Abstract
Serratia marcescens culture filtrates have been reported to be cytotoxic to mammalian cells. Using biochemical and genetic approaches, we have identified a major source of this cytotoxic activity. Both heat and protease treatments abrogated the cytotoxicity of S. marcescens culture filtrates towards HeLa cells, suggesting the involvement of one or more protein factors. A screen for in vitro cytotoxic activity revealed that S. marcescens mutant strains that are deficient in production of a 56-kDa metalloprotease are significantly less cytotoxic to mammalian cells. Cytotoxicity was significantly reduced when culture filtrates prepared from wild-type strains were pretreated with either EDTA or 1,10-phenanthroline, which are potent inhibitors of the 56-kDa metalloprotease. Furthermore, cytotoxic activity was restored when the same culture filtrates were incubated with zinc divalent cations, which are essential for enzymatic activity of the 56-kDa metalloprotease. Finally, recombinant expression of the S. marcescens 56-kDa metalloprotease conferred a cytotoxic phenotype on the culture filtrates of a nonpathogenic Escherichia coli strain. Collectively, these data suggest that the 56-kDa metalloprotease contributes significantly to the in vitro cytotoxic activity commonly observed in S. marcescens culture filtrates.
Serratia marcescens is a gram-negative enteric bacterium that can function as an opportunistic pathogen within immunocompromised hosts (7, 14, 23, 24, 41). S. marcescens is a source of nosocomial infections, in part because the organism readily adheres to invasive hospital instrumentation, such as catheters, endoscopes, and intravenous tubing (1, 17, 27, 32, 34), and is relatively resistant to standard sterilization and disinfection protocols (3, 8, 52, 53). Resistances to β-lactams, cephalosporins, and aminoglycosides have been reported, thereby complicating treatment of S. marcescens nosocomial infections (5, 6, 16, 35, 56).
Upon introduction into the host, S. marcescens can infect numerous sites, including the urinary (42, 60) and respiratory (2) epithelia, muscle and subcutaneous tissues (17), the kidneys (25, 42), the lungs (13, 47), and also the heart and pericardium (58, 59). In addition, S. marcescens eye infections are common and are a frequent cause of keratitis (4, 29-31, 36, 65). In general, S. marcescens infections induce inflammation and fever, but fatal bacteremia can develop in patients weakened by previous infection, surgery, or immunosuppression (24, 59, 64, 67). Despite numerous reported S. marcescens infections and the emergence of antibiotic-resistant strains (7, 14, 23, 62), the virulence mechanisms of this organism are poorly understood.
Carbonell and coworkers reported that S. marcescens culture filtrates exhibited pronounced in vitro cytotoxicity to cultured mammalian cells (11, 12). Importantly, cytotoxicity was detected to various extents in all the strains that were tested, regardless of biotype or serotype (12). However, the cytotoxic factor or factors in S. marcescens culture filtrates were not identified in these studies. S. marcescens secretes many known extracellular proteins, including chitinases, a lecithinase, a hemolysin, siderophores, lipases, proteases, and a nuclease (5, 28). Because a number of these extracellular factors are hydrolytic in nature, it is reasonable to hypothesize that one or more of the factors may directly contribute to cellular cytotoxicity by exerting their damaging effects upon host cells. Alternatively, an unidentified factor may be responsible for inducing cellular cytotoxicity.
In this study, we have used genetic and biochemical approaches to investigate multiple S. marcescens isolates with the objective of identifying the source of cytotoxicity towards mammalian cells that has been previously reported (11, 12). Collectively, our data support the idea that within the culture filtrates of each of the S. marcescens strains screened, the factor primarily responsible for cytotoxicity towards mammalian cells is a zinc-dependent 56-kDa metalloprotease.
MATERIALS AND METHODS
Preparation of culture filtrates.
The S. marcescens and Escherichia coli strains used in this study are listed in Table 1. All bacterial strains were cultured into stationary phase at 37°C in liquid Luria-Bertani medium (Difco, Detroit, Mich.) while being shaken on a rotary platform shaker at 250 rpm. The cultures were harvested by centrifugation at 16,000 × g. The supernatants were immediately filter sterilized by passage through Millipore 0.2-μm-pore-size syringe filters. The culture filtrates were divided into aliquots and stored at 4°C, under which conditions cytotoxic activity did not detectably diminish for at least 2 weeks. The supernatants were assayed for total protein content using the Coomassie protein assay (Pierce, Rockford, Ill.).
TABLE 1.
Bacterial strains screened for cytotoxic activity
| Strain | Source or genotype | Reference |
|---|---|---|
| S. marcescens | ||
| MB835 | Wild-type strain Sr41-8000 (Japan) | 43 |
| MB841 | Wild-type strain Sm6 | 18 |
| MB848 | Wild-type strain 24 (Russia) | 19 |
| MB849 | Wild-type strain HY | 66 |
| MB1065 | Sm6 nucA::Mu-L (Nuc−) | 28 |
| MB1066 | Sm6 prt::Tn 5-1 (MtPrt−) | 28 |
| MB1069 | Sm6 prt::Tn 5-3 (MtPrt−) | 28 |
| MB1911 | Clinical isolate (Houston, Tex.) | |
| E. coli | ||
| MB531 | 594 rpsh galK2 gclTi | 10 |
| MB568 | JM101(pGSD6) | 61 |
| MB2031 | MB568(pUC 19 prt CE Δ16) | 61 |
| MB2033 | MB568(pXclu) | 61 |
Tissue culture.
HeLa cells were cultured at 37°C under 5% CO2 and 90% humidity in 25-cm2 tissue culture flasks (Corning Costar Corp, Cambridge, Mass.) using minimal essential medium supplemented with 10% (vol/vol) fetal bovine serum, 1% (vol/vol) l-glutamine, 5,000 U of penicillin/ml, and 0.85% streptomycin (Invitrogen, Carlsbad, Calif.). Twenty-four hours prior to each experiment, 96-well tissue culture plates (Corning Costar Corp, Cambridge, Mass.) were seeded with 4.0 × 104 cells/well.
Cytotoxicity assay.
Bacterial culture filtrates were applied to monolayers of HeLa cells as indicated for each experiment and were incubated for 24 h at 37°C under 5% CO2. The monolayers were washed with phosphate-buffered saline (PBS) and incubated with 5 mg of 3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyl tetrazolium bromide (MTT) (Amersham Life Sciences, Arlington Heights, Ill.)/ml of RPMI 1240 medium (Sigma, Detroit, Mich.) for 3 to 4 h at 37°C. The HeLa cells were washed with PBS, and the colored formazan product was solubilized by treating the cells with a lysis solution composed of 90% isopropanol containing 40.6 mM HCl and 0.5% sodium dodecyl sulfate (SDS). Conversion of MTT to formazan was quantified by measuring the optical density at 570 nm with subtraction of background absorbance at 690 nm using a Dynatech MR 5000 plate reader. The relative cytotoxicity was calculated by subtracting from a value of 1.0 the fraction of total metabolic activity detected in a monolayer of cells treated with bacterial culture filtrates relative to control monolayers treated with PBS.
Heat treatment of culture filtrates.
Culture filtrates were heated at 100°C for 15 min. The filtrates were then immediately placed on ice until they were applied to monolayers of HeLa cells.
Pronase treatments of culture filtrates.
E. coli or S. marcescens culture filtrates (10 mg of total protein/ml of filtrate) were treated with 1% (wt/wt) pronase (CalBiochem Inc., San Diego, Calif.) in ammonium bicarbonate buffer (pH 8) for 24 h at 37°C. The treated filtrates were immediately added to monolayers of HeLa cells.
Biochemical evaluation of the role of zinc in the cytotoxic activity of S. marcescens culture filtrates.
S. marcescens culture filtrates were treated with the metalloprotease inhibitors 50 mM EDTA and 50 mM 1,10-phenanthroline (Sigma, St. Louis, Mo.) for 24 h at 4°C and then for 1 h at 37°C. As controls, the filtrates were treated with PBS (pH 7.2) for 24 h at 4°C and then for 1 h at 37°C. Each treated filtrate was dialyzed into PBS (pH 7.2) at 4°C, with three changes of buffer (100× volume). Culture filtrates were treated in an identical fashion with the highest manufacturer (Calbiochem, La Jolla, Calif.)-recommended concentrations of Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK) (100 μM), 4-(2-aminoethyl) benzenesulfonylfluoride-HCl (AEBSF) (1 mM), E-64 (10 μM), and (2S, 3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane ethyl ester (EST) (100 μM). For restoration of cytotoxic activity, culture filtrates previously treated with metalloprotease inhibitors were incubated with 2.1 mM ZnSO4 at 22°C for 1 h.
Western blots.
Aliquots of culture filtrates were denatured in SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer and heated for 5 min at 90°C. The denatured proteins were fractionated by SDS-12% PAGE and electrotransferred to a polyvinylidene difluoride (PVDF) membrane (Osmonics, Westborough, Mass.). The membrane was probed with antiserum raised against the S. marcescens 56-kDa metalloprotease (28, 61) and then with alkaline phosphatase-conjugated secondary antibodies (Sigma). The Western blots were visualized by chemiluminescence (Genor Technology, St. Louis, Mo.).
Statistical analysis.
Data analyses were conducted using a Student's paired t test. A P value of less than 0.05 was considered statistically significant.
RESULTS
Effects of S. marcescens on HeLa cell viability.
To test the effects of S. marcescens-derived secreted products on mammalian cells, culture filtrates were prepared from S. marcescens strains derived from different genetic and geographic sources (Table 1). All strains were cultivated to stationary phase in Luria-Bertani broth at 37°C for 24 h, followed by centrifugal harvesting and immediate passage of the supernatants through 0.2-μm-pore-size filters. Serial dilutions of each culture filtrate were applied to monolayers of HeLa cells and incubated at 37°C for 24 h. Consistent with previous reports (11, 12), culture filtrates from each of the strains caused a change in the overall morphology of the mammalian cells, as observed by phase-contrast microscopy (data not shown). At lower concentrations (approximately 0.1 to 1.0 mg of total protein/ml of culture filtrate), the cells became markedly distended and elongated, while at higher concentrations (>1 mg of total protein/ml of culture filtrate), significant rounding of individual cells was observed. Although we used HeLa cells to generate these data, we have found that multiple epithelium-derived cells (CHO-K1 and Vero cells) are similar in their sensitivity to S. marcescens culture filtrates (data not shown).
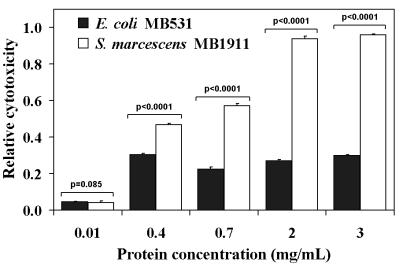
S. marcescens culture filtrate cytotoxicity towards mammalian cells was quantified using the well-established MTT assay (9, 15, 21, 22), as described in Materials and Methods. Figure 1 shows that the culture filtrate of one recently obtained clinical isolate, MB1911, demonstrated a dose-dependent cytotoxicity to HeLa cells, with maximum effects observed at concentrations of approximately 1 to 2 mg of protein/ml of culture filtrate. In contrast, identical concentrations of culture filtrates prepared from a nonpathogenic E. coli K-12 strain (MB531) did not induce morphological changes within HeLa monolayers (data not shown) and were significantly less cytotoxic towards HeLa cells (Fig. 1).
FIG. 1.
S. marcescens MB1911 culture filtrates are cytotoxic to HeLa cell monolayers. Culture filtrates prepared from S. marcescens MB1911 and E. coli MB531 were incubated with a monolayer of HeLa cells (4.0 × 104) for 24 h at 37°C. The relative cytotoxicity of each sample was determined versus control cells treated with an equal volume of PBS, as described under Materials and Methods. The data from three separate experiments performed in replicates of at least six were averaged. The error bars indicate standard deviations. P values are reported directly above the data.
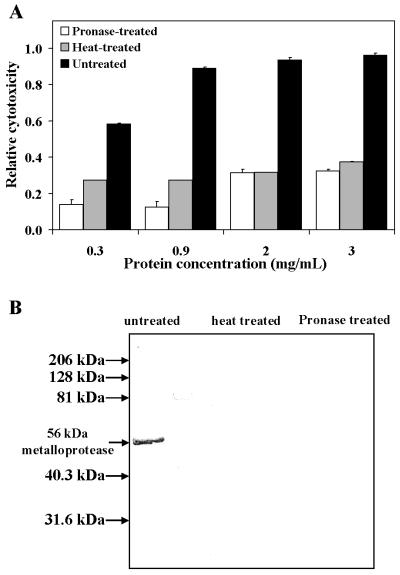
To investigate the molecular nature of the S. marcescens cytotoxic activity, we tested whether the activity is heat labile and/or sensitive to protease treatment. S. marcescens MB1911 culture filtrates were treated with pronase for 24 h. In parallel, culture filtrates were heated at 100°C for 15 min. Filtrates from both treatments were applied to HeLa cell monolayers and incubated at 37°C for 24 h prior to being assayed for cytotoxicity. As shown in Fig. 2, protease or heat treatments drastically attenuated the in vitro cytotoxicity of S. marcescens MB1911 culture filtrates, to about the same level as a nonpathogenic E. coli filtrate (Fig. 1). Collectively, these data suggest that S. marcescens secretes one or more proteinaceous factors with cytotoxic activity.
FIG. 2.
The cytotoxic factor in S. marcescens culture filtrates is sensitive to heat and proteolytic digestion. Culture filtrates prepared from S. marcescens MB1911 were heated for 15 min at 100°C (heat treated), treated for 24 h with pronase (1% [wt/wt]) (pronase treated), or untreated, as described under Materials and Methods. Each sample was tested for cytotoxicity as described in the legend to Fig. 1 (A) or analyzed by Western blot analysis (B). (A) The data from three separate experiments performed in replicates of at least six were averaged. The error bars indicate standard deviations. (B) The culture filtrates were fractionated by SDS-PAGE and electrotransferred onto a PVDF membrane. The membrane was probed with antiserum prepared against the S. marcescens 56-kDa metalloprotease and then with secondary antibodies conjugated to alkaline phosphatase. The blot was visualized by chemiluminescence using an alkaline phosphatase substrate and exposed in the dark to autoradiograph film. In panel A, for all pronase-treated and heated-treated samples, P was <0.0001 compared to the untreated culture filtrates.
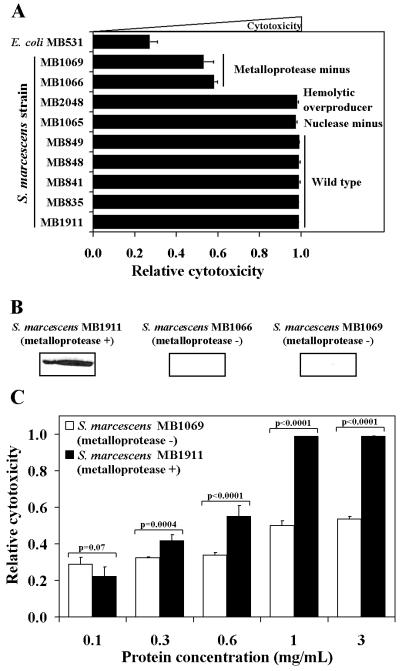
Culture filtrates prepared from S. marcescens mutants defective in metalloprotease production demonstrate reduced cytotoxicity.
In an attempt to identify which factor or factors are responsible for the in vitro cytotoxicity towards cultured mammalian cells, we screened culture filtrates prepared from a panel of wild-type and mutant strains of S. marcescens for cytotoxic activity. Each culture filtrate (2 mg of total protein/ml of filtrate) was applied to HeLa cells and incubated for 24 h at 37°C. S. marcescens isolates from different geographical areas, as well as a nuclease-deficient mutant (MB1065) and a strongly hemolytic strain (MB2048), demonstrated nearly identical cytotoxicities to HeLa cell monolayers (Fig. 3A). However, culture filtrates prepared from two S. marcescens mutant strains deficient in metalloprotease production (MB1066 and MB1069) were found to be markedly less cytotoxic to HeLa cells. MB1066 was previously demonstrated to be devoid of protease activity (28). We also found that culture filtrates prepared from both MB1066 and MB1069 did not contain detectable cross-reacting material when probed with antiserum raised against the S. marcescens 56-kDa metalloprotease (Fig. 3B). Moreover, the MB1069 filtrate did not exhibit the dose-dependent cytotoxicity to HeLa cells demonstrated by the MB1911 filtrate (Fig. 3C). Western blot analysis of protease- or heat-treated culture filtrates prepared from MB1911 that were attenuated in cytotoxic activity (as shown in Fig. 2) also lacked detectable cross-reacting material against 56-kDa metalloprotease antiserum (Fig. 2B), further correlating the presence of the S. marcescens 56-kDa metalloprotease to the observed cytotoxic activity. Collectively, these results suggest that the 56-kDa metalloprotease contributes significantly to the in vitro cytotoxicity elaborated in S. marcescens culture filtrates.
FIG. 3.
Metalloprotease-minus mutants of S. marcescens are attenuated in cellular cytotoxicity. (A and C) The relative cytotoxicities of culture filtrates (2 mg of total protein/ml of filtrate) prepared from the indicated bacterial strains (A) and the dose responses of a metalloprotease-minus strain (MB1069) and a recently acquired clinical isolate (MB1911) (C) were determined, as described in the legend to Fig. 1. The data from three separate experiments performed in replicates of at least six were averaged. The error bars indicate standard deviations. (B) Western blot analysis of culture filtrates prepared from S. marcescens strains MB1911, MB1066, and MB1069 was conducted as described in the legend to Fig. 2. In panel A, P > 0.05 for MB835, MB841, MB848, and MB849; P = 0.02 for MB1065; P = 0.05 for MB2048; and P < 0.0001 for MB1066, MB1069, and MB531 compared to MB1911. In panel C, all P values are printed directly above the data.
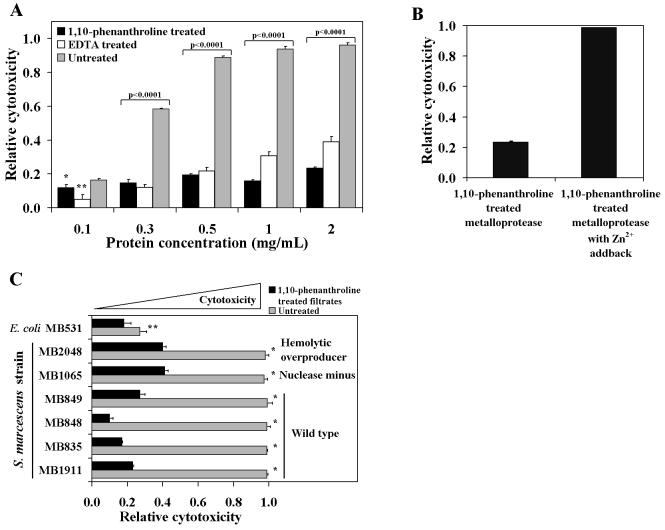
Metalloprotease inhibitors reduce the cytotoxicity of S. marcescens MB1911 culture filtrates.
The enzymatic properties of the S. marcescens 56-kDa metalloprotease have been studied, indicating that an active-site Zn2+ is critical for the protein's catalytic activity (44). The purified 56-kDa metalloprotease was shown to be inactivated by reagents that chelate Zn2+, such as EDTA or 1,10-phenanthroline (44). To further explore the possibility that the S. marcescens 56-kDa metalloprotease contributes to cellular cytotoxicity, culture filtrates prepared from S. marcescens MB1911 were extensively incubated with either EDTA or 1,10-phenanthroline. In the case of EDTA, a final concentration (50 mM) was used that was previously shown to be sufficient to completely inhibit the 56-kDa metalloprotease enzymatic activity (44). The samples were dialyzed to remove excess reagents and then applied to HeLa cells. After 24 h, the relative cytotoxic activities of the treated culture filtrates were analyzed (Fig. 4A). Pretreatment with either EDTA or 1,10-phenanthroline significantly reduced the cytotoxicity of S. marcescens MB1911 culture filtrates to levels exhibited by nonpathogenic E. coli (Fig. 1). 1,10-Phenanthroline was effective in blocking the cytotoxic effects of even the highest concentration of culture filtrate tested (2 mg/ml). In additional experiments, culture filtrates prepared from MB1066 and MB1069 S. marcescens strains defective in metalloprotease production were pretreated with either EDTA or 1,10-phenanthroline as described above. Treatment with these chelating reagents did not result in a further decrease in the cytotoxic activity of either MB1066 or MB1069 (data not shown), suggesting that the majority of divalent-cation-dependent cytotoxic activity observed in S. marcescens culture filtrates is due to the activity of the 56-kDa metalloprotease. In contrast to the inhibitory effects of known metalloprotease inhibitors, we found in preliminary experiments that the cytotoxic activity within S. marcescens culture filtrates was not blocked by pretreatment with AEBSF (1 mM) or TLCK (100 μM), irreversible inhibitors of serine proteases (data not shown). In addition, the cytotoxicity within S. marcescens culture filtrates was not blocked by pretreatment with EST (100 μM) and E-64 (10 μM), which are irreversible inhibitors of cysteine-protease inhibitors (data not shown).
FIG. 4.
The role of divalent ions in the cytotoxic activity from S. marcescens culture filtrates. (A and C) The relative cytotoxicity of each sample was determined versus control cells treated with an equal volume of PBS, as described in the legend to Fig. 1. Culture filtrates prepared from S. marcescens MB1911 (A) or multiple strains of different origins (C) were treated at 4°C for 24 h and then at 37°C for 1 h with either 50 mM EDTA or 1,10-phenanthroline or in the absence of additional reagents (untreated). Excess protease inhibitors were removed by dialysis into PBS. Serial dilutions of the dialyzed filtrates were assayed. (B) Culture filtrates from S. marcescens MB1911 were prepared as described for panel A and then incubated with 2.1 mM Zn2+ for 1 h at 22°C prior to application to HeLa cell monolayers. The data from three separate experiments performed in replicates of at least six were averaged. The error bars indicate standard deviations. Statistical significance is as follows: (A) ∗, P = 0.007, and ∗∗, P < 0.0001; (B) P < 0.0001 for the 1,10-phenanthroline-treated metalloprotease compared to the sample with the zinc addback; (C) ∗, P < 0.0001, and **, P = 0.025 compared to the untreated culture filtrates.
Because the purified S. marcescens 56-kDa apometalloprotease requires Zn2+ for catalytic activity (44), we tested whether adding Zn2+ back to S. marcescens culture filtrates previously detoxified with the metalloprotease inhibitor 1,10-phenanthroline would restore cytotoxic activity. MB1911 culture filtrates were pretreated with the metalloprotease inhibitor 1,10-phenanthroline as described above. The dialyzed culture filtrates were then incubated with 2 mM ZnCl2 at 22°C for 1 h. The treated culture filtrates were applied to HeLa cells and incubated for 24 h at 37°C prior to analysis. As expected, S. marcescens MB1911 culture filtrates pretreated with 1,10-phenanthroline lacked cytotoxic activity (Fig. 4B). However, adding Zn2+ back to the culture filtrates restored the in vitro cytotoxic activity towards HeLa cells. In control experiments, we confirmed that ZnCl2 was not cytotoxic towards HeLa cells at the concentration used in these studies.
Culture filtrates prepared from a number of different strains were pretreated with 1,10-phenanthroline, dialyzed, and applied to HeLa cells for 24 h at 37°C, as described above. For each of the strains tested, the cytotoxic activity in the culture filtrates was reduced significantly after pretreatment with the Zn2+-chelating compound (Fig. 4C), further supporting the role of the 56-kDa metalloprotease in S. marcescens-induced in vitro cytotoxicity.
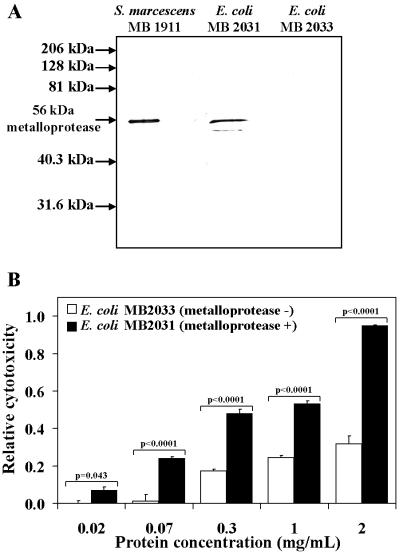
Recombinant S. marcescens metalloprotease potentiates the cytotoxicity of E. coli culture filtrates.
The S. marcescens 56-kDa metalloprotease was expressed as a recombinant protein to determine whether a cytotoxic phenotype would be conferred on a nonpathogenic E. coli strain. E. coli MB568, which has plasmid pGSD6 that carries the genes encoding an ABC transporter necessary for activation and secretion of the metalloprotease, was transformed with a plasmid harboring the gene encoding the 56-kDa metalloprotease, resulting in a strain (MB2031) that secretes the 56-kDa metalloprotease (61). The same pGSD6-carrying E. coli strain transformed with only the parent plasmid (pUC19) was also prepared (MB2033). Both strains were cultivated, and culture filtrates were prepared and analyzed for the presence of the 56-kDa metalloprotease. Western blot analysis using antiserum raised against the metalloprotease revealed significant cross-reacting material in the culture filtrates of E. coli MB2031 but not in the control strain, MB2033 (Fig. 5A), demonstrating that the 56-kDa metalloprotease was produced and secreted in the heterologous host. Culture filtrates from both strains normalized for total protein concentration were incubated with HeLa cells for 24 h at 37°C. Significantly, culture filtrates prepared from E. coli MB2031 demonstrated significantly more cytotoxicity towards HeLa cells than those prepared from MB2033 (Fig. 5B). The cytotoxic activity of E. coli MB2031 was inhibited by pretreatment of culture filtrates with either EDTA or 1,10-phenanthroline as described above (data not shown), further supporting the idea that the recombinant metalloprotease was responsible for the cytotoxic activity. These data indicate that the presence of S. marcescens 56-kDa metalloprotease in the culture filtrates of a nonpathogenic E. coli strain is sufficient to induce cytotoxicity towards cultured HeLa cells.
FIG. 5.
Heterologous expression of the S. marcescens metalloprotease in E. coli confers a cytotoxic phenotype on culture filtrates. (A) Western blot analysis of culture filtrates prepared from an E. coli strain expressing the 56-kDa metalloprotease (MB2031) and an E. coli strain containing the parent plasmid alone (MB2033). The culture filtrates were fractionated by SDS-PAGE and electrotransferred onto a PVDF membrane. The membrane was probed with primary antibodies to the S. marcescens 56-kDa metalloprotease and secondary antibodies conjugated to alkaline phosphatase. The blot was visualized by chemiluminescence using an alkaline phosphatase substrate and exposed in the dark to autoradiograph film. (B) Culture filtrates were added in serial dilutions to HeLa cell monolayers, and the relative cytotoxic activity of each sample was determined as for Fig. 1. The data from three separate experiments performed in replicates of at least six were averaged. The error bars indicate standard deviations. All P values are printed directly above the data.
DISCUSSION
Because S. marcescens infections occur at many target tissues within the host, it is likely that the bacterium exhibits virulence strategies common to bacterial pathogens known as “generalists” (56). These pathogens, which include Staphylococcus aureus, Pseudomonas aeruginosa, and many Streptococcus species, elaborate virulence factors that facilitate colonization of multiple niches within the host and encompass different cell types and overall environments. A common strategy used by bacterial pathogens is to secrete toxins and other factors that modulate the properties of host cell tissues (20, 57). The identification of bacterial factors that are important for host cell interactions will be critical to our understanding of S. marcescens pathogenesis.
Carbonell and coworkers (11, 12) demonstrated that culture filtrates prepared from strains of S. marcescens caused cytotoxic effects on both HeLa and Vero cells. Importantly, cytotoxicity was found in the culture filtrates of all S. marcescens strains that were screened. Based on the cytotoxicity observed in these studies, S. marcescens strains were proposed to secrete one or more cytotoxic factors that induced morphological changes in cultured mammalian cells and also reduced the viability of cellular monolayers. S. marcescens secretes a broad array of factors, including a hemolysin, a nuclease, chitinases, a metalloprotease, serine proteases, siderophores, and lipases (5, 28). Each of these factors by itself has the potential to exert a cytotoxic effect on mammalian cells. Our primary objective focused on identifying which, if any, of these secreted factors within culture filtrates contribute to the previously established cytotoxic activity.
In this investigation, we used both genetic and biochemical approaches to identify the previously described 56-kDa metalloprotease as a significant, and perhaps the dominant, source of in vitro cytotoxicity within S. marcescens culture filtrates. S. marcescens mutant strains that were deficient in metalloprotease production demonstrated decreased cytotoxicity to HeLa cells. Importantly, the 56-kDa metalloprotease has been reported to be secreted from essentially every S. marcescens strain and is, in fact, a marker for identifying S. marcescens isolates (28). This is consistent with our finding, and those previously published (11, 12), that cytotoxic activity is present in all culture filtrates that have been screened.
The S. marcescens metalloprotease is known to contain a bound Zn2+ that is essential for enzymatic activity (44). It was previously shown that when the active-site Zn2+ was extracted with strong divalent-cation chelating agents, the enzymatic activity of the 56-kDa metalloprotease was inhibited (44). Significantly, we eliminated the cytotoxic activity by treating S. marcescens culture filtrates with either EDTA or 1,10-phenanthroline. Moreover, we restored the cytotoxic activity to previously detoxified culture filtrates by reintroducing Zn2+, which reactivates the catalytic activity of the apoenzyme (44). Because we could modulate cytotoxic activity by treating S. marcescens culture filtrates in a manner that directly affects metalloprotease enzymatic activity, it is likely that the catalytic activity of the 56-kDa metalloprotease is required for cytotoxicity. These data, however, do not by themselves rule out the possibility that EDTA or 1,10-phenanthroline inactivates another unknown factor produced by S. marcescens that exerts cytotoxic activity towards HeLa cells.
Perhaps the strongest evidence implicating the S. marcescens 56-kDa metalloprotease as the primary cytotoxic factor is the dramatic elevation in cytotoxicity demonstrated by culture filtrates prepared from a nonpathogenic E. coli strain transformed with a plasmid harboring the gene encoding the 56-kDa metalloprotease. This is the first evidence that when expressed in a different genetic background, the 56-kDa metalloprotease is sufficient to confer a cytotoxic phenotype on culture filtrates.
Our results indicate, for the first time, that among the broad array of potentially hydrolytic and cytotoxic factors secreted by S. marcescens, the 56-kDa metalloprotease is a dominant source of observed cytotoxicity toward mammalian cells. Interestingly, the 56-kDa metalloprotease has previously been proposed to be involved in pathogenesis (37, 38, 40, 45). The purified enzyme has been used in a model system to study keratitis (33), and its enzymatic activity has been characterized and shown to rapidly degrade a wide range of structural and serum proteins (48). Moreover, purified 56-kDa metalloprotease demonstrated a marked cytotoxic effect when applied to human fibroblast cells (48). Thus, our identification of the 56-kDa metalloprotease as a dominant source of cytotoxicity within culture filtrates strongly supports the hypothesis that this secreted factor could play a role in pathogenesis.
Zinc-dependent metalloprotease activity is exhibited by some of the most potent toxins produced by bacterial pathogens (26), including the lethal botulinum, tetanus, and anthrax toxins. Interestingly, each of these toxins functions from an intracellular site of action, thus requiring entry into host cells (46, 49). These intracellularly acting toxins generally possess an A-B domain structure, with the B fragment binding the toxin to sensitive cells and facilitating translocation of a catalytic A fragment into the cytosol. It is not yet clear whether the S. marcescens metalloprotease also possesses a B fragment for transporting the catalytic fragment into the cell. Importantly, the purified 56-kDa metalloprotease was previously shown to be internalized in fibroblasts but required the formation of a complex with α-macroglobulin for successful entry (39). These results suggest that the 56-kDa metalloprotease may possess a binding site for specific host proteins that are internalized by an endocytic mechanism, which represents an interesting mechanism for active transport of a cytotoxic factor into host cells. In the previous study, it was not established that upon entry into sensitive mammalian cells, the 56-kDa metalloprotease acts upon a specific intracellular target, as in the case of anthrax, botulinum, and tetanus toxins (50, 51, 54, 55, 63). However, the possibility exists that the 56-kDa metalloprotease could specifically target a host cellular protein, thereby altering its function to result in the modulation of cellular properties during S. marcescens infection.
In summary, we have confirmed previous reports that culture filtrates prepared from S. marcescens strains are cytotoxic to mammalian cells. Significantly, we employed genetic and biochemical approaches to identify the secreted 56-kDa metalloprotease, common to all S. marcescens strains, as a dominant contributor to in vitro cytotoxicity. The loss of cytotoxicity in S. marcescens strains deficient in metalloprotease production, as well as the gain of a cytotoxic phenotype in E. coli strains expressing and secreting the recombinant 56-kDa metalloprotease, strongly suggests that this extracellular factor could be important for S. marcescens pathogenesis within immunocompromised hosts. Additional investigations will not only reveal the cytotoxic mechanism of the 56-kDa metalloprotease but will be necessary to assess the role of this secreted factor in S. marcescens pathogenesis within the host.
Acknowledgments
We thank A. Vailas and D. Martinez, who provided access to their laboratory's Dynatech MR5000 microtiter plate reader.
This work was supported by the Robert A. Welch Foundation (E-1311), the American Heart Association (98BG472), and an Oak Ridge Junior Faculty Enhancement Award to S.R.B and Welch Foundation E-1310 and N.I.H./NIAID AI46340-03 grants to M.J.B.
REFERENCES
- 1.Acar, J. F. 1986. Serratia marcescens infections. Infect. Control 7:273-278. [DOI] [PubMed] [Google Scholar]
- 2.Albers, M. J., J. W. Mouton, and D. Tibboel. 2001. Colonization and infection by Serratia species in a paediatric surgical intensive care unit. J. Hosp. Infect. 48:7-12. [DOI] [PubMed] [Google Scholar]
- 3.Archibald, L. K., et al. 1997. Serratia marcescens outbreak associated with extrinsic contamination of 1% chlorxylenol soap. Infect. Control Hosp. Epidemiol. 18:704-709. [DOI] [PubMed] [Google Scholar]
- 4.Atlee, W. E., R. P. Burns, and M. Oden. 1970. Serratia marcescens keratoconjunctivitis. Am. J. Ophthalmol. 70:31-33. [DOI] [PubMed] [Google Scholar]
- 5.Aucken, H. M., and T. L. Pitt. 1998. Antibiotic resistance and putative virulence factors of Serratia marcescens with respect to O and K serotypes. J. Med. Microbiol. 47:1105-1113. [DOI] [PubMed] [Google Scholar]
- 6.Berlanga, M., J. L. Vazquez, J. Hernandez-Borrell, M. T. Montero, and M. Vinas. 2000. Evidence of an efflux pump in Serratia marcescens. Microb. Drug Resist. 6:111-117. [DOI] [PubMed] [Google Scholar]
- 7.Boquete, T., A. Vindel, C. Martin-Bourgon, L. Azanedo, and J. A. Saez-Nieto. 1996. Epidemiological markers of Serratia marcescens isolates causing nosocomial infections in Spain (1981-1991). Microbiologia 12:607-612. [PubMed] [Google Scholar]
- 8.Bosi, C., A. Davin-Regli, R. Charrel, B. Rocca, D. Monnet, and C. Bollet. 1996. Serratia marcescens nosocomial outbreak due to contamination of hexetidine solution. J. Hosp. Infect. 33:217-224. [DOI] [PubMed] [Google Scholar]
- 9.Buttke, T. M., J. A. McCubrey, and T. C. Owen. 1993. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J. Immunol. Methods 157:233-240. [DOI] [PubMed] [Google Scholar]
- 10.Campbell, A. 1961. Sensitive mutants of bacteriophage λ. Virology 14:22-32. [DOI] [PubMed] [Google Scholar]
- 11.Carbonell, G. V., A. F. Alfieri, A. A. Alfieri, M. C. Vidotto, C. E. Levy, A. L. Darini, and R. M. Yanaguita. 1997. Detection of cytotoxic activity on Vero cells in clinical isolates of Serratia marcescens. Braz. J. Med. Biol. Res. 30:1291-1298. [DOI] [PubMed] [Google Scholar]
- 12.Carbonell, G. V., B. A. Fonseca, L. T. Figueiredo, A. L. Darini, and R. M. Yanaguita. 1996. Culture conditions affect cytotoxin production by Serratia marcescens. FEMS Immunol. Med. Microbiol. 16:299-307. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri, A. K., and C. F. Booth. 1992. Outbreak of chest infections with Serratia marcescens. J. Hosp. Infect. 22:169-170. [DOI] [PubMed] [Google Scholar]
- 14.Cimolai, N., C. Trombley, D. Wensley, and J. LeBlanc. 1997. Heterogeneous Serratia marcescens genotypes from a nosocomial pediatric outbreak. Chest 111:194-197. [DOI] [PubMed] [Google Scholar]
- 15.Cory, A. H., T. C. Owen, J. A. Barltrop, and J. G. Cory. 1991. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 3:207-212. [DOI] [PubMed] [Google Scholar]
- 16.Edgar, P., R. Mlcak, M. Desai, H. A. Linares, L. G. Phillips, and J. P. Heggers. 1997. Containment of a multiresistant Serratia marcescens outbreak. Burns 23:15-18. [DOI] [PubMed] [Google Scholar]
- 17.Egebo, K., P. Toft, and C. J. Jakobsen. 1996. Contamination of central venous catheters. The skin insertion wound is a major source of contamination. J. Hosp. Infect. 32:99-104. [DOI] [PubMed] [Google Scholar]
- 18.Falkow, S., J. Marmur, W. Carey, W. Spilman, and L. Baron. 1961. Episomic transfer between Salmonella typhosa and Serratia marcescens. Genetics 46:703-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filimonova, M. N., N. P. Balaban, F. P. Sharipova, and I. B. Leshchinskaia. 1980. Isolation and physico-chemical properties of homogenous nuclease from Serratia marcescens. Biokhimiia. 45:2096-2104. [PubMed] [Google Scholar]
- 20.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gieni, R. S., Y. Li, and K. T. HayGlass. 1995. Comparison of [3H]thymidine incorporation with MTT- and MTS-based bioassays for human and murine IL-2 and IL-4 analysis. Tetrazolium assays provide markedly enhanced sensitivity. J. Immunol. Methods 187:85-93. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin, C. J., S. J. Holt, S. Downes, and N. J. Marshall. 1995. Microculture tetrazolium assays: a comparison between two new tetrazolium salts, XTT and MTS. J. Immunol. Methods 179:95-103. [DOI] [PubMed] [Google Scholar]
- 23.Goullet, P., and B. Picard. 1997. An epidemiological study of Serratia marcescens isolates from nosocomial infections by enzyme electrophoresis. J. Med. Microbiol. 46:1019-1028. [DOI] [PubMed] [Google Scholar]
- 24.Haddy, R. I., B. L. Mann, D. D. Nadkarni, R. F. Cruz, D. J. Elshoff, F. C. Buendia, T. A. Domers, and A. M. Oberheu. 1996. Nosocomial infection in the community hospital: severe infection due to Serratia species. J. Fam. Pract. 42:273-277. [PubMed] [Google Scholar]
- 25.Haraoka, M., T. Matsumoto, Y. Mizunoe, N. Ogata, K. Takahashi, S. Kubo, M. Tanaka, and J. Kumazawa. 1993. Effect of prednisolone on renal scarring in rats following infection with Serratia marcescens. Ren. Fail. 15:567-571. [DOI] [PubMed] [Google Scholar]
- 26.Hase, C. C., and R. A. Finkelstein. 1993. Bacterial extracellular zinc-containing metalloproteases. Microbiol. Rev. 57:823-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hejazi, A., and F. R. Falkiner. 1997. Serratia marcescens. J. Med. Microbiol. 46:903-912. [DOI] [PubMed] [Google Scholar]
- 28.Hines, D. A., P. N. Saurugger, G. M. Ihler, and M. J. Benedik. 1988. Genetic analysis of extracellular proteins of Serratia marcescens. J. Bacteriol. 170:4141-4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hume, E. B., L. L. Conerly, J. M. Moreau, B. M. Cannon, L. S. Engel, D. W. Stroman, J. M. Hill, and R. J. O'Callaghan. 1999. Serratia marcescens keratitis: strain-specific corneal pathogenesis in rabbits. Curr. Eye Res. 19:525-532. [DOI] [PubMed] [Google Scholar]
- 30.Hume, E. B., and M. D. Willcox. 1997. Adhesion and growth of Serratia marcescens on artificial closed eye tears soaked hydrogel contact lenses. Aust. N. Z. J. Ophthalmol. 25(Suppl. 1):S39-S41. [DOI] [PubMed]
- 31.Hume, E. B., M. D. Willcox, D. F. Sweeney, and B. A. Holden. 1996. An examination of the clonal variants of Serratia marcescens that infect the eye during contact lens wear. J. Med. Microbiol. 45:127-132. [DOI] [PubMed] [Google Scholar]
- 32.Janknecht, P., and I. Kappstein. 1998. Bacterial contamination of the pressure receiver of a vitrectomy machine. Ophthalmol. Surg. Lasers 29:345-347. [PubMed] [Google Scholar]
- 33.Kamata, R., K. Matsumoto, R. Okamura, T. Yamamoto, and H. Maeda. 1985. The serratial 56K protease as a major pathogenic factor in serratial keratitis. Clinical and experimental study. Ophthalmology 92:1452-1459. [DOI] [PubMed] [Google Scholar]
- 34.Kappstein, I., C. M. Schneider, H. Grundmann, R. Scholz, and P. Janknecht. 1999. Long-lasting contamination of a vitrectomy apparatus with Serratia marcescens. Infect. Control Hosp. Epidemiol. 20:192-195. [DOI] [PubMed] [Google Scholar]
- 35.Luzzaro, F., M. Perilli, R. Migliavacca, G. Lombardi, P. Micheletti, A. Agodi, S. Stefani, G. Amicosante, and L. Pagani. 1998. Repeated epidemics caused by extended-spectrum beta-lactamase-producing Serratia marcescens strains. Eur. J. Clin. Microbiol. Infect. Dis. 17:629-636. [DOI] [PubMed] [Google Scholar]
- 36.Lyerly, D., L. Gray, and A. Kreger. 1981. Characterization of rabbit corneal damage produced by Serratia keratitis and by a Serratia protease. Infect. Immun. 33:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda, H. 1996. Role of microbial proteases in pathogenesis. Microbiol. Immunol. 40:685-699. [DOI] [PubMed] [Google Scholar]
- 38.Maeda, H., and A. Molla. 1989. Pathogenic potentials of bacterial proteases. Clin. Chim. Acta 185:357-367. [DOI] [PubMed] [Google Scholar]
- 39.Maeda, H., A. Molla, T. Oda, and T. Katsuki. 1987. Internalization of serratial proteases into cells as an enzyme-inhibitor complex with α2-macroglobulin, and regeneration of protease activity and cytotoxicity. J. Biol. Chem. 262:10946-10950. [PubMed] [Google Scholar]
- 40.Maeda, H., and T. Yamamoto. 1996. Pathogenic mechanisms induced by microbial proteases in microbial infections. Biol. Chem. Hoppe-Seyler 377:217-226. [DOI] [PubMed] [Google Scholar]
- 41.Manfredi, R., A. Nanetti, M. Ferri, and F. Chiodo. 2000. Clinical and microbiological survey of Serratia marcescens infection during HIV disease. Eur. J. Clin. Microbiol. Infect. Dis. 19:248-253. [DOI] [PubMed] [Google Scholar]
- 42.Marre, R., J. Hacker, and V. Braun. 1989. The cell-bound hemolysin of Serratia marcescens contributes to uropathogenicity. Microb. Pathog. 7:153-156. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto, H., T. Tazaki, and S. Hosogaya. 1973. A generalized transducing phage of Serratia marcescens. Jpn. J. Microbiol. 17:473-479. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto, K., H. Maeda, K. Takata, R. Kamata, and R. Okamura. 1984. Purification and characterization of four proteases from clinical isolate of Serratia marcescens kums 3958. J. Bacteriol. 157:225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumoto, K., T. Yamamoto, R. Kamata, and H. Maeda. 1984. Pathogenesis of serratial infection: activation of the Hageman factor-prekallikrein cascade by serratial protease. J. Biochem. (Tokyo) 96:739-749. [DOI] [PubMed] [Google Scholar]
- 46.Menestrina, G., G. Schiavo, and C. Montecucco. 1994. Molecular mechanisms of action of bacterial protein toxins. Mol. Aspects Med. 15:79-193. [DOI] [PubMed] [Google Scholar]
- 47.Mizota, M., K. Kawakami, O. Ijichi, T. Takezaki, and K. Miyata. 1995. Serratia marcescens lung abscess in a child with autoimmune neutropenia. Acta Paediatr. Jpn. 37:377-380. [DOI] [PubMed] [Google Scholar]
- 48.Molla, A., K. Matsumoto, I. Oyamada, T. Katsuki, and H. Maeda. 1986. Degradation of protease inhibitors, immunoglobulins, and other serum proteins by Serratia protease and its toxicity to fibroblast in culture. Infect. Immun. 53:522-529. [DOI] [PMC free article] [PubMed]
- 49.Montecucco, C., E. Papini, and G. Schiavo. 1994. Bacterial protein toxins penetrate cells via a four-step mechanism. FEBS Lett. 346:92-98. [DOI] [PubMed] [Google Scholar]
- 50.Montecucco, C., G. Schiavo, and O. Rossetto. 1996. The mechanism of action of tetanus and botulinum neurotoxins. Arch. Toxicol. Suppl. 18:342-354. [DOI] [PubMed] [Google Scholar]
- 51.Montecucco, C., G. Schiavo, V. Tugnoli, and D. de Grandis. 1996. Botulinum neurotoxins: mechanism of action and therapeutic applications. Mol. Med. Today 2:418-424. [DOI] [PubMed] [Google Scholar]
- 52.Nakashima, A., M. McCarthy, W. Martone, and R. Anderson. 1987. Epidemic septic arthritis caused by Serratia marcescens and associated with a benzalkanium chloride antiseptic. J. Clin. Microbiol. 25:1014-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakashima, A. K., A. K. Highsmith, and W. J. Martone. 1987. Survival of Serratia marcescens in benzalkonium chloride and in multiple-dose medication vials: relationship to epidemic septic arthritis. J. Clin. Microbiol. 25:1019-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pellizzari, R., C. Guidi-Rontani, G. Vitale, M. Mock, and C. Montecucco. 2000. Lethal factor of Bacillus anthracis cleaves the N-terminus of MAPKKs: analysis of the intracellular consequences in macrophages. Int. J. Med. Microbiol. 290:421-427. [DOI] [PubMed] [Google Scholar]
- 55.Pellizzari, R., O. Rossetto, G. Schiavo, and C. Montecucco. 1999. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Phil. Trans. R. Soc. Lond. B 354:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfennig, K. S. 2001. Evolution of pathogen virulence: the role of variation in host phenotype. Proc. R. Soc. Lond. B 268:755-760. [DOI] [PMC free article] [PubMed]
- 57.Raupach, B., J. Mecsas, U. Heczko, S. Falkow, and B. B. Finlay. 1999. Bacterial epithelial cell cross talk. Curr. Top. Microbiol. Immunol. 236:137-161. [DOI] [PubMed] [Google Scholar]
- 58.Riberi, A., T. Caus, T. Mesana, A. Goudard, A. Mouly, G. Habib, and J. R. Monties. 1997. Aortic valve or root replacement with cryopreserved homograft for active infectious endocarditis. Cardiovasc. Surg. 5:579-583. [DOI] [PubMed] [Google Scholar]
- 59.Rudnick, J. R., C. M. Beck-Sague, R. L. Anderson, B. Schable, J. M. Miller, and W. R. Jarvis. 1996. Gram-negative bacteremia in open-heart-surgery patients traced to probable tap-water contamination of pressure-monitoring equipment. Infect. Control Hosp. Epidemiol. 17:281-285. [DOI] [PubMed] [Google Scholar]
- 60.Shupp Byrne, D. E., J. F. Sedor, M. Soroush, P. A. McCue, and S. G. Mulholland. 2001. Interaction of bladder glycoprotein GP51 with uropathogenic bacteria. J. Urol. 165:1342-1346. [DOI] [PubMed] [Google Scholar]
- 61.Suh, Y., and M. J. Benedik. 1992. Production of active Serratia marcescens metalloprotease from Escherichia coli by alpha-hemolysin HlyB and HlyD. J. Bacteriol. 174:2361-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Ogtrop, M. L., D. van Zoeren-Grobben, E. M. Verbakel-Salomons, and C. P. van Boven. 1997. Serratia marcescens infections in neonatal departments: description of an outbreak and review of the literature. J. Hosp. Infect. 36:95-103. [DOI] [PubMed] [Google Scholar]
- 63.Vitale, G., R. Pellizzari, C. Recchi, G. Napolitani, M. Mock, and C. Montecucco. 1998. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248:706-711. [DOI] [PubMed] [Google Scholar]
- 64.Wilfert, J., F. Barret, and E. Kass. 1968. Bacteremia due to Serratia marcescens. N. Engl. J. Med. 279:286-289. [DOI] [PubMed] [Google Scholar]
- 65.Willcox, M. D., and E. B. Hume. 1999. Differences in the pathogenesis of bacteria isolated from contact-lens-induced infiltrative conditions. Aust. N. Z. J. Ophthalmol. 27:231-233. [DOI] [PubMed] [Google Scholar]
- 66.Winkler, U. 1968. Mutants of Serratia marcescens defective or superactive in the release of a nuclease, p. 187-201. In H. Wittman and H. Schuster (ed.), Molecular genetics. Springer, Berlin, Germany.
- 67.Yu, W. L., C. W. Lin, and D. Y. Wang. 1998. Serratia marcescens bacteremia: clinical features and antimicrobial susceptibilities of the isolates. J. Microbiol. Immunol. Infect. 31:171-179. [PubMed] [Google Scholar]