Abstract
Untreated infections with Chlamydia trachomatis commonly result in ascending infection to fallopian tubes and subsequent immune-mediated tubal pathology in females. The proposed immune-mediated injury may be associated with the increased recruitment of CD4 cells to the upper genital tract (GT) (oviducts) in comparison to the lower GT (cervix) during infection, as shown in animal models. To understand the mechanisms responsible for this biased recruitment of CD4 cells within the GT, we characterized chemokine expression patterns in the upper and lower GTs in mice during infection with the murine pneumonitis biovar of Chlamydia trachomatis. Enzyme-linked immunosorbent assays of supernatants from GT homogenates revealed that the levels of the Th1-associated chemokines CXCL9 (monokine induced by gamma interferon), CXCL10 (interferon-inducible protein 10), and CCL5 (RANTES) were significantly higher in the upper GT than in the lower GT after infection, while the CCL3 (macrophage inflammatory protein 1α) level was not increased. In contrast, the level of chemokine CCL11 (eotaxin) was significantly elevated in the lower GT later in the course of infection. Increased levels of mRNA confirmed the selective differences in chemokine expression within the upper and lower GTs. The increased levels of Th1-inducible chemokines in the upper GT were not due to differences in the magnitude of infection or progesterone pretreatment. These data demonstrate that the upper and lower regions of the GT respond differently to Chlamydia infection.
The gram-negative bacterium Chlamydia trachomatis is sexually transmitted and infects squamous epithelial cells in the female cervix. From here, the bacteria ascend and establish infection in columnar cells of human fallopian tubes or oviducts in mice. Left untreated, human infection can lead to pelvic inflammatory disease, fallopian tube injury, and infertility (22). Oviduct injury, characterized by hydrosalpinx, and infertility are also seen in murine infections (33). The development of T-cell-mediated immunity is one of the most crucial elements required for the effective clearance of this pathogen. A CD4 Th1 response is necessary for Chlamydia eradication, and this finding has been demonstrated by prolonged infection in gamma interferon knockout mice (23) as well as in mice injected with blocking antibodies against the Th1-inducing cytokine interleukin 12 (IL-12) (23). Additionally, the ability to clear infection in nude mice is restored following the adoptive transfer of an anti-C. trachomatis murine pneumonitis biovar (MoPn) CD4 Th1 clone (15). Similarly, IL-10 knockout mice exhibit a shorter course of infection (37). Thus, the regulation of Th1 and Th2 responses in the genital tract (GT) during Chlamydia infection is a crucial factor controlling the duration of infection and subsequent tubal pathology.
Chemokines are a rapidly growing family of small chemotactic molecules that are specific for various subsets of lymphocytes as well as other types of leukocytes. Increasing evidence suggests that chemokines play an important role in the regulation of Th1 and Th2 responses in vivo. These responses appear to be directed by the differential expression of chemokine receptors on Th1- and Th2-cell subsets (21). Many studies have demonstrated patterns of either Th1- or Th2-associated chemokines in diseased tissues previously shown to contain large infiltrates of either Th1 or Th2 cells (9). For instance, in the Th1-mediated disease multiple sclerosis, high levels of chemokines CXCL10 (interferon-inducible protein 10 [IP-10]), CXCL9 (monokine induced by gamma interferon [MIG]), and CCL5 (RANTES) are found in the cerebrospinal fluid (30). These data suggest that the chemokine profile plays a central role in determining the predominant T-cell subset associated with a particular disease or infection.
Chemokines also provide fine specificity for the direction of cellular recruitment to discrete anatomical regions within a given tissue. For example, site specificity has been noted at mucosal surfaces, where CCL25 (thymus-expressed chemokine) has been shown to localize to the epithelium of the small intestine but not the large intestine (19). Many chemokines have been detected in the endometrial epithelium within the female GT in humans, including CCL3 (macrophage inflammatory protein 1α [Mip-1α]) (1), CCL5 (RANTES) (2), CCL2 (monocyte chemotactic protein 1 [MCP-1]) (4), and CCL11 (eotaxin) (13). However, it is not known whether chemokine expression differs within functionally discrete regions of the GT. It was previously shown that a significantly larger number of CD4 cells are recruited to the oviducts (upper GT) than to the cervical-vaginal region (lower GT) of mice infected with MoPn (18). To investigate the basis for the increased recruitment of CD4 cells to the upper GT, we evaluated the expression of chemokines associated with Th1 and Th2 responses in the upper and lower GTs during infection.
MATERIALS AND METHODS
Infection.
Female BALB/c mice, 4 to 6 weeks old, were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.) and were housed according to American Association of Accreditation of Laboratory Animal Care guidelines. Experimental procedures were approved by the UCLA Institutional Animal Care and Use Committee. All mice were first injected subcutaneously with 2.5 mg of medroxyprogesterone acetate (Depo-Provera; Upjohn, Kalamazoo, Mich.) in 100 μl of sterile phosphate-buffered saline. Medroxyprogesterone acetate drives mice into a state of anestrus, thus eliminating the variability in the rate and severity of infection due to the estrous cycle (26). Seven days later, while under sodium pentobarbital anesthesia, all mice were inoculated with 107 inclusion-forming units (IFU) (50% infective dose, 1.5 × 103 IFU) of MoPn grown in McCoy cells. Mice were killed on days 3, 7, 14, 21, and 35 after inoculation. Infection was monitored by examining cervical-vaginal swab samples (Dacroswab type 1; Spectrum Labs, Houston, Tex.) obtained immediately before mice were killed and homogenates of tissue samples obtained from the upper and lower GTs. Swab samples were stored in sucrose-phosphate buffer at −70°C until analyzed. Tissue homogenate samples were also frozen at −70°C until analyzed.
Tissue homogenates.
GT tissues were divided into the cervical-vaginal region (lower GT) and oviducts (upper GT) with the ovaries removed. Uterine horns were not included in our analysis. Tissue sections from individual mice were placed in 1 ml of a protease inhibitor buffer (1 μg each of antipain, aprotinin, leupeptin, and pepstatin A/ml and 2 mM phenylmethylsulfonyl fluoride in sterile phosphate-buffered saline) (Sigma, St. Louis, Mo.) and homogenized as previously described (3) by using a hand-held homogenizer (Omni International, Warrenton, Va.). Aliquots of each homogenate were removed for isolation of chlamydiae. The remaining homogenate volumes were sonicated at 20 kHz for 1 min and then centrifuged at 900 × g for 15 min at 10oC to remove cellular debris. Supernatants were filtered through 0.2-μm-pore-size Acrodisks (Gelman Sciences, Ann Arbor, Mich.) to remove chlamydiae, and samples were stored at −70oC until analyzed.
Isolation of chlamydiae from cervical-vaginal swab and tissue homogenate samples.
Swab samples were prepared as previously described. McCoy cell monolayers in individual wells of 96-well plates were inoculated with 200 μl of the solution from vaginal swabs or homogenized GT tissue (18), followed by centrifugation at 1,900 × g for 1 h. The plates were incubated for 2 h at 37°C. At this time, the isolation solutions were removed, fresh cycloheximide medium was added, and the plates were incubated for an additional 32 h. The cultures were then fixed with methanol. MoPn inclusions were identified by the addition of anti-MoPn immune sera and anti-mouse immunoglobulin G conjugated to fluorescein isothiocyanate (ICN Immunobiologicals, Irvine, Calif.). The inclusion bodies within 20 fields (×40) were counted under a fluorescence microscope, and numbers of IFU per milliliter were calculated (17). Data were adjusted for IFU per milligram of crude homogenized GT tissue (upper or lower).
Chemokine ELISAs.
Recombinant protein and antibodies against CCL3 (Mip-1α), CCL11 (eotaxin), CXCL9 (MIG), CXCL10 (IP-10), and CCL5 (RANTES) were purchased from R&D Systems (Minneapolis, Minn.) and those against CCL2 (MCP-1) were purchased from PharMingen (San Diego, Calif.) for use in enzyme-linked immunosorbent assays (ELISAs). Upper and lower GT homogenates were added to duplicate wells of microtiter enzyme immunoassay plates (Costar/Corning, Acton, Mass.) and assayed according to the manufacturer's protocol with the following exceptions. CXCL10 and CXCL9 primary antibody concentrations were 1 and 2 μg/ml, respectively, and secondary antibody concentrations were 0.5 μg/ml. The recommended substrate was replaced with 1-StepTM Turbo TMB-ELISA substrate (Pierce Chemical Co., Rockford, Ill.). The optical densities were read at 450 nm with a microplate reader (model 550; Bio-Rad, Hercules, Calif.). Chemokine values were determined from a standard curve generated with recombinant chemokines by using microplate reader software. Chemokine values were corrected for total protein by using a micro-bicinchoninic acid protein assay kit (Pierce).
Serum progesterone levels.
Serum was collected from mice administered or not administered medroxyprogesterone acetate. Progesterone levels were determined by a competitive electrochemiluminescence immunoassay with an Elecsys 2010 automated analyzer (Roche, Berkeley, Calif.).
mRNA isolation and SuperArray analysis.
Total RNA was isolated from paired GT tissues of mice according to the manufacturer's protocol following homogenization of tissues in RNAzol B (Tel-Test, Inc., Friendswood, Tex.) and stored at −80°C until use. Nonrad-GEArray kits specific for chemokine analysis were purchased from SuperArray Inc. (Bethesda, Md.). Each kit provides a matched set of membranes containing 23 chemokines plus controls. Probe synthesis was carried out by using 10 or 7 μg of mRNA per sample. The manufacturer's protocol was followed for all steps. Following substrate addition, membranes were exposed to X-ray film (Fuji, Tustin, Calif.) for 5 to 10 min. Data were quantified by using a laser densitometer and ImageQuaNT software (Molecular Dynamics, Sunnyvale, Calif.) to calculate the average integrated volumes of dots. Data were expressed as the average integrated volume of a sample relative to the average integrated volume of a positive control (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]).
Immunohistochemical analysis.
Tissues were harvested and prepared as previously described (18). Staining was carried out as previously described with the following exceptions. After a tissue blocking step with rabbit serum, the primary antibodies (goat anti-mouse CXCL10 [IP-10] and goat anti-mouse CCL11 [eotaxin]) (R&D Systems) were incubated on tissue sections for 45 min at room temperature in a humidified chamber, and then the sections were washed. A rabbit anti-goat immunoglobulin G antibody conjugated to biotin at 30 μg/ml (Antibodies Inc., Davis, Calif.) and streptavidin conjugated to horseradish peroxidase (Zymed, San Francisco, Calif.) were added next, and the tissue sections were incubated for 45 min. Slides were developed as previously described (18). Photographs were generated by scanning the microscope slides with an Olympus DP10 color digital video camera.
Statistics.
Statistical differences in chemokine protein levels were tested by using two-way analysis of variance (ANOVA) and Tukey's post hoc test. Statistical differences in chemokine message levels were determined by using a paired Student t test. The above statistical tests were suggested by and performed with SigmaStat software based on the distribution of the data and sample size (Jandel Scientific, San Rafael, Calif.). Groups were considered statistically different at a P value of <0.05.
RESULTS
Differential expression of chemokines in the upper and lower GTs in response to C. trachomatis infection.
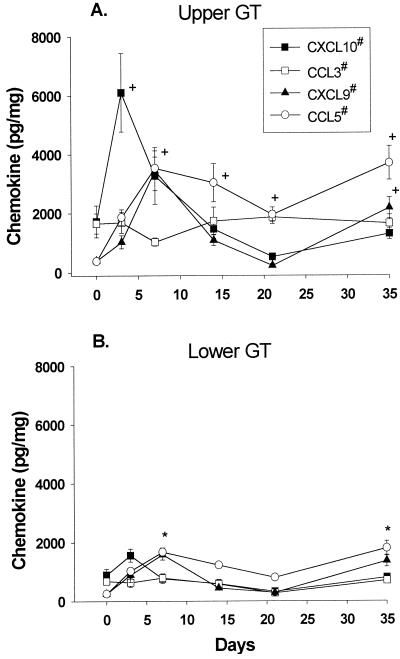
To determine whether chemokine production differed between the upper and lower GTs during MoPn infection, we measured protein levels in tissue homogenates at weekly intervals that spanned the induction phase (0 to 14 days) and the resolution phase (14 to 35 days) of infection. We evaluated the expression of chemokines that are generally associated with a Th1 response, CXCL10 (IP-10), CXCL9 (MIG), CCL3 (Mip-1α), and CCL5 (RANTES), or a Th2 response, CCL11 (eotaxin). We also evaluated CCL2 (MCP-1), which has not been shown to associate with any particular T-cell subset. As shown in Fig. 1A, the Th1-associated chemokines CXCL10 (IP-10), CXCL9 (MIG), and CCL5 (RANTES) were all induced by infection in the upper GT. CXCL10 (IP-10) was measured at a significantly elevated level on day 3 compared to the level in uninfected mice. The level of CXCL9 (MIG) was significantly elevated on day 7 and again later in infection, on day 35, whereas an elevated level of CCL5 (RANTES) was maintained in the upper GT from day 7 throughout the course of infection. In the lower GT (Fig. 1B), the levels of both CXCL9 (MIG) and CCL5 (RANTES) were elevated early in the course of infection and the CCL5 (RANTES) level was significantly increased late in the course of infection compared to the results for controls. Finally, CCL3 (Mip-1α) was not induced by infection in either the upper or the lower GT. However, CCL3 (Mip-1α) was expressed at a constitutively higher level in the upper GT than in the lower GT.
FIG. 1.
Th1-associated chemokine protein levels during MoPn infection. Chemokines were measured in upper and lower GT tissue homogenates in uninfected (day 0) and infected (days 3, 7, 14, 21, and 35) mice by ELISAs. The data are compiled from two separate experiments and are expressed as the mean and standard error of the mean (SEM) for four mice per group. Number signs indicate values that were significantly elevated in the upper GT versus the lower GT (CXCL10, days 3 and 7; CXCL9, days 3 and 35; CCL5, days 7, 14, 21, and 35; and CCL3, days 0, 3, 14, 21, and 35). Plus signs indicate values that were significantly different from those on day 0 in the upper GT (CXCL10, day 3; CXCL9, days 7 and 35; and CCL5, days 7, 14, 21, and 35). Asterisks indicate values that were significantly different from those on day 0 in the lower GT (CXCL9, day 7; and CCL5, days 7 and 35). The P value determined by ANOVA was <0.005; the P value determined by the post hoc Tukey test was <0.05 ± SEM.
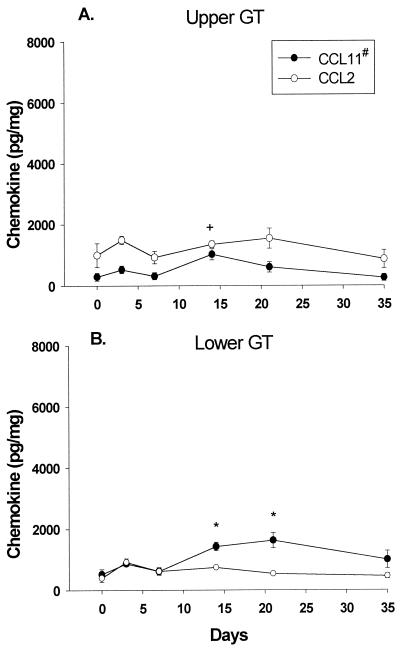
We next examined the protein levels of CCL11 (eotaxin) and CCL2 (MCP-1) in the upper and lower GTs with ELISAs. As shown in Fig. 2A, CCL11 (eotaxin) expression was induced by infection in the upper GT but only later in the course of infection (day 14). Furthermore, the level of CCL11 (eotaxin) was comparatively lower than the peak levels of Th1-associated chemokines in the upper GT (Fig. 1A). CCL11 (eotaxin) was also induced in the lower GT (Fig. 2B) by day 14 and, interestingly, was expressed to a significantly higher degree in the lower GT than in the upper GT during the resolution phase of infection (Fig. 2). There was no difference in CCL2 (MCP-1) expression between uninfected and infected tissues and between the upper and lower GTs. Taken together, these data show that the overall production of chemokines was greater in the upper GT than in the lower GT during infection. Furthermore, higher concentrations of Th1-associated chemokines were found in the upper GT, while the concentration of the Th2-associated chemokine CCL11 (eotaxin) was higher in the lower GT than in the upper GT.
FIG. 2.
CCL11 and CCL2 protein levels during MoPn infection. Chemokines were measured in upper and lower GT tissue homogenates in uninfected (day 0) and infected (days 3, 7, 14, 21, and 35) mice by ELISAs. The data are compiled from two separate experiments and are expressed as the mean and standard error of the mean (SEM) for four mice per group. A number sign indicates a value that was significantly elevated in the lower GT versus the upper GT (CCL11, days 21 and 35). A plus sign indicates a value that was significantly different from those on day 0 in the upper GT (CCL11, day 14). Asterisks indicate values that were significantly different from those on day 0 in the lower GT (CCL11, days 14 and 21). The P value determined by ANOVA was <0.005; the P value determined by the post hoc Tukey test was <0.05 ± SEM.
Course of C. trachomatis infection.
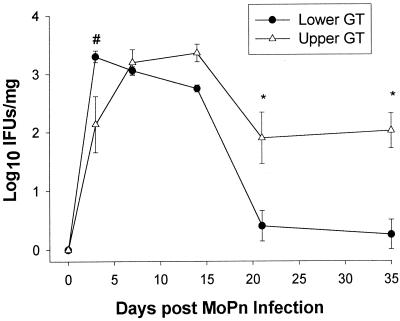
To rule out the possibility that a larger bacterial load in the upper GT could account for differences in chemokine production, we quantitated chlamydiae from GT homogenates. As shown in Fig. 3, the magnitudes of infection were similar for both GT regions. In fact, larger numbers of chlamydiae were detected in the lower GT 3 days following infection, a time when elevated chemokine levels were seen in the upper GT. Resolution of infection began in both regions between days 14 and 21 but appeared to occur more rapidly in the lower GT than in the upper GT. Thus, the levels of infection were similar in the upper and lower GTs during the induction phase of the infection, when marked differences in chemokine levels appeared.
FIG. 3.
Quantitation of viable chlamydiae in different regions of the GT throughout the course of MoPn infection. The lower and upper GT regions from individual mice were homogenized and cultured for the isolation of chlamydiae. The data are compiled from two separate experiments and are expressed as the mean and standard error of the mean (SEM) for seven or eight mice per group. In the upper GT, protein levels ranged from 0.25 mg/ml in uninfected mice to 2 mg/ml between days 7 and 14 of infection. Lower GT protein levels ranged from 1.1 to 2.2 mg/ml in uninfected and infected mice. A number sign indicates a value that was significantly elevated in the lower GT versus the upper GT (the ANOVA P value was <0.001; the post hoc Tukey test P value was <0.05 ± SEM). Asterisks indicate values that were significantly elevated in the upper GT versus the lower GT (the ANOVA P value was <0.001; the post hoc Tukey test P value was <0.05 ± SEM).
Effect of progesterone on chemokine expression.
To ensure that the administration of progesterone 7 days prior to MoPn infection did not influence chemokine expression profiles in the upper and lower GTs, we used ELISAs to measure the levels of chemokines CXCL10 (IP-10) and CCL11 (eotaxin) in the upper and lower GTs in medroxyprogesterone acetate-treated, uninfected mice at weekly intervals matching those of infected mice. We found that baseline levels of CXCL10 (IP-10) and CCL11 (eotaxin) remained unchanged for at least 6 weeks after progesterone injection (data not shown). We also measured serum hormone levels in uninfected mice following the medroxyprogesterone acetate treatment; serum progesterone levels were elevated and fluctuated between 300 and 1,500 pg/ml, while serum estradiol concentrations were less than 10 pg/ml during the same time period (data not shown). Therefore, the increases in chemokine expression seen in Fig. 1 and 2 are a result of inoculation with MoPn and not hormonal variations.
Measurement of chemokine message levels by SuperArray analysis.
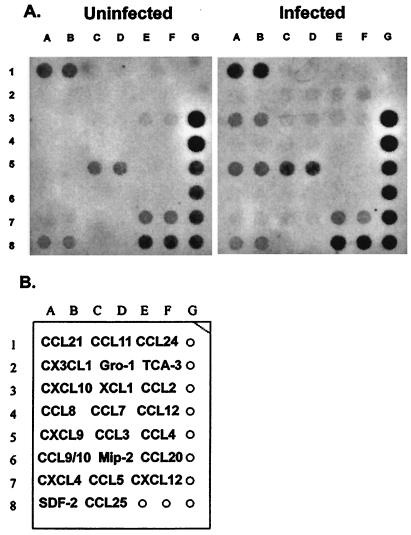
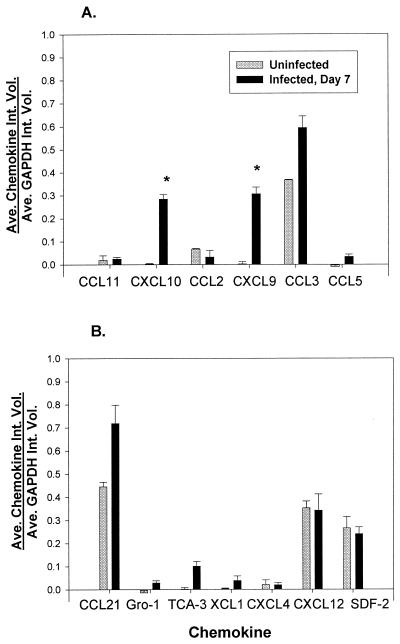
We further confirmed our finding of differential chemokine expression between the upper and lower GTs by measuring mRNA levels by SuperArray analysis. The SuperArray system is designed to semiquantitatively compare the levels of mRNA expression of two matched samples by using paired membranes containing equal amounts of each probe. In addition, the SuperArray assay enabled us to measure mRNA expression for 17 additional chemokines. The comparison of mRNAs isolated from infected (day 7) and uninfected upper GT tissues showed the expression of a number of chemokines (Fig. 4). Notable increases were seen in the intensities of spots for CXCL10 (IP-10) (spots 3A and 3B) and CXCL9 (MIG) (spots 5A and 5B) in infected tissues compared to uninfected tissues.
FIG. 4.
SuperArray analysis of uninfected and infected (day 7) upper GT tissues. (A) Total RNA was isolated from the oviducts of three mice, and a 10-μg pool of RNAs (3.3 μg/mouse) was converted to cDNA by using biotinylated dUTP. Hybridized products were detected by using avidin-alkaline phosphatase and a chemiluminescent substrate. (B) Chemokine template. GAPDH and β-actin served as internal controls (spots 3G to 8G and 8E and F, respectively). Bacterial plasmid pUC18 (1G and 2G) served as a negative control.
To quantitate the data, a laser densitometer was used to determine the average integrated intensity of each dot. Average integrated intensities of duplicate chemokine dots were compared with the average integrated intensity of six GAPDH dots for each paired hybridization experiment. As shown in Fig. 5A, a significant increase in CXCL10 (IP-10) and CXCL9 (MIG) mRNA levels was found in infected upper GT tissues compared to uninfected tissues and was consistent with significantly elevated protein levels. The level of CCL3 (Mip-1α) mRNA was also increased in infected tissues, although not significantly. Surprisingly, only a moderate increase in the level of CCL5 (RANTES) mRNA was detected, despite an elevated protein level. Finally, we did not observe a difference in mRNA expression for CCL11 (eotaxin) and CCL2 (MCP-1) in the upper GT following infection. These data further support the differential expression of Th1-associated chemokines CXCL10 (IP-10) and CXL9 (MIG) following C. trachomatis infection in the upper GT.
FIG. 5.
Quantitation of mRNA data obtained by SuperArray analysis of uninfected and infected (day 7) upper GT tissues as pictured in Fig. 4. Spot intensities were determined by laser densitometry and by using ImageQuaNT software. Data are expressed as the mean (Ave.) integrated volume (Int. Vol.) of duplicate chemokine spots relative to the mean integrated volume of six GAPDH spots; error bars indicate the standard error of the mean (SEM). (A) Chemokines previously measured by ELISAs. Asterisks indicate values that were significantly elevated in infected upper GTs (the Student's t test P value was <0.05 ± SEM). (B) Other chemokines with detectable message levels.
The microarray analysis revealed the expression of other chemokines not evaluated at the protein level. The levels of chemokines CCL21 (secondary lymphoid tissue chemokine [SLC]), growth-regulated oncogene 1 (Gro-1), T-cell activation gene 3 (TCA-3), and XCL1 (lymphotactin) were elevated in upper GT tissues from infected mice compared to uninfected mice (Fig. 5B). We also detected mRNA expression of CXCL4 (platelet factor 4), CXCL12 (stroma-derived factor 1α/β [SDF-1α/β]), and SDF-2. However, the levels of expression were similar between infected and uninfected tissues, suggesting that these chemokines may be constitutively expressed in murine oviducts.
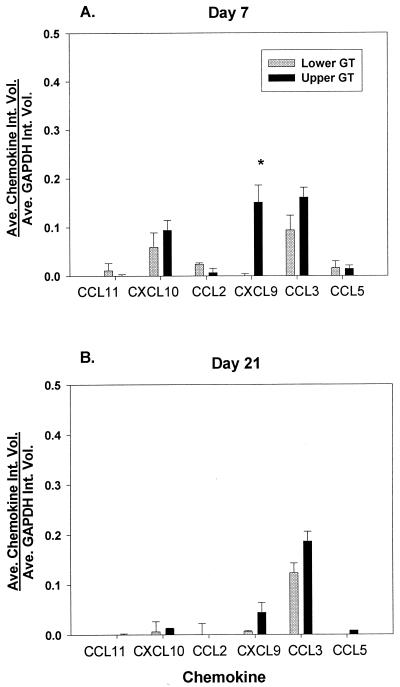
We compared the levels of chemokine mRNAs between the upper and lower GTs during infection (Fig. 6A). Data compiled from two experiments showed a significant increase in the CXCL9 (MIG) mRNA level in the upper GT compared to the lower GT 7 days after infection. Similarly, CXCL10 (IP-10) and CCL3 (Mip-1α) mRNA levels were elevated in the upper GT in comparison to the lower GT. We again observed only low levels of CCL5 (RANTES) mRNA, which did not differ between the upper and lower GTs. Also, the levels of mRNA for CCL11 (eotaxin) and CCL2 (MCP-1) were slightly higher in the lower GT than in the upper GT of infected mice. As noted for the previous microarray analysis, CCL21 (SLC), CXCL12 (SDF-1α/β), and SDF-2 mRNAs were again found in the upper and lower GTs (data not shown), but the levels did not differ significantly between the tissues.
FIG. 6.
Quantitation of mRNA data obtained by SuperArray analysis from infected upper and lower GT tissues. Spot intensities were determined by laser densitometry and by using ImageQuaNT software. Data are expressed as the mean (Ave.) integrated volume (Int. Vol.) of duplicate chemokine spots relative to the mean integrated volume of six GAPDH spots; error bars indicate the standard error of the mean (SEM). (A) Day 7 of infection. Results are an average from two experiments with 10 or 7 μg of mRNA. An asterisk indicates a value that was significantly elevated in the upper GT versus the lower GT (the ANOVA P value was <0.001; the post hoc Tukey P value was <0.05 ± SEM). (B) Day 21 of infection.
Finally, we compared mRNA expression levels in the upper and lower GTs of day 21 infected mice (Fig. 6B). In general, we found that mRNA levels had decreased by this time point, as was observed for chemokine protein levels. Specifically, message levels for CXL10 (IP-10) and CXCL9 (MIG), which peaked between days 3 and 7 of infection, returned to the levels seen in uninfected mice by day 21. Likewise, mRNA levels for CCL5 (RANTES), CCL11 (eotaxin), and CCL2 (MCP-1) also dropped to almost undetectable values in both the upper and the lower GTs. CCL3 (Mip-1α) was the only chemokine with message levels that remained elevated in both the upper and the lower GTs on day 21. Together, these data support the notion of differential chemokine expression between the upper and lower GTs during Chlamydia infection.
Localization of chemokines by immunohistological analysis.
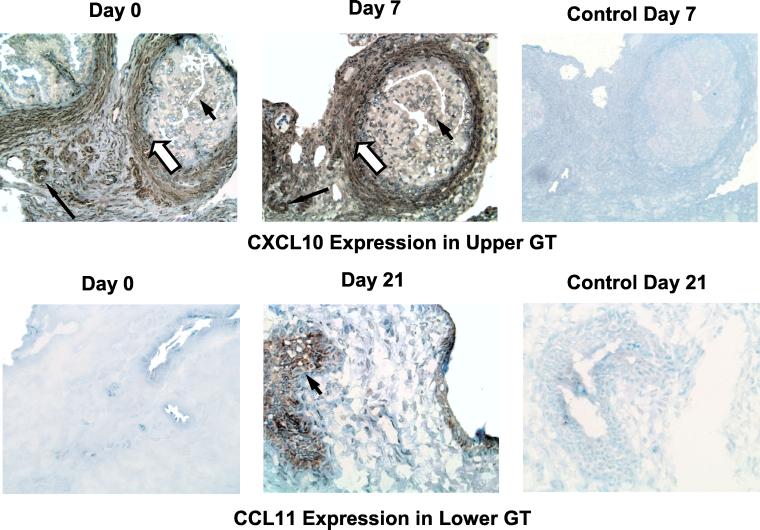
To determine which cells within the GT are responsible for chemokine production, we used immunohistochemical analysis to identify CXCL10 (IP-10)- and CCL11 (eotaxin)-producing cells in the upper and lower GTs. As shown in Fig. 7, CXCL10 (IP-10) was found on columnar epithelial cells, endothelial cells, and stromal cells within the oviduct (Fig. 7, upper left panel). Following infection, the same cell types stained positively for CXCL10 (IP-10) but with greater intensity on day 7 (Fig. 7, upper middle panel). In the lower GT region, CCL11 (eotaxin) staining was not found in uninfected mice (Fig. 7, lower left panel), but squamous epithelial cells stained positively on days 7 (data not shown) and 21 (Fig. 7, lower middle panel). Interestingly, CXCL10 (IP-10) staining in the lower GT was also confined to squamous epithelial cells (data not shown). These data suggest that the high CXCL10 (IP-10) protein levels noted in the upper GT may result from increased production by multiple cell types that are not associated with an inflammatory response, while in the lower GT, chemokine production is confined to the epithelium following infection.
FIG. 7.
Cellular localization of CXCL10 and CCL11. Immunohistochemical staining of CXCL10 in the upper GT (upper panels) and CCL11 in the lower GT (lower panels) in uninfected (day 0) and infected (day 7 or 21) mice is shown. Arrowheads denote columnar (upper GT) or squamous (lower GT) epithelial cells, closed arrows denote endothelial cells, and open arrows denote stromal cells. Magnification, ×400. A low magnification was used to emphasize the differences in cell types producing CXCL10 and CCL11.
DISCUSSION
The expression of chemokines within tissues regulates the recruitment of specific subsets of lymphocytes to distinct tissue sites. Chemokines are therefore responsible, in part, for directing the immune response that ensues following bacterial invasion. Our results are the first to demonstrate that there are regional differences in chemokine expression within the female reproductive tract in response to Chlamydia infection. Although homeostatic differences in CCL25 (thymus-expressed chemokine) expression have been demonstrated within regions of the intestinal tract (19), this is the first report demonstrating regional chemokine differences in response to infection. Studies measuring cellular influx and adhesion molecule expression during Chlamydia infection first suggested that there were regional differences in the immune response between the cervical-vaginal region and oviducts of mice (18, 25). Our data further support this theory, as we found that chemokines associated with Th1 responses were present at significantly higher levels in the oviducts than in the cervical-vaginal tissues of mice during infection. Namely, CXCL10 (IP-10) and CXCL9 (MIG) protein levels peaked early in infection in the upper GT and then returned to the baseline, whereas the CCL5 (RANTES) level remained elevated for the duration of infection. These results were confirmed at the mRNA level, although CXCL9 (MIG) but not CXCL10 (IP-10) mRNA levels were significantly higher in the upper GT than in the lower GT. This finding may have been due to the fact that the CXCL10 (IP-10) protein level in the upper GT peaked at a time point earlier than the one at which we measured mRNA. Although we have not yet confirmed the functions of these chemokines, there are data to suggest that the concentrations reached during infection are sufficient for lymphocyte recruitment (29, 32). Experiments are under way to demonstrate that the induction of CXCL9 (MIG), CXCL10 (IP-10), and/or CCL5 (RANTES) is responsible for the selective recruitment of Th1 cells to the upper GT during infection.
Compared to the results for the upper GT, the chemokine expression patterns differed quantitatively and kinetically in the cervical-vaginal region during infection. First, only low levels of Th1-associated chemokines were present in the lower GT. Second, CCL11 (eotaxin) levels were significantly increased late in the course of infection. Immunohistochemical staining supported these findings by showing that CCL11 (eotaxin) expression was confined to epithelial cells during the resolution phase of infection (day 21). However, the mRNA expression of CCL11 (eotaxin) increased in the lower GT relative to the upper GT early after infection but not at later time points, when the expression of CCL11 (eotaxin) protein was significantly elevated. Although mice cleared infection in the lower GT, the diminished production of Th1-associated chemokines in that region may have been responsible for the reduced numbers of CD4 cells observed in the lower GT. It is possible that ascending infection correlates with smaller numbers of CD4 Th1 cells in the lower GT.
Considering the anatomical and functional differences between the oviducts and cervical regions of the GT, it is not unanticipated to find immunologically distinct responses at these sites. For instance, epithelial cells are different at the two sites. Squamous epithelial cells are found in the cervical region, while ciliated columnar epithelial cells line the oviducts. Epithelial cells play a central role in directing the immune response, since they host Chlamydia and secrete cytokines, such as IL-8, early after infection (27). Moreover, endocervical but not endometrial cell lines secrete IL-8 in response to Chlamydia infection (35), suggesting that epithelial cells at these discrete sites respond differently to infection. In addition, we found that CXCL10 (IP-10) was expressed on a wider array of cell types in the upper GT than in the lower GT, further supporting the concept that chemokine secretion differs between the upper and lower GTs.
The differences in chemokine expression in the upper and lower GTs cannot be explained by simple differences in the level of infection between these two regions. Our data show that the level of infection was significantly higher in the lower GT early in infection, at a time when the levels of Th1-associated chemokines were significantly higher in the upper GT. Likewise, chlamydia levels were similar in the upper and lower GTs during the second week of infection, although resolution of infection occurred more quickly in the lower GT. By day 35, the lower GT was negative for chlamydiae, while the upper GT was either negative or had minimal numbers of inclusions. These results are similar to those previously reported (18), although our data indicate more variability in the numbers of chlamydiae detected in the upper and lower GTs throughout infection and suggest that there may be a small lag in the clearance of chlamydiae from the upper GT. Also, to rule out the possible influence of inoculating dose on chemokine levels, we found no differences in the levels of CXCR10 (IP-10) and CCL11 (eotaxin) in mice infected with 1.5 × 105 IFU of MoPn (data not shown). These data, coupled with the results of the immunohistochemical analysis showing that chemokine expression occurs in noninflammatory cell types early after infection, suggest that chemokine expression in the upper GT precedes the recruitment of inflammatory cells and is not influenced by the inoculating dose. These conclusions are not surprising, since all somatic cells produce chemokines and in other models, chemokine production has been shown to precede the influx of inflammatory cells (20).
Our results showing that steady, basal levels of CXCL10 (IP-10) and CCL11 (eotaxin) are maintained in the upper and lower GTs of uninfected mice treated with medroxyprogesterone acetate indicate that the chemokine differences seen between the upper and lower GTs of infected mice are not due to progesterone treatment. Female reproductive hormones have been reported to alter cytokine production (10, 24), and other data have shown that the expression of some chemokines varies with hormonal fluctuations during normal menstruation. For example, increased immunoreactivity to CCL11 (eotaxin) has been observed in endometrial epithelium during the luteal phase (high progesterone) of the mouse menstrual cycle compared to the follicular phase (low progesterone) (13). In contrast, Saavedra and colleagues (28) reported that estrogen treatment did not alter CCL2 (MCP-1), Mip-2, or CCL5 (RANTES) levels over a 21-day period. In our model, progesterone treatment did not appear to influence CXCL10 (IP-10) and CCL11 (eotaxin) levels, verifying that the increases observed were produced in response to infection.
CCL5 (RANTES) was the only chemokine to stay at significantly elevated levels in the upper GT throughout the course of infection. However, mRNA expression was low on all days that were evaluated. The presence of CCL5 (RANTES) protein in tissue has generally been shown to correlate with mRNA expression, making our results somewhat surprising. A possible explanation is that CCL5 (RANTES) protein is delivered to the tissue from other sites. CCL5 (RANTES) is found at picogram levels in the blood of healthy humans (8) and is known to be released from thrombin-stimulated platelets (16). It is therefore possible that the CCL5 (RANTES) protein measured in the GT following Chlamydia infection is blood derived rather than locally produced. Upon secretion, CCL5 (RANTES) may then directly bind to the activated genital endothelium (34).
SuperArray analysis allowed the evaluation of additional chemokines in the local genital mucosa of infected mice. Other chemokines detected by this analysis include CCL21 (SLC), Gro-1, TCA-3, XCL1 (lymphotactin), CXCL4 (platelet factor 4), CXCL12 (SDF-1α/β), and SDF-2. Most notably, there was an increase in the level of CCL21 (SLC) mRNA in infected upper GT tissues compared to uninfected tissues. CCL21 (SLC) is important for T-cell migration across high endothelial venules within secondary lymphoid tissues, as demonstrated for mice deficient in CCL21 (SLC) (11) or the chemokine receptor CCR7 (38). However, CCL21 (SLC) has also been shown to bind to CXCR3, the receptor for CXCL9 (MIG) and CXCL10 (IP-10) in mice but not humans (31). Preliminary data obtained with reverse transcription-PCR for whole GT homogenates have shown that CXCR3 and CCR5 are expressed only in the GTs of infected mice and not until 14 days after infection (unpublished observations). In addition, we found that the levels of CXCL12 (SDF-1α/β) mRNA expression were similar between infected and uninfected tissues but were approximately twofold higher in upper GT tissue than in lower GT tissue (data not shown). CXCL12 (SDF-1α/β) induces rapid adhesion of CD4 cells to CD54 (5). Thus, CXCL10 (IP-10), CXCL9 (MIG), CCL5 (RANTES), CCL21 (SLC), and CXCL12 (SDF-1α/β) are most likely involved in the chemotaxis of Th1 cells to the upper GT during Chlamydia infection.
To date, there have been very few reports of chemokine induction in response to Chlamydia infection in the GT. Previous reports have examined chemokine induction in vitro and have focused on chemokines of the CXC class, which are important for neutrophil chemotaxis. Namely, IL-8 (27, 35), CXCL1 (Gro-α), and CXCL5 (epithelial neutrophil activating protein 78 [ENA-78]) (35) were produced by epithelial cells infected with human serovars of C. trachomatis. Interestingly, IL-8 was not found in vaginal secretions of women with C. trachomatis infection (12). However, Mip-2 and CCL2 (MCP-1) were found at increased levels in the lungs of mice during infection with Chlamydia psittaci (14). In this study, we noted an increase in Gro-1 but not Mip-2, the functional homolog of murine IL-8. We also found that CCL2 (MCP-1) mRNA expression was consistently low in both the upper and the lower GTs, supporting our protein data. CCL2 (MCP-1) has been shown to be upregulated in vaginal tissues of mice following infection with Candida albicans in vivo (28). These differences in Mip-2 and CCL2 (MCP-1) expression may reflect differences between tissue sites or specific features of the pathogens.
The factors that lead to ascending Chlamydia infection in a subset of individuals are currently unknown. Our data showing differential chemokine expression in the upper and lower GTs support increasing evidence that the inflammatory response in the lower GT may be prematurely terminated even in the presence of an active C. trachomatis infection. Perhaps Chlamydia-infected cells secrete immunosuppressive factors which hamper antichlamydial immunity in the lower GT. Alternatively, early termination of inflammatory responses in the lower GT may be an inherent response of a site that is commonly exposed to nonpathogenic organisms. For example, using another mucosal tissue that is exposed to commensal flora, Yamamoto et al. showed that intestinal epithelial cells inhibit T-cell responses through a novel, non-transforming growth factor β-dependent mechanism (36). Interestingly, the early production of gamma interferon (6) and tumor necrosis factor α (7) in vaginal secretions and the expression of adhesion molecules in the lower GT early after infection (18) diminished to nearly baseline levels by day 7 in the presence of viable chlamydiae. Therefore, we hypothesize that delayed eradication of chlamydiae in the lower GT early after infection may facilitate upper GT infection. Future studies will therefore be aimed at selectively boosting the antichlamydial immune response in the cervical-vaginal region.
Acknowledgments
We thank Robert Strieter for helpful discussions and advice and Ann Chan for histochemical staining.
This work was supported by PHS grant AI26328 from NIH. H.K.M. was supported by Microbial Pathogenesis training grant 5-T32-AI-07323.
Editor:J. D. Clements
REFERENCES
- 1.Akiyama, M., H. Okabe, K. Takakura, Y. Fujiyama, and Y. Noda. 1999. Expression of macrophage inflammatory protein-1alpha (MIP-1alpha) in human endometrium throughout the menstrual cycle. Br. J. Obstet. Gynaecol. 106:725-730. [DOI] [PubMed]
- 2.Altman, G. B., A. M. Gown, D. L. Luchtel, and C. Baker. 1999. RANTES production by cultured primate endometrial epithelial cells. Am. J. Reprod. Immunol. 42:168-174. [DOI] [PubMed] [Google Scholar]
- 3.Arenberg, D. A., S. L. Kunkel, P. J. Polverini, S. B. Morris, M. D. Burdick, M. C. Glass, D. T. Taub, M. D. Iannettoni, R. I. Whyte, and R. M. Strieter. 1996. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J. Exp. Med. 184:981-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher, A., W. Mourad, J. Mailloux, A. Lemay, and A. Akoum. 2000. Ovarian hormones modulate monocyte chemotactic protein-1 expression in endometrial cells of women with endometriosis. Mol. Hum. Reprod. 6:618-626. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, J. J., J. Hedrick, A. Zlotnik, M. A. Siani, D. A. Thompson, and E. C. Butcher. 1998. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 279:381-384. [DOI] [PubMed] [Google Scholar]
- 6.Darville, T., C. W. Andrews, Jr., and R. G. Rank. 2000. Does inhibition of tumor necrosis factor alpha affect chlamydial genital tract infection in mice and guinea pigs? Infect. Immun. 68:5299-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darville, T., J. Sikes, C. W. Andrews, Jr., and R. Rank. 2000. Cytokine response profile that determines early eradication of chlamydial genital tract infection in a murine model, p. 171. In P. Saikku (ed.), Immunology and pathogenesis. Proceedings for the Meeting of the European Society for Chlamydia Research. Universitas Helsingiensis, Helsinke, Finland.
- 8.Fiore, J., M. Di Stefano, L. Lepera, A. Saracino, L. Monno, G. Angarano, and G. Pastore. 1999. Evidence for a local synthesis of B-chemokines within the genital tract of both HIV-1-infected and uninfected women. J. Acquir. Immune Defic. Syndr. 21:255-257. [DOI] [PubMed] [Google Scholar]
- 9.Gerard, C., and B. J. Rollins. 2001. Chemokines and disease. Nat. Immunol. 2:108-115. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore, W., L. P. Weiner, and J. Correale. 1997. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J. Immunol. 158:446-451. [PubMed] [Google Scholar]
- 11.Gunn, M. D., S. Kyuwa, C. Tam, T. Kakiuchi, A. Matsuzawa, L. T. Williams, and H. Nakano. 1999. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189:451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedges, S. R., D. A. Sibley, M. S. Mayo, E. W. Hook, and M. W. Russell. 1998. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J. Infect. Dis. 178:742-751. [DOI] [PubMed] [Google Scholar]
- 13.Hornung, D., K. Dohrn, K. Sotlar, R. R. Greb, D. Wallwiener, L. Kiesel, and R. N. Taylor. 2000. Localization in tissues and secretion of eotaxin by cells from normal endometrium and endometriosis. J. Clin. Endocrinol. Metab. 85:2604-2608. [DOI] [PubMed] [Google Scholar]
- 14.Huang, J., M. D. Wang, S. Lenz, D. Gao, and B. Kaltenboeck. 1999. IL-12 administered during Chlamydia psittaci lung infection in mice confers immediate and long-term protection and reduces macrophage inflammatory protein-2 level and neutrophil infiltration in lung tissue. J. Immunol. 162:2217-2226. [PubMed] [Google Scholar]
- 15.Igietseme, J. U., K. H. Ramsey, D. M. Magee, D. M. Williams, T. J. Kincy, and R. G. Rank. 1993. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific TH1 lymphocyte clone. Reg. Immunol. 5:317-324. [PubMed] [Google Scholar]
- 16.Kameyoshi, Y., A. Dorschner, A. L. Mallet, E. Chrisophers, and J. M. Schroder. 1992. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J. Exp. Med. 176:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly, K. A., and R. G. Rank. 1997. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect. Immun. 65:5198-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly, K. A., J. C. Walker, S. H. Jameel, H. L. Gray, and R. G. Rank. 2000. Differential regulation of CD4 lymphocyte recruitment between the upper and lower regions of the genital tract during Chlamyida infection. Infect. Immun. 68:1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunkel, E. J., J. J. Campbell, G. Haraldsen, J. Pan, J. Boisvert, A. I. Roberts, E. C. Ebert, M. A. Vierra, S. B. Goodman, M. C. Genovese, A. J. Wardlaw, H. B. Greenberg, C. M. Parker, E. C. Butcher, D. P. Andrew, and W. W. Agace. 2000. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunkel, S. L., T. Standiford, K. Kasahara, and R. M. Strieter. 1991. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Exp. Lung Res. 17:17-23. [DOI] [PubMed] [Google Scholar]
- 21.O'Garra, A., McEvoy, L. M., and A. Zlotnik. 1998. T cell subsets: chemokine receptors guide the way. Curr. Biol. 8:R646-R649. [DOI] [PubMed]
- 22.Paavonen, J., and M. Lehtinen. 1996. Chlamydial pelvic inflammatory disease. Hum. Reprod. Update 2:519-529. [DOI] [PubMed] [Google Scholar]
- 23.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 24.Piccinni, M. P., M. G. Giudizi, R. Biagiotti, L. Beloni, L. Giannarini, S. Sampognaro, P. Parronchi, R. Manetti, F. Annunziato, C. Livi, et al. 1995. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J. Immunol. 155:128-133. [PubMed] [Google Scholar]
- 25.Rank, R. G., A. K. Bowlin, and K. A. Kelly. 2000. Characterization of lymphocyte response in the female genital tract during ascending chlamydial genital infection in the guinea pig model. Infect. Immun. 68:5293-5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rank, R. G., M. M. Sanders, and A. T. Kidd. 1993. Influence of the estrous cycle on the development of upper genital tract pathology as a result of chlamydial infection in the guinea pig model of pelvic inflammatory disease. Am. J. Pathol. 142:1291-1296. [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen, S., L. Eckmann, A. J. Quayle, L. Shen, Y. Zhang, D. J. Anderson, J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Investig. 99:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saavedra, M., B. Taylor, N. Lukacs, and P. L. Fidel, Jr. 1999. Local production of chemokines during experimental vaginal candidiasis. Infect. Immun. 67:5820-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sebastiani, S., P. Allavena, C. Albanesi, F. Nasorri, G. Bianchi, C. Traidl, S. Sozzani, G. Girolomoni, and A. Cavani. 2001. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory acitivity. J. Immunol. 166:996-1002. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen, T. L., M. Tani, J. Jensen, V. Pierce, C. Lucchinetti, V. A. Folcik, S. Qin, J. Rottman, F. Sellebjerg, R. M. Strieter, J. L. Frederiksen, and R. M. Ransohoff. 1999. Expresion of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Investig. 103:807-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soto, H., W. Wang, R. M. Strieter, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, J. Hedrick, and A. Zlotnik. 1998. The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc. Natl. Acad. Sci. USA 95:8205-8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taub, D. D., K. Conlon, A. R. Lloyd, J. J. Oppenheim, and D. J. Kelvin. 1993. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1alpha and MIP-1beta. Science 260:355-358. [DOI] [PubMed] [Google Scholar]
- 33.Tuffrey, M., F. Alexander, C. Inman, and M. E. Ward. 1990. Correlation of infertility with altered tubal morphology and function in mice with salpingitis induced by a human genital-tract isolate of Chlamydia trachomatis. J. Reprod. Fertil. 88:295-305. [DOI] [PubMed] [Google Scholar]
- 34.Wiedermann, C. J., E. Kowald, N. Reinish, C. M. Kaehler, I. von Luettichau, J. M. Pattison, P. Huie, R. K. Sibley, P. J. Nelson, and A. M. Krensky. 1993. Monocyte haptotaxis induced by the RANTES chemokine. Curr. Biol. 3:735-739. [DOI] [PubMed] [Google Scholar]
- 35.Wyrick, P. B., S. T. Knight, T. R. Paul, R. G. Rank, and C. S. Barbier. 1999. Persistent chlamydial envelope antigens in antibiotic-exposed infected cells trigger neutrophil chemotaxis. J. Infect. Dis. 179:954-966. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto, M., K. Fujihashi, K. Kawabata, J. R. McGhee, and H. Kiyono. 1998. A mucosal intranet: intestinal epithelial cells down-regulate intraepithelial, but not peripheral, T lymphocytes. J. Immunol. 160:2188-2196. [PubMed] [Google Scholar]
- 37.Yang, X., J. Gartner, L. Zhu, S. Wang, and R. C. Brunham. 1999. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J. Immunol. 162:1010-1017. [PubMed] [Google Scholar]
- 38.Yoshida, R., M. Nagira, M. Kitaura, N. Imagawa, T. Imai, and O. Yoshie. 1998. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J. Biol. Chem. 273:7118-7122. [DOI] [PubMed] [Google Scholar]