Abstract
Arcanobacterium pyogenes, a common inhabitant of the upper respiratory and urogenital tracts of economically important animals, such as cattle and swine, is also an opportunistic pathogen associated with suppurative infections in these animals. A. pyogenes expresses neuraminidase activity encoded by the nanH gene, and previously, construction of a nanH mutant of A. pyogenes BBR1 indicated that a second neuraminidase is present in this strain. A 5,112-bp gene, nanP, was cloned and sequenced, and this gene conferred neuraminidase activity on an Escherichia coli host strain. The predicted 186.8-kDa NanP protein exhibited similarity to a number of bacterial neuraminidases and contained the RIP/RLP motif and five copies of the Asp box motif found in all bacterial neuraminidases. As expected, insertional inactivation of the nanP gene in A. pyogenes BBR1 resulted in a mutant with reduced neuraminidase activity. However, insertional inactivation of the nanP gene in the nanH mutant strain resulted in a complete lack of neuraminidase activity. Like NanH, NanP was localized to the A. pyogenes cell wall. However, unlike the nanH gene, which was present in 100% of the strains examined, nanP was present in only 64.2% of the isolates (n = 53). A. pyogenes adheres to HeLa cells, and a nanP mutant displayed a wild-type adhesion phenotype with these cells. In contrast, the ability of a nanH nanP double mutant to bind to HeLa cells was reduced by 53%. The wild-type adhesion phenotype was restored by providing nanP in trans. These data indicate that the neuraminidases of A. pyogenes play a role in adhesion of this organism to host epithelial cells.
Adhesion to epithelial cells is necessary for bacteria to colonize host mucosal surfaces. This adhesion is the result of interaction of a number of surface-exposed or secreted bacterial proteins with host cells and molecules. Neuraminidases can be important factors in promoting adhesion to host epithelial cells in Streptococcus pneumoniae (10, 37) and oral Actinomyces spp. (4, 7). Neuraminidase treatment of whole-organ perfusion cultures of chinchilla tracheae increased S. pneumoniae adhesion and reversed inhibition of glycoconjugate analogs of known S. pneumoniae receptors (37). In Actinomyces naeslundii, adhesion to human epithelial or skin fibroblast cells mediated by type 2 fimbriae could be increased by pretreatment of the cells with neuraminidase (4). Similarly, pretreatment of human buccal epithelial cells with neuraminidase significantly increased the adhesion of Actinomyces israelii, A. naeslundii, and Actinomyces viscosus (7). In addition, the action of neuraminidase can decrease mucus viscosity (11), possibly enhancing bacterial colonization of the underlying tissues. Furthermore, the susceptibility of mucosal immunoglobulin A to bacterial proteases is increased when sialic acid moieties are removed from this molecule (9, 28).
Arcanobacterium pyogenes is a common inhabitant of the upper respiratory, urogenital (6, 35), and gastrointestinal 19; B. H. Jost, K. W. Post, and S. J. Billington, unpublished data) tracts of many domestic animal species. However, this organism can cause disease, usually following a physical or microbial insult to the host. A. pyogenes causes a variety of suppurative infections of the skin, joints, and visceral organs in economically important animals and birds, such as mastitis in dairy cows (14) and goats (1), liver abscesses in feedlot cattle (17, 18), pneumonia in pigs (12), and osteomyelitis in turkeys (5).
A. pyogenes expresses neuraminidase activity, and this activity is in part due to NanH, a cell wall-bound neuraminidase found in all isolates (16). Construction of a nanH insertion deletion mutant in A. pyogenes resulted in a strain with reduced neuraminidase activity, but neuraminidase activity was still present, suggesting that a second enzyme was present (16). The nanH mutant was not defective for adhesion to epithelial cells (16), but the role of NanH in adhesion of A. pyogenes to host cells may have been masked by the presence of the second neuraminidase.
In this paper we describe cloning and characterization of NanP, a second neuraminidase expressed by A. pyogenes. In addition, we constructed a nanH nanP double mutant and determined that neuraminidase activity plays a role in the adhesion of A. pyogenes to host epithelial cells.
MATERIALS AND METHODS
Bacteria and growth conditions.
A. pyogenes strain BBR1 was isolated from a bovine abscess. A. pyogenes strain NANH-1, in which the entire nanH gene was deleted and replaced with an erythromycin resistance cassette, was described previously (16). Other A. pyogenes strains used in this study were obtained from veterinary diagnostic laboratories or personal collections. A. pyogenes strains were grown on brain heart infusion (Difco) agar plates supplemented with 5% bovine blood at 37°C in the presence of 5% CO2 or in brain heart infusion broth supplemented with 5% bovine calf serum (Omega Scientific Inc.) at 37°C with shaking. Escherichia coli DH5αMCR strains (Gibco-BRL) were grown at 37°C on Luria-Bertani (Difco) agar or in Luria-Bertani broth with shaking. The following antibiotics (Sigma) were added as appropriate: for A. pyogenes strains, chloramphenicol (5 μg/ml), erythromycin (15 μg/ml), and kanamycin (30 μg/ml); and for E. coli strains, chloramphenicol (30 μg/ml) and kanamycin (50 μg/ml).
Preparation of CSF, CWE, and protoplasts.
Culture supernatant fluid (CSF) was prepared from liquid cultures of A. pyogenes grown overnight to optical densities at 600 nm of approximately 3.0 to 4.0. Cells were removed by centrifugation at 5,000 × g, and the CSF was filtered through a 0.22-μm-pore-size filter. A. pyogenes cell wall extract (CWE) and protoplasts were prepared as previously described for S. pneumoniae (20). Protoplasts were resuspended in distilled water and were lysed by several cycles of freezing and thawing.
DNA techniques.
Preparation of plasmid DNA and electroporation-mediated transformation of A. pyogenes strains were performed as previously described (15). Genomic DNA from A. pyogenes was isolated by the method of Pospiech and Neumann (24). The methods used for growth and purification of bacteriophage were essentially the methods described by Ausubel et al. (3). DNA was prepared from bacteriophage as previously described (32) and was further purified by using a Wizard DNA Clean-Up system (Promega). E. coli plasmid DNA extraction, transformation, DNA restriction, ligation, agarose gel electrophoresis, and Southern transfer of DNA to nitrocellulose membranes were performed essentially as described previously (3). Preparation of DNA probes, DNA hybridization, and probe detection were performed by using a DIG DNA labeling and detection kit (Roche Molecular Biochemicals) as recommended by the manufacturer. PCR DNA amplification was performed by using Taq DNA polymerase (Promega) with the supplied reaction buffer for 35 cycles consisting of 1 min at 94°C, 1 min at 55°C, and 1 min/kb at 72°C, followed by a final extension step of 72°C for 5 min.
Nucleotide sequence determination.
The sequence of nanP was determined with plasmid pJGS360 by automated DNA sequencing. Sequencing was performed for both strands; all restriction sites were crossed, and KS or T7 sequencing primers or oligonucleotide primers (Sigma-Genosys) designed to sequence nanP were used. Sequencing reactions were performed at the DNA Sequencing Facility of The University of Arizona with a 377 DNA sequencer (Applied Biosystems Inc.).
Computer sequence analysis.
Nucleotide sequence data were compiled by using the Sequencher program (GeneCodes). Database searches were performed by using the BlastX and BlastP algorithms (2). Sequence analysis was performed by using the suite of programs available through the Genetics Computer Group, Inc. (University of Wisconsin). Signal sequence prediction was performed by using SignalP (22). Multiple sequence alignments were performed by using CLUSTAL W (34).
Detection of neuraminidase activity.
Neuraminidase activity was assayed by using the fluorogenic substrate 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUAN) (Sigma), essentially as described by Winter et al. (38), in 100 mM citrate phosphate buffer (pH 6.0) at 37°C for 1 h.
A λGEM-12 library of A. pyogenes BBR1 genomic DNA was plated, as described above, so that approximately 300 plaques were present on an 8.5-cm-diameter petri dish. An overlay consisting of 2.5 ml of top agar (3), 75 μl of 1.0 M sodium acetate (pH 4.5), and 500 μl of 0.35% MUAN was poured over the plate and allowed to set. The plate was incubated at 4°C for 30 min prior to excitation with a UV source (254 nm), and the presence of neuraminidase activity was indicated by cyan fluorescence.
Screening of bacterial strains for neuraminidase activity was performed by using a filter paper assay. No. 2 filter paper (Whatman) was wetted with 100 μM MUAN in 0.1 M sodium acetate (pH 4.5). Colonies were patched onto the filter paper and incubated at 37°C for 15 min prior to excitation with UV light, as described above.
Epithelial cell adhesion assay.
Human cervical epithelial cells (HeLa cells) were cultured in Iscove's modified Dulbecco's medium (Life Technologies) supplemented with 10% fetal bovine serum (Omega Scientific Inc.) and with 100 μg of gentamicin (Sigma) per ml in a humidified, 5% CO2 atmosphere at 37°C. For adhesion assays, HeLa cells in Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum without gentamicin were seeded into 24-well plates at a concentration of 2 × 105 cells per well in 1-ml volumes. The cells were incubated at 37°C in the presence of 5% CO2 for 18 h prior to addition of 2 × 106 bacteria (freshly grown to an optical density at 600 nm of 1.0). Bacterial adhesion was assessed after 1 h of incubation at 37°C in the presence of 5% CO2. Cell monolayers were washed three times with 0.01 M phosphate-buffered saline (pH 7.2) to remove nonadherent bacteria. Bacteria were recovered by treatment of the cell monolayers with 1 ml of 0.1% Triton X-100 (Sigma) for 10 min at 0°C, and viable bacteria were enumerated by dilution plating. All experiments were performed in triplicate on three separate occasions.
Statistical analysis.
A one-way analysis of variance was performed with the data from the epithelial cell adhesion assays by using the Minitab statistical software (Minitab Inc.).
Nucleotide sequence accession number.
The nanP sequence has been deposited in the GenBank database under accession number AY045771.
RESULTS
Cloning and determination of the nucleotide sequence of nanP.
An A. pyogenes BBR1 nanH insertion deletion mutant exhibited neuraminidase activity (16), suggesting that a second neuraminidase gene was present in this strain. Plaques from a λGEM-12 library of A. pyogenes BBR1 genomic DNA (16) were overlaid with MUAN-containing top agar and were visualized under UV light. Several strongly fluorescent plaques were selected and used for PCR analysis. One of these bacteriophages, λJGS37, was not amplified with nanH-specific primers (data not shown) and contained an approximately 15-kb partial Sau3AI fragment. DNA purified from λJGS37 was digested with NotI and cloned into similarly digested pBC KS (Stratagene). Plasmid DNA from one of the recombinants contained an approximately 10-kb NotI fragment (pJGS360) encompassing the entire nanP gene region (Fig. 1). pJGS360 conferred neuraminidase activity on the E. coli host, as determined by the MUAN filter paper assay.
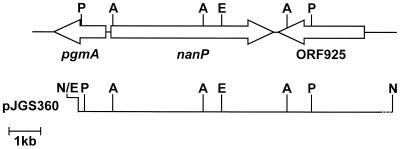
FIG. 1.
Map of the nanP gene region. A map of plasmid pJGS360 is shown below the gene region map. Asp700I (A), EcoRI (E), NotI (N), and PstI (P) sites used to clone portions of the nanP gene are shown.
The DNA sequence of the nanP gene region was deduced from pJGS360. There are several in-frame ATG codons at the 5′ end of the nanP gene. The first and second ATG codons appear to be equal candidates, as they both have consensus ribosome binding sites. If it was assumed that translation occurred from the first ATG, the 5,211-bp nanP gene encoded a protein with a predicted molecular mass of 186.8 kDa. A gram-positive signal sequence, with a cleavage site between Ala-50 and Glu-51, was predicted by SignalP (22). A putative rho-independent terminator (ΔG = −18.4 kcal/mol) was identified 25 bases downstream of the nanP stop codon. No E. coli σ70-like promoter sequences were apparent upstream of the nanP gene.
Upstream of nanP was an open reading frame (ORF), pgmA, whose protein product was similar to phosphoglucomutase from Streptomyces coelicolor (26). Downstream sequences encoded an ORF designated ORF925, whose protein product was similar to a putative integral membrane protein from S. coelicolor (26). pgmA and ORF925 were transcribed in the direction opposite the direction of nanP transcription (Fig. 1), suggesting that nanP is monocistronic.
Analysis of the primary structure of NanP.
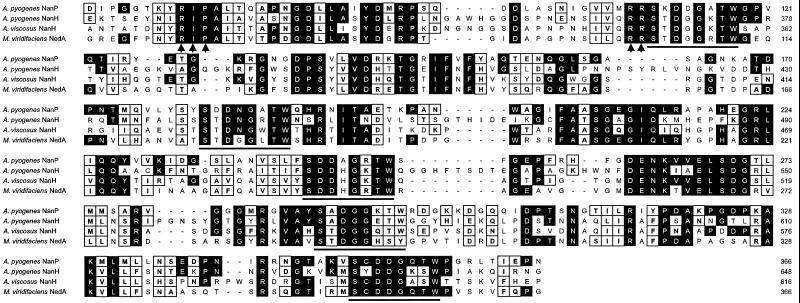
Cleavage at the predicted signal peptide sequence of NanP should result in a mature protein with a predicted molecular mass of 181.6 kDa and a pI of 5.4. The NanP protein contained the conserved catalytic RIP/RLP motif, as well as five copies of the Asp box motif (Ser-x-Asp-x-Gly-x-Thr-Trp) associated with bacterial neuraminidases (29) (Fig. 2). NanP was most similar to NedA from Micromonospora viridifaciens (45.3% identity, 61.6% similarity), NanH from A. viscosus (43.1% identity, 59.4% similarity), and NanH from A. pyogenes (38.8% identity, 53.8% similarity) (Fig. 2).
FIG. 2.
Amino acid alignment for the active site regions of A. pyogenes NanP and NanH (16), A. viscosus NanH (39), and M. viridifaciens NedA (31). Amino acids identical to amino acids in the NanP sequence are indicated by black boxes. Conservative substitutions are indicated by open boxes. The RIP/RLP motif and the two additional catalytic Arg residues are indicated by arrows. The Ser-x-Asp-x-Gly-x-Thr-Trp (Asp box) motifs are underlined. Amino acid numbers for each protein are indicated on the right.
At the C terminus of the NanP protein was a sequence similar to the cell wall sorting signals found in surface-expressed proteins of gram-positive bacteria (33). The terminal 32 amino acids of NanP (LAWTGAAVVGLAVMSLVFLLAGFVLTVRRRKA) consisted of an LPxTG cleavage motif (boldface), a hydrophobic domain (underlined), and a positively charged stop transfer sequence (italics). However, in the A. pyogenes NanP protein, the cleavage motif was LAWTG instead of LPxTG. In addition, a Pro-rich repetitive region, which is thought to facilitate spanning of the cell wall peptidoglycan (33), was found directly upstream of the LAWTG cleavage motif.
Determination of the prevalence of the nanP gene by DNA dot blotting.
In order to determine whether nanP was present in all A. pyogenes strains, genomic DNAs were prepared from 53 A. pyogenes strains and were subjected to hybridization under high-stringency conditions with a nanP-specific probe that spanned bases 143 to 1385 of the nanP ORF, encompassing the active site region of the molecule. As a control, pJGS306, encoding the entire nanH ORF except the signal sequence, was tested (16). The nanP probe did not hybridize to nanH sequences (data not shown), indicating that the probe was specific for nanP. Genomic DNA from 34 of the 53 strains hybridized strongly to the probe (data not shown), indicating that the nanP gene is present in 64.2% of the A. pyogenes strains tested. Furthermore, nanP was found preferentially in A. pyogenes isolates of bovine origin. Of 31 bovine A. pyogenes isolates, 24 (77.4%) were nanP positive, compared with only 5 of 17 porcine isolates (29.4%). In addition, 7 of the 53 strains hybridized weakly, but consistently, to the nanP probe (data not shown), suggesting that a different but related neuraminidase may be present in these A. pyogenes strains.
Construction and characterization of nanP mutants.
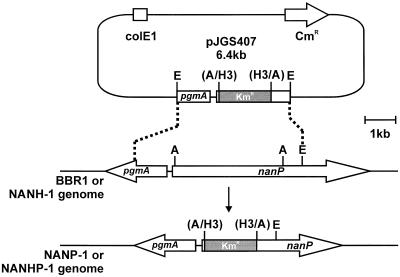
To construct nanP mutants, we used an allelic exchange plasmid in which the nanP gene was inactivated by deletion of the active site region of the nanP ORF and insertion of a kanamycin resistance gene (Fig. 3). The 4.5-kb EcoRI fragment of pJGS360 (Fig. 1) was cloned into pHSS21 (21) to form the recombinant plasmid pJGS401. A 1.4-kb HindIII fragment containing the kanamycin resistance gene from pKRP11 (27) was treated with T4 DNA polymerase (Promega). The internal 2.9-kb Asp700I fragment (containing the active site region) of pJGS401 was replaced with a kanamycin resistance gene fragment, resulting in pJGS403. The EcoRI insert of pJGS403 was then cloned into similarly digested pBC KS to construct pJGS407 (Fig. 3). As pJGS407 was based on a ColE1 replicon, it acted as a suicide plasmid in A. pyogenes (15). pJGS407 plasmid DNA was introduced into A. pyogenes BBR1 or NANH-1 cells by electroporation, and recombinants were selected on brain heart infusion blood agar containing kanamycin. Kmr Cms colonies were chosen and used for further analysis.
FIG. 3.
Scheme for insertional inactivation of the A. pyogenes nanP gene. An Asp700I fragment containing the active site region of nanP was replaced with a Kmr cassette to construct pJGS407. This plasmid was introduced into A. pyogenes strains BBR1 and NANH-1 by electroporation. Reciprocal recombination, indicated by the dashed lines, resulted in replacement of the active site region of the nanP gene in the BBR1 and NANH-1 chromosomes with the Kmr cassette to construct NANP-1 and NANHP-1, respectively. A, Asp700I; E, EcoRI; H3, HindIII. Only the insert portion of pJGS407 is to scale. The EcoRI site in pJGS407 was derived from the vector portion of pJGS360.
Southern blotting of A. pyogenes BBR1 genomic DNA digested with PstI revealed a hybridizing band at 7.2 kb in BBR1 when the preparation was probed with a nanP-specific probe (spanning bases 143 to 1385 of the nanP ORF). No hybridizing bands were observed for the nanP mutant (NANP-1) or the nanH nanP double mutant (NANHP-1) when they were probed similarly, indicating that the nanP active site region was not present in these strains. A 5.7-kb band was apparent in PstI-digested NANP-1 or NANHP-1 DNA but not BBR1 genomic DNA when a kanamycin resistance gene-specific probe was used. None of the strains hybridized with a pBC KS-specific (vector) probe (data not shown). These data confirmed that deletion or inactivation of the nanP gene occurred in NANP-1 and NANHP-1 by double-crossover events.
Complementation of the NANHP-1 double mutant.
In order to construct a complementing plasmid, the 7.2-kb PstI fragment of pJGS360, containing nanP, was cloned into similarly digested pJGS180 to construct pJGS398. pJGS180 is a derivative of pEP2 (25) carrying a chloramphenicol resistance gene, and this plasmid can replicate in A. pyogenes (S. J. Billington, unpublished data). pJGS398 was introduced into NANHP-1 by electroporation with selection on chloramphenicol. The neuraminidase activities of NANP-1, NANHP-1, NANHP-1(pJGS180), and NANHP-1(pJGS398) were compared to the neuraminidase activity of wild-type strain BBR1 by using a MUAN-filter paper assay. BBR1 and the NANP-1 mutant expressed neuraminidase activity, but neither NANHP-1 nor NANHP-1(pJGS180) had detectable neuraminidase activity. NANHP-1(pJGS398) expressed neuraminidase activity, indicating that neuraminidase activity could be restored by providing nanP in trans on a replicating plasmid (data not shown).
Localization of NanP.
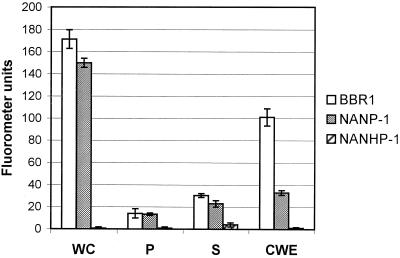
Whole cells, CSF, and CWE were prepared from wild-type strain BBR1 and the NANP-1 and NANHP-1 mutants, and these preparations were tested for the presence of neuraminidase activity with the fluorometric assay by using MUAN as a substrate. For BBR1 and NANP-1 the majority of the neuraminidase activity was detected in whole cells and CWE. While BBR1 cells had only slightly higher neuraminidase activity than NANP-1 cells, BBR1 CWE had significantly higher neuraminidase activity than the CWE from the NANP-1 mutant (Fig. 4). Some neuraminidase activity was detected in the CSF and in lysed protoplasts of either BBR1 or NANP-1 (Fig. 4), and this activity may have resulted from fragments of cell wall material that were present in the CSF or were still associated with the protoplasts. These data indicate that the majority of NanP-specific neuraminidase activity was associated with the cell wall. No significant neuraminidase activity was detected in any of the NANHP-1 fractions (Fig. 4).
FIG. 4.
Cell localization of neuraminidase activity. A total of 4 × 107 whole cells (WC), 4 × 107 lysed protoplasts (P), 20 μl of culture supernatant (S), and 200 μg of purified CWE from wild-type BBR1 (open bars), the NANP-1 mutant (gray bars), and the NANHP-1 mutant (cross-hatched bars) were assayed for neuraminidase activity by using the fluorometric assay with MUAN as a substrate. The error bars indicate 1 standard deviation of the mean calculated by using the averages from at least three independent experiments.
Adhesion of A. pyogenes neuraminidase mutants to HeLa cells.
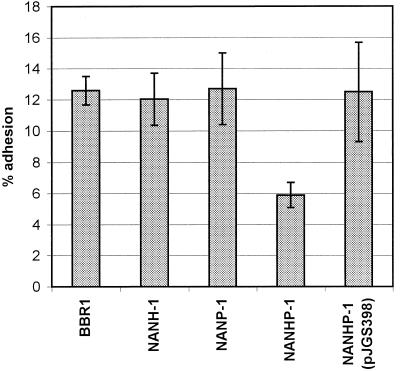
The abilities of BBR1, NANH-1, NANP-1, NANHP-1, and the complemented NANHP-1 mutant to adhere to HeLa cells were determined. In the initial experiments centrifugation following addition of bacteria to HeLa cells was used to increase bacterium-host cell interactions (16). However, this step could potentially result in bacterium-host cell adhesion and mask subtle differences between wild-type and neuraminidase-deficient A. pyogenes strains, so subsequent experiments were performed without centrifugation. The NANHP-1 double mutant exhibited impaired adhesion compared to wild-type strain BBR1; the average levels of adhesion were 5.9 and 12.6%, respectively (Fig. 5). The lower level of adhesion was reproducible and statistically significant (P < 0.001). The adhesion phenotypes of the NANH-1 and NANP-1 mutants were the same as the adhesion phenotype of BBR1, and the average levels of adhesion were 12.0 and 12.7%, respectively (Fig. 5). Complementation of the NANHP-1 mutant with pJGS398, carrying nanP, resulted in a wild-type adhesion phenotype (average level of adhesion, 12.5%). If centrifugation was used, no significant difference in adhesion between BBR1 and NANHP-1 was observed (data not shown). These findings suggest that a complete absence of neuraminidase activity does impair the ability of A. pyogenes to adhere to HeLa cells.
FIG. 5.
Adhesion of A. pyogenes strains to HeLa cells. A. pyogenes strains were added to cell monolayers and allowed to adhere for 1 h at 37°C prior to washing and recovery of cell-associated bacteria. Adhesion is expressed as a percentage of the number of bacteria originally added to the cells. The error bars indicate 1 standard deviation of the mean calculated by using the averages from at least three independent experiments performed in triplicate.
DISCUSSION
We previously characterized NanH, a neuraminidase from A. pyogenes BBR1, and demonstrated that a nanH mutant exhibited neuraminidase activity and showed no reduction in adhesion to HeLa cells. In this paper we describe cloning and sequencing of NanP, a second neuraminidase from A. pyogenes BBR1. Like the NanH neuraminidase, NanP is present in the cell wall. However, unlike the nanH gene, nanP is carried by only 64.2% of the A. pyogenes isolates examined.
Sequence analysis of the nanP gene region indicated that nanP was monocistronic and flanked by pgmA, a phosphoglucomutase, and ORF925, which encodes a putative integral membrane protein, which were identified by their similarity to S. coelicolor genes (26). NanP contained sequences consistent with its activity as a neuraminidase, including the RIP/RLP and Asp box motifs (29). Like NanH, NanP is most closely related to NedA of M. viridifaciens (31) and NanH of A. viscosus (39). Surprisingly, the A. pyogenes NanH and NanP proteins exhibit less similarity to each other than either of them exhibits to the neuraminidases of other species. In addition, the nanH and nanP genes do not align, nor do they hybridize under high-stringency conditions, suggesting that it is unlikely that these genes arose through gene duplication in A. pyogenes.
The finding that NanP is localized to the A. pyogenes cell wall is consistent with the presence of an N-terminal signal peptide and C-terminal cell sorting signals, including an LPxTG-like cell anchor (33). NanP, like NanH, has a variant cell wall anchor motif, LAWTG. It is now clear that many cell wall-sorted proteins from other gram-positive bacteria also have divergent LPxTG motifs (23).
As expected, a nanP mutant, NANP-1, exhibited significant neuraminidase activity due to the presence of nanH. However, a nanH nanP double mutant, NANHP-1, completely lacked neuraminidase expression, a defect which could be reversed by providing nanP in trans on a replicating plasmid, pJGS398. Like NanH, NanP was localized to the cell wall, as NANP-1 CWE had significantly reduced neuraminidase activity (Fig. 4). The neuraminidase activity of whole cells of NANP-1 was only slightly lower than the neuraminidase activity of wild-type BBR1 (Fig. 4). This was expected, as deletion of the nanH gene resulted in an approximately 80% reduction in the total amount of neuraminidase activity compared with the total amount of neuraminidase activity in BBR1 (16).
In contrast to nanH, which was present in all A. pyogenes isolates, nanP was found in only 64.2% of the strains examined. Interestingly, nanP appeared to be preferentially associated with A. pyogenes isolates of bovine origin; 77.4% of bovine strains carried nanP, compared to 29.4% of porcine isolates. In addition, some strains hybridized weakly, but consistently, to the nanP probe (data not shown), suggesting that a different but related neuraminidase is present in these A. pyogenes strains. Whether the presence of different neuraminidases suggests different host specificities requires further investigation. However, while there is some diversity in the number and types of neuraminidase genes present in A. pyogenes, all strains do express neuraminidase activity due to the presence of at least nanH.
Attachment to mammalian cells via specific recognition structures is the first step in bacterial colonization of a host. Adherence of A. naeslundii to epithelial cells (4) and polymorphonuclear leukocytes (30) was enhanced by pretreatment of the cells with neuraminidase. Similarly, neuraminidase treatment of tracheal organ cultures increased the adherence of S. pneumoniae (37), and S. pneumoniae mutants deficient in neuraminidase activity had reduced abilities to colonize and persist in the nasopharynx (36). A nanH mutant of A. pyogenes was not deficient in adhesion to HeLa cells (16), presumably due to the presence of the NanP neuraminidase. Similarly, the nanP mutant NANP-1 had a wild-type adhesion phenotype. In contrast, the ability of NANHP-1, which had no detectable neuraminidase activity, to adhere to HeLa cells was reduced 53%, indicating that neuraminidase activity was required for complete adhesion. The level of adhesion of NANHP-1 could be increased to the wild-type level by introducing nanP on a plasmid. No effect of nanP copy number was observed. In fact, the NANH-1 or NANP-1 mutant or complemented NANHP-1 adhered as well as BBR1, which suggests that only a defined amount of neuraminidase is required for complete adhesion.
While our results demonstrate that the neuraminidases play a role in adhesion to host cells, adhesion of A. pyogenes to host epithelial cells is probably a complex process, and several adhesins or ligands may contribute to it. The finding that adhesion of a neuraminidase-deficient mutant was reduced but was not absent suggests that there are adhesins which act in a neuraminidase-independent manner. Some of these adhesins could be cell wall proteins, including fibronectin- or collagen-binding proteins, such as the microbial surface components recognizing adhesive matrix molecules present in Streptococcus pyogenes and Staphylococcus aureus (8, 13). Furthermore, the data do not preclude the possibility that the neuraminidase activity that promotes adhesion of A. pyogenes to host cells plays a more prominent role in vivo.
Acknowledgments
We thank Stefani Gilbert for construction of the λGEM-12 library and Hien Trinh and Dawn Bueschel for their excellent technical assistance.
Partial support for this work was provided by USDA/NRICGP award 99-35204-7818.
REFERENCES
- 1.Al-Graibawi, M. A. A., V. K. Sharma, and A. J. Al-Shammari. 1986. Microbial pathogens from goat mastitis and phage-typing of Staphylococcus aureus isolates. Comp. Immunol. Microbiol. Infect. Dis. 9:23-28. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1994. Current protocols in molecular biology, vol. 1. Greene Publishing Associates and John Wiley and Sons, Inc., New York, N.Y.
- 4.Brennan, M. J., J. O. Cisar, A. E. Vatter, and A. L. Sandberg. 1984. Lectin-dependent attachment of Actinomyces naeslundii to receptors on epithelial cells. Infect. Immun. 46:459-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinton, M. K., L. C. Schellberg, J. B. Johnson, R. K. Frank, D. A. Halvorson, and J. A. Newman. 1993. Description of osteomyelitis lesions associated with Actinomyces pyogenes infection in the proximal tibia of adult male turkeys. Avian Dis. 37:259-262. [PubMed] [Google Scholar]
- 6.Carter, G. R., and M. M. Chengappa. 1991. Essentials of veterinary bacteriology and mycology, 4th ed. Lea and Febiger, Philadelphia, Pa.
- 7.Childs, W. C., and R. J. Gibbons. 1990. Selective modulation of bacterial attachment to oral epithelial cells by enzyme activities associated with poor oral hygiene. J. Periodontal Res. 25:172-178. [DOI] [PubMed] [Google Scholar]
- 8.Foster, T. J., and M. Höök. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 9.Frandsen, E. V. G. 1994. Carbohydrate depletion of immunoglobulin A1 by oral species of gram-positive rods. Oral Microbiol. Immunol. 9:352-358. [DOI] [PubMed] [Google Scholar]
- 10.Giebink, G. S. 1999. Otitis media: the chinchilla model. Microb. Drug Resist. 5:57-72. [DOI] [PubMed] [Google Scholar]
- 11.Gottschalk, A. 1960. Correlation between composition, structure, shape, and function of a salivary mucoprotein. Nature 186:949-951. [DOI] [PubMed] [Google Scholar]
- 12.Høie, S., K. Falk, and B. M. Lium. 1991. An abattoir survey of pneumonia and pleuritis in slaughter weight swine from 9 selected herds. IV. Bacteriological findings in chronic pneumonic lesions. Acta Vet. Scand. 32:395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joh, D., E. R. Wann, B. Kreikemeyer, P. Speziale, and M. Höök. 1999. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18:211-223. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson, P., S.-E. Olsson, A.-S. Olofson, C. Fälth, O. Holmberg, and H. Funke. 1991. Bacteriological investigations of clinical mastitis in heifers in Sweden. J. Dairy Res. 58:179-185. [DOI] [PubMed] [Google Scholar]
- 15.Jost, B. H., S. J. Billington, and J. G. Songer. 1997. Electroporation-mediated transformation of Arcanobacterium (Actinomyces) pyogenes. Plasmid 38:135-140. [DOI] [PubMed] [Google Scholar]
- 16.Jost, B. H., J. G. Songer, and S. J. Billington. 2001. Cloning, expression and characterization of a neuraminidase gene from Arcanobacterium pyogenes. Infect. Immun. 69:4430-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lechtenberg, K. F., T. G. Nagaraja, H. W. Leipold, and M. M. Chengappa. 1988. Bacteriologic and histologic studies of hepatic abscesses in cattle. Am. J. Vet. Res. 49:58-62. [PubMed] [Google Scholar]
- 18.Nagaraja, T. G., S. B. Laudert, and J. C. Parrott. 1996. Liver abscesses in feedlot cattle. Part I. Causes, pathogenesis, pathology, and diagnosis. Comp. Cont. Educ. Pract. Vet. 18:S230-S241, S256.
- 19.Narayanan, S., T. G. Nagaraja, N. Wallace, J. Staats, M. M. Chengappa, and R. D. Oberst. 1998. Biochemical and ribotypic comparison of Actinomyces pyogenes and A. pyogenes-like organisms from liver abscesses, ruminal wall, and ruminal contents of cattle. Am. J. Vet. Res. 59:271-276. [PubMed] [Google Scholar]
- 20.Neeleman, C., S. P. M. Geelen, P. C. Aerts, M. R. Daha, T. E. Mollnes, J. J. Roord, G. Posthuma, H. van Dijk, and A. Fleer. 1999. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect. Immun. 67:4517-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickoloff, J. A., and R. J. Reynolds. 1991. Subcloning with new ampicillin- and kanamycin-resistant analogs of pUC19. BioTechniques 10:469-472. [PubMed] [Google Scholar]
- 22.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 23.Pallen, M. J., A. C. Lam, M. Antonio, and K. Dunbar. 2001. An embarrassment of sortases--a richness of substrates? Trends Microbiol. 9:97-101. [DOI] [PubMed] [Google Scholar]
- 24.Pospiech, A., and B. Neumann. 1995. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet. 11:217-218. [DOI] [PubMed] [Google Scholar]
- 25.Radford, A. J., and A. L. Hodgson. 1991. Construction and characterization of a Mycobacterium-Escherichia coli shuttle vector. Plasmid 25:149-153. [DOI] [PubMed] [Google Scholar]
- 26.Redenbach, M., H. M. Kieser, D. Denapaite, A. Eichner, J. Cullum, H. Kinashi, and D. A. Hopwood. 1996. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol. Microbiol. 21:77-96. [DOI] [PubMed] [Google Scholar]
- 27.Reece, K. S., and G. J. Phillips. 1995. New plasmids carrying antibiotic resistance cassettes. Gene 165:141-142. [DOI] [PubMed] [Google Scholar]
- 28.Reinholdt, J., M. Tomana, S. B. Mortensen, and M. Kilian. 1990. Molecular aspects of immunoglobulin A1 degradation by oral streptococci. Infect. Immun. 58:1186-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roggentin, P., B. Rothe, J. B. Kaper, J. Galen, L. Lawrisuk, E. C. Vimr, and R. Schauer. 1989. Conserved sequences in bacterial and viral sialidases. Glycoconjugate J. 6:349-353. [DOI] [PubMed] [Google Scholar]
- 30.Ruhl, S., J. O. Cisar, and A. L. Sandberg. 2000. Identification of polymorphonuclear leukocyte and HL-60 cell receptors for adhesins of Streptococcus gordonii and Actinomyces naeslundii. Infect. Immun. 68:6346-6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakurada, K., T. Ohta, and M. Hasegawa. 1992. Cloning, expression and characterization of the Micromonospora viridifaciens neuraminidase gene in Streptomyces lividans. J. Bacteriol. 174:6896-6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos, M. A. 1991. An improved method for the small scale preparation of bacteriophage DNA based on phage precipitation by zinc chloride. Nucleic Acids Res. 19:5442.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneewind, O., D. Mihaylova-Petkov, and P. Model. 1993. Cell wall sorting signals in surface proteins of Gram-positive bacteria. EMBO J. 12:4803-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timoney, J. F., J. H. Gillespie, F. W. Scott, and J. E. Barlough. 1988. Hagan and Bruner's microbiology and infectious diseases of domestic animals, 8th ed. Cornell University Press, Ithaca, N.Y.
- 36.Tong, H. H., L. E. Blue, M. A. James, and T. F. DeMaria. 2000. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68:921-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong, H. H., M. A. McIver, L. M. Fisher, and T. F. DeMaria. 1999. Effect of lacto-N-neotetraose, asialoganglioside-GM1 and neuraminidase on adherence of otitis media-associated serotypes of Streptococcus pneumoniae to chinchilla tracheal epithelium. Microb. Pathog. 26:111-119. [DOI] [PubMed] [Google Scholar]
- 38.Winter, A. J., S. D. Comis, M. P. Osborne, M. J. Tarlow, J. Stephen, P. W. Andrew, J. Hill, and T. J. Mitchell. 1997. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect. Immun. 65:4411-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeung, M. 1993. Complete nucleotide sequencing of the Actinomyces viscosus T14V sialidase gene: presence of a conserved repeating sequence among strains of Actinomyces spp. Infect. Immun. 61:109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]