Abstract
Chlamydia trachomatis is an obligate intracellular bacterium that infects the oculogenital mucosae. C. trachomatis infection of the eye causes trachoma, the leading cause of preventable blindness. Infections of the genital mucosae are a leading cause of sexually transmitted diseases. A vaccine to prevent chlamydial infection is needed but has proven difficult to produce by using conventional vaccination approaches. Potent immunity to vaginal rechallenge in a murine model of chlamydial genital infection has been achieved only by infection or by immunization with dendritic cells (DC) pulsed ex vivo with whole inactivated organisms. Immunity generated by infection or ex vivo antigen-pulsed DC correlates with a chlamydia-specific interleukin 12 (IL-12)-dependent CD4+ Th1 immune response. Because of the potent antichlamydial immunizing properties of DC, we hypothesized that DC could be a powerful vehicle for the delivery of individual chlamydial antigens that are thought to be targets for more conventional vaccine approaches. Here, we investigated the recombinant chlamydial major outer membrane protein (rMOMP) as a target antigen. The results demonstrate that DC pulsed with rMOMP secrete IL-12 and stimulate infection-sensitized CD4+ T cells to proliferate and secrete gamma interferon. These immunological properties implied that rMOMP-pulsed DC would be potent inducers of MOMP-specific CD4+ Th1 immunity in vivo; however, we observed the opposite result. DC pulsed ex vivo with rMOMP and adoptively transferred to naive mice generated a Th2 rather than a Th1 anti-MOMP immune response, and immunized mice were not protected following infectious challenge. We conclude from these studies that the immunological properties of ex vivo pulsed DC are not necessarily predictive of the immune response generated in vivo following adoptive transfer. These findings suggest that the nature of the antigen used to pulse DC ex vivo influences the Th1-Th2 balance of the immune response in vivo.
Chlamydiae are successful and complex obligate intracellular parasites with a unique biphasic life cycle comprised of an infectious elementary body (EB) and a noninfectious reticulate body (22, 26, 43). Chlamydia trachomatis is an epithelium-tropic parasite of humans, and infections of the oculogenital mucosae are the cause of trachoma, the leading cause of preventable blindness in developing countries, and a major cause of sexually transmitted diseases (25, 47). Antibiotic treatment effectively eradicates infection. However, asymptomatic infections are common (>50%) and, when left untreated, can progress to chronic disease, an impasse that questions whether effective intervention can be managed by anti-infective agents alone. It is likely that the most effective form of intervention for diseases caused by C. trachomatis will be a vaccine (6, 19, 28, 44, 45). However, there has been little progress in the development of an efficacious vaccine because of a lack of understanding of adaptive protective immunity against C. trachomatis infection of the genital tract. Only recently has an understanding of adaptive immunity to infection become apparent as a result of independent studies from a number of laboratories using a murine model of female genital tract infection (1, 42). Collectively, these independent investigations have resulted in highly corroborative findings providing compelling evidence that protective immunity against C. trachomatis infection of the genital mucosae is mediated by a cooperative interaction of CD4+ Th1 T cells and B cells (8, 13, 18, 20, 21, 24, 32, 36, 46, 48). Although the exact mechanism(s) that mediates the eradication of chlamydiae from the genital mucosae has yet to be precisely defined, it is evident that gamma interferon (IFN-γ) is an important effector cytokine, particularly in the clearance of infections caused by human strains (11, 12). Despite this knowledge, there has been only minimal progress in the development of a conventional vaccine that is effective in preventing chlamydial genital infection.
Presently, the only proven means of eliciting potent protective immunity against C. trachomatis infection in murine genital mucosae is infection itself or adoptive immunization with dendritic cells (DC) pulsed ex vivo with killed chlamydial EBs (37). Interestingly, immunization with antigen-pulsed DC elicits levels of protective immunity equivalent to those generated by infection. The potent adjuvant properties of DC, particularly their ability to generate Th1 immune responses (29) and their propensity to home to follicular sites of systemic and mucosal lymphatic tissues (30), likely account for their superior antichlamydial immunizing properties. It is unrealistic to propose a therapeutic role for ex vivo antigen-pulsed DC as a practical antichlamydial vaccine for use in humans. However, the exceptional antichlamydial immunizing properties of DC-based cellular vaccines make them preeminent vehicles for screening candidate chlamydial vaccine antigens that have potential for more conventional vaccine development.
The C. trachomatis major outer membrane protein (MOMP) (4) is regarded as the most promising vaccine candidate (3, 5, 44, 49). It is surface accessible and highly immunogenic, eliciting both neutralizing antibodies and T-cell responses (33, 35). Moreover, the MOMP has been implicated as a chlamydial cytoadhesin (39, 40) that mediates attachment by binding to host glycoaminoglycan receptors (38). Attempts to vaccinate mice against chlamydial infection of the genital tract by using recombinant MOMP (rMOMP) (41), synthetic peptides corresponding to MOMP T- and B-cell epitopes (33, 34), or DNA-based immunogens (23) have met with only marginal success. The inability to generate potent protective immunity by using these MOMP-based immunogens likely is multifactorial, but it is possible that an optimum protective immune response against the MOMP requires the use of an immunogen that more closely mimics the native conformation of the protein (31). It has been shown that, following purification, a recombinant maltose binding protein (MBP)-rMOMP fusion protein exists as ca. 30-nm colloidal particles that effectively compete with viable chlamydial EBs for attachment to HeLa 229 cells (38); this finding implies that rMOMP possesses sufficient native structural characteristics to mediate its cytoadhesin function and hence may represent a more native antigenic structure.
We hypothesized that the rMOMP particles might represent a rational antigen for pulsing DC and for targeting a potent anti-MOMP Th1 response in vivo. In this report, we test this hypothesis. The results showed that although DC pulsed ex vivo with rMOMP secreted interleukin 12 (IL-12) and presented processed MOMP antigen to infection-sensitized CD4+ T cells that secreted IFN-γ, they elicited a nonprotective Th2 rather than a protective Th1 immune response following adoptive transfer. These results imply that the in vitro immunological profiling of antigen-pulsed DC cannot be used to consistently predict their in vivo immunizing characteristics, suggesting that a more thorough understanding of DC maturation following adoptive transfer is required to optimally use their potent immunizing properties as cellular vaccines.
MATERIALS AND METHODS
Chlamydiae.
The C. trachomatis mouse pneumonia strain (MoPn) was grown in HeLa 229 cells and infectious EBs were purified by density gradient centrifugation as previously described (3).
Mice.
Female C57BL/10 (H-2b) mice between 8 and 12 weeks of age were purchased from Jackson Laboratory (Bar Harbor, Maine). Animals were housed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care in filter-top cages under standard environmental conditions and provided food and water ad libitum.
DC.
Bone marrow-derived DC were generated from female C57BL/10 mice (6 to 12 weeks old) by using a modified version of the method of Inaba et al. (9). Briefly, femurs were removed from mice, and bone marrow cells were flushed from femurs and cultured in IMDM-10 (GIBCO BRL) supplemented with 10% fetal bovine serum, 10 μg of gentamicin sulfate/ml, 10 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF)/ml, and 103 U of IL-4 (PharMingen)/ml at 2 × 106 cells/ml in 100-mm tissue culture dishes. On day 3 of culturing, the nonadherent cells were removed and fresh medium containing GM-CSF and IL-4 was added. On day 5 of culturing, DC were isolated by transferring the nonadherent and loosely adherent cells to new culture plates (leaving behind the adherent macrophages), incubating the plates at 37°C for at least 2 h, and then repeating the procedure to remove any contaminating macrophages. The DC were further enriched (>95% purity) by density gradient centrifugation with 14.5% metrizamide solution (Sigma) in culture medium. DC purity was characterized by cell size, dendritic morphology was characterized by phase-contrast microscopy, and viability was assessed by trypan blue exclusion. DC populations were characterized for purity by fluorescence-activated cell sorting analysis after staining with anti-I-Ab, anti-CD86, anti-CD40, and anti-CD11b antibodies. DC cultures were also analyzed by fluorescence-activated cell sorting following staining with anti-Gr1, anti-CD3, anti-CD19, and anti-pan-NK monoclonal antibodies (MAbs) for enumerating contaminating granulocytes, T cells, B cells, and NK cells, respectively. In general, CD11b-positive DC showed high levels of MHC class II, CD86, and CD40 expression. Isolated DC contained less than 5% contamination with T, B, or NK cells (results not shown).
Pulsing DC with rMOMP.
Day 5 DC were panned twice and purified by density gradient centrifugation. DC (5 × 105 DC in 1 ml of IMDM-10) were incubated in the presence 1.2 mg of rMOMP for 1 h at 37°C with gentle mixing every 15 min. The biological and physical properties of the C. trachomatis MoPn rMOMP particles were previously described (38). The purified fusion protein exists as ca. 30-nm colloidal spheres that specifically bind to and efficiently compete with infectious EBs for cultured HeLa 299 cells. Purified MBP was used as a control antigen. The cells were centrifuged, washed twice with IMDM-10, and plated on IMDM-10-GM-CSF (10 ng/ml) overnight. On the following day, the cells were fixed in methanol, stained with murine MAb 33B (specific for the MoPn MOMP), and then stained with a secondary fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (IgG) antibody. DC were viewed by fluorescent-antibody staining and confocal microscopy for internalization of rMOMP.
T-cell proliferation assay.
Spleens were harvested from immune mice (animals that had resolved primary genital infections), and a single-cell suspension in phosphate-buffered balanced salt solution-5% fetal bovine serum was made. Magnetic CD4+ (L3T4) and CD8+ (Ly-2) microbeads (Miltenyi Biotec) were used for positive selection of CD4+ T cells and CD8+ T cells, respectively (37). Immune T cells were then plated in round-bottom 96-well plates at 3 × 105 cells/well. DC that had been pulsed overnight with rMOMP were harvested, and 4 × 103 DC were incubated with 1.2 × 104 T cells in 100 μl of medium. Forty-eight hours later, 1 μCi of 3H-thymidine (78.4 Ci/mmol; LIFESCIENCE-NEN) was added to each well. Radioactive cultures were incubated overnight, and counts per minute were measured by using a TopCount NXT microplate scintillation counter (Packard Instrument Company, Meriden, Conn.) to assess thymidine uptake or incorporation.
Antibody and cytokine ELISAs.
Chlamydia-specific serum antibodies (IgG1 and IgG2a) and IgA in mouse vaginal secretions were assayed by an enzyme-linked immunosorbent assay (ELISA) with formalin-fixed MoPn EB and alkaline phosphatase-conjugated goat anti-mouse IgG antibodies (Southern Biotechnology Associates, Birmingham, Ala.) as described previously (18, 37). Cytokines were measured by an ELISA with the corresponding specific capture and detection antibodies, and cytokine quantities were calculated by using standard curves constructed with recombinant murine cytokines as described by the manufacturer (Pharmingen).
Adoptive immunization with antigen-pulsed DC.
Female C57BL/10 mice (6 to 12 weeks old) were used as bone marrow donors. DC were propagated and enriched as described above. Mice (four to eight per treatment group) were adoptively immunized by intravenous (retro-orbital) injection with 4 × 106 to 7 × 106 DC pulsed with either MBP, rMOMP, or heat-killed (HK) EBs suspended in Hanks balanced salt solution (37). A booster immunization was administered 14 days after the initial immunization, and mice were bled and vaginal wash specimens were collected 5 to 8 days following the booster immunization. One week following the booster immunization, mice were injected subcutaneously with 2.5 mg of medroxyprogesterone acetate (Depo-Provera; Pharmacia and Upjohn Company, Kalamazoo, Mich.) per mouse to synchronize the estrous cycle prior to the infectious challenge. One week following the medroxyprogesterone acetate injection, mice were challenged intravaginally with 20 μl of MoPn (150 infection-forming units [IFU] are equivalent to 10 50% infective doses). Protection was assessed by quantifying the chlamydial burden isolated from cervicovaginal swab samples taken at 7 days postchallenge.
RESULTS
Phagocytosis of rMOMP by DC.
We first investigated whether DC efficiently ingested rMOMP particles. Bone marrow-derived DC were pulsed with a range of concentrations (0 to 60 μg of protein) of rMOMP, washed, and reseeded on glass coverslips. At different times after pulsing, the medium was removed and the cells were washed, fixed in methanol, and examined for MOMP antigen by fluorescent-antibody staining. DC exhibiting the highest frequency and intensity of staining for rMOMP were those pulsed with 1.2 μg of rMOMP/ml at a DC density of 105 cells/ml for 1 h (Fig. 1). Under these conditions, the majority (>95%) of cultured DC stained positive for MOMP antigen 24 h after pulsing. Fluorescence was consistently observed as a particulate staining pattern localized at the cell periphery. Confocal microscopy of pulsed DC confirmed the intracellular location of immunoreactive material (results not shown).
FIG. 1.
Endocytosis of rMOMP by DC. (A) Phase-contrast photomicrograph of rMOMP-pulsed DC. (B) Fluorescent-antibody staining of rMOMP-pulsed DC. DC (5 × 105 cells) were pulsed with 1.2 μg of rMOMP in 1 ml of medium for 1 h at 37°C, washed, and incubated overnight at 37°C with 10 ng of GM-CSF/ml. DC were fixed in methanol and stained with an anti-mouse MAb specific for C. trachomatis MoPn MOMP (MAb 33B). A goat anti-mouse antibody conjugated to fluorescein isothiocyanate was used for secondary staining. Arrows indicate the punctate staining of antigen along the perimeter of the DC surface. No staining was observed when unpulsed DC were stained with MAb 33B or when rMOMP-pulsed DC were stained with a negative control MAb (EVI-H1) specific for chlamydial LPS (data not shown).
DC pulsed with rMOMP secrete IL-12.
It was previously shown that DC pulsed ex vivo with HK EBs secrete IL-12, a potent Th1-polarizing cytokine, and that following adoptive transfer to naive mice, these DC generated a potent chlamydia-specific protective CD4+ Th1 immune response. It was therefore of interest to compare the cytokine profiles found in the supernatants of DC pulsed with rMOMP and DC pulsed with HK EBs. The culture supernatants of DC pulsed with HK EBs or rMOMP for 48 h were assayed for IL-12 (p40), IL-10, IL-6, and IL-4. We found that DC pulsed with either HK EBs or rMOMP secreted IL-12 (Fig. 2). Neither DC population secreted IL-10 or IL-4, and only DC pulsed with HK EBs secreted IL-6.
FIG. 2.
DC pulsed ex vivo with rMOMP secrete IL-12. DC were cultured alone or following pulsing with HK EBs or rMOMP. After 48 h of incubation, culture supernatants were collected and assayed by an ELISA for cytokines IL-12, IL-10, IL-6, and IL-4. A representative from two independent experiments is shown.
Immune CD4+ T cells proliferate in response to DC pulsed with rMOMP.
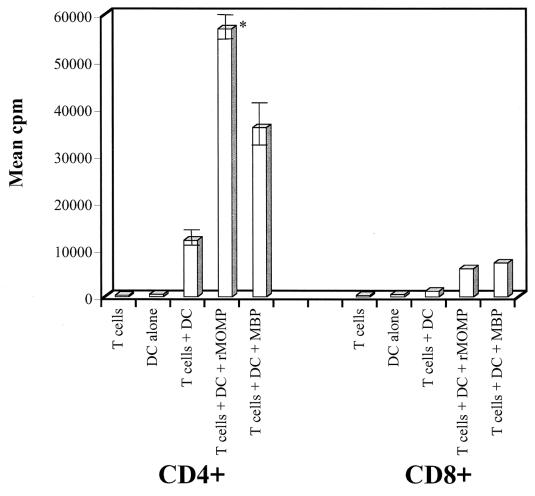
We next investigated whether DC pulsed with rMOMP presented antigen to either CD4+ or CD8+ T cells isolated from the spleens of mice that had resolved a primary infection of the genital tract and were immune to rechallenge. We found that CD4+ but not CD8+ T cells proliferated in response to DC pulsed with rMOMP (Fig. 3). A vigorous proliferative response occurred following incubation of CD4+ T cells with rMOMP-pulsed DC. DC pulsed with the MBP fusion partner alone also stimulated immune CD4+ T cells to proliferate. Importantly, however, immune CD4+ T cells showed a significantly greater response to DC pulsed with the rMOMP fusion protein (P = 0.01) than to DC pulsed with MBP alone, demonstrating that rMOMP was specifically recognized by CD4+ T cells in the context of chlamydial genital tract infection.
FIG. 3.
Infection-sensitized CD4+ T cells proliferate in response to DC pulsed with rMOMP. CD4+ and CD8+ T cells were collected from mice that had resolved a C. trachomatis (MoPn) genital infection. Infection-sensitized T cells were incubated for 48 h in the presence of T cells alone, DC alone, T cells plus DC, T cells plus DC and rMOMP, or T cells plus MBP. T-cell proliferation was measured by pulsing cells with 3H-thymidine overnight, and cell-associated radioactivity was determined by using a TopCount NXT microplate scintillation counter. Experiments were performed in triplicate, and counts per minute are expressed as the mean and standard deviation. The asterisk denotes a P value of 0.01 (Student's two-tailed t test) for a comparison of CD4+ T cells pulsed with rMOMP fusion protein or with MBP alone.
Immune CD4+ T cells secrete IFN-γ in response to DC pulsed with rMOMP.
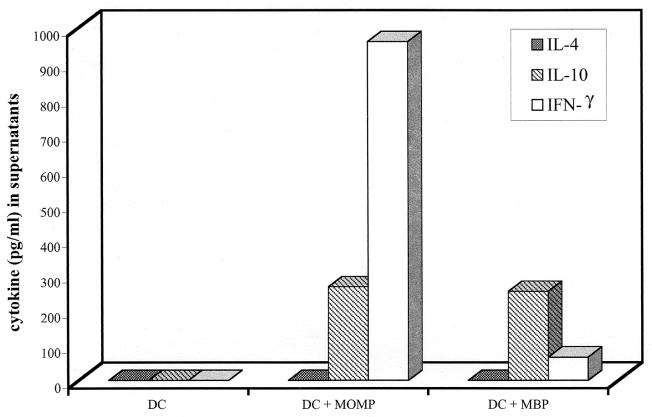
To determine the type of MOMP-specific CD4+ T cells (Th1 or Th2) that were being activated in response to infection, we assayed IL-4, IL-10, and IFN-γ in the culture supernatants of DC alone, DC pulsed with MBP, and DC pulsed with rMOMP by an ELISA (Fig. 4). No detectable cytokines were found in culture supernatants of unpulsed DC propagated with immune CD4+ T cells. IFN-γ was detected in high concentrations (900 pg/ml) relative to IL-10 and IL-4 (280 and 0 pg/ml, respectively) in culture supernatants obtained from CD4+ T cells incubated with DC pulsed with rMOMP. Both cytokines were detected, but at much lower levels, in supernatants of CD4+ T cells cultured with MBP-pulsed DC. Moreover, the cytokine profile was reversed compared to that observed for rMOMP-pulsed DC, with IL-10 (270 pg/ml) being present at higher concentrations than IFN-γ (53 pg/ml). These finding indicate that the reactivity found in the immune CD4+ T-cell population against the MBP fusion partner was mediated by a CD4+ Th2 rather than a Th1 subset of cells.
FIG. 4.
Infection-sensitized CD4+ T cells secrete IFN-γ in response to DC pulsed with rMOMP. Infection-sensitized CD4+ T cells were incubated in the presence of unpulsed DC (DC), DC pulsed with rMOMP, and DC pulsed with MBP for 48 h, and supernatants were collected. Levels of IL-4, IL-10, and IFN-γ in supernatants were measured by an ELISA. A representative from two independent experiments is shown.
The following conclusions can be drawn from the above results. (i) DC cultured in vitro efficiently ingest rMOMP particles; (ii) pulsed DC secrete IL-12 following uptake of rMOMP particles; (iii) pulsed DC present processed MOMP antigen to CD4+ T cells; and (iv) the responding CD4+ T cells secrete IFN-γ, indicative of a Th1 phenotype. Thus, genital infection generates an MOMP-specific CD4+ Th1 immune response. Therefore, we hypothesized that it should be possible to immunize mice with rMOMP-pulsed DC to generate an MOMP-specific CD4+ Th1 immune response and then to challenge mice to ascertain if this immunity was protective.
Immunization of mice with rMOMP-pulsed DC generates a CD4+ Th2 immune response.
Naive mice were immunized with DC alone, DC pulsed with HK EBs, or DC pulsed with rMOMP. The anti-MOMP humoral and CD4+ T-cell immune responses were tested following immunization. Serum antichlamydial IgG1 and IgG2a antibodies were assayed by an ELISA with formalin-fixed MoPn EBs as the antigen. CD4+ T-cell immunity was assessed by determining the concentrations of IL-4, IL-10, and IFN-γ in the supernatants of splenocytes cultured with formalin-treated MoPn EB antigen. Infected immune mice and mice immunized with DC pulsed with HK EBs produced a mixed IgG1 and IgG2a antichlamydial response (Fig. 5). In contrast, mice immunized with DC pulsed with rMOMP showed a predominant IgG1 response. Vaginal wash specimens obtained from mice immunized with rMOMP-pulsed DC were negative for chlamydia-specific IgG and IgA (results not shown). No antichlamydial antibodies were detected in the sera of mice immunized with MBP-pulsed DC. These findings showed that rMOMP immunization generated an antibody response capable of reacting with intact protein on the chlamydial cell surface, strongly implying that rMOMP retained at least some structural characteristics in common with the native protein. Conversely, the predominant IgG1 antibody response indicated that the anti-MOMP response was Th2 rather than Th1 biased.
FIG. 5.
Mice immunized with rMOMP-pulsed DC produce a chlamydia-specific IgG1 antibody response. Sera were collected from mice that had resolved a C. trachomatis (MoPn) genital infection (infected), mice immunized with chlamydia-pulsed DC (DC + HK EB), mice immunized with rMOMP-pulsed DC, and mice immunized with MBP-pulsed DC. An ELISA was used to measure the levels of chlamydia-specific IgG1 and IgG2a antibodies. Mice previously infected with chlamydiae or immunized with chlamydia-pulsed DC generated high titers of IgG2a; however, mice immunized with rMOMP-pulsed DC produced only IgG1. Mice immunized with MBP-pulsed DC (control) did not produce chlamydia-specific antibodies. A representative from two independent experiments is shown. A 1:256 dilution of serum was used for all assays.
To more directly test this hypothesis, CD4+ T cells isolated from rMOMP-immunized mice were assayed for antigen-specific production of IL-4, IL-10, and IFN-γ (Fig. 6). CD4+ T cells from infected immune mice or mice immunized with DC pulsed with HK EBs generated a Th1 cytokine profile characterized by elevated concentrations of IFN-γ, moderate levels of IL-10, and no detectable IL-4. In contrast, CD4+ T cells from mice immunized with rMOMP-pulsed DC produced no IFN-γ but did secrete moderate levels of both IL-4 and IL-10, a cytokine profile characteristic of a Th2 response.
FIG. 6.
CD4+ T cells isolated from mice immunized with rMOMP-pulsed DC secrete IL-4 and IL-10. CD4+ T cells were harvested from mice that had resolved a C. trachomatis (MoPn) genital infection (infected), mice immunized with chlamydia-pulsed DC (DC + HK EB), and mice immunized with rMOMP-pulsed DC. T cells were incubated in the presence of antigen-presenting cells pulsed with whole chlamydial EB antigen (EB), and supernatants were collected 72 h later. An ELISA was used to measure the levels of IL-4, IL-10, and IFN-γ in culture supernatants. A representative from two individual experiments is shown.
Mice immunized with rMOMP-pulsed DC are not protected against chlamydial challenge.
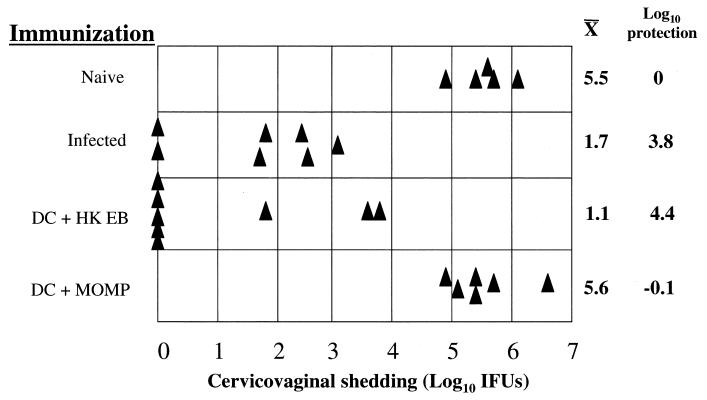
We next challenged mice immunized with rMOMP-pulsed DC intravaginally to assess protective immunity. Naive mice, infected immune mice, and mice immunized with HK EB-pulsed DC were included as negative and positive controls. As previously shown, both infected immune mice and mice immunized with DC pulsed with HK EBs were significantly more resistant to intravaginal challenge than naive controls, with a reduction in the levels of recoverable organisms of 3.8 and 4.4 log10 units, respectively (Fig. 7). On the other hand, mice immunized with rMOMP-pulsed DC were not protected, as shown by equivalent levels of shedding of infectious organisms in these mice and in intravaginally challenged immunologically naive mice. Thus, in contrast to infected immune mice or mice immunized with HK EB-pulsed DC, mice immunized with rMOMP-pulsed DC produced a CD4+ Th2 immune response that did not protect against chlamydial challenge of the genital tract.
FIG. 7.
Mice immunized with rMOMP-pulsed DC are not protected following chlamydial challenge. Mice were given no immunization (naive), 150 IFU intravaginally (infected), 7 × 106 DC pulsed with whole inactivated chlamydial EBs (DC + HK EB), and 7 × 106 DC pulsed with rMOMP (DC + MOMP) and then challenged intravaginally with 150 IFU of chlamydiae. Recoverable IFU were determined from cervicovaginal swab samples 7 days following challenge. Triangles represent individual mice. Log10 mean organisms shed from the cervicovaginal region and log10 protection are shown to the right of the graph. Mice immunized with unpulsed DC, HK EB alone (no DC vehicle), and MBP-pulsed DC did not show protection (data not shown).
DISCUSSION
We have proposed that because of their potent adjuvant properties and capability of immunizing against chlamydial genital tract mucosal colonization and infection (37), ex vivo antigen-pulsed DC will be useful as an antigen delivery system for screening the immunogenicity and protective efficacy of candidate chlamydial vaccine antigens. In the work described here, we investigated this hypothesis by using the chlamydial MOMP as a target antigen. Although there have been numerous attempts to immunize female mice by using rMOMP (41), MOMP peptides (34), or naked DNA encoding MOMP (23) incorporated in a number of adjuvants, these approaches have yielded either negative findings or only partial protective immunity following intravaginal challenge. There are likely many possible explanations for the inability of MOMP-based vaccines to generate potent protective immunity of the genital mucosae. One possibility, suggested by Stephens (31) and Zhang et al. (49), is that in order to achieve optimum utility of MOMP as a vaccine, the protein must be delivered in a nondenatured native conformation to elicit a recall response to infection-specific T- and B-cell epitopes. Validation of native MOMP is not possible, because its crystal structure has yet to be determined; therefore, the native conformation can be implied only through indirect analysis, such as its interaction with antibodies that bind to conformationally dependent epitopes or maintenance of biological activity. Toward this end, it was previously shown that the rMOMP fusion protein forms colloidal particles of about 30 nm and that the rMOMP particles specifically bind to HeLa 229 cells and, most importantly; compete with the binding of infectious EBs (38). Based on these findings, we suggested that MOMP binding to its cognate host receptor was dependent on its native configuration. Therefore, because of the superior ability of ex vivo pulsed DC to generate a Th1 immune response and the evidence supporting MOMP as a promising vaccine candidate, it was logical to investigate whether rMOMP-pulsed DC could function in mediating an anti-MOMP Th1 response and, if so, whether this response would be protective.
We found that rMOMP was efficiently ingested by DC, a finding likely due to the colloidal nature of the recombinant protein. Of further interest, however, was the observation that rMOMP-pulsed DC produced IL-12 and stimulated infection-sensitized CD4+ T cells to proliferate and secrete IFN-γ. These findings demonstrate that rMOMP-pulsed DC present antigen in the context of class II to CD4+ T cells generated in response to natural infection. Moreover, the secretion by rMOMP-pulsed DC of IL-12, a potent stimulator of Th1 immunity, suggests that rMOMP-pulsed DC had the potential to generate an anti-MOMP CD4+ Th1 immune response in vivo. Furthermore, our finding that antibodies induced by rMOMP-pulsed DC reacted with the surface of intact EBs provides additional evidence that rMOMP retained certain native structural characteristics. In all, these results were very encouraging, leading us to believe that rMOMP-pulsed DC would be efficient inducers of CD4+ Th1 immunity. In fact, we observed the opposite result. Mice adoptively immunized with rMOMP-pulsed DC generated a CD4+ Th2 response, as shown by the predominant serum IgG1 antibody response and the secretion of IL-4 and IL-10 but not IFN-γ by cultured CD4+ T cells. Not unexpectedly and consistent with the immune response elicited by rMOMP-pulsed DC, immunized mice were not protected following chlamydial genital challenge. This finding was unlike that observed following adoptive immunization with HK EB-pulsed DC, which elicited potent protective CD4+ Th1 immunity.
What are some of the possible explanations for these contradictory findings? Culturing murine bone marrow cells with GM-CSF and IL-4 gives rise to immature DC. Immature DC differentiate into subclasses of CD8α+ or CD8α−, which direct the development of distinct Th-cell populations in vivo (16, 17). Antigen-pulsed CD8α+ cells induce a Th1 response, whereas CD8α− cells induce a Th2 response. It is therefore possible that pulsing of DC ex vivo with inactivated intact chlamydiae and rMOMP differentially drives DC populations to the CD8α+ and CD8α− phenotypes, respectively. Chlamydiae are similar to gram-negative bacteria and possess lipopolysaccharide (LPS) (2). Chlamydial LPS differs structurally from gram-negative bacterial LPS molecules, and these structural alterations are known to reduce its endotoxin activities (15). Nevertheless, highly purified chlamydial LPS and chlamydial heat shock protein 60 have been shown to bind the CD14 LPS receptor and activate CD14 signal transduction pathways (10, 14). Therefore, it is possible that chlamydial LPS or heat shock protein 60 or other activating EB moieties influence DC maturation toward the CD8α+ phenotype, which then induces a protective antichlamydial Th1 immune response. In contrast, rMOMP that lacks similar inflammatory molecules may preferentially drive the development of the CD8α− phenotype, with the subsequent generation of a nonprotective Th2 immune response.
The potent in vivo immunizing properties of chlamydia-pulsed DC (27, 37) make them useful delivery vehicles for screening promising candidate chlamydial vaccine antigens. However, it is evident from the results reported here that further manipulation of DC populations to regulate their capacity to skew Th1-like immunological properties in vivo will be necessary to achieve an optimum outcome of vaccination. Future experiments with rMOMP-pulsed DC should focus on culture conditions that induce DC maturation toward the CD8α+ phenotype which, in theory, would generate an anti-MOMP Th1 immune response. Perhaps this goal can be accomplished by using MOMP-pulsed IL-10-deficient DC (F. O. Eko, W. Lubitz, and J. U. Igietseme, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., p. 341, 2001) or by formulating MOMP vaccines to include CD8α+-inducing molecules. It is also possible that more traditional vaccine strategies designed to target anti-MOMP Th1 immunity to mucosal surfaces 7; Eko et al., Abstr. 101st Gen. Meet. Am. Soc. Microbiol.) will yield effective vaccines against this most important pathogen; however, these approaches have not yielded the superior level of protective immunity at the genital mucosae that is conferred by antigen-pulsed DC.
REFERENCES
- 1.Barron, A. L., H. J. White, R. G. Rank, B. L. Soloff, and E. B. Moses. 1981. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J. Infect. Dis. 143:63-66. [DOI] [PubMed] [Google Scholar]
- 2.Brade, L., S. Schramek, U. Schade, and H. Brade. 1986. Chemical, biological, and immunochemical properties of the Chlamydia psittaci lipopolysaccharide. Infect. Immun. 54:568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldwell, H. D., and R. C. Judd. 1982. Structural analysis of chlamydial major outer membrane proteins. Infect. Immun. 38:960-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell, H. D., and J. Schachter. 1982. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect. Immun. 35:1024-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Maza, M. A., and L. M. de la Maza. 1995. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine 13:119-127. [DOI] [PubMed] [Google Scholar]
- 7.Igietseme, J. U., and A. Murdin. 2000. Induction of protective immunity against Chlamydia trachomatis genital infection by a vaccine based on major outer membrane protein-lipophilic immune response-stimulating complexes. Infect. Immun. 68:6798-6806. [DOI] [PMC free article] [PubMed]
- 8.Igietseme, J. U., K. H. Ramsey, D. M. Magee, D. M. Williams, T. J. Kincy, and R. G. Rank. 1993. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg. Immunol. 5:317-324. [PubMed] [Google Scholar]
- 9.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingalls, R. R., P. A. Rice, N. Qureshi, K. Takayama, J. S. Lin, and D. T. Golenbock. 1995. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect. Immun. 63:3125-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson, M., K. Schon, M. Ward, and N. Lycke. 1997. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect. Immun. 65:1032-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson, M., K. Schon, M. Ward, and N. Lycke. 1997. Studies in knockout mice reveal that anti-chlamydial protection requires TH1 cells producing IFN-γ: is this true for humans? Scand. J. Immunol. 46:546-552. [DOI] [PubMed] [Google Scholar]
- 13.Johansson, M., M. Ward, and N. Lycke. 1997. B-cell-deficient mice develop complete immune protection against genital tract infection with Chlamydia trachomatis. Immunology 92:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kol, A., A. H. Lichtman, R. W. Finberg, P. Libby, and E. A. Kurt-Jones. 2000. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 164:13-17. [DOI] [PubMed] [Google Scholar]
- 15.Kosma, P. 1999. Chlamydial lipopolysaccharide. Biochim. Biophys. Acta 1455:387-402. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Heirman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maldonado-Lopez, R., C. Maliszewski, J. Urbain, and M. Moser. 2001. Cytokines regulate the capacity of cd8alpha(+) and cd8alpha(−) dendritic cells to prime th1/th2 cells in vivo. J. Immunol. 167:4345-4350. [DOI] [PubMed] [Google Scholar]
- 18.Morrison, R. P., K. Feilzer, and D. B. Tumas. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison, R. P., D. S. Manning, and H. D. Caldwell. 1992. Immunology of Chlamydia trachomatis infections: immunoprotective and immunopathogenetic responses, p. 57-84. In T. C. Quinn (ed.), Advances in host defense mechanisms, 8th ed. Raven Press, Ltd., New York, N.Y.
- 20.Morrison, S. G., and R. P. Morrison. 2001. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect. Immun. 69:2643-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moulder, J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55:143-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal, S., K. M. Barnhart, Q. Wei, A. M. Abai, E. M. Peterson, and L. M. de la Maza. 1999. Vaccination of mice with DNA plasmids coding for the Chlamydia trachomatis major outer membrane protein elicits an immune response but fails to protect against a genital challenge. Vaccine 17:459-465. [DOI] [PubMed] [Google Scholar]
- 24.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 25.Schachter, J. 1978. Chlamydial infections (first of three parts). N. Engl. J. Med. 298:428-434. [DOI] [PubMed] [Google Scholar]
- 26.Schachter, J., and H. D. Caldwell. 1980. Chlamydiae. Annu. Rev. Microbiol. 34:285-309. [DOI] [PubMed] [Google Scholar]
- 27.Shaw, J. H., V. R. Grund, L. Durling, and H. D. Caldwell. 2001. Expression of genes encoding Th1 cell-activating cytokines and lymphoid homing chemokines by Chlamydia-pulsed dendritic cells correlates with protective immunizing efficacy. Infect. Immun. 69:4667-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stagg, A. J. 1998. Vaccines against Chlamydia: approaches and progress. Mol. Med. Today 4:166-173. [DOI] [PubMed] [Google Scholar]
- 29.Steinman, R. M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271-296. [DOI] [PubMed] [Google Scholar]
- 30.Steinman, R. M., M. Pack, and K. Inaba. 1997. Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 156:25-37. [DOI] [PubMed] [Google Scholar]
- 31.Stephens, R. S. 1999. Advances, challenges, and changing paradigms, p. xi-xxiii. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 32.Su, H., and H. D. Caldwell. 1995. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 63:3302-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su, H., and H. D. Caldwell. 1992. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J. Exp. Med. 175:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su, H., and H. D. Caldwell. 1993. Immunogenicity of a synthetic oligopeptide corresponding to antigenically common T-helper and B-cell neutralizing epitopes of the major outer membrane protein of Chlamydia trachomatis. Vaccine 11:1159-1166. [DOI] [PubMed] [Google Scholar]
- 35.Su, H., and H. D. Caldwell. 1991. In vitro neutralization of Chlamydia trachomatis by monovalent Fab antibody specific to the major outer membrane protein. Infect. Immun. 59:2843-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su, H., K. Feilzer, H. D. Caldwell, and R. P. Morrison. 1997. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect. Immun. 65:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su, H., R. Messer, W. Whitmire, E. Fischer, J. C. Portis, and H. D. Caldwell. 1998. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J. Exp. Med. 188:809-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su, H., L. Raymond, D. D. Rockey, E. Fischer, T. Hackstadt, and H. D. Caldwell. 1996. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc. Natl. Acad. Sci. USA 93:11143-11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su, H., N. G. Watkins, Y. X. Zhang, and H. D. Caldwell. 1990. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect. Immun. 58:1017-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su, H., Y. X. Zhang, O. Barrera, N. G. Watkins, and H. D. Caldwell. 1988. Differential effect of trypsin on infectivity of Chlamydia trachomatis: loss of infectivity requires cleavage of major outer membrane protein variable domains II and IV. Infect. Immun. 56:2094-2100. [DOI] [PMC free article] [PubMed]
- 41.Tuffrey, M., F. Alexander, W. Conlan, C. Woods, and M. Ward. 1992. Heterotypic protection of mice against chlamydial salpingitis and colonization of the lower genital tract with a human serovar F isolate of Chlamydia trachomatis by prior immunization with recombinant serovar L1 major outer-membrane protein. J. Gen. Microbiol. 138:1707-1715. [DOI] [PubMed] [Google Scholar]
- 42.Tuffrey, M., P. Falder, J. Gale, and D. Taylor-Robinson. 1986. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br. J. Exp. Pathol. 67:605-616. [PMC free article] [PubMed] [Google Scholar]
- 43.Ward, M. E. 1988. The chlamydial developmental cycle, p. 71-95. In A. L. Barron (ed.), Microbiology of Chlamydia. CRC Press, Inc., Boca Raton, Fla.
- 44.Ward, M. E. 1992. Chlamydial vaccines—future trends. J. Infect. 25(Suppl. 1):11-26. [DOI] [PubMed] [Google Scholar]
- 45.Ward, M. E., G. M. Webber, and A. K. Shahani. 1986. Computer modelling of trachoma control strategies, p. 154-157. In D. Oriel, G. Ridgway, J. Schachter, D. Taylor-Robinson, and M. Ward (ed.), Chlamydial infections. Cambridge University Press, Cambridge, England.
- 46.Williams, D. M., B. G. Grubbs, E. Pack, K. Kelly, and R. G. Rank. 1997. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect. Immun. 65:2876-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. 1996. Global prevalence and incidence of selected curable sexually transmitted diseases: overview and estimates. World Health Organization, Geneva, Switzerland.
- 48.Yang, X., and R. C. Brunham. 1998. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. [PubMed]
- 49.Zhang, Y. X., S. Stewart, T. Joseph, H. R. Taylor, and H. D. Caldwell. 1987. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J. Immunol. 138:575-581. [PubMed] [Google Scholar]