Abstract
Following entry into the host cytosol, the bacterial pathogen Listeria monocytogenes dramatically increases the expression of several key virulence factors. The expression of actA, whose protein product is required for L. monocytogenes actin-based intracellular motility, is increased by more than 200-fold in cytosolic bacteria in comparison to broth-grown cultures. Two distinct promoter elements have been reported to regulate actA expression. One promoter is located immediately upstream of actA coding sequences, while the second promoter is contributed by the upstream mpl gene via the generation of an mpl-actA-plcB transcript. A series of L. monocytogenes mutants were constructed to define the contributions of individual promoter elements to actA expression. The intracellular induction of actA expression was found to be dependent upon the actA proximal promoter; the mpl promoter appeared to contribute to the extracellular induction of actA but did not affect intracellular levels of expression. The actA promoter is dependent upon a regulatory factor known as PrfA for transcriptional activation; however, no increase in actA expression was detected following the introduction of a high-affinity PrfA binding site within the actA promoter. The presence of a mutationally activated form of PrfA, known as PrfA*, increased overall actA expression in broth-grown cultures of both wild-type and actA promoter mutant strains, but the levels of induction observed were still approximately 50-fold lower than those observed for intracellularly grown L. monocytogenes. Collectively, these results indicate that the dramatic induction of actA expression that occurs in the host cell cytosol is mediated through a single promoter element. Furthermore, intracellular induction of actA appears to require additional steps or factors beyond those necessary for the activation and binding of PrfA to the actA promoter.
Listeria monocytogenes is a gram-positive facultative intracellular pathogen that is responsible for serious infections in immunocompromised individuals, pregnant women, and neonates (25, 26). The bacteria infect a wide variety of host cells, and a number of gene products that participate in the processes of invasion, intracellular replication, and cell-to-cell spread have been identified (reviewed in references 46 and 56). Following internalization, L. monocytogenes escapes from the host cell phagosome through the activity of a pore-forming hemolysin known as listeriolysin O, encoded by hly (11, 16, 31, 41, 47). Once within the host cell cytosol, the bacteria begin to replicate and to express a surface-associated protein, ActA, that is required for host cell actin polymerization-based bacterial movement and for spread of the bacteria into adjacent cells (12, 17, 34, 44, 52).
L. monocytogenes has been demonstrated to specifically induce the expression of selected genes within host cells (7, 8, 18, 21, 22, 24, 33, 43). For example, the expression of actA is highly induced in the mammalian cell cytosol (226-fold), whereas hly shows more moderate levels of intracellular induction (20-fold) (43). The host environmental signals that lead to the induction of intracellular bacterial gene expression have not been identified, and the mechanisms by which that induction occurs are unknown. To begin to define the mechanisms that govern the intracellular induction of bacterial gene expression, we have chosen to focus on the transcriptional activation of actA, as the product of this gene is one of the most abundant bacterial proteins expressed within the cytosol but is expressed minimally in broth cultures (6). Two promoters appear to contribute to actA expression, a proximal promoter located 198 bp upstream from the start of actA translation and a promoter from the upstream mpl gene (Fig. 1). Northern analysis with RNA isolated from L. monocytogenes grown in broth cultures detected the presence of an approximately 3-kb transcript corresponding to actA-plcB and a 5.4-kb transcript corresponding to mpl-actA-plcB (5).
FIG. 1.
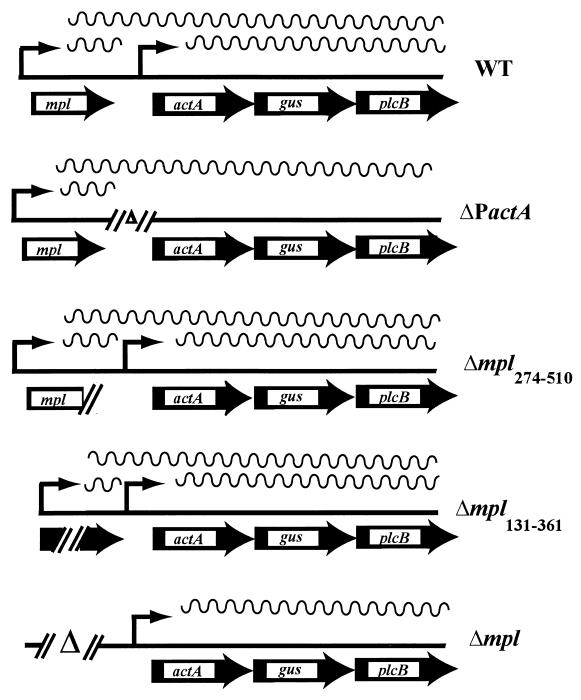
Chromosomal actA and mpl deletion mutants in the presence of actA-gus-plcB transcriptional reporter gene fusions in L. monocytogenes. The mpl and actA promoters are shown as thin arrows with associated transcripts (wavy lines). WT, wild type. ΔP actA contains a complete deletion of the actA proximal promoter but maintains the ribosome binding site immediately upstream of the actA coding sequences. Δmpl274-510 contains a frameshift mutation at codon 274 of mpl resulting in the loss of the protease active site and approximately 50% of the protein. Δmpl131-361 contains an in-frame deletion of amino acids 131 through 361 of Mpl resulting in the loss of the protease active site. Δmpl contains a complete deletion of the mpl promoter and coding regions. A promoterless copy of gus was inserted between the actA and plcB coding regions in the chromosomes of all mutants via homologous recombination.
The actA and hly genes are both members of a 10-kb gene cluster that is positively regulated by a 27-kDa transcriptional activator known as PrfA (38, 40). PrfA belongs to the cyclic AMP receptor protein (CRP)-FNR family of transcriptional activators and binds to a conserved 14-bp region of dyad symmetry present within the −40 region of target promoters (2, 15, 23, 36, 55). Certain PrfA-regulated promoters, such as actA and mpl, contain PrfA DNA binding sites that are imperfect palindromes, and the activation of transcription by PrfA at these promoters is less efficient than the PrfA-dependent activation of hly and plcA, which share a perfect palindromic PrfA binding site (54, 60).
In this report, the contributions of the actA and mpl promoter elements to the intracellular induction of actA expression are further investigated. Mutational analysis of actA and mpl promoter and coding regions indicates that the actA proximal promoter is the primary element that contributes to the intracellular induction of actA expression, whereas the mpl promoter contributes to patterns of expression of actA outside host cells. Additional analysis of the actA proximal promoter, which has been reported to be completely dependent on PrfA activation (2), indicates that no increase in actA expression occurs following the introduction of a high-affinity PrfA DNA binding site within the actA promoter. The failure of a high-affinity PrfA DNA binding site to alter actA expression patterns is also seen in the presence of the mutationally activated prfA* allele (49, 59), suggesting that the modified form of the protein does not discriminate between high-affinity and low-affinity binding sites. The intracellular induction of actA expression must therefore require additional factors or events beyond those leading to the activation and binding to the actA promoter of PrfA. Finally, our results suggest that the Mpl protease contributes an additional role to L. monocytogenes pathogenesis that is distinct from the activation of the PlcB lecithinase.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains used in this study are listed in Table 1. L. monocytogenes (serotype 1/2a) is resistant to streptomycin and has a 50% lethal dose (LD50) for mice of 2 × 104 (23). L. monocytogenes was stored at −70°C in brain heart infusion (BHI; Difco Laboratories, Detroit, Mich.) broth containing 20% glycerol. Escherichia coli HB101 or DH5α was used as the host strain for recombinant plasmids. Antibiotics were used at the following concentrations, unless otherwise noted: carbinicillin, 50 μg/ml; chloramphenicol, 10 μg/ml; and streptomycin, 200 μg/ml.
TABLE 1.
Bacterial strains and relevant characteristics
| Straina | Relevant characteristic(s) | Reference or source |
|---|---|---|
| 10403S | Wild type | 23 |
| DP-L1942 | ΔactA164-465b | 6 |
| DP-L1465 | Δmpl274-510c | Dan Portnoy |
| DP-L2296 | Δmpl131-361d | 39 |
| DP-L2343 | Δmple | Dan Portnoy |
| NF-L559 | P(hly)-actA | This work |
| NF-L476 | actA-gus-plcB | This work |
| NF-L767 | ΔPactA-gus-plcB | This work |
| NF-L707 | Δmpl274-510actA-gus-plcB | This work |
| NF-L861 | Δmpl131-361actA-gus-plcB | This work |
| NF-L862 | Δmpl actA-gus-plcB | This work |
| NF-L572 | P(hly)-actA-gus-plcB | This work |
| NF-L753 | actA-gus-plcB prfA* | This work |
| NF-L754 | P(hly)-actA-gus-plcB prfA* | This work |
| NF-L904 | ΔPactA-gus-plcB comK::actA | This work |
All DP and NF strains were derived from L. monocytogenes 10403S.
In-frame deletion of actA.
mplframeshift mutation.
In-frame deletion of mpl.
Deletion of mpl promoter and coding region.
The thermosensitive plasmid vectors pKSV7 (56) and pCON1-ΔprfA7973 (1) were described previously.
Construction of L. monocytogenes actA and mpl promoter and gene deletion mutants.
An internal out-of-frame mpl deletion was introduced into strain 10403S by allelic exchange with vector pDP1434 (39). Vector pDP1434 contains a copy of the mpl gene that has been digested with BamHI and XbaI, treated with the Klenow fragment to produce blunt ends, and religated, resulting in a deletion of 83 bp followed by three new codons and a stop codon. The resulting open reading frame encoded a product that was 274 amino acids long, corresponding to approximately 50% of the length of the original protein and lacking the putative active site of the protease. Strain DP-L2296 contains a 684-bp in-frame deletion of mpl in 10403S, encompassing 45% of the open reading frame, including the putative propeptide cleavage site and the putative active site of Mpl (39). A complete deletion of the mpl coding region and promoter sequences was generated by ligating the 3′ end of the hly structural gene and terminator to the 5′ end of the actA promoter and structural gene. A 637-bp DNA fragment encompassing the actA promoter region and an N-terminal portion of the structural gene was amplified by PCR with primers DP-2276 (5′GGGGTACCTTAACAAATGTTAGAGAAAAA3′) and DP-2277 (5′GGAATTCCGCTGCGCTATCC3′) (the underlined sequences designate the introduced KpnI and EcoRI restriction sites, respectively). The resulting PCR fragment was ligated into vector pKSV7 (56) digested with KpnI and EcoRI. A 638-bp fragment encompassing the 3′ end of hly and its terminator was amplified by PCR with primers DP-2295 (5′-GGCTCTAGACTTACGCGATATTTTG-3′) and DP-2275 (5′-GGGGTACCTTCTTCTAAAAAAATTAAAAAAT-3′) (the underlined sequences designate the introduced XbaI and KpnI restriction sites, respectively). The resulting PCR fragment was ligated into the XbaI and KpnI cloning sites of vector pKSV7 containing the 5′-end actA fragment described above. The new construct was designated DP-2308 and was verified by DNA sequencing. The 10403S chromosomal allele of hly-mpl-actA was replaced with the hly-actA allele (or Δmpl) of DP-2308 by allelic exchange (20) to generate strain DP-L2343. The Δmpl mutation of DP-L2343 was verified by Southern analysis.
Deletion of the actA promoter region was achieved by using the gene splicing by overlap extension (SOEing) method of PCR (27) with the following primers: actAΔ1 (5′-GGCGAATTCCATGCGCCAAAACTATTGTTG-3′), actAΔ4 (5′-GGCCTGCAGAGCCGCATTCTCAGTTTGTTC-3′), actAΔ5 (5′-TTAATCCCACTTATACTCCCTCCTTCAGTTAACCCCAACTG-3′), and actAΔ6 (5′-TTAATCCCACTTATACTCCCTCCTTCAGTTAACCCCAACTG-3′). Primers actAΔ1 and actAΔ4 contained EcoRI and PstI sites, respectively (underlined), to facilitate cloning of the final PCR product into allelic exchange shuttle vector pKSV7 (57). Initial PCRs were carried out with separate reaction mixtures, primers actAΔ1 and actAΔ5, and primers actAΔ4 and the actAΔ6; the products were purified and combined in a second PCR with primers actAΔ1 and actAΔ4. The resulting PCR product contained a deletion of the actA promoter region beginning directly downstream of the mpl stop codon and extending to the Shine-Delgarno region of actA, with a flanking sequence on either side to facilitate homologous recombination. The final PCR product was digested with EcoRI and PstI and subcloned into vector pKSV7 to yield vector pNF765. Transfer of the actA promoter deletion to the L. monocytogenes chromosome in a single copy was carried out by allelic exchange as previously described (20) to generate strain NF-L767, and the deletion was verified by Southern analysis and DNA sequencing of PCR products derived from the L. monocytogenes chromosome.
Construction of L. monocytogenes actA-gus-plcB transcriptional gene fusion mutants.
Primers GUS-XBA (5′-GCTCTAGAAGGAGGAAAAATATGTTTACGTCCTGTAGAAA-3′) and GUS-PST (5′-GGCTGCAGTCATTGTTTGCCTCCCTGC-3′) were designed to amplify gus coding sequences from plasmid pMLK100 (30) by PCR (28) and to introduce a gram-positive ribosome binding site derived from Shine-Delgarno sequence 1 (SD1) of ermC (14) (underlined sequence of GUS-XBA) upstream of gus. Plasmid pNF333, containing a transcriptional fusion of actA to the wild-type gfp allele of Aquorea victoria, was described previously (21). Plasmid pNF333 was digested with XbaI and PstI to remove the wild-type copy of gfp, and the appropriately digested gus PCR product was subcloned into pNF333 to generate plasmid pNF470. Plasmid pNF470 thereby contains a transcriptional fusion of actA to gus as well as flanking L. monocytogenes regions for introduction of the actA-gus-plcB fusion into the L. monocytogenes chromosome. Transfer of the actA-gus-plcB transcriptional fusion to the L. monocytogenes chromosome in a single copy was carried out by allelic exchange as previously described (20) with the following L. monocytogenes strains (Table 1): 10403S, to generate NF-L476 (actA-gus-plcB); DP-L1465, to generate NF-L707 (Δmpl274-510 actA-gus-plcB); DP-L2296, to generate NF-L861 (Δmpl131--361 actA-gus-plcB); and DP-L2343, to generate NF-L862 (Δmpl actA-gus-plcB). Introduction of the actA-gus-plcB reporter gene fusion into L. monocytogenes parent strain 10403S did not affect extracellular growth, intracellular growth, cell-to-cell spread, or virulence of the strain in mice (N. Freitag and H. Bouwer, unpublished data).
Complementation analysis of ΔPactA and Δmpl deletion mutants with actA supplied in a single copy in trans.
The introduction of the complete actA promoter and coding sequence in a single copy within the comK locus of the L. monocytogenes chromosome in strain NF-L767 to generate strain NF-L904 was accomplished with the assistance of Richard Calendar, Peter Lauer, and Daniel Portnoy, University of California at Berkeley, by using a method based on bacteriophage integration (unpublished data) (see also reference 37).
Metabolic labeling of ActA in infected J774 cells.
J774 mouse macrophage-like cells were infected with L. monocytogenes as described previously (39). At 2.5 h postinfection, the infected cells were starved for methionine and cysteine, host protein synthesis was blocked with anisomycin (30 μg/ml) and cycloheximide (22.5 μg/ml), and cell-to-cell spread was inhibited with cytochalasin D (0.25 μM). Twenty minutes later, the infected cells were pulse-labeled for 5 min with 35S-methionine as described previously (39). The cells were rapidly chilled on ice, washed four times in cold phosphate-buffered saline, and lysed in 200 μl of 2× sample buffer (0.06 M Tris HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 5% 2-mercaptoethanol, 0.01% bromophenol blue). The lysates were rapidly frozen in dry ice-ethanol and stored at −80°C for 1 day. Bacterial counts were determined in triplicate for each bacterial strain tested as described previously (39). Samples were resolved on an SDS-8% polyacrylamide gel. The amount of sample loaded per lane was normalized for the number of bacteria. After electrophoresis, the gel was processed for phosphorimaging analysis.
Substitution of the actA promoter PrfA binding site with that of hly.
L. monocytogenes genomic DNA was isolated and used in conjunction with PCR (28) to amplify a fragment containing the actA promoter and a portion of the N-terminal coding region of actA with the following primers: ActA-hlyP (5′-GCGGATCCTGATTAACATTTGTTAAAGAAAAATT-3′), designed to substitute the PrfA box of actA with that of hly (underlined nucleotides), and ActA-2 (5′-GCTCTAGAGTGTTTTTAATTATTTTTTC-3′). The resulting PCR product was digested with XbaI and BamHI and subcloned into pKSV7 (57) to generate plasmid pNF468. Primers mpl-12/97 (5′-GCAGATCTTCAGTTAACCCCAACTGC-3′) and mpl-4 (5′-GCGAATTCGCCTGGTAACGCGAGAAA-3′) were used in conjunction with PCR and L. monocytogenes genomic DNA to amplify a product containing the C-terminal coding region of mpl. This PCR product was digested with EcoRI and BglII and subcloned into pNF468 to produce plasmid pNF517. Plasmid pNF517 thus contains the modified actA promoter with the hly PrfA box substitution [designated P(hly)-actA] and regions of flanking homology for introduction of the P(hly)-actA promoter mutation into the L. monocytogenes chromosome via homologous recombination.
Plasmid pNF517 was introduced into L. monocytogenes strain NF-L476 by electroporation, and transformants were isolated following growth at 30°C on BHI agar containing chloramphenicol. L. monocytogenes strains containing the P(hly)-actA promoter mutation in a single copy in the chromosome were isolated following allelic exchange as previously described (20) to generate strain NF-L572 [P(hly)-actA-gus-plcB] (Table 1). Southern analysis (51) was used to verify the presence of the P(hly)-actA promoter in a single copy in the correct L. monocytogenes chromosomal location by confirmation of a novel BstYI restriction site. The sequence of the P(hly)-actA mutant promoter was confirmed by DNA sequencing of PCR products amplified from chromosomal DNA by using Thermosequenase (Amersham Life Science, Arlington Heights, Ill.).
Introduction of prfA7973 (prfA*) into NF-L476 and NF-L572.
L. monocytogenes strain NCTC7973, a natural isolate, was described elsewhere (1, 42, 45). The NCTC7973 prfA allele, which encodes a serine in place of a glycine at position 145, produces increased expression of virulence genes in NCTC7973 in comparison to wild-type L. monocytogenes and is thought to encode a transcriptionally active, cofactor-independent form of PrfA (PrfA*) (49). NCTC7973 PrfA also has a second amino acid change (in comparison to 10403S PrfA), a Cys to Tyr change at position 229; however, this substitution has not been demonstrated to influence PrfA-dependent gene expression (1). pCON1-ΔprfA7973 was conjugated into 10403S derivatives NF-L476 and NF-L572 to generate NF-L753 [prfA* actA-gus-plcB] and NF-L754 [prfA* P(hly)-actA-gus-plcB] as described by Behari and Youngman (1), with the following modifications. Transconjugants were selected on BHI agar containing chloramphenicol (5 μg/ml) after 24 h of incubation at 30°C. Selected colonies were inoculated into BHI medium containing chloramphenicol and streptomycin and grown overnight at 40°C with shaking to force chromosomal integration of the vector. Overnight 40°C cultures were diluted 1:1,000 into fresh BHI medium containing chloramphenicol and streptomycin and incubated overnight at 40°C with shaking. Appropriate dilutions were then plated on BHI agar containing chloramphenicol and incubated at 40°C. Integration of the vector into the chromosome was verified and the prfA7973 (prfA*) sequences were confirmed by PCR amplification of prfA and sequencing of the PCR products.
Assay for hemolytic activity.
Stationary-phase bacteria were diluted 1:10 into BHI medium and grown at 37°C for 5 h with shaking. The supernatant fluid was assayed for hemolytic activity as previously described (9).
Plaque formation in L2 cells.
Plaque assays were performed as previously described by Sun et al. (58). Plaque size was measured by using a micrometer, and the average diameter of at least 10 plaques from three independent experiments was determined.
Intracellular growth assays.
The cell line used in these studies was the J774 mouse macrophage-like cell line maintained as previously described (6). Intracellular growth in J774 cells and in mouse bone marrow-derived macrophages was monitored by using cell monolayers grown on acid-washed glass coverslips in tissue culture dishes as previously described (20, 48, 58).
β-Glucuronidase (GUS) assays of bacteria grown in liquid cultures.
For experiments with BHI broth, BHI broth treated with 0.2% charcoal (charcoal removed by filtration of autoclaved medium), or Luria broth (LB), overnight cultures of bacteria grown in the indicated broth media were diluted 1:10 into fresh media and grown for 5 h with shaking at 37°C. For cultures incubated in Dulbecco's minimal essential medium (MEM; GIBCO-BRL, Rockville, Md.), overnight cultures of bacteria were diluted into BHI broth as described above and grown for 3 h with shaking at 37°C. Aliquots (3 ml) of cultures were removed and centrifuged briefly to recover bacteria, and then the bacterial pellets were washed in phosphate-buffered saline and resuspended in 3 ml of MEM. Bacterial suspensions in MEM were incubated at 37°C for 2 h. Bacterial pellets from 1-ml culture aliquots were collected following centrifugation and quickly frozen on dry ice. The optical density at 595 nm was measured for each culture by using a Spectronic 20 spectrophotometer (Milton Roy, Rochester, N.Y.). In some experiments, aliquots of bacterial cultures were diluted and plated on LB agar plates to determine CFU per milliliter.
For GUS enzymatic assays, bacterial cell pellets were thawed, washed once with ABT buffer (0.1 M potassium phosphate [pH 7.0], 0.1 M NaCl, 0.1% Triton) and resuspended in 200 μl of ABT buffer. GUS activity was measured as described by Youngman (61), with the substitution of 4-methylumbilliferyl-β-d-glucuronide in place of 4-methylumbilliferyl-β-d-galactoside. Units were normalized to bacterial CFU to enable direct comparison of GUS activity between broth-grown cultures and bacteria isolated from infected tissue culture cells. In some experiments, in which units of GUS activity were compared for broth-grown cultures, the units were normalized to the optical density at 595 nm. Background activity from negative control samples was subtracted from all samples.
Intracellular GUS assays.
Measurement of intracellular GUS activity was carried out by using the protocol described by Moors et al. (43) and J774 cells, with the substitution of 4-methylumbelliferyl-β-d-glucuronide as a substrate in place of 4-methylumbilliferyl-β-d-galactoside.
Virulence phenotype.
LD50s were determined by intravenous injection of BALB/c mice as previously described (47).
RESULTS
Measurement of extracellular and intracellular actA expression levels.
To better define the promoter elements that contribute to the intracellular induction of actA expression, a series of actA and mpl promoter and coding region mutants were constructed and introduced into the L. monocytogenes chromosome. The constructs were designed such that the loss of promoter function (promoter deletions) could be phenotypically contrasted with the loss of gene function (coding sequence deletions). To facilitate the measurement of actA expression levels, transcriptional fusions between actA and the reporter gene gus were constructed and introduced into each L. monocytogenes mutant strain (Fig. 1). gus encodes the enzyme β-glucuronidase (GUS) (29) and has been used successfully to generate reporter gene fusions in a variety of bacteria (19, 30, 53), including L. monocytogenes (1).
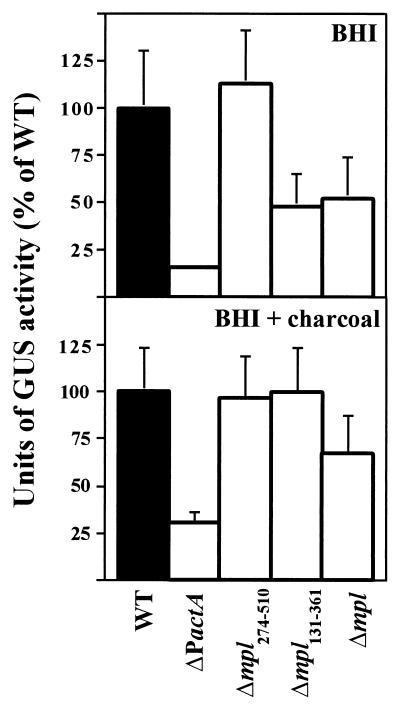
As shown in Fig. 2, the ΔPactA mutant strain lacking the actA proximal promoter showed a significant, sixfold decrease compared to the wild type in the levels of actA expression in standard broth media (BHI) (Fig. 2). The Δmpl131-361 and Δmpl mutants also showed reduced expression levels, but to a lesser degree (approximately twofold lower than the wild-type expression level). Levels of actA expression were not reduced in the mpl frameshift mutant, Δmpl274-510. It was previously demonstrated that actA expression is up-regulated following the growth of L. monocytogenes in BHI broth treated with 0.2% activated charcoal (5, 50) (Fig. 2). The ΔPactA mutant demonstrated lower overall levels of actA expression in comparison to the wild type and the mpl deletion mutants following growth in BHI broth treated with 0.2% charcoal; however, expression was still induced approximately 11-fold over the levels in BHI broth (mean and standard error [SE], 3 ± 1 U in BHI broth versus 33 ± 6 U in BHI broth-charcoal). The remaining mutants did not differ significantly from the wild type in actA expression levels. Taken together, these data suggest that the actA proximal promoter is the major regulatory element contributing to the levels of actA expression observed in broth cultures. A modest contribution from the mpl promoter could be observed in BHI broth but was not evident following treatment of BHI broth with 0.2% activated charcoal. Interestingly, the induction of actA expression in BHI broth-0.2% charcoal occurred in all of the mutant strains, but the highest level of induction (11-fold) was observed in the ΔPactA mutant, which contains the complete mpl promoter and coding sequences.
FIG.2.
Examination of actA expression in L. monocytogenes mpl and actA mutant strains grown in broth media. GUS activity was measured following 5 h of growth in BHI broth or BHI broth treated with 0.2% charcoal. Units of GUS were normalized for optical density at 595 nm as described by Youngman (61) for the measurement of β-galactosidase activity but with the appropriate substrate substitution of 4-methylumbilliferyl-β-d-glucuronide. Units are expressed as a percentage of NF-L476 (wild-type [WT]) activity in BHI broth or BHI broth treated with 0.2% charcoal. Each assay was done in triplicate, and the data represent the mean and SE for at least three individual experiments.
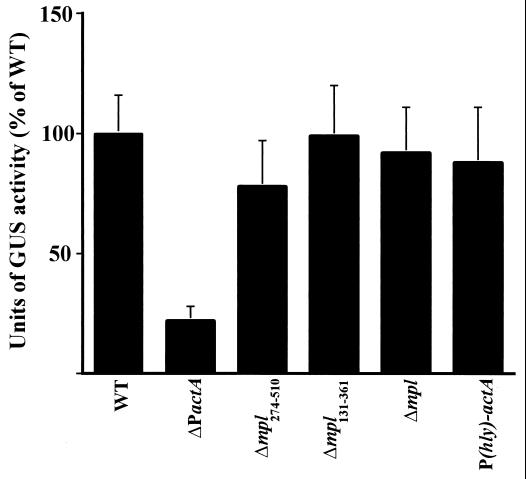
ActA is one of the most abundant surface proteins expressed by L. monocytogenes within the host cell cytosol (5, 6). Moors et al. (43), using an actA-lacZ reporter gene fusion, recently demonstrated that actA expression is highly induced (226-fold) in bacteria grown in the mouse macrophage-like cell line J774. To compare actA expression by use of gus transcriptional fusions in cytosolic bacteria, J774 cells were infected with L. monocytogenes; at 5 h postinfection, the cells were lysed, aliquots were removed to determine the numbers of viable bacteria, and GUS activity was measured (Fig. 3). We found that the amount of actA-gus expression observed for wild-type L. monocytogenes following intracellular growth was approximately 150 times the amount produced by bacteria grown in LB (mean and SE, 61.0 ± 1.1 U/106 CFU in J774 cells and 0.4 ± 0.1 U/106 CFU in LB); this high-level induction of actA expression agrees well with the 226-fold level of induction reported by Moors et al. (43). The amount of actA expression observed in the host cytosol was 47 times the amount produced by bacteria grown in BHI broth treated with 0.2% charcoal (1.3 ± 0.5 U/106 CFU in BHI broth treated with 0.2% charcoal). The levels of actA expression were approximately fourfold lower in the ΔPactA mutant than in the wild type; all other mutants showed no statistically significant difference in actA expression following cytosolic growth.
FIG. 3.
Intracellular actA expression in mpl and actA mutant strains. Tissue culture dishes containing monolayers of J774 cells were infected with the indicated L. monocytogenes strains for 5 h as described in Materials and Methods. GUS activity was determined following lysis of the monolayers. The number of CFU per dish was determined by lysing infected J774 cells grown on coverslips in duplicate dishes and plating a portion of the lysates on LB agar. The total number of CFU per dish was extrapolated by multiplication by a factor that corrected for the area of the coverslip relative to that of the 60-mm dish. Units of GUS are as described by Youngman (61) for the measurement of β-galactosidase activity but with the appropriate substrate substitution of 4-methylumbilliferyl-β-d-glucuronide. Units are expressed as a percentage of NF-L476 (wild-type [WT]) activity. Each assay was done in triplicate, and the data represent the mean and SE for at least three individual experiments. Background activities from 10403S-infected monolayers and from uninfected cells were equivalent and never represented greater than 2% of the activity detected for NF-L476.
To confirm the actA expression patterns observed for the L. monocytogenes mutant strains with GUS reporter assays, metabolic labeling of bacteria grown in the macrophage-like cell line J774 was carried out, and ActA was extracted and detected as described in Materials and Methods (Fig. 4). In agreement with the reporter gene fusion data, the ΔPactA mutant demonstrated a significant reduction in ActA protein levels in comparison to the wild type, as little to no protein could be detected. With the exception of the actA coding region deletion mutant, ΔactA164-465, the remaining mutants produced ActA at levels comparable to those seen with the wild type. These results indicate that the primary promoter responsible for the induction of actA expression within the cytosol of infected host cells is the actA proximal promoter and that the mpl promoter contributes little, if any, to the intracellular induction of actA expression.
FIG. 4.
Intracellular expression of ActA by L. monocytogenes in J774 cells. J774 mouse macrophage-like cells were infected with L. monocytogenes; at 2.5 h postinfection, the infected cells were starved for methionine and cysteine, host protein synthesis was blocked, and cell-to-cell spread was inhibited as described in Materials and Methods. Infected cells were pulse-labeled with 35S-methionine prior to host cell lysis, and SDS-extractable bacterial proteins were resolved on an SDS-8% polyacrylamide gel. The amount of sample loaded per lane was normalized for the number of bacteria. The three species of ActA, representing different phosphorylation states of the protein, are the major protein bands observed by using this approach (6). WT and WT*, proteins derived from 10403S- and NF-L476-infected cells, respectively.
To assess the effects of the actA and mpl deletion mutations on the capacity of L. monocytogenes to grow and spread within infected host cells, the mutants were examined for their ability to form plaques in monolayers of mouse L2 cells (Table 2). All of the actA and mpl deletion mutants formed smaller plaques than wild-type L. monocytogenes, indicative of defects in intracellular growth and/or cell-to-cell spread. The most severe defects were observed for the ΔPactA and ΔactA164-465 mutants, which formed tiny plaques representing a 93% reduction in plaque size in comparison with the plaques formed by wild-type L. monocytogenes. The defect in the plaque formation of ΔPactA could be partially complemented by the introduction of a single copy of actA into the comK locus of L. monocytogenes (Table 2). The degree of complementation of the ΔPactA mutant (78%) was similar to that observed for the ΔactA164-465 mutant following the introduction of actA::comK (37). The ΔactA164-465 mutant was previously demonstrated to be defective for the nucleation of actin filaments and impaired for cell-to-cell spread (6). The ΔPactA mutant was also defective for cell-to-cell spread within monolayers of J774 cells and formed microcolonies within infected cells that were similar in appearance to those formed by the ΔactA164-465 mutant (H. Marquis, unpublished data). It therefore appears that the loss of the actA proximal promoter results in a phenotype equivalent to that observed for mutants that lack a functional ActA protein.
TABLE 2.
Plaque formation in L2 cells and virulence phenotype analysis
Deletion of the mpl promoter and coding region resulted in plaques that were 60% the size of wild-type L. monocytogenes plaques (Table 2). The Δmpl mutant defect in plaque formation was less severe than that observed for the ΔPactA or ΔactA164-465 mutant; however, the Δmpl mutant did exhibit a dramatic decrease in virulence in mice (2 orders of magnitude), similar to the decreases observed for the ΔPactA and ΔactA164-465 mutants. In contrast, deletion of the Mpl protease active site (Δmpl131-361) did not reduce virulence in mice (LD50, <5 × 104), although plaque size was moderately reduced (70%). The reduction in virulence for the Δmpl mutant does not appear to result from decreased expression of actA, as ActA protein levels were not significantly reduced in this mutant (Fig. 3 and 4). Interestingly, the Δmpl274-510 mutant, which contains a functional mpl promoter but lacks the C-terminal half of the protein, shows a 2-log-unit reduction in virulence in mice (LD50, 2 × 106). The virulence defect observed for Δmpl274-510 but not for Δmpl131-361 suggests that the C-terminal portion of the Mpl protein, beyond the active site for the protease, contributes a significant role toward the function of Mpl and its role in L. monocytogenes pathogenesis.
Introduction of a high-affinity PrfA DNA binding site within the actA promoter.
The experiments described above defined the actA proximal promoter as the major regulatory element contributing to the induction of actA expression within the cytosol of infected host cells. To further investigate what nucleotide motifs are important for promoter activation, we directed our focus on the PrfA binding site of the actA promoter. actA is strictly dependent upon PrfA for expression (2). The actA proximal promoter contains a PrfA DNA binding site in the −40 region that is an imperfect palindrome and that is thought to represent a low-affinity binding site for the transcriptional activator (22, 23, 54). To determine if the introduction of a high-affinity PrfA DNA binding site within the actA promoter would result in an increased level of actA expression, the PrfA box of the actA promoter was replaced with a high-affinity site derived from hly by allelic exchange as described in Materials and Methods. The actA promoter mutation [P(hly)-actA] was introduced in a single copy into the chromosome of L. monocytogenes strain NF-L476, containing the actA-gus-plcB transcriptional reporter gene fusion (Table 1). The wild-type actA gene and promoter are referred to from this point on as PWT-actA to distinguish this construct from the P(hly)-actA promoter mutant.
Previous studies demonstrated that hly transcription occurs at levels 10-fold higher than those for actA following bacterial growth in LB cultures (43). To determine if actA transcriptional activation in broth cultures was increased by the introduction of the P(hly)-actA promoter mutation, we measured actA expression in L. monocytogenes strains containing chromosomal actA-gus transcriptional reporter gene fusions following growth under a variety of broth culture conditions (Fig. 5). Consistent with results reported in previous studies (5, 6, 50), only low levels of actA expression were detected for wild-type L. monocytogenes following 5 h of growth in BHI broth (Fig. 5). Significantly higher levels of actA expression were seen following growth in LB or after 2 h of incubation in tissue culture medium (MEM), as first reported by Bohne et al. (4, 5). The highest levels of actA expression were achieved following 5 h of growth in BHI broth treated with 0.2% activated charcoal (Fig. 5). For all culture media tested, there was no significant difference in actA expression levels between L. monocytogenes strains containing either PWT-actA or P(hly)-actA. In agreement with data obtained from the gus reporter gene fusion experiments, similar amounts of ActA were detected in the PWT-actA and P(hly)-actA strains by SDS-polyacrylamide gel electrophoresis analysis of surface proteins after 5 h of bacterial growth in LB (L. Shetron-Rama and N. Freitag, unpublished data). These data indicate that substitution of the actA promoter PrfA binding site with that of the hly promoter produced no significant changes in the levels of extracellular actA expression.
FIG. 5.
Examination of actA expression in P(hly)-actA and PWT-actA L. monocytogenes strains grown in various broth media. GUS activity was measured following 5 h of growth in BHI broth (B), MEM (M), BHI broth treated with 0.2% charcoal (BC), and LB. Units of GUS are as described by Youngman (61) for the measurement of β-galactosidase activity but with the appropriate substrate substitution of 4-methylumbilliferyl-β-d-glucuronide. Each assay was done in triplicate, and the data represent the mean and SE for at least three individual experiments.
It was demonstrated previously that multiple copies of the hly PrfA DNA binding site may reduce the transcription of PrfA-dependent genes in L. monocytogenes by titrating available PrfA (4, 10). However, the PWT-actA and P(hly)-actA strains produced similar levels of hly-encoded listeriolysin O, as measured by secreted-hemolysin assays [mean and SE, 18 ± 2 U for 10403S, 20 ± 1 U for the PWT-actA strain, and 25 ± 5 U for the P(hly)-actA strain], thus indicating that the single extra copy of the hly PrfA box resulted in no significant titration effects.
To compare PWT-actA and P(hly)-actA expression by using gus transcriptional fusions in cytosolic bacteria, J774 cells were infected with L. monocytogenes; at 5 h postinfection, the cells were lysed and GUS activity was measured (Fig. 3). As observed for bacteria grown in broth cultures, the expression of actA in the L. monocytogenes P(hly)-actA promoter mutant was not significantly different from wild-type actA expression (Fig. 3). Furthermore, no defect was observed for this mutant with regard to intracellular growth and/or cell-to-cell spread in mouse L2 cells, macrophage cell lines, or primary mouse bone marrow-derived macrophages (Table 2) (Shetron-Rama and Freitag, unpublished). Thus, the introduction of a high-affinity PrfA binding site into the actA promoter resulted in no change in either extracellular or intracellular patterns of actA expression.
actA promoter activation in L. monocytogenes strains containing PrfA*.
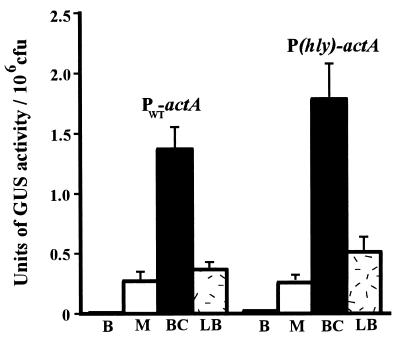
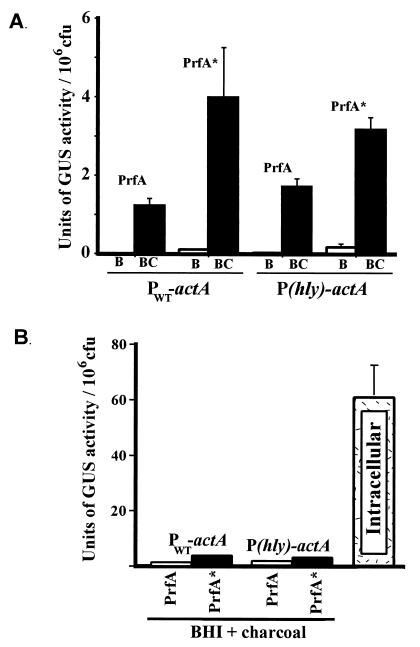
The prfA* mutant allele appears to encode a constitutively activated form of the PrfA protein (PrfA*) which is thought to resemble CRP*, which is active in the absence of cofactor binding (32, 35, 49). PrfA* has been demonstrated to have an increased binding affinity for target DNA (59). To quantitate and compare the effects of PrfA* on PWT-actA and P(hly)-actA promoter activation, the prfA* allele from L. monocytogenes strain NCTC7973 was introduced into the chromosome in place of wild-type prfA in strains NF-L476 and NF-L572 as described in Materials and Methods. NCTC7973 PrfA also has a second amino acid change (in comparison to 10403S PrfA), a Cys-to-Tyr change at position 229; however, this substitution has not been demonstrated to influence PrfA-dependent gene expression (1). actA-gus expression was measured following bacterial growth in BHI broth and BHI broth treated with 0.2% charcoal (Fig. 6). The introduction of prfA* resulted in an 11-fold stimulation of actA expression for bacteria grown in BHI broth (mean and SE, 0.01 ± 0.004 U/106 CFU for NF-L476 versus 0.11 ± 0.02 U/106 CFU for NF-L752) and an approximately 3-fold stimulation for bacteria grown in BHI broth treated with 0.2% activated charcoal. No significant difference was seen in expression levels between PWT-actA and P(hly)-actA L. monocytogenes mutant strains, indicating that the transcriptional activation of actA by PrfA* was not influenced by the introduction of a high-affinity PrfA binding site within the actA promoter (Fig. 6A). Interestingly, the introduction of the prfA* allele resulted in levels of actA expression following growth in BHI broth that were 555-fold lower than those observed for intracellular bacteria (0.11 ± 0.02 U/106 CFU versus 61.0 ± 10.6 U/106 CFU) (Fig. 6B). The expression of actA in prfA*-containing bacteria grown in BHI broth-charcoal was highly induced in comparison to the results for strains grown in BHI broth, yet the absolute level of induction was still approximately 20-fold lower than the level achieved by L. monocytogenes within the host cytosol (Fig. 6B). No significant difference was seen between intracellular levels of either PWT-actA or P(hly)-actA expression for L. monocytogenes strains containing wild-type prfA or prfA* (data not shown).
FIG. 6.
Comparison of PWT-actA and P(hly)-actA promoter activation in L. monocytogenes strains containing PrfA or PrfA*. GUS activity was measured following 5 h of growth in BHI broth or BHI broth treated with 0.2% charcoal or following bacterial growth in J774 cells. Units of GUS are as described by Youngman (61) for the measurement of β-galactosidase activity but with the appropriate substrate substitution of 4-methylumbilliferyl-β-d-glucuronide. Each assay was done in triplicate, and the data represent the mean and SE for at least three individual experiments. (A) Measurement of actA-gus expression in cultures following growth in either BHI broth (B) or BHI broth treated with 0.2% charcoal (BC). (B) Comparison of the levels of actA-gus expression achieved under optimal extracellular conditions versus the level of activity observed for L. monocytogenes located within the host cell cytosol (intracellular). The intracellular value shown is for NF-L476. Background activities from 10403S-infected monolayers and from uninfected cells were equivalent and never represented greater than 2% of the activity detected for NF-L476.
DISCUSSION
L. monocytogenes provides an excellent model system for defining the responses of an intracellular pathogen to the host cell environment. In this study, we have chosen to focus on identifying the promoter regions that contribute to the intracellular induction of actA, a gene that is highly induced once L. monocytogenes reaches the cytosol of infected host cells (5, 6, 21, 43). Although two promoters have been reported to contribute to actA expression (5), the data presented in this study indicate that only the actA proximal promoter is required for intracellular induction. The increase in actA expression mediated by the actA proximal promoter is impressive, as cytosolic expression levels were found to be 150 times greater than the levels of expression observed for cultures grown in LB and 46 times greater than the levels observed for cultures grown in BHI broth treated with activated charcoal. The large increase observed in actA expression for intracellular bacteria could not be duplicated in broth cultures by the introduction of a higher-affinity PrfA binding site within the actA promoter or the mutationally activated form of PrfA (PrfA*), suggesting that additional steps or cofactors are required for the intracellular induction of actA expression.
The upstream mpl promoter functions as a minor contributor to patterns of actA expression, but its presence is required for full virulence. Comparative studies of the Δmpl274-510, Δmpl131-361, and Δmpl mutant strains indicate that the Mpl protein may play an additional role in L. monocytogenes pathogenesis beyond the processing of proPC-PLC to mature PC-PLC (a broad-spectrum phospholipase C). It has been demonstrated that the processing of proPC-PLC to the mature form in infected cells occurs via both Mpl-dependent and Mpl-independent pathways, the latter being mediated by host cell cysteine proteases (39). The mutant strain containing the in-frame deletion of the protease active site (Δmpl131-361) was not attenuated for virulence in a mouse model of infection (38) (Table 2). In contrast to the Δmpl131-361 mutant, a mutant lacking the C-terminal half of Mpl (Δmpl274-510) or containing a complete deletion of the mpl coding sequences (Δmpl) exhibited a dramatic 2-log-unit decrease in virulence. Complementation of the mpl promoter and gene deletion mutant could not be achieved by providing a copy of actA in trans, suggesting that the defect in virulence was not due to insufficient synthesis of actA (Shetron-Rama and Freitag, unpublished). These results were further supported by experiments demonstrating that actA expression occurs at levels similar to those in wild-type strains in the absence of the mpl promoter (Fig. 3 and 4). The results presented here therefore suggest that the functional roles of Mpl in L. monocytogenes pathogenesis have yet to be completely defined. Experiments designed to more closely examine the contributions of Mpl to L. monocytogenes intracellular growth and cell-to-cell spread are currently in progress.
The ΔPactA promoter deletion mutant was found to closely resemble the ΔactA164-465 gene deletion mutant in tissue culture infection assays, and both mutants were highly attenuated for virulence in mice (Table 2). However, the LD50 for the actA promoter deletion mutant was approximately fourfold lower than that for the ΔactA164-465 mutant. It is possible that the low level of intracellular actA expression contributed by the upstream mpl promoter (Fig. 3) accounts for the difference in virulence between the ΔactA164-465 and ΔPactA mutants. Low-level actA expression may provide enough functional ActA for some cell-to-cell spread, although this was not apparent in L2 cell plaque assays (Table 2). Alternatively, it is possible that PlcB activity is toxic for host cells in the absence of cell-to-cell spread of the bacteria. Attenuation of virulence as a result of host cell toxicity has been reported for L. monocytogenes hly mutants lacking a PEST-like sequence (13). It is likely that normal levels of PlcB are expressed in the ΔactA164-465 mutant strain, whereas the loss of the actA promoter should greatly diminish plcB expression.
Previous studies suggested that the timing of PrfA-dependent promoter activation and the levels of transcriptional induction achieved are both closely tied to the binding affinity of PrfA for specific target promoters (22, 23, 54). The data presented here, in agreement with a recent study carried out with Bacillus subtilis (60), suggest that the presence of a high-affinity PrfA DNA binding site does not significantly influence the PrfA-dependent activation of all target promoters. Intracellular induction of virulence gene expression in L. monocytogenes therefore appears to be a complex process requiring multiple events, including increased production of PrfA protein, activation of PrfA, and induction or activation of a second bacterial factor (or factors) within the host cytosol. The existence of a PrfA-activating factor has been reported (3, 15), but thus far, other than RNA polymerase (2), no other cofactors that participate in L. monocytogenes virulence gene regulation have been identified. It is, of course, possible that the product of the mutationally activated prfA* allele does not fully mimic the environmentally activated form of the wild-type protein. Work in progress, however, indicates that it is possible to isolate L. monocytogenes mutants which have increased actA expression in broth culture and which contain mutations mapping outside the prfA regulon (Shetron-Rama and Freitag, unpublished). Characterization of these mutants will aid in the identification of additional factors that contribute to the regulation of L. monocytogenes virulence gene expression. Indeed, it is possible that the induction of actA expression within the host cell cytosol does not require PrfA but instead relies on the activity of other factors. This possibility will be explored in future studies.
It has been shown that hly and actA differ in their patterns of expression within infected host cells (8, 43). The hly and plcA promoters have been reported to be predominantly activated in the phagosomal compartment, while those for actA and inlC are predominantly activated in the host cell cytosol (8). We investigated whether the P(hly)-actA promoter mutation resulted in altered patterns of L. monocytogenes actA expression with respect to cell compartment location by introducing the mutation into L. monocytogenes strains containing a transcriptional fusion of gfp to actA in the chromosome. gfp was successfully used in previous studies as a reporter gene system to monitor the timing and intracellular location of actA expression through the use of fluorescence microscopy (8, 21). No significant difference was observed for either the timing or the location of intracellular actA expression with the P(hly)-actA mutant (Shetron-Rama and Freitag, unpublished).
We have focused our attention on the mechanisms of induction of actA expression as a means of characterizing the events that lead to intracellular gene expression in L. monocytogenes. The high-level induction of actA expression that occurs within the host cytosol is impressive, and it accents the ability of L. monocytogenes to respond to specific host cell environments. By isolating and analyzing L. monocytogenes mutants with altered patterns of intracellular gene expression, we hope to be able to identify additional components of the regulatory machinery and perhaps to gain insight into the nature of the intracellular signals used by this pathogen to guide its infectious processes.
Acknowledgments
We thank Patrick Piggot for the gift of plasmid pMLK100 and Jai Behari and Philip Youngman for the gift of plasmid pCON1-ΔprfA7973. We thank Dwayne Baxa and Audelia Munguia for assistance in strain constructions. We thank Dan Portnoy, Peter Lauer, and Richard Calendar for the construction of actA-complemented L. monocytogenes strains. We thank Jerry Cangelosi, Daniel Portnoy, and Richard Calendar for critical comments and helpful discussions. We also thank the reviewers of the manuscript for insightful criticism and helpful comments.
This work was supported by Public Health Service grants AI41816 (to N.E.F.), AI42800 (to H.M.), and AI40698 and AI44376 (to H.G.A.B.) from the National Institutes of Health and by VA Merit Review Funds (to H.G.A.B.).
REFERENCES
- 1.Behari, J., and P. Youngman. 1998. Regulation of hly expression in Listeria monocytogenes by carbon sources and pH occurs through separate mechanisms mediated by PrfA. Infect. Immun. 66:3635-3642. [DOI] [PMC free article] [PubMed]
- 2.Böckmann, R., C. Dickneite, W. Goebel, and J. Buhne. 2000. PrfA mediates specific binding of RNA polymerase of Listeria monocytogenes to PrfA-dependent virulence gene promoters resulting in a transcriptionally active complex. Mol. Microbiol. 36:487-497. [DOI] [PubMed] [Google Scholar]
- 3.Böckmann, R., C. Dickneite, B. Middendorf, W. Goebel, and Z. Sokolovic. 1996. Specific binding of the Listeria monocytogenes transcriptional regulator PrfA to target sequences requires additional factor(s) and is influenced by iron. Mol. Microbiol. 22:643-653. [DOI] [PubMed] [Google Scholar]
- 4.Bohne, J., H. Kestler, C. Uebele, Z. Sokolovic, and W. Goebel. 1996. Differential regulation of the virulence genes of Listeria monocytogenes by the transcriptional activator PrfA. Mol. Microbiol. 20:1189-1198. [DOI] [PubMed] [Google Scholar]
- 5.Bohne, J., Z. Sokolovic, and W. Goebel. 1994. Transcriptional regulation of prfA and PrfA-regulated virulence genes in Listeria monocytogenes. Mol. Microbiol. 11:1141-1150. [DOI] [PubMed] [Google Scholar]
- 6.Brundage, R. A., G. A. Smith, A. Camilli, J. A. Theriot, and D. A. Portnoy. 1993. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc. Natl. Acad. Sci. USA 90:11890-11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bubert, A., H. Kestler, M. Gotz, R. Bockmann, and W. Goebel. 1997. The Listeria monocytogenes iap gene as an indicator gene for the study of PrfA-dependent regulation. Mol. Gen. Genet. 256:54-62. [DOI] [PubMed] [Google Scholar]
- 8.Bubert, A., Z. Sokolovic, S.-K. Chun, L. Papatheodorou, A. Simm, and W. Goebel. 1999. Differential expression of Listeria monocytogenes virulence genes in mammalian host cells. Mol. Gen. Genet. 261:323-336. [DOI] [PubMed] [Google Scholar]
- 9.Camilli, A., C. R. Paynton, and D. A. Portnoy. 1989. Intracellular methicillin selection of Listeria monocytogenes mutants unable to replicate in a macrophage cell line. Proc. Natl. Acad. Sci. USA 86:5522-5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cossart, P., M. F. Vincente, J. Mengaud, F. Baquero, J. C. Perez-Diaz, and P. Berche. 1989. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57:3629-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabiri, G. A., J. M. Sanger, D. A. Portnoy, and F. S. Southwick. 1990. Listeria monocytogenes moves rapidly through the host cytoplasm by inducing directional actin assembly. Proc. Natl. Acad. Sci. USA 87:6068-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decatur, A. L., and D. A. Portnoy. 2000. A PEST-like sequence in Listeriolysin O essential for Listeria monocytogenes pathogenecity. Science 290:992-995. [DOI] [PubMed] [Google Scholar]
- 14.Denoya, C. D., D. H. Bechhofer, and D. Dubnau. 1986. Translational autoregulation of ermC 23S rRNA methyltransferase expression in Bacillus subtilis. J. Bacteriol. 168:1133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickneite, C., R. Böckmann, A. Spory, W. Goebel, and Z. Sokolovic. 1998. Differential interaction of the transcription factor PrfA and the PrfA-activating factor (Paf) of Listeria monocytogenes with target sequences. Mol. Microbiol. 27:915-928. [DOI] [PubMed] [Google Scholar]
- 16.Domann, E., and T. Chakraborty. 1989. Nucleotide sequence of the listeriolysin gene from a Listeria monocytogenes serotype 1/2a strain. Nucleic Acids Res. 17:6406.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domann, E., J. Wehland, M. Rohde, S. Pistor, M. Hartl, W. Goebel, M. Leimeister-Wachter, M. Wuenscher, and T. Chakraborty. 1992. A novel bacterial gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 11:1981-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubail, I., P. Berche, T. E. L. G. Consortium, and A. Charbit. 2000. Listeriolysin O as a reporter to identify constitutive and in vivo inducible promoters in the pathogen Listeria monocytogenes. Infect. Immun. 68:3242-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldhaus, M. J., V. Hwa, Q. Cheng, and A. Salyers. 1991. Use of an Escherichia coli β-glucuronidase gene as a reporter gene for investigation of Bacteroides promoters. J. Bacteriol. 173:4540-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freitag, N. E. 2000. Genetic tools for use with Listeria monocytogenes, p. 488-498. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 21.Freitag, N. E., and K. E. Jacobs. 1999. Examination of Listeria monocytogenes intracellular gene expression by using the green fluorescent protein of Aequorea victoria. Infect. Immun. 67:1844-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freitag, N. E., and D. A. Portnoy. 1994. Dual promoters of the Listeria monocytogenes prfA transcriptional activator appear essential in vitro but are redundant in vivo. Mol. Microbiol. 12:845-853. [DOI] [PubMed] [Google Scholar]
- 23.Freitag, N. E., L. Rong, and D. A. Portnoy. 1993. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect. Immun. 61:2537-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gahan, C. G. M., and C. Hill. 2000. The use of listeriolysin to identify in vivo induced genes in the Gram-positive intracellular pathogen Listeria monocytogenes. Mol. Microbiol. 36:498-507. [DOI] [PubMed] [Google Scholar]
- 25.Gellin, B. G., and C. V. Broome. 1989. Listeriosis. JAMA 261:1313-1320. [PubMed] [Google Scholar]
- 26.Gray, M. L., and A. H. Killinger. 1966. Listeria monocytogenes and listeric infections. Bacteriol. Rev. 30:309-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horton, R. 1993. In vitro recombination and mutagenesis of DNA, p. 251-260. In B. White (ed.), PCR protocols: current methods and applications. Humana Press Inc., Totowa, N.J.
- 28.Innis, M. A., and D. H. Gelfand. 1990. Optimization of PCRs, p. 3-12. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols. A guide to methods and applications. Academic Press, Inc., San Diego, Calif.
- 29.Jefferson, R. A. 1989. The GUS reporter gene system. Nature 342:837-838. [DOI] [PubMed] [Google Scholar]
- 30.Karow, M., and P. J. Piggot. 1995. Construction of gusA transcriptional fusion vectors for Bacillus subtilis and their utilization for studies of spore formation. Gene 163:69-74. [DOI] [PubMed] [Google Scholar]
- 31.Kathariou, S., P. Metz, H. Hof, and W. Goebel. 1987. Tn 916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J. Bacteriol. 169:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, J., S. Adhya, and S. Garges. 1992. Allosteric changes in the cAMP receptor protein of Escherichia coli: hinge reorientation. Proc. Natl. Acad. Sci. USA 89:9700-9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klarsfeld, A., P. L. Goossens, and P. Cossart. 1994. Five Listeria monocytogenes genes preferentially expressed in infected mammalian cells: plcA, purH. purD, pyrE and an arginine ABC transporter gene, arpJ. Mol. Microbiol. 13:585-597. [DOI] [PubMed] [Google Scholar]
- 34.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521-531. [DOI] [PubMed] [Google Scholar]
- 35.Kolb, A., S. Busby, H. Buc, S. Garges, and S. Adhya. 1993. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62:749-795. [DOI] [PubMed] [Google Scholar]
- 36.Lampidis, R., R. Gross, Z. Sokolovic, W. Goebel, and J. Kreft. 1994. The virulence regulator protein of Listeria ivanovii is highly homologous to PrfA from Listeria monocytogenes and both belong to the Crp-Fnr family of transcription regulators. Mol. Microbiol. 13:141-151. [DOI] [PubMed] [Google Scholar]
- 37.Lauer, P. M. 2000. Systematic mutational analysis of the charged amino acids in the amino-terminal domain of the Listeria monocytogenes ActA protein. Ph.D. thesis. University of California, Berkeley.
- 38.Leimeister-Wachter, M., C. Haffner, E. Domann, W. Goebel, and T. Chakraborty. 1990. Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 87:8336-8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marquis, H., H. Goldfine, and D. A. Portnoy. 1997. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J. Cell Biol. 137:1381-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mengaud, J., S. Dramsi, E. Gouin, J. A. Vazquez-Boland, G. Milon, and P. Cossart. 1991. Pleiotropic control of Listeria monocytogenes virulence factors by a gene that is autoregulated. Mol. Microbiol. 5:2273-2283. [DOI] [PubMed] [Google Scholar]
- 41.Mengaud, J., M. Vincente, J. Chenevert, J. M. Pereira, C. Geoffroy, B. Gicquel-Sanzey, F. Baquero, J. Perez-Diaz, and P. Cossart. 1988. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect. Immun. 56:766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milenbachs, A. A., D. P. Brown, M. Moors, and P. Youngman. 1997. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol. Microbiol. 23:1075-1085. [DOI] [PubMed] [Google Scholar]
- 43.Moors, M. A., B. Levitt, P. Youngman, and D. A. Portnoy. 1999. Expression of listeriolysin O and ActA by intracellular and extracellular Listeria monocytogenes. Infect. Immun. 67:131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mounier, J., A. Ryter, M. Coquis-Rondon, and P. J. Sansonetti. 1990. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F actin in the enterocytelike cell line Caco-2. Infect. Immun. 58:1048-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park, S. F., and R. G. Kroll. 1993. Expression of listeriolysin and phosphatidylinositol-specific phospholipase C is repressed by the plant-derived molecule cellobiose in Listeria monocytogenes. Mol. Microbiol. 8:653-661. [DOI] [PubMed] [Google Scholar]
- 46.Portnoy, D. A., T. Chakraborty, W. Goebel, and P. Cossart. 1992. Molecular determinants of Listeria monocytogenes pathogenesis. Infect. Immun. 60:1263-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Portnoy, D. A., R. D. Schreiber, P. Connelly, and L. G. Tilney. 1989. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J. Exp. Med. 170:2141-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ripio, M.-T., G. Dominguez-Bernal, M. Lara, M. Suarez, and J.-A. Vazquez-Boland. 1997. A Gly145Ser substitution in the transcriptional activator PrfA causes constitutive overexpression of virulence factors in Listeria monocytogenes. J. Bacteriol. 179:1533-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ripio, M. T., G. Dominguez-Bernal, M. Suarez, K. Brehm, P. Berche, and J. A. Vazquez-Boland. 1996. Transcriptional activation of virulence genes in wild-type strains of Listeria monocytogenes in response to a change in the extracellular medium composition. Res. Microbiol. 147:371-384. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 52.Sanger, J. M., J. W. Sanger, and F. S. Southwick. 1992. Host cell actin assembly is necessary and likely to provide the propulsive force for intracellular movement of Listeria monocytogenes. Infect. Immun. 60:3609-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma, S. B., and E. R. Singer. 1990. Temporal and spatial regulation of the symbiotic genes of Rhizobium meliloti in plants revealed by transposon Tn5-gusA. Genes Dev. 4:344-356. [DOI] [PubMed] [Google Scholar]
- 54.Sheehan, B., A. Klarsfeld, T. Msadek, and P. Cossart. 1995. Differential activation of virulence gene expression by PrfA, the Listeria monocytogenes virulence regulator. J. Bacteriol. 177:6469-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheehan, B., A. Klarsfeld, T. Msadek, and P. Cossart. 1995. A single substitution in the putative helix-turn-helix motif of the pleiotropic activator PrfA attenuates Listeria monocytogenes virulence. Mol. Microbiol. 20:785-797. [DOI] [PubMed] [Google Scholar]
- 56.Sheehan, B., C. Kocks, S. Dramsi, E. Gouin, A. D. Klarsfeld, J. Mengaud, and P. Cossart. 1994. Molecular and genetic determinants of the Listeria monocytogenes infectious process. Curr. Top. Microbiol. Immunol. 192:187-216. [DOI] [PubMed] [Google Scholar]
- 57.Smith, K., and P. Youngman. 1992. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie 74:705-711. [DOI] [PubMed] [Google Scholar]
- 58.Sun, A. N., A. Camilli, and D. A. Portnoy. 1990. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 58:3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vega, Y., C. Dickneite, M.-T. Ripio, R. Böckmann, B. Gonzalez-Zorn, S. Novella, G. Gominguez-Bernal, W. Goebel, and W. Vazquez-Boland. 1998. Functional similarities between the Listeria monocytogenes virulence regulator PrfA and cyclic AMP receptor protein: the PrfA* (Gly145Ser) mutation increases binding affinity for target DNA. J. Bacteriol. 180:6655-6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams, J. R., C. Thayyullathil, and N. E. Freitag. 2000. Sequence variations within PrfA DNA binding sites and effects on Listeria monocytogenes virulence gene expression. J. Bacteriol. 182:837-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Youngman, P. 1987. Plasmid vectors for recovering and exploiting Tn 917 transpositions in Bacillus and other gram-positive bacteria, p. 79-103. In K. Hardy (ed.), Plasmids: a practical approach. IRL Press, Oxford, England.