 min. However, two of the mutants, DCS03 and STS02, grew to an approximately 20% lower final density than the other 12 strains (data not shown).
FIG. 5.
Kidney abscess infections with selected S. aureus mutants. Infections were performed as described in Materials and Methods. Each data point represents the logarithm of the number of bacterial CFU recovered from the kidneys of a single mouse at 5 days postinfection. The horizontal bars represent the geometric means. The limit of detection in the experiment is 40 CFU.
DISCUSSION
We have established a high-throughput method of using DFI to identify S. aureus genes induced under culture conditions likely to be encountered in vivo and demonstrated that many of these genes are required for full infectivity. The bacterial culture conditions selected include some shown previously to induce the expression of virulence determinants in both gram-negative and gram-positive bacterial species (5, 17, 24, 25). In this way, we have attempted to dissect the infection environment encountered by a pathogen as a means of identifying bacterial virulence determinants that are suitable as novel antimicrobial targets. The premises of this study were that virulence genes are likely to be induced in an infection and that some virulence genes do not affect in vitro growth. When the genes identified in our DFI screens were mutated, 39% of the mutant strains were attenuated in at least one of the animal infection models, demonstrating the validity of this approach.
While some of the mutants showed a modest effect of 10-fold or less, six mutants showed substantial attenuation in an infection model: LpH01, LpH06, DCS02, DCS03, DCS015, and STS02 ORF mutants were each attenuated 30-fold or more compared to the wild-type strain. ORFs of LpH01 and STS02 and ORF2 of DCS015 all encode hypothetical proteins with potentially novel functions in virulence. The LpH06 ORF encodes lytM, whose product is involved in autolysis, a process known to affect the virulence of Streptococcus pneumoniae. The DCS02 ORF encodes coenzyme A disulfide reductase, and the DCS03 ORF encodes molybdenum cofactor biosynthesis protein C; the effects of a mutation in either function could potentially be multiple, resulting in an attenuated phenotype. DCS015 ORF1 encodes a likely virulence determinant, an exotoxin homolog.
DFI is an attractive approach for several reasons. Analysis is done with intact wild-type bacteria, in which promoter activity can be evaluated in the context of the target bacterium's entire genetic repertoire and its growth environment. Differentially activated promoters can be identified regardless of their basal activity. The reporter gene, gfpmut2, is suitable for use in flow cytometry and is not toxic in S. aureus (6; our unpublished data), thereby eliminating bias against highly active promoters. Furthermore, DFI does not disrupt chromosomal copies of virulence factors and does not exclude identification of genes essential for growth in culture. Lastly, it easily allows analysis of the expression of genes of interest that are identified.
By using DFI, we have identified promoters of genes whose products are involved in a broad range of cellular functions: cell wall biosynthesis, nucleic acid biosynthesis, and translation, among others. Genes putatively essential both in vivo and in vitro were identified, as well as several genes encoding proteins of unknown function. These unknown-function genes are potentially the most interesting in terms of new target development. Interestingly, there appears to be very little overlap between the genes shown in this work to be important for infection and genes affecting infection that have been identified in studies in which other techniques were used (14, 19). The induced genes identified here by DFI include those for beta-hemolysin, urease, and fibrinogen-binding protein, which have been previously shown by others to be important for S. aureus pathogenesis (16, 18, 22). However, of these, only the urease mutant showed attenuation of virulence in this study. The lack of attenuation of the other two mutants may be a function of the type of infection model used, including the particular bacterial or mouse strains.
It is noteworthy that several genes were identified in more than one screen, peptidoglycan hydrolase, elongation factor Tu, coenzyme A disulfide reductase, and a hypothetical protein. These gene products may be involved in the overall response of S. aureus to stress or may indicate common pathways in different environmental sensing strategies. The hypothetical protein YqeU was identified in both the aerobic and STS screens; therefore, it may be that this is an as yet uncharacterized heat shock protein. Thus, a potential application of DFI can be to address the function of unknown induced genes based on their induction profiles. Undoubtedly, further work is required before a function can be assigned to a gene such as yqeU, but some understanding of its expression could facilitate the direction of future experiments.
We were unable to identify operons downstream of four clones with induced promoter activity. This may be due to gratuitous promoter activity, incomplete annotation of the S. aureus genome sequence, or perhaps an inducible promoter regulating an RNA molecule of unknown function.
We chose two different murine infection models to analyze these mutants, systemic and kidney abscess infections. Both models measure the overall ability of the organism to survive and replicate within the host, with the kidney abscess infection model perhaps being more stringent in that it requires the organism to survive in the bloodstream, colonize the kidneys, and maintain infection for a longer time. Systemic infection is a shorter-term lethality model in which bacteria are injected i.p. and cause bacteremia and grow at several organ sites. We found that 13 of the induced clones control genes that play a role in virulence, as mutants were attenuated in at least one model. Because the models we used are quantitative, different levels of attenuation among mutants could be detected, indicating either different levels of importance for a given infection or differential timing of activity. Further studies of the in vivo activity of specific promoters are necessary to address this issue. That we obtained mutants with different levels of attenuation in either model indicates the complexity of the virulence process under these conditions.
Mechanisms by which bacterial pathogens cause disease are under intensive investigation, due in no small part to the drive to develop novel antibiotics. Whereas tissue culture and other surrogate models have yielded some understanding of the molecular mechanisms used by bacteria to establish and maintain infections, real progress has been stymied by the difficulties imposed by limitations of in vivo experimentation. Because animal infection models do not lend themselves to high-throughput approaches, investigators have developed strategies such as STM that allow analysis of large pools of mutants at once and IVET, DFI, and DNA microarray technologies to study gene expression in vitro or in vivo. Each of these approaches has advantages, as well as limitations. That the set of virulence factors identified for a given pathogen often differs with the type of identification approach used shows that these approaches should be considered complementary and are best used collectively to further our understanding of pathogenic mechanisms.
Acknowledgments
We thank Stanley Falkow and Rafael Valdivia for advice; Patricia Lekas, Dixie Polakoff, Cedric Wiesner, Christine Johnson, and Jon Yang for technical assistance; and Richard Novick, Tim Foster, and Brendan Cormack for advice and gifts of plasmids and strains.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed]
- 2.Ang, O., M. Gungor, F. Aricioglu, D. Inanc, H. Sagduyu, V. Uysal, and M. Kucuker. 1990. The effect of parenteral iron administration on the development of Staphylococcus aureus-induced experimental pyelonephritis in rats. Int. J. Exp. Pathol. 71:507-511. [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1998. Current methods in molecular biology, vol. 1. John Wiley & Sons, Inc., New York, N.Y.
- 4.Bartilson, M., A. Marra, J. Christine, J. S. Asundi, W. P. Schneider, and A. E. Hromockyj. 2000. Differential fluorescence induction reveals Streptococcus pneumoniae loci regulated by competence peptide. Mol. Microbiol. 39:126-135. [DOI] [PubMed] [Google Scholar]
- 5.Burne, R. A. 1998. Oral streptococci…products of their environment. J. Dent. Res. 77:445-452. [DOI] [PubMed] [Google Scholar]
- 6.Cheung, A. L., C. C. Nast, and A. S. Bayer. 1998. Selective activation of sar promoters with the use of green fluorescent protein transcriptional fusions as the detection system in the rabbit endocarditis model. Infect. Immun. 66:5988-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 8.de Saizieu, A., C. Gardes, N. Flint, C. Wagner, M. Kamber, T. J. Mitchell, W. Keck, K. E. Amrein, and R. Lange. 2000. Microarray-based identification of a novel Streptococcus pneumoniae regulon controlled by an autoinduced peptide. J. Bacteriol. 182:4696-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hensel, M., J. E. Shea, C. Gleeson, M. D. Jones, E. Dalton, and D. W. Holden. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400-403. [DOI] [PubMed] [Google Scholar]
- 10.Horinouchi, S., and B. Weisblum. 1982. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J. Bacteriol. 150:804-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 12.Lazarevic, V., and D. Karamata. 1995. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol. Microbiol. 16:345-355. [DOI] [PubMed] [Google Scholar]
- 13.Lofdahl, S., J. E. Sjostrom, and L. Philipson. 1978. Characterization of small plasmids from Staphylococcus aureus. Gene 3:145-159. [PubMed] [Google Scholar]
- 14.Lowe, A. M., D. T. Beattie, and R. L. Deresiewicz. 1998. Identification of novel staphylococcal virulence genes by in vivo expression technology. Mol. Microbiol. 27:967-976. [DOI] [PubMed] [Google Scholar]
- 15.Mahan, M. J., J. M. Slauch, and J. J. Mekalanos. 1993. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259:686-688. [DOI] [PubMed] [Google Scholar]
- 16.Marshall, M. J., G. A. Bohach, and D. F. Boehm. 2000. Characterization of Staphylococcus aureus beta-toxin induced leukotoxicity. J. Nat. Toxins 9:125-138. [PubMed] [Google Scholar]
- 17.Maurelli, A. T., A. E. Hromockyj, and M. L. Bernardini. 1992. Environmental regulation of shigella virulence. Curr. Top. Microbiol. Immunol. 180:95-116. [DOI] [PubMed] [Google Scholar]
- 18.McLean, R. J., J. C. Nickel, K. J. Cheng, and J. W. Costerton. 1988. The ecology and pathogenicity of urease-producing bacteria in the urinary tract. Crit. Rev. Microbiol. 16:37-79. [DOI] [PubMed] [Google Scholar]
- 19.Mei, J. M., F. Nourbakhsh, C. W. Ford, and D. W. Holden. 1997. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol. Microbiol. 26:399-407. [DOI] [PubMed] [Google Scholar]
- 20.Novick, R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 21.Novick, R. P. 1991. Genetic systems in staphylococci. Methods Enzymol. 204:587-636. [DOI] [PubMed] [Google Scholar]
- 22.Palma, M., S. Nozohoor, T. Schennings, A. Heimdahl, and J. I. Flock. 1996. Lack of the extracellular 19-kilodalton fibrinogen-binding protein from Staphylococcus aureus decreases virulence in experimental wound infection. Infect. Immun. 64:5284-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156.
- 24.Skorupski, K., and R. K. Taylor. 1997. Control of the toxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol. Microbiol. 25:1003-1009. [DOI] [PubMed] [Google Scholar]
- 25.Smith, H., H. Buijs, R. R. de Vries, H. Wisselink, N. Stockhofe-Zurwieden, and M. Smits. 2001. Environmentally regulated genes of Streptococcus suis: identification by the use of iron-restricted conditions in vitro and by experimental infection of piglets. Microbiology 147:271-280. [DOI] [PubMed] [Google Scholar]
- 26.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 27.Valdivia, R. H., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 28.Wei, Y., J. M. Lee, C. Richmond, F. R. Blattner, J. A. Rafalski, and R. A. LaRossa. 2001. High-density microarray-mediated gene expression profiling of Escherichia coli. J. Bacteriol. 183:545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye, R. W., W. Tao, L. Bedzyk, T. Young, M. Chen, and L. Li. 2000. Global gene expression profiles of Bacillus subtilis grown under anaerobic conditions. J. Bacteriol. 182:4458-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]