Abstract
The airway epithelium represents a primary site for the entry of pathogenic bacteria into the lungs. It has been suggested for many respiratory pathogens, including Klebsiella pneumoniae, that adhesion and invasion of the lung epithelial cells is an early stage of the pneumonia process. We observed that poorly encapsulated K. pneumoniae clinical isolates and an isogenic unencapsulated mutant invaded lung epithelial cells more efficiently than highly encapsulated strains independent of the K type. By contrast, the unencapsulated mutant was completely avirulent in a mouse model of pneumonia, unlike the wild-type strain, which produced pneumonia and systemic infection. Furthermore, the unencapsulated mutant bound more epithelially produced complement component C3 than the wild-type strain. Our results show that lung epithelial cells play a key role as a host defense mechanism against K. pneumoniae pneumonia, using two different strategies: (i) ingestion and control of the microorganisms and (ii) opsonization of the microorganisms. Capsular polysaccharide avoids both mechanisms and enhances the virulence of K. pneumoniae.
Klebsiella pneumoniae is an opportunistic pathogen that frequently causes nosocomial infections, mainly in immunocompromised patients. K. pneumoniae infections range from mild urinary infections to severe bacteremia and pneumonia with a high rate of mortality and morbidity (5, 12, 16). Pulmonary infections due to K. pneumoniae are often characterized by a rapid progressive clinical course complicated by lung abscesses and multilobular involvement which leaves little time to establish an effective antibiotic treatment.
Experimental evidence suggests that capsular polysaccharide (CPS) may be important for the establishment of pneumonia caused by K. pneumoniae, since active immunization with purified CPS protected rats against lethal experimentally induced Klebsiella pneumonia (7). Furthermore, in a more recent study, monoclonal antibodies against Klebsiella CPS reduced the severity and hematogenic spread of K. pneumoniae pneumonia (13). Although these results are in agreement with the clinical observation that the encapsulated strains are usually highly virulent, they contradict recent studies showing that unencapsulated strains invaded lung epithelial cells more efficiently than encapsulated strains (10).
Entry into host cells is critical for the survival of bacterial pathogens that replicate in an intracellular milieu. For organisms that replicate at extracellular sites, as occurs with K. pneumoniae, the significance of bacterial entry into host cells is less well defined. It has been suggested, but not demonstrated, that K. pneumoniae entry into epithelial cells may produce a reservoir or a persistent infection in a site where the bacteria are protected from the actions of antibiotics and the immune system (25).
The goal of this study was to investigate the role of lung epithelial cells in K. pneumoniae pneumonia and to determine whether the interaction between the bacteria and the epithelial cells contributes to the pathogenesis of K. pneumoniae pneumonia.
MATERIALS AND METHODS
Bacteria, plasmids, and media.
K. pneumoniae strain 52145 is a clinical isolate (serotypes O1 and K2) that has been described previously (20). Strain 52145R is a spontaneous mutant resistant to rifampin and derived from strain 52145. Other K. pneumoniae clinical isolates with different K types included in this study are listed in Table 1. Escherichia coli strain S17-1 λpir (8) harboring plasmid pUTmini-Tn5 Km1, a conjugative plasmid containing the mini-Tn 5 Km1 transposon (8), was used to generate mutants derived from K. pneumoniae 52145. Bacterial cells were grown in Luria Bertani (LB) broth at 37°C with shaking or solidified with 1.5% agar. When necessary, media were supplemented with the following antibiotics at the indicated concentrations: kanamycin, 50 μg/ml; rifampin, 40 μg/ml.
TABLE 1.
Internalization of K. pneumoniae clinical isolates by human lung epithelial cells (A549)
| Strain | K type | CPS (fg/CFU) | Intracellular bacteria (mean CFU)a |
|---|---|---|---|
| 52145 | K2 | 400 | 775 |
| 2330 | K2 | 26 | 4,575 |
| 1555 | K35 | 200 | 442 |
| 1850 | K35 | <10 | 1,360 |
| 0352 | K47 | 200 | 603 |
| 2073 | K47 | 13 | 1,268 |
Mean of triplicate determinations.
Determination of CPS production.
CPS production was quantified by a competitive enzyme-linked immunosorbent assay (ELISA). CPS containing extracts from 4-ml overnight cultures was obtained by a phenol-water minipreparation method (2). Phenol was eliminated by chloroform extraction and ethanol precipitation. Precipitates containing CPS extracts were resuspended in distilled water and used in the inhibition step in the competitive ELISA.
For the ELISA, plates were coated with 1 μg of either purified CPS type 2, type 47, or type 35 per well. After a blocking step with phosphate-buffered saline containing 1% bovine serum albumine (PBS-BSA), the plates were incubated with serial dilutions of CPS extracts and antiserum against CPS type 2, type 47, or type 35. Antibodies bound to the CPS-coated plates were detected with alkaline phosphatase-labeled goat anti-rabbit immunoglobulin G and developed with p-nitrophenyl phosphate. Incubations with antisera diluted in 1% PBS-BSA were carried out at 37°C for 1 h and were always followed by PBS washes. Known amounts of CPS purified by the method of Wilkinson and Sutherland (31) were used to construct a standard curve.
DNA manipulation.
Plasmid DNA was isolated using the Wizard Miniprep kit (Promega) according to the manufacturer's instructions. Isolation of genomic DNA and transformation were carried out by standard techniques (4). T4 DNA ligase and restriction endonucleases (Pharmacia) were used following the manufacturer's recommendations. DNA fragments prepared by restriction enzyme digestion were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. DNA sequencing was performed using an automated sequencing apparatus (Applied Biosystems).
Generation of capsule mutant derived from K. pneumoniae strain 52145R.
To derive a capsular mutant from K. pneumoniae strain 52145R, we conjugated the strain with E. coli strain S17-1 λpir carrying plasmid pUTmini-Tn5 Km1. A set of 900 mutants whose genomes were interrupted by a single copy of the transposon were screened by their resistance to bacteriophage φ2, a bacteriophage specific for type 2 CPS. One mutant resistant to φ2, designated K. pneumoniae 52K0, was selected for further characterization.
Determination of LPS and outer membrane protein (OMP) expression.
Lipopolysaccharide (LPS) O side chain expression was analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. LPS was obtained by the method of Hitchcock and Brown (14) and run in 15% polyacrylamide resolving gel, using 25 mM Tris-0.1% SDS-0.19 M glycine (pH 8.3) as the running buffer, and silver stained as described by Tsai and Frasch (30).
OMPs were isolated as sodium lauryl sarcosinate-insoluble material (11) and analyzed by SDS-polyacrylamide gel electrophoresis and Coomassie blue staining.
Adhesion and internalization assays.
Monolayers of human lung carcinoma cells (A549; ATCC CCL185) derived from type II pneumocytes were grown to confluence in RPMI medium supplemented with 10% fetal bovine serum plus penicillin and streptomycin in 24-well tissue culture plates (≈5 × 105 cells per well). For the adhesion assays, cells were washed three times with PBS and incubated for 1 h at 37°C in 5% CO2 with a suspension of 108 bacterial cells in RPMI medium. After incubation, the wells were washed three times with PBS, and adherent bacteria were released by the addition of 0.5% Triton X-100 (Sigma) and quantified by plating appropiate dilutions on LB agar plates. In the internalization assays, after the incubation of the epithelial cells with the bacterial suspension, the wells were washed with PBS and then incubated with fresh RPMI medium containing gentamicin (100 μg/ml) to kill extracellular bacteria. At different times after the initial inoculation, an aliquot of the medium was plated to confirm killing of extracellular bacteria, and the gentamicin-containing medium was washed again. The epithelial cells were lysed, and intracellular bacteria were quantified as described above. Cytotoxicity was estimated by assessing the abilities of epithelial cells from replicate wells to exclude trypan blue.
Murine model of pneumonia.
Male (16- to 20-g) ICR:CD-1 mice (Harlan Ibérica) were anesthetized and intubated intratracheally using a blunt-ended feeding needle. Approximately 106 CFU of K. pneumoniae from an early-log-phase broth culture were suspended in 50 μl of Ringer's solution and inoculated through the needle. The animals were observed daily, and bacteremia was assessed on days 2, 4, and 6 by culturing 10 to 30 μl of tail vein blood on LB agar plates. The lungs and spleens from animals surviving at the end of the experiment (7 days) or animals that died during the experiment were immediately removed under aseptic conditions and homogenized for quantitative bacterial cultures.
Microscopy.
Blocks of lung tissue were dissected from representative animals. The tissues were fixed in 10 volumes of 10% neutral buffered formalin, embedded in paraffin, and sectioned at 4 to 6μm. The tissue sections were stained with hematoxylin and eosin by standard techniques (23). Bacteria were detected by Gram staining of the tissue sections.
For electron microscopy, monolayers of lung epithelial cells infected as described above or lungs from infected animals were washed, fixed with glutaraldehyde, and processed for transmission electron microscopy as described previously (18).
C3 binding to bacterial cells.
C3 binding assays were carried out by ELISA as described previously (1). Bacterial strains alone (negative controls) or incubated with A549 epithelial cells were incubated in serum-free medium. After 24 h at 37°C, the supernatants were collected and the bacterial cells were washed twice with PBS. Microtiter plate wells were coated with serial dilutions of the bacterial suspension and dried overnight at 37°C. After 2 h of incubation with PBS-1% BSA, the plates were incubated with anti-human C3, then with alkaline phosphatase-labeled goat anti-rabbit immunoglobulin G, and finally with p-nitrophenyl phosphate in 50 mM carbonate-bicarbonate buffer (pH 9.6) plus 5 mM MgCl2 to develop alkaline phosphatase. Incubations with antisera diluted in PBS-BSA were carried out at 37°C for 1 h and were always followed by PBS washes.
RESULTS
Internalization of K. pneumoniae clinical isolates by human lung epithelial cells.
We determined the internalization by human lung epithelial cells of K. pneumoniae clinical isolates belonging to different K types and with different degrees of encapsulation (Table 1). Similar numbers of microorganisms were used for each strain in the internalization assays, so the number of intracellular microorganisms recovered from the A549 cells represents a direct measure of internalization efficiency. We observed a wide range of internalization efficiencies among strains, although the results obtained in different experiments were quite reproducible. We detected an inverse relationship between the amount of CPS produced by the strain and its capacity to enter the lung epithelial cells. This phenomenon was observed in all strains independent of their K types. Thus, we recovered 1,268 to 4,575 intracellular microorganisms from K. pneumoniae strains that produce little capsule but only 442 to 775 microorganisms from the encapsulated strains. These results suggest that the degree of encapsulation modulates bacterial entry into lung epithelial cells.
Adhesion and internalization of an isogenic unencapsulated mutant.
To study directly the role of CPS in adhesion to and internalization in the lung epithelial cells, we constructed an unencapsulated mutant by transposon mutagenesis. Cloning and sequencing of the chromosomal minitransposon-containing fragment from the unencapsulated mutant 52K0 revealed that it was integrated within the orf14 gene of the operon required for the synthesis of type 2 CPS in K. pneumoniae (3). We demonstrated by competitive ELISA with capsule extracts from the mutant that this insertion completely abolished capsule expression in the mutant. Furthermore, LPS and OMP expression in the mutant 52K0 was not altered by the transposon insertion and was identical to that in wild-type strain 52145R (data not shown).
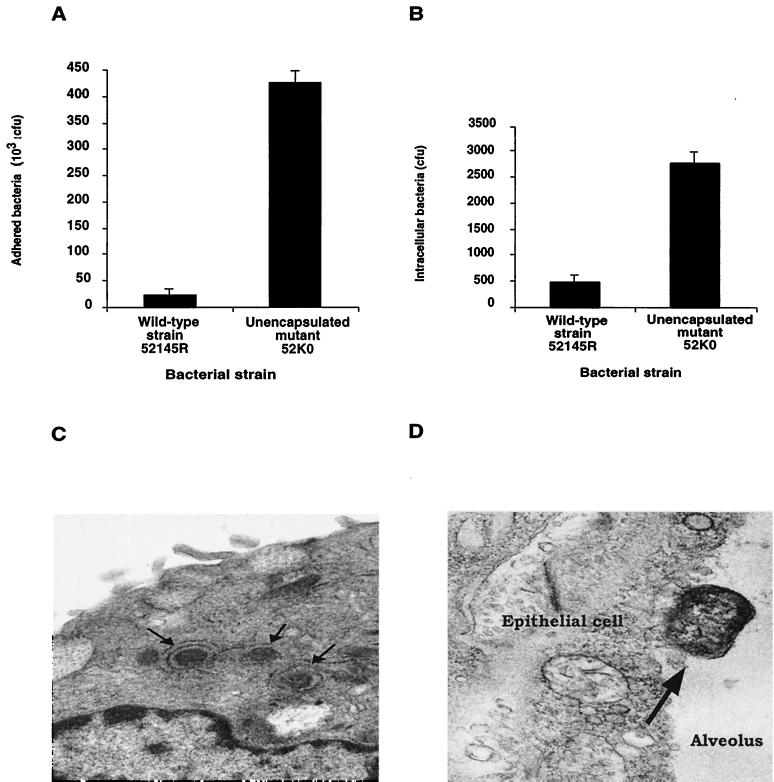
We investigated the ability of the wild-type strain 52145R and its derived unencapsulated mutant 52K0 to attach to and invade human lung epithelial cells (Fig. 1). The unencapsulated mutant 52K0 attached to and invaded epithelial cells with high efficiency compared to the wild-type strain 52145R. The unencapsulated mutant showed approximately 10-fold-greater efficiency of adhesion and internalization than the wild-type strain. These results confirm that CPS modulates the interactions between K. pneumoniae and human lung epithelial cells.
FIG. 1.
Adhesion (A) and internalization (B) of K. pneumoniae wild-type strain 52145R and its derived isogenic unencapsulated mutant 52K0 by human lung epithelial cells. The results are expressed in CFU per monolayer. The data are the mean plus standard deviation of at least three independent experiments (P < 0.01 for comparison between the unencapsulated mutant and the encapsulated wild-type strain for the adhesion and internalization experiments; two-tailed t test). (C) Transmission electron micrograph of an A549 human lung epithelial cell infected by the unencapsulated K. pneumoniae strain 52K0. The arrows indicate three bacterial cells surrounded by vacuolar membranes. (D) Transmission electron micrograph of a lung section from a mouse infected with the unencapsulated strain 52K0. The arrow indicates a bacterial cell adhering to lung epithelial cells.
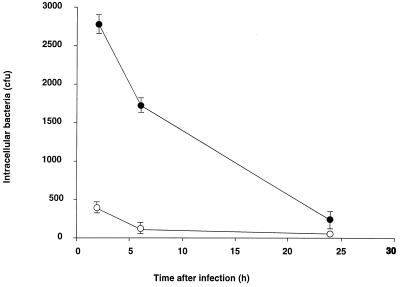
We studied recovery over time of the intracellular wild-type strain 52145R and the unencapsulated mutant 52K0 from infected lung epithelial cells. After 6 h of incubation, the number of viable bacteria had decreased to 50% of the intracellular bacteria recovered after 2 h of incubation (Fig. 2). Viable intracellular bacteria continued to decline over time, and after 24 h, only 8% of the bacteria recovered after 2 h of incubation were viable. Reduction of bacterial viability over time was not due to the exposure of the cells to gentamicin, since we obtained identical results in parallel experiments without gentamicin. After 24 h of incubation, we determined the viability of the epithelial cells by measuring the number of cells that failed to exclude trypan blue. More than 80% of the epithelial cells remained viable, suggesting that epithelial cell death accounted for some, but not all, of the decline in the number of intracellular bacteria. Thus, intracellular bacteria, both the wild-type strain and the unencapsulated mutant, were not able to multiply in the epithelial cells, and after 24 h of incubation, 92% of the bacteria were nonviable.
FIG. 2.
Survival of K. pneumoniae within human lung epithelial cells. After inoculation of A549 cells with K. pneumoniae strain 52145R (open circles) and 52K0 (solid circles), the cells were incubated in the presence of gentamicin for various periods and then washed and lysed for quantitative culture. The data are the mean ± standard deviation of at least three independent experiments.
Role of K. pneumoniae entry into lung epithelial cells.
The results described above present an apparent paradox: reduction of capsule expression increased adhesion to and invasion of human lung epithelial cells. However, clinical and experimental observations suggest that encapsulated strains are more virulent than unencapsulated strains. If adhesion to and invasion of lung epithelial cells were a virulence mechanism for lower respiratory tract infections, we would expect that unencapsulated strains would be virulent in an animal model of pneumonia. In contrast, if the entry of K. pneumoniae into epithelial cells were not a virulence mechanism but rather represented a mechanism to contain the infection, unencapsulated strains would be expected to exhibit reduced virulence.
To investigate this hypothesis, we studied the ability of the unencapsulated mutant to cause pneumonia in a mouse model. We inoculated the wild-type strain 52145R and the unencapsulated mutant 52K0 intratracheally into mice. Animals inoculated with the wild-type strain showed an important increment in the weight and size of the lung compared to healthy control animals due to the presence of abscesses and edema (Table 2). Histopathologic examination of the lung lesions demonstrated extensive polymorphonuclear infiltration, with pleuritis, vasculitis, and edema. Gram staining confirmed the presence of gram-negative rods throughout the inflamed tissue (data not shown). In addition, a total of 17 of 32 animals inoculated with 52145R developed bacteremia (table 2). The mortality rate was 58.3%.
TABLE 2.
K. pneumoniae pneumonia in micea
| Strain | Phenotype | No. of animals | Mean lung weight (g) | Mean log CFU/g of lung | Blood or spleen culture positiveb | Mortalityc |
|---|---|---|---|---|---|---|
| 52145R | Wild type | 36 | 0.29 ± 0.11 | 6.11 | 17/32 (52) | 21/36 (58.3) |
| 52K0 | Unencapsulated | 18 | 0.18 ± 0.06 | 0.44 | 0/18 (0) | 0/18 (0) |
P < 0.01 for all comparisons (lung weight, log CFU/gram of lung, blood or spleen culture positive, and mortality) between the wild-type and the unencapsulated mutant; two-tailed t test.
Number of animals positive/total (percent); blood or spleen culture was not obtained from four animals.
Number of animals dead/total (%).
In contrast with the lesions observed in the lungs of the animals infected with the wild-type strain 52145R, the lungs of the mice infected with the unencapsulated mutant 52K0 were apparently normal. Histopathology of the lungs did not identify differences compared to those of the healthy control animals, and Gram staining did not detect bacteria in the lungs (data not shown). None of the animals infected with strain 52K0 had bacteria in blood or spleen samples. Furthermore, no bacteria were recovered from lung homogenates 7 days after infection, and none of the animals inoculated with the unencapsulated mutant 52K0 died (Table 2). In addition, when we studied the lower respiratory tract infections caused by the unencapsulated mutant over time, we observed that all of the animals had bacteria in their lungs the first day (Fig. 1D). However, host defenses in the lung were sufficient to eliminate this mutant efficiently, since on day 2 none of the animals inoculated with the unencapsulated mutant presented bacteria in their lungs (data not shown). Thus, the unencapsulated mutant was completely avirulent in a mouse model of pneumonia. Based on these results, we concluded that CPS impedes entry into lung epithelial cells. Internalization of the bacteria by lung epithelial cells represents a strategy of the host to avoid the capacity of K. pneumoniae to produce pneumonia and systemic dissemination after introduction of the microorganism into the lower respiratory tract.
Binding of C3 to K. pneumoniae wild-type strain and the unencapsulated isogenic mutant.
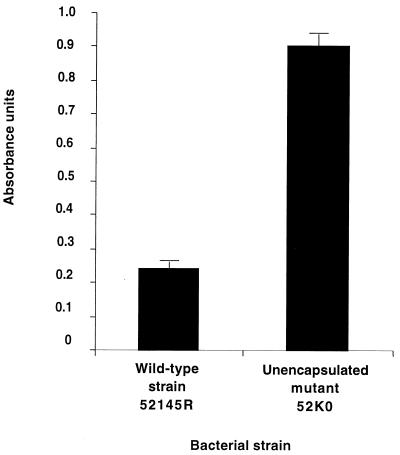
Our results demonstrate that entry into epithelial cells represents a mechanism to contain bacterial infection. However, our internalization assays demonstrated that most of the microorganisms remained outside of the epithelial cells, in particular the wild-type strain that was virulent in the animal model. Thus, we investigated the role of the epithelial cells in the clearance of the bacterial cells that do not invade them. It has been reported that resting A549 lung epithelial cells produce complement component C3, while C3 secretion increases in activated cells (27). To investigate if this C3 interacts with K. pneumoniae, we studied the binding of epithelially produced C3 to the wild-type strain and its derived mutants. As is shown in Fig. 3, the unencapsulated strain 52K0 bound approximately twofold more C3 than the wild-type strain. These results indicate that C3 produced by the epithelial cells interacts with K. pneumoniae and that CPS is critical to modulate the levels of C3 deposited on the bacterial surface.
FIG. 3.
Binding of epithelially produced C3 to K. pneumoniae wild-type strain 52145R and its derived isogenic unencapsulated mutant 52K0. After inoculation of A549 cells with K. pneumoniae, the C3 deposited on the bacterial cells was determined by ELISA. The values are arbitrary optical densities at 405 nm (OD405). The data are the mean plus standard deviation of at least three independent experiments (P < 0.01 for comparison between the unencapsulated mutant and the encapsulated wild-type strain; two-tailed t test). Values from negative controls were always lower than 0.1 OD405 units.
Since bacteria opsonized with C3 are cleared from the lung either by alveolar macrophages or by recruited neutrophils more efficiently than nonopsonized bacteria (G. Cortés and S. Albertí, unpublished data), interaction of epithelially produced C3 with K. pneumoniae cells helps to eliminate the microorganism.
DISCUSSION
The airway epithelium represents a primary route of entry for pathogenic bacteria into the host. Many bacteria pathogenic for humans have been shown to enter eukaryotic cells in culture, including lung epithelial cells (6, 19, 24, 29). For certain bacteria, entry into eukaryotic cells represents an initial step in establishing infection. However, the biological significance of the internalization of certain bacterial pathogens by nonphagocytic cells, such as epithelial cells, is not known. K. pneumoniae has the ability to invade human ileocecal, bladder, and lung epithelial cells in vitro (10, 25). Some authors have suggested that K. pneumoniae internalization represent a virulence mechanism because it enables the bacteria to avoid host defenses and antimicrobial drugs and therefore might represent a reservoir of the pathogen. In contrast, our results clearly demonstrate that bacterial entry into epithelial cells is not necessarily associated with invasive infection. Our data indicate that K. pneumoniae entry into lung epithelial cells represents a host defense mechanism and not a bacterial virulence mechanism. These results are highly consistent with the data reported by other authors with other respiratory pathogens (15, 21, 22, 26, 28) showing that decreased epithelial cell adherence and uptake is associated with virulence and that this is likely a host defense, not microbial virulence. As has been reported by other authors (10, 25), K. pneumoniae isolates that enter human lung epithelial cells more efficiently are those that lack CPS expression. These results have been confirmed in our investigations showing that poorly encapsulated clinical isolates or an isogenic unencapsulated mutant invades A549 cells more efficiently than the highly encapsulated clinical isolates.
Once K. pneumoniae interacted with the epithelial cells, some microorganisms entered into the cells and did not proliferate but rather exhibited a steady decline in viability over the 24 h after cell entry. Sahly et al. observed a similar phenomenon over a period of 72 h in the recovery of viable K. pneumoniae organisms from bladder epithelial cells (25). These observations suggested that K. pneumoniae entry into human lung epithelial cells might not represent a virulence mechanism, a conclusion that was supported by the results obtained in the animal model of pneumonia, where the unencapsulated mutant was cleared in 2 days (data not shown).
Our results support the data obtained by other authors for the entry of different bacterial pathogens into different cell lines (15, 21, 22, 26, 28). These authors demonstrated that internalization of the bacterial pathogens did not promote subsequent invasive infection but rather represented a mechanism to contain infection.
Thus, as occurs in the interaction with phagocytic blood leukocytes, where CPS blocks the uptake of the bacteria, CPS acts as a virulence factor preventing the ingestion of the bacteria by the epithelial cells. The mechanisms that allow the epithelial cells to contain the infection remain unclear. Perhaps bacterial invasion of lung epithelial cells induces apoptosis, as occurs with other pathogens that invade intestinal epithelial cells (17), and promotes the elimination of infected cells from the airway epithelium via cellular desquamation. On the other hand, it is not known if there are other celullar responses subsequent to bacterial ingestion that could participate in the clearance of K. pneumoniae from the lung epithelium. We are investigating both hypotheses.
Although internalization of bacterial cells represents a mechanism to control infection, in our experiments, most of the bacterial cells remained in the extracellular milieu, particularly those strains that expressed capsule and were virulent in the animal model. Alveolar macrophages and complement are the first lung defenses against lower respiratory tract infections. Complement levels in the lung are low, and one of the sources of this central component of the complement is the lung epithelial cells. We have demonstrated that K. pneumoniae binds C3 produced by the epithelial cells and that unencapsulated strains bind more C3 than the wild-type strain. Thus, CPS modulates the amount of C3 deposited on the bacterial surface, as other authors have previously suggested (9). Opsonization of the bacterial cell by complement components is a crucial step for the elimination of the microorganism via phagocytosis by alveolar macrophages or neutrophils recruited from peripheral blood.
In summary, our results demonstrate that internalization of K. pneumoniae by human alveolar epithelial cells represents a mechanism to contain infection. Furthermore, C3 produced by the epithelial cells interacts with the bacteria and facilitates its elimination. CPS allows the bacteria to evade both mechanisms, leading to pneumonia and dissemination of the infection.
Acknowledgments
We thank D. S. Hansen (Statens Serum Institute, Copenhagen, Denmark) for donating specific K-type antisera, M. Pocoví for technical assistance in electron microscopy, and V. J. Benedi for critical reading of the manuscript.
This work was supported by Fondo de Investigaciones Sanitarias (FIS).
REFERENCES
- 1.Albertí, S., D. Álvarez, S. Merino, M. T. Casado, F. Vivanco, J. M. Tomás, and V. J. Benedí. 1996. Analysis of complement C3 deposition and degradation on Klebsiella pneumoniae. Infect. Immun. 64:4726-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albertí, S., J. Imperial, J. M. Tomás, and V. J. Benedí. 1991. Bacterial lipopolysaccharide extraction in silica gel-containing tubes. J. Microbiol. Methods 14:63-69. [Google Scholar]
- 3.Arakawa, Y., R. Wacharotayankun, T. Nagatsuka, H. Ito, N. Kato, and M. Ohta. 1995. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. Infect. Immun. 177:1788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Current protocols in molecular biology. Wiley Interscience, New York, N.Y.
- 5.Bartlett, J. G., P. O'Keefe, F. P. Tally, T. J. Louie, and S. L. Gorbach. 1986. Bacteriology of hospital-acquired pneumonia. Arch. Intern. Med. 146:868-871. [PubMed] [Google Scholar]
- 6.Burns, J. L., M. Jonas, E. Y. Chi, D. K. Clark, A. Berger, and A. Griffith. 1996. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect. Immun. 64:4054-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryz, S. J., Jr., E. Fürer, and R. Germanier. 1986. Immunization against fatal experimental Klebsiella pneumoniae pneumonia. Infect. Immun. 54:403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Transposon derivates for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domenico, P., J. M. Tomás, S. Merino, X. Rubires, and B. A. Cunha. 1999. Surface antigen exposure by bismuth dimercaprol supression of Klebsiella pneumoniae capsular polysaccharide. Infect. Immun. 67:664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Favre-Bonte, S., B. Joly, and C. Forestier. 1999. Consequences of reduction of Klebsiella pneumoniae capsule expression on interactions of this bacterium with epithelial cells. Infect. Immun. 67:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filip, C., G. Fletcher, J. L. Wulf, and C. F. Earhart. 1973. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J. Bacteriol. 115:717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García de la Torre, M., J. Romero-Vivas, J. Martínez-Beltrán, A. Guerrero, M. Messeguer, and E. Bouza. 1985. Klebsiella bacteremia: an analysis of 100 episodes. Rev. Infect. Dis. 7:143-150. [DOI] [PubMed] [Google Scholar]
- 13.Held, T. K., M. Trautmann, M. E. Mielke, H. Neudeck, S. J. Cryz, and A. Cross. 1992. Monoclonal antibody against Klebsiella capsular polysaccharide reduces severity and hematogenic spread of experimental Klebsiella pneumoniae pneumonia. Infect. Immun. 60:1771-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulse, M. L., S. Smith, E. Y. Chi, A. Pham, and C. E. Rubens. 1993. Effect of type III group B streptococcal capsular polysaccharide on invasion of respiratory epithelial cells. Infect. Immun. 61:4835-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis, W. R., V. P. Munn, A. K. Highsmith, D. H. Culver, and J. M. Hughes. 1985. The epidemiology of nosocomial infections caused by Klebsiella pneumoniae. Infect. Control 6:68-74. [DOI] [PubMed] [Google Scholar]
- 17.Jung Mogg, K., L. Eckmann, T. C. Savidge, D. C. Lowe, T. Witthöft, and M. F. Kagnoff. 1998. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Investig. 102:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruskal, B. A., K. Sastry, A. B. Warner, C. E. Mathieu, and R. A. B. Ezekowitz. 1992. Phagocyte chimeric receptors require both transmembrane and cytoplasmic domains from the mannose receptor. J. Exp. Med. 176:1673-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaPenta, D., C. Rubens, E. Chi, and P. P. Cleary. 1994. Group A streptococci efficently invade human respiratory epithelial cells. Proc. Natl. Acad. Sci. USA 91:12115-12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassif, X., J. M. Fournier, J. Arondel, and P. J. Sansonetti. 1989. Mucoid phenotype of Klebsiella pneumoniae is a plasmid-encoded virulence factor. Infect. Immun. 57:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pier, G. B., M. Grout, T. S. Zaidi, J. C. Olsen, L. G. Johnson, J. R. Yankaskas, and J. B. Goldberg. 1996. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science 5:64-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pöhlmann-Dietze, P., M. Ulrich, K. B. Kiser, G. Döring, J. C. Lee, J.-M. Fournier, K. Botzenhart, and C. Wolz. 2000. Adherence of Staphylococcus aureus to endothelial cells: influence of capsular polysaccharide, global regulator agr, and bacterial growth phase. Infect. Immun. 68:4865-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prophet, E. B., B. Mills, J. B. Arrington, and L. H. Sobin (ed.). 1992. Laboratory methods in histotechnology. American Registry of Pathology, Washington, D.C.
- 24.Rubens, C. E., S. Smith, M. Hulse, E. Y. Chi, and G. van Belle. 1992. Respiratory epithelial cell invasion by group B streptococci. Infect. Immun. 60:5157-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahly, H., R. Podschun, T. A. Oelschalaeger, M. Greiwe, H. Parolis, D. Hastly, J. Kekow, U. Ullman, I. Ofek, and S. Sela. 2000. Capsule impedes adhesion to an invasion of epithelial cells by Klebsiella pneumoniae. Infect. Immun. 68:6744-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrager, H. M., J. G. Rheinwald, and M. R. Wessels. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Investig. 98:1954-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, B. L., and M. K. Hostetter. 2000. C3 as substrate for adhesion of Streptococcus pneumoniae. J. Infect. Dis. 182:497-508. [DOI] [PubMed] [Google Scholar]
- 28.Stephens, D. S., P. A. Spellman, and J. S. Swartley. 1993. Effect of the (alpha 2→8)-linked polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J. Infect. Dis. 167:475-479. [DOI] [PubMed] [Google Scholar]
- 29.Talbot, U. M., A. W. Paton, and J. C. Paton. 1996. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect. Immun. 64:3772-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson, J. F., and I. W. Sutherland. 1971. Chemical extraction methods of microbial cells. Methods Microbiol. 5B:345-383.