Abstract
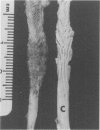
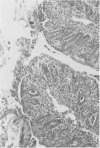
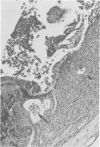
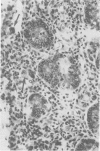
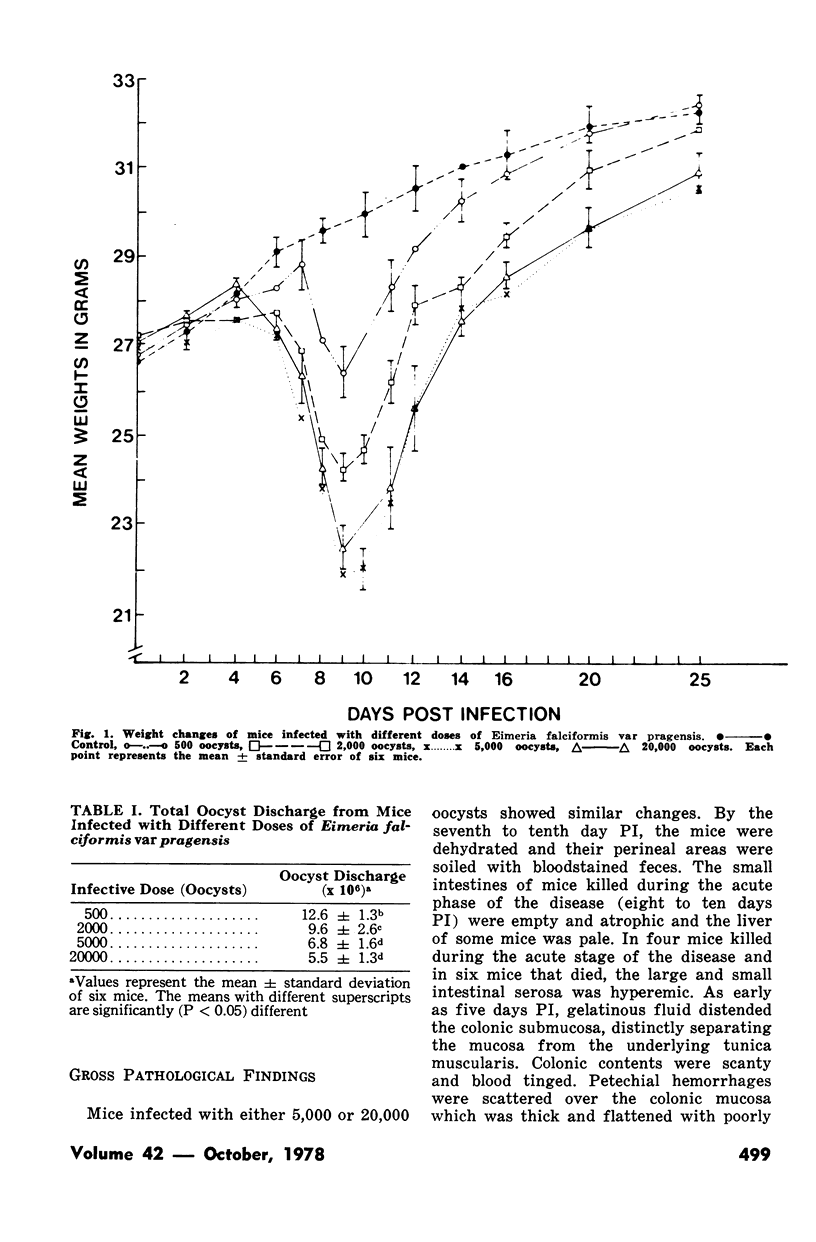
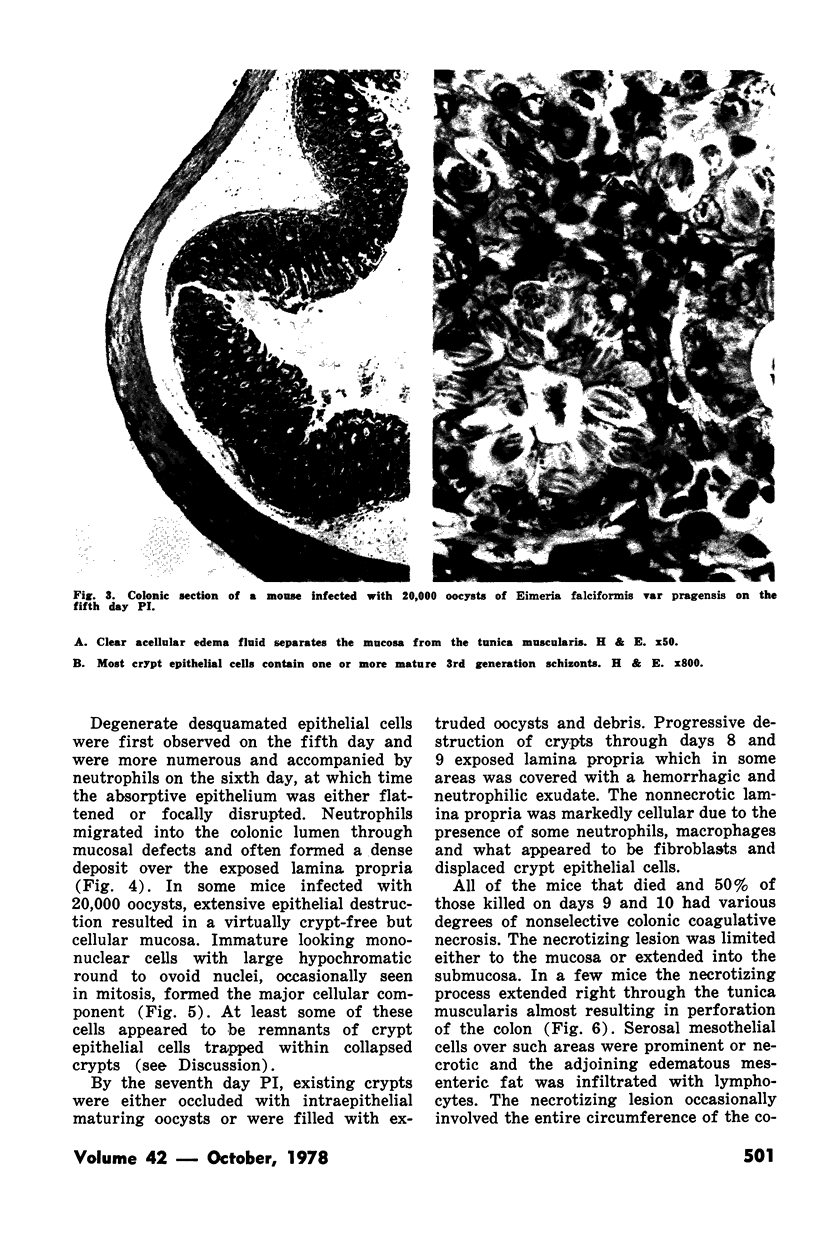
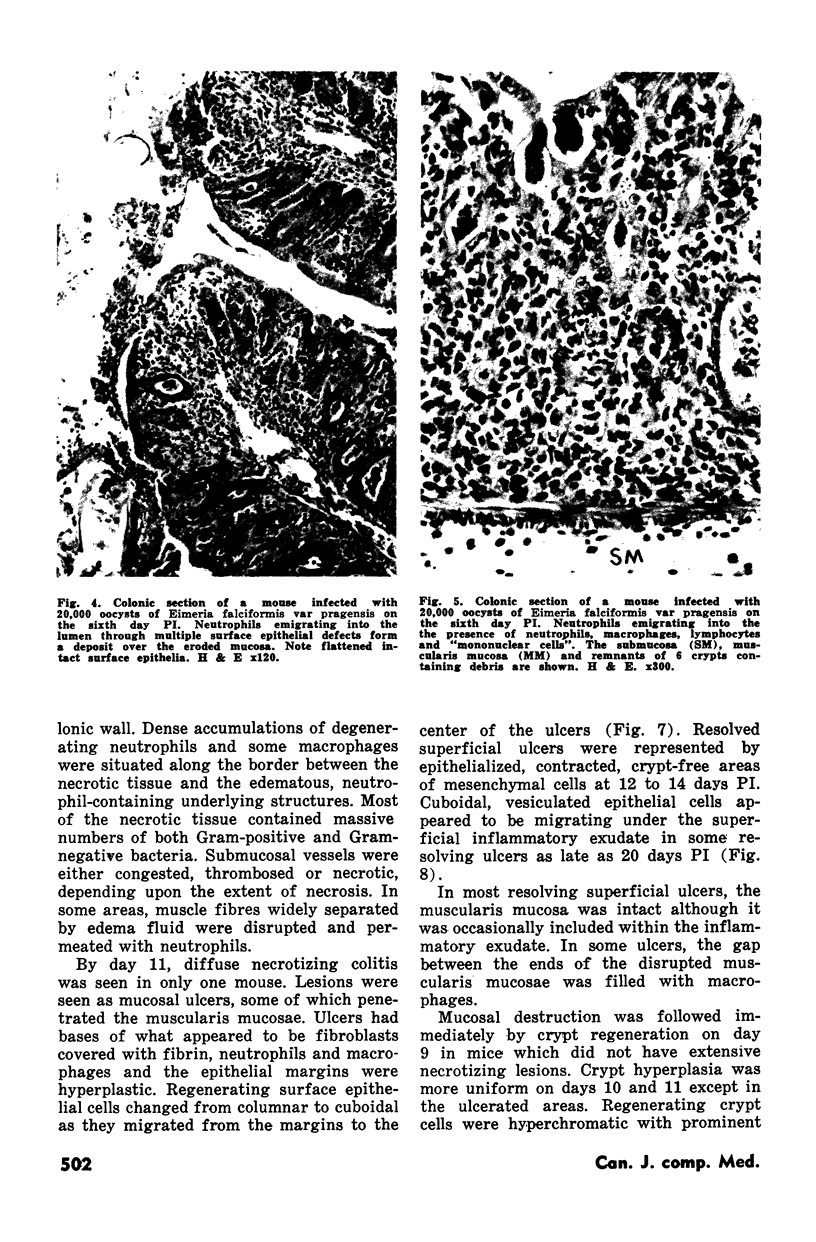
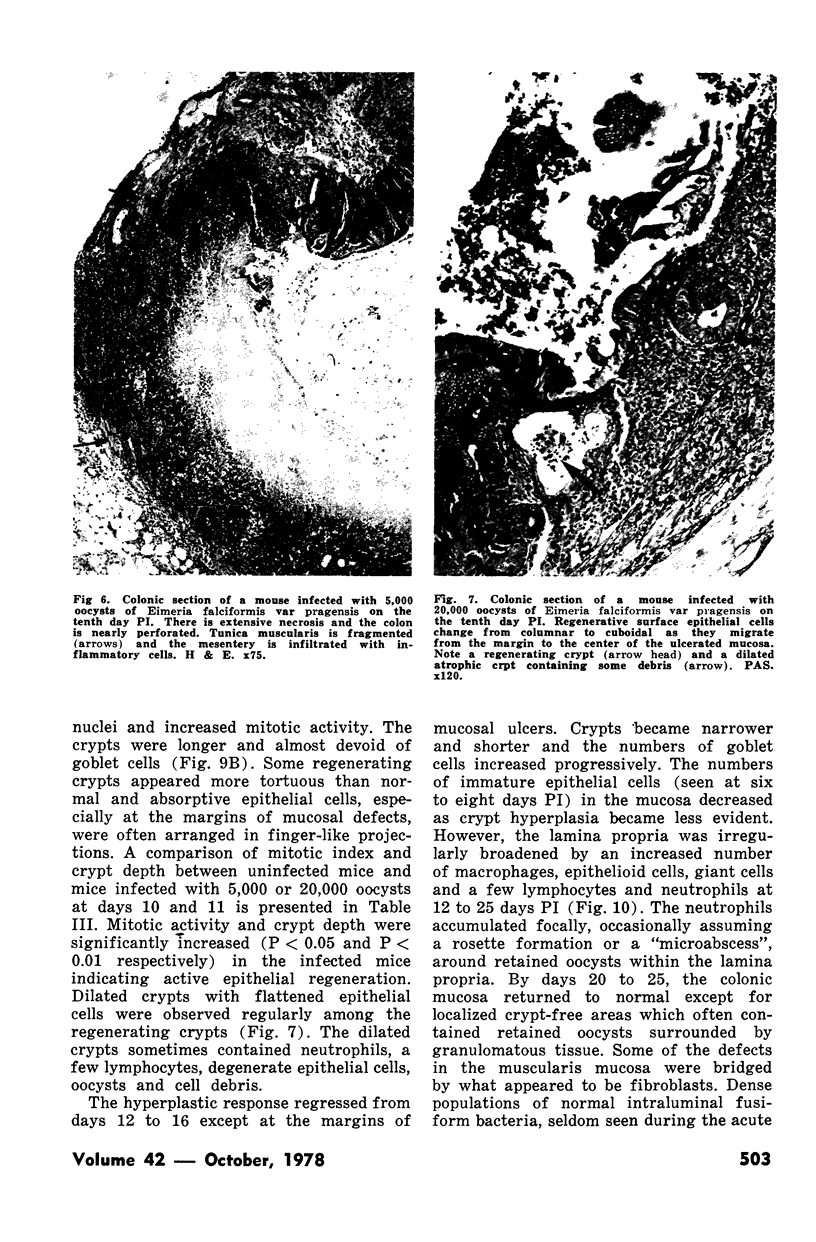
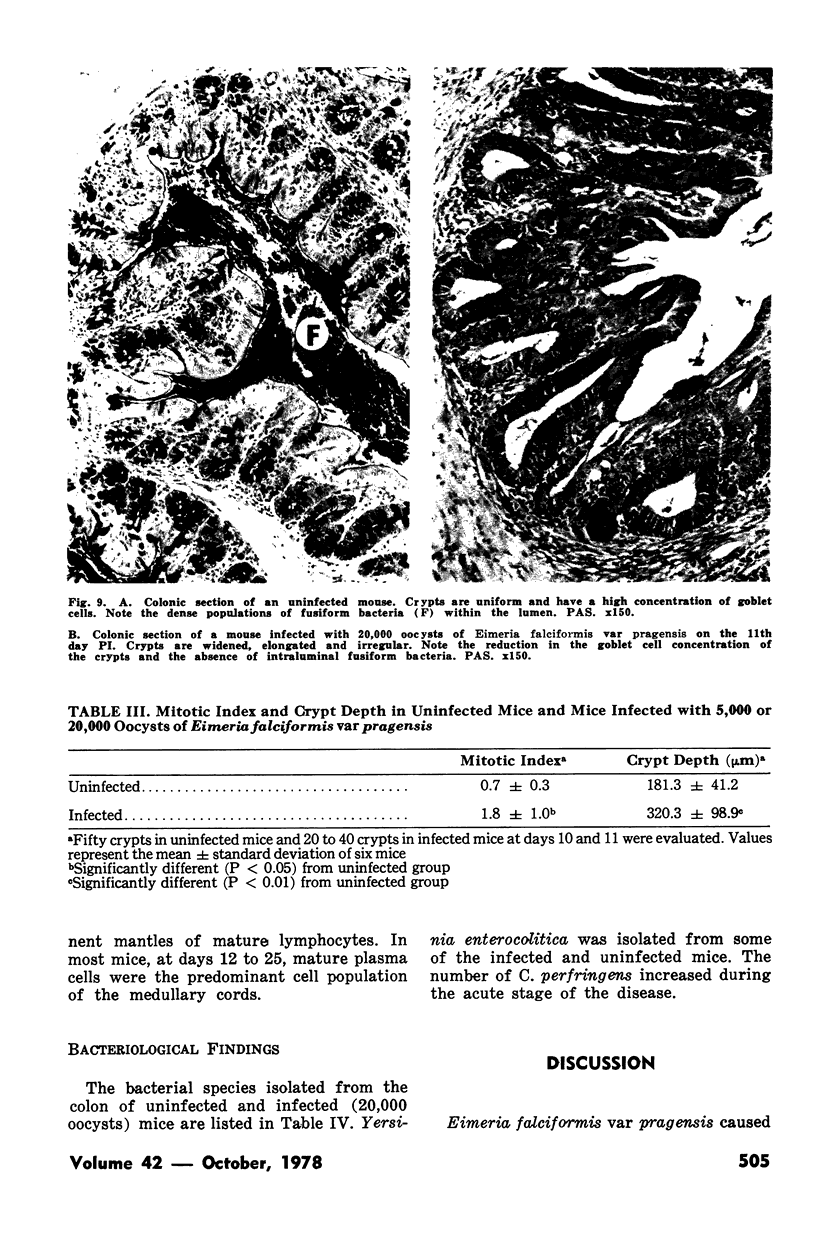
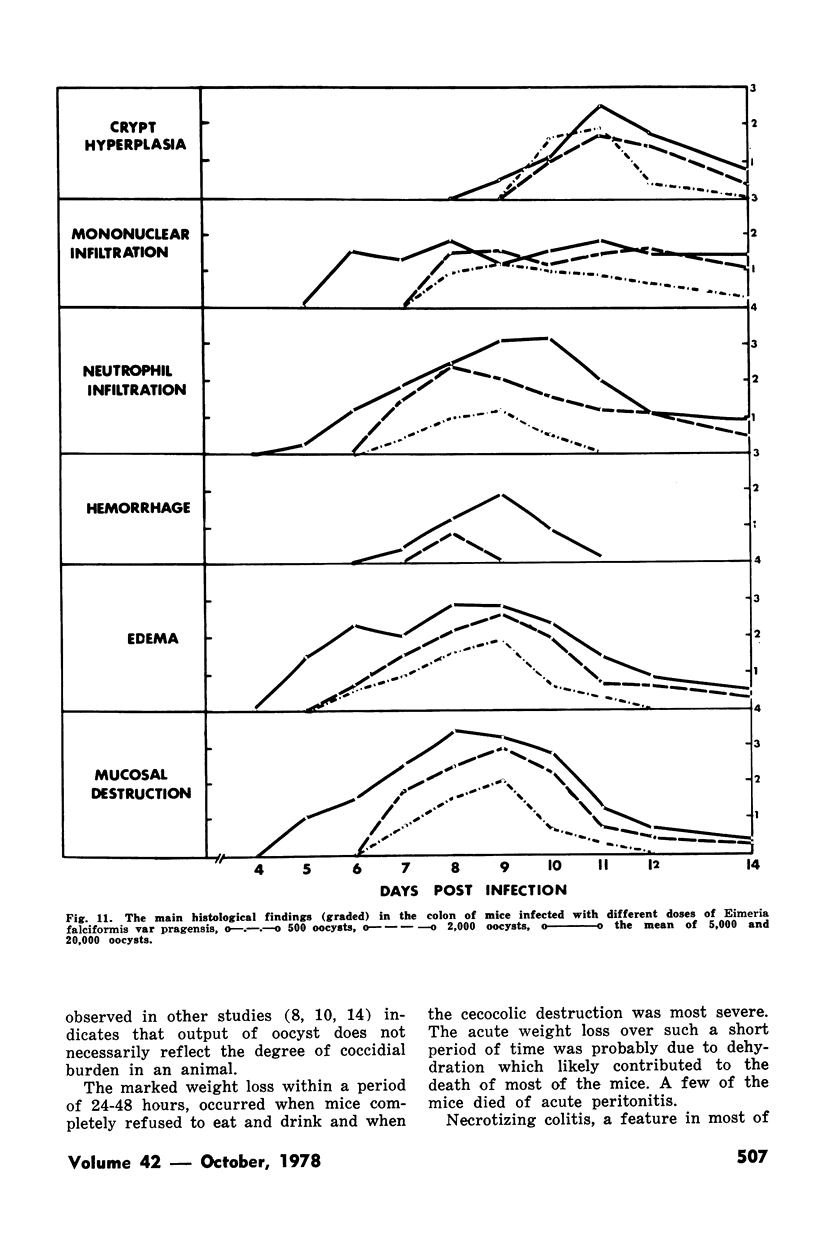
Groups of Swiss white mice weighing 25-28 grams were infected orally with 500, 2,000, 5,000 or 20,000 oocysts of Eimeria falciformis var pragensis. Depression, anorexia, weight loss, diarrhea or dysentery, and dehydration were most pronounced at eight to ten days postinfection. The highest mortality, 31%, occurred in mice infected with 20,000 oocysts. None of the mice infected with 500 oocysts died. The pathological findings were equally severe in mice infected with 5,000 and 20,000 oocysts. The enteric lesions, most pronounced at eight to ten days postinfection, were restricted mainly to the large intestine and consisted initially of both cryptal and absorptive epithelial cell destruction and submucosal edema. These changes were followed in 12 to 24 hours by a transient influx of neutrophils into the lamina propria followed by mononuclear cell infiltration which lasted for five to ten days. As the infective dose decreased, the inflammatory response occurred later and was less extensive. When seen, hemorrhage occurred seven to 11 days postinfection. In 50% of the mice infected with 5,000 and 20,000 oocysts, varying degrees of a nonselective mucosal necrosis were seen at eight to 12 days postinfection. In mice infected with 500 oocysts, mucosal destruction was restricted to the epithelium. Neutrophils predominated when necrosis was extensive, otherwise, mononuclear cells were the main inflammatory cells. Two to three days following necrosis, crypt hyperplasia was marked and mucosal integrity was restored. Ulcers, some of which extended into the submucosa, healed by days 14 to 20. Localized granulomatous colitis, induced by trapped oocysts within the lamina propria, was seen until the experiment was terminated at 25 days postinfection. Infection was followed by lymphoid hyperplasia in the lymph nodes and the spleen.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS W. C. The lethal effect of Eimeria tenella extracts on rabbits. J Parasitol. 1959 Feb;45(1):38–46. [PubMed] [Google Scholar]
- Bergmann V. Elektronenmikroskopische Untersuchungen zur Pathogenese der Blinddarmkokzidiose der Hühnerkülken. Arch Exp Veterinarmed. 1970;24(5):1169–1184. [PubMed] [Google Scholar]
- Bradford W. D., Noce P. S., Gutman L. T. Pathologic features of enteric infection with Yersinia enterocolitica. Arch Pathol. 1974 Jul;98(1):17–22. [PubMed] [Google Scholar]
- Cerná Z., Sénaud J., Mehlhorn H., Scholtyseck E. Etude comparée des rélations morphologiques et immunologiques chez les coccidies de la souris: Eimeria falciformis et Eimeria pragensis (Coccidia, Eimeriidae) Folia Parasitol (Praha) 1974;21(4):301–309. [PubMed] [Google Scholar]
- Fernando M. A., McCraw B. M. Changes in the generation cycle of duodenal crypt cells in chickens infected with Eimeria acervulina. Z Parasitenkd. 1977 Jul 29;52(3):213–218. doi: 10.1007/BF00380540. [DOI] [PubMed] [Google Scholar]
- Fernando M. A., McCraw B. M. Mucosal morphology and cellular renewal in the intestine of chickens following a single infection of Eimeria acervulina. J Parasitol. 1973 Jun;59(3):493–501. [PubMed] [Google Scholar]
- Fernando M. A., Pasternak J., Barrell R., Stockdale P. H. Induction of host nuclear DNA synthesis in coccidia-infected chicken intestinal cells. Int J Parasitol. 1974 Jun;4(3):267–276. doi: 10.1016/0020-7519(74)90082-4. [DOI] [PubMed] [Google Scholar]
- Hegde K. S., Reid W. M., Johnson J., Womack H. E. Pathogenicity of Eimeria brunetti in bacteria-free and conventional chickens. J Parasitol. 1969 Apr;55(2):402–405. [PubMed] [Google Scholar]
- Hein H. Eimeria brunetti: pathogenic effects in young chickens. Exp Parasitol. 1974 Dec;36(3):333–341. doi: 10.1016/0014-4894(74)90073-3. [DOI] [PubMed] [Google Scholar]
- Kimura N., Mimura F., Nishida S., Kobayashi A. Studies on the relationship between intestinal flora and cecal coccidiosis in chicken. Poult Sci. 1976 Jul;55(4):1375–1383. doi: 10.3382/ps.0551375. [DOI] [PubMed] [Google Scholar]
- Kouwenhoven B. The significance of infection dose, body weight and age of the host on the pathogenic effect of Eimeria acervulina in the fowl. Z Parasitenkd. 1972;39(3):255–268. doi: 10.1007/BF00329462. [DOI] [PubMed] [Google Scholar]
- Lee D. L., Long P. L. An electron microscopal study of Eimeria tenella grown in the liver of the chick embryo. Int J Parasitol. 1972 Mar;2(1):55–58. doi: 10.1016/0020-7519(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Melnyk C. S., Braucher R. E., Kirsner J. B. Colon mucosa response to injury. I. Morphological study. Gastroenterology. 1966 Jul;51(1):43–49. [PubMed] [Google Scholar]
- Melnyk C. S., Braucher R. E., Kirsner J. B. Regeneration of the human colon mucosa. Morphological and histochemical study. Gastroenterology. 1967 Jun;52(6):985–987. [PubMed] [Google Scholar]
- O'CONNOR R. J. The healing of localised areas of necrosis in the colon of the mouse. Br J Exp Pathol. 1954 Dec;35(6):545–550. [PMC free article] [PubMed] [Google Scholar]
- Owen D. Eimeria falciformis (Eimer, 1870) in specific pathogen free and gnotobiotic mice. Parasitology. 1975 Oct;71(2):293–303. doi: 10.1017/s0031182000046734. [DOI] [PubMed] [Google Scholar]
- PARISH W. E. Necrotic enteritis in the fowl. III. The experimental disease. J Comp Pathol. 1961 Oct;71:405–413. doi: 10.1016/s0368-1742(61)80045-3. [DOI] [PubMed] [Google Scholar]
- Pout D. D. Coccidiosis of lambs. 3. The reaction of the small intestinal mucosa to experimental infections with E. arloingi "B" and E. crandallis. Br Vet J. 1974 Jan-Feb;130(1):45–53. doi: 10.1016/s0007-1935(17)35989-4. [DOI] [PubMed] [Google Scholar]
- SHARMA N. N., FOSTER J. W. TOXIC SUBSTANCE IN VARIOUS CONSTITUENTS OF EIMERIA TENELLA OOCYSTS. Am J Vet Res. 1964 Jan;25:211–216. [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale P. H., Fernando M. A. The development of the lesions caused by second generation schizonts of Eimeria necatrix. Res Vet Sci. 1975 Sep;19(2):204–208. [PubMed] [Google Scholar]
- Stockdale P. H. The pathogenesis of the lesions produced by Eimeria zuernii in calves. Can J Comp Med. 1977 Jul;41(3):338–344. [PMC free article] [PubMed] [Google Scholar]
- Visco R. J., Burns W. C. Eimeria tenella in bacteria-free and conventionalized chicks. J Parasitol. 1972 Apr;58(2):323–331. [PubMed] [Google Scholar]