Abstract
One of the major problems related with anticancer chemotherapy is resistance against anticancer drugs. The ATP-binding cassette (ABC) transporters are a family of transporter proteins that are responsible for drug resistance and a low bioavailability of drugs by pumping a variety of drugs out cells at the expense of ATP hydrolysis. One strategy for reversal of the resistance of tumor cells expressing ABC transporters is combined use of anticancer drugs with chemosensitizers. In this review, the physiological functions and structures of ABC transporters, and the development of chemosensitizers are described focusing on well-known proteins including P-glycoprotein, multidrug resistance associated protein, and breast cancer resistance protein.
Keywords: ABC transporter, bioavailability, chemosensitizer, drug resistance, P-glycoprotein, multidrug resistance associated protein, breast cancer resistance protein.
Background
One of the major problems related with anticancer chemotherapy is resistance against anticancer drugs. Some cancers such as non-small cancer, lung cancer, and rectal cancer show what is called primary resistance or natural resistance in which they do not respond to standard chemotherapy drugs from the beginning. On the other hand, many types of sensitive tumors respond well to chemotherapy drugs in the beginning but show acquired resistance later. Experimentally, drug resistance could be very specific to the drug used due to abnormal genetic machinery such as gene amplification within tumor cells in many cases. Multidrug resistance (MDR) is especially problematic in acquired drug resistance. MDR is the phenomenon in which cancer cells exposed to one anticancer drug show resistance to various anticancer drugs that are structurally and functionally different from the initial anticancer drug. The most investigated mechanisms with known clinical significance are: a) activation of transmembrane proteins effluxing different chemical substances from the cells; b) activation of the enzymes of the glutathione detoxification system; c) alterations of the genes and the proteins involved into the control of apoptosis (especially p53 and Bcl-2). The cell membrane, cytoplasm, and nuclear protein participate in these resistance mechanisms [1]. The resistance mechanism is called typical MDR or classical MDR when overexpression of the membrane efflux pumps is involved in MDR. The classical MDR is due mostly to increased efflux pumps in the cell membrane of cells pumping anticancer drugs out of cells. The most typical efflux pumps in the cell membrane is P-glycoprotein (Pgp) [2] having the molecular weight of 170 KD, due to the gene amplification of the normal human gene, MDR1. The efflux pump Pgp is responsible for transporting various xenobiotics (not limited to anticancer drugs) out of cells by using ATP (Fig. 1) [3]. Pgp is one of the membrane transporter superfamily having the ATP-binding cassette (ABC) with well-preserved homology of the site where ATP binds. There are more than 100 ABC transporters distributed from prokaryotes to humans. Forty-eight ABC genes have been reported in humans, among which the functions of 16 genes have been determined and 14 genes are related with diseases present in humans (cystic fibrosis, adrenoleukodystrophy, Stargardt's disease, drug-resistant tumors, Dubin-Johnson syndrome, Byler's disease, progressive familiar intrahepatic cholestasis, X-linked sideroblastic anemia, ataxia, and persistent and hyperinsulimenic hypoglycemia in children) http://www.nutrigene.4t.com/ humanabc.htmc[4,5].
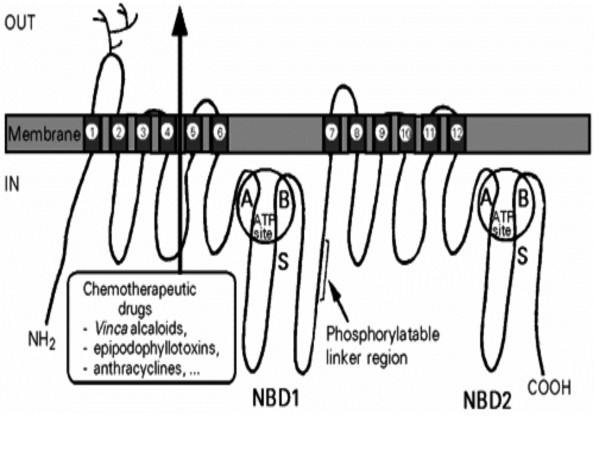
Figure 1.
Schematic structural organization of P-glycoprotein. Each half contains a highly hydrophobic domain with 6 transmembrane α-helices involved in chemotherapeutic drug efflux, and a hydrophilic domain located at the cytoplasmic face of the membrane, nucleotide binding domain 1(NBD1) or NMD 2, containing an ATP-binding site with cheracteristic Walker motifs A and B and the S signature of ABC transporters. The two half molecules are separated by a highly charged "linker region which is phosphorylated at several sites by protein kinase C and the first extracellular loop is heavily N-glycosylated [3].
Other efflux pumps of the mammalian cell membrane in ABC superfamily include multidrug resistance-associated proteins (MRP) [6] and breast cancer resistance proteins (BCRP; mitoxantrone resistance proteins, MXR) [7,8]. Other than the fact that these resistant proteins belong to the ABC superfamily, they are quite different with respect to gene locus, amino acid sequence, structure and substrate (Table 1 and 2). In this review, the physiological functions and structures of ABC transporters, and development of chemosensitizers are described focusing on well-known proteins including Pgp, MRP, and BCRP.
Table 1.
Gene locus and tissue distribution of ABC transporters
| Name | Alternate name | Gene locus | Tissue distribution |
| MDR1 | ABCB1, P-GP | 7q36 [9] | Gut (apical membrane), liver (canalicular membrane), kindey (apical membrane of epithelial cells of proximal tubule), blood brain barrier (luminal membrane of endothelial cells), testis (endothelial cells of capillary), placenta (trophoblast) |
| MRP1 | ABCC1 | 16p13.1 [6] | Many tissues (brain etc) |
| MRP2 | ABCC2, cMOAT | 10q24 [10] | Liver, gut, kidney, placenta |
| MRP3 | ABCC3 | 17q21.3 [11] | Liver, gut, adrenal cortex, placenta |
| MRP4 | ABCC4 | 13q32 [11] | Many tissues |
| MRP5 | ABCC5 | 3q27 [11] | Many tissues(brain etc) |
| MRP6 | ABCC6 | 16p13.1 [12] | Liver, kidney |
| MRP7 | ABCC10 | 6p12-21 [13] | Many tissues |
| MRP8 | ABCC11 | 16q12.1 [14] | Breast, testes |
| BCRP | ABCG2, MXR1, ABCP | 4q22 [15] | Placenta (syncytiotrophoblasts), intestine (epithelium), liver (canalicular membrane), breast (ducts and lobules), endometrium (vein and capillary but not artery), gut |
Table 2.
Endogenous and exogenous substrates for ABC transporters
| Name | Endogenous substrate | Exogenous cytotoxic substance |
| MDR1 | Estrogen glucuronide conjugates (estradiol, estriol), endorphin, glutamate, steroids (cortisol, aldosterone, corticosterone), beta-amyloid, 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (generically platelet-activating factor, PAF) | Anthracyclines (doxorubucin, daunorubicin, epirubicin), actinomycin D, colchicine, podophyllotoxin (etoposide, teniposide), methotrexate (only in carrier-deficient cells), mitomycin C, mitoxantrone, taxenes (paclitaxel, docetaxel), vinca alkaloids (vincristine, vinblastine) |
| MRP1 | Estradiol-17beta(beta-D-glucuronide) glutathione, glutathione S-conjugate leukoetriene C4, glucuronosyl bilirubin | Anthracyclines, cochicine, etoposide, heavy metals (arsenite, arsenate, antimonials), vincristine, vinblastine, paclitaxel |
| MRP2 | Estradiol-17beta(beta-D-glucuronide), glutathione, glutathione S-conjugate Leukoetriene C4, glucuronosyl bilirubin, | Cisplatin, CPT-11, doxorubicin, etoposide, methotrexate, SN-38, vincristine, vinblastine |
| MRP3 | S-(2,4-dinitrophenyl)glutathione | Cisplatin, doxorubicin, etoposide, methotrexate, teniopside, vincristine, |
| MRP4 | Glucuronide and glutathione conjugates | Methotrexate, nucleotide analogs, PMEA* |
| MRP5 | Glutamate and phosphate conjugates | Doxorubicin, methotrexate, nucleotide analogs, topotecan, |
| MRP6 | Cyclic nucleotides (cAMP, cGMP), glutathione conjugate | Doxorubicin, etoposide, teniposide |
| MRP7 | ? | ? |
| MRP8 | 17beta-estradiol-(17-beta-D-glucuronide), leukotriene C4, cyclic nucleotides | 5'-Fluorouracil, 5'-fluoro-2'-deoxyuridine, 5'-fluoro-5'-deoxyuridine, PMEA* |
| BCRP | Heme or porphyrin | Anthracyclines, bisantrene, camptothecin, epirubicin, flavopiridol, mitoxantrone, S-38, topotecan |
* PMEA, 2',3'-dideoxycytidine 9'-(2'-hosphonylmethoxynyl)adenine
Functions of ABC transporters
Although the physiologic functions of ABC transporters are not well known, they are expressed constitutively in not only tumor cells but also normal cells in the digestive system including the small intestine, large intestine, liver, and pancreas; epithelial cells in the kidneys, adrenals, brain, and testes; and endothelial cells (Table 1). From the aspect of the tissue distribution, ABC transporters are thought to participate in the absorption and secretion of endogenous and exogenous substances. Endogenous and exogenous substrates for ABC transporters reported so far are summarized in Table 2. Especially, the ABC transporters have shown to function as an efflux pump for lipid, multiple drugs, natural products and peptides. It is proposed to operate as a hydrophobic vacuum cleaner, expelling non-polar compounds from the membrane bilayer to the exterior, driven by the energy of ATP hydrolysis [143]. ATP-dependent transbilayer lipid transporters are classified into cytofacially-directed flippases and exofacially-directed floppases. Floppase activity has been associated with the ABC transporters although not all ABC transporters are floppases [144]. Endogenous substrates for Pgp include corticosterone [145], beta-estradiol 17beta-D-glucuronide, an endogenous cholestatic metabolite of estradiol [146], 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (generically platelet-activating factor, PAF) [147], glutamate [148] and endorphin [149]. It was also recently reported that Pgp has the function of removing beta-amyloid, which was reported as the causal substance of Alzheimer's disease [150,151]. MRP1 effluxes various conjugated substrates such as leukotriene C4 conjugates [152], steroid conjugates [153] and the GSH conjugate of aflatoxin B1, which is a mycotoxin [154]. Cells can, upon hypoxic demand, use BCRP to reduce heme or porphyrin accumulation, which can be detrimental to cells [155]. When cancer originates not only from cells normally expressing efflux pump but also cells having genes but not expressing, gene expression is initiated due to the exposure to anticancer drugs, resulting in resistance to anticancer drugs, eventually interfering with chemotherapy.
Pgp is mainly present in the apical membrane of intestinal mucosal membrane and lowers bioavailability of drugs by preventing the absorption of the drugs. Digoxin, which shows a low bioavailability and is mainly excreted through stool in normal mice due to poor absorption in the mouse intestine, shows a high bioavailability and mainly excreted through urine in mice with mdr1 knocked out[156]. The bioavailability of the substrate of Pgp, paclitaxel, also increased significantly in mice with mdr1 knocked out and in mice administered with the Pgp inhibitor, PSC-833 [157].
Recently, multiple MDR1 polymorphisms including more than 20 single nucleotide polymorphism (SNP) have been identified. The mutations at positions 2677(G→T) and 2995(G →A) of MDR1 in normal cells were firstly reported [158]. MDR1 polymorphism could be not only associated with alteration of Pgp expression and/or function, drug disposition and treatment outcome but also increase the risk of diseases such as Parkinson's disease and ulcerative colitis [159]. The influence of MDR1 SNP(C3435T and G2677T) on disposition of Pgp substrates or treatment outcome has been examplified in digoxin, phenytoin, fexofenadine, nelfinarvir, cyclosporine, talinolol and loperamide [159]. Polymorphisms of other ABC transporters have been reported [160-163].
If a substance in food affects Pgp, this substance also could affect the bioavailability of substrate drugs for Pgp. It was reported that substances present in grape juice or orange juice could increase the bioavailability of a drug being the substrate of Pgp by inhibiting it. [164]. These substances could also affect pharmacokinetics of other drugs [160,165]. On the contrary, some drugs could increase the expression of Pgp. St John's Wort used as an antidepressant increases the expression of Pgp, so it could significantly lower the serum concentration of indinavir or cyclosporin [166]. Digoxin is the substrate of Pgp and induces paclitaxel resistance by increasing Pgp [166]. Not only Pgp but also MRP and BCRP could affect the bioavailability of drugs.
One of the important physiological functions of efflux pump present in the cell membrane is to provide a pharmacological sanctuary for tissues present in the blood-tissue barriers such as in the case of blood-brain barrier (BBB), blood-placental barrier and blood-testes barrier. Hydrophilic substances present in blood could not go into tissues when they are not small enough to pass through the tight junction with simple diffusion. Nonetheless, various hydrophobic substances could not enter these tissues because they are effluxed out by efflux pumps. Actually, Pgp effluxes neurotransmitters or neuromodulators such as glutamate [148] and opioids [149,167] into blood from the brain. Compared with wild-type mice, drugs beings the substrate of Pgp were significantly increased in the brain of fetus when the mdr1 gene is knocked out in mice [168-171]. When the BCRP inhibitor, GF120918, was introduced to pregnant mice, the topotecan level was increased by two-folds in mouse fetus, suggesting that BCRP would function as the maternal-fetal barrier in the placenta [172]. Thus, quantitative and qualitative changes of transporters present in the membrane could affect pharmacokinetics such as the distribution of endogenous and exogenous substances.
Structure of ABC transporters
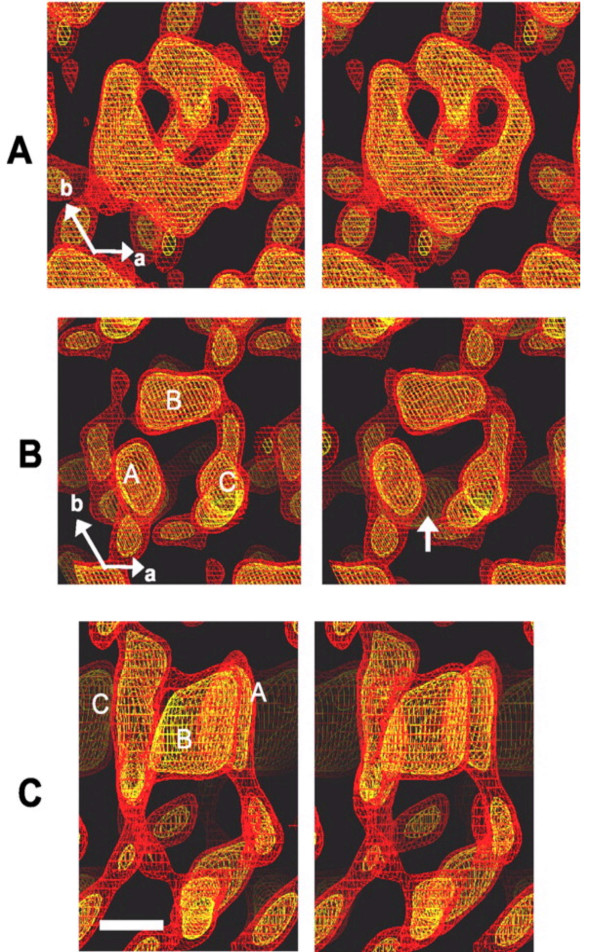
Pgp is a 170-kDa membrane protein glycosylated at the first extracellular loop (Fig. 1). Pgp is composed of 12 hydrophobic transmembrane domains (TMDs) and 2 nucleotide-binding domain (NBD). One NBD connects two TMDs with a hydrophilic NBD loop. TDMs form channels for substrate drugs, determine the characteristics of substrate, and efflux substrate drugs whereas NBDs are located in the interior of cytoplasm, and participate in ATP binding and hydrolysis [173]. Pgp undergoes conformational changes upon binding of nucleotide to the NBDs [174]. Rosenberg et al. have analyzed the three-dimensional structures of Pgp and its conformational change in the presence and absence of nucleotide [175-177]. The projection of the protein perpendicular to the membrane is roughly rectangular with a maximum depth of 8 nm, a pore size of 2.5 nm and two 3-nm lobes exposed at the cytoplasmic face of the membrane. The conformational change revealed a major reorganization of the TMDs throughout the entire depth of the membrane upon binding of nucleotide (Fig. 2A). In the absence of nucleotide, the two TMDs form a single barrel 5–6 nm in diameter and about 5 nm deep with a central pore that is open to the extracellular surface and spans much of the membrane depth. Upon binding nucleotide, the TMDs reorganize into three compact domains that are each 2–3 nm in diameter and 5–6 nm deep (Fig. 2B). This reorganization opens the central pore along its length in a manner that could allow access of hydrophobic drugs (transport substrates) directly from the lipid bilayer to the central pore of the transporter [176].
Figure 2.
Comparison of nucleotide free-Pgp (nf-Pgp) and Pgp-AMP-PNP (Pgp-AMP-PNP) three-dimensional structures. A, stereo pair of the nf-Pgp three-dimensional structure, displayed using netting at 1.0 σ (red) and 1.5 σ (yellow) above the mean density level and viewed perpendicular to the crystal plane from the more heavily stained side (corresponding to the extracellular surface). B, equivalent views of the Pgp-AMP-PNP structure. The arrow indicates the gap along one side of the central pore. The locations of the three discrete densities A, B, and C are indicated. C, stereo pair of a side view of Pgp-AMP-PNP with the same color scheme as above. The directions of the principle crystallographic axes a and b are shown. Scale bar = 2.2 nm. AMP-PNP, non-hydralizable ATP analogue [176].
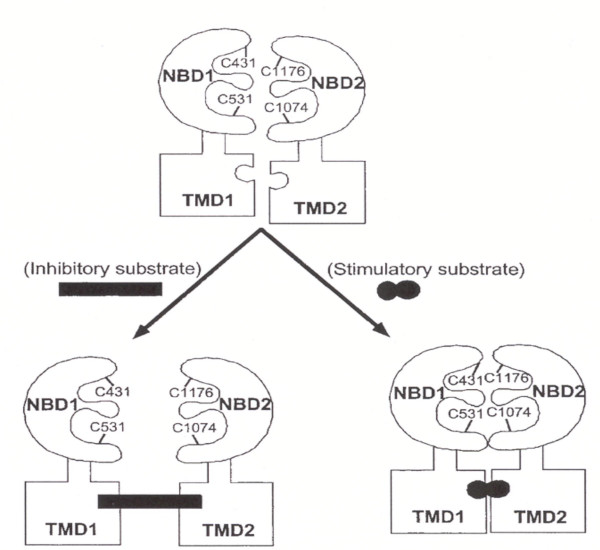
When one of two NBDs of Pgp is inactivated, not only drug transport but also ATP hydrolysis of normal NBD is inhibited. This result indicates that two NBDs would function cooperatively and they could not hydrolyze ATP independently [178]. It was recently reported that structural changes of NBDs are brought about when a drug binds to TMD so that the distance between NBDs is changed to affect the activity of ATPase as shown in Fig. 3[179]. Unlike in Pgp, however, the substrate leukotriene C4 could not be transported once NBD2 is inactivated but the substrate transport could not be inhibited when NBD1 is inactivated in MRP1 [180]. This result suggests that among ABC transporters, interactions of NBDs are not simple but function differently for every transport. Although the exact site and number of Pgp binding with drugs have not yet been determined, the important binding sites such as TMD 4, 5, 6, 10, 11 and 12 have been determined [181] whereas substrate drugs do not bind to NBDs [182].
Figure 3.
Model of the NBD conformational change by the drug binding to TDM. [178].
Development of chemosensitizers to overcome resistance
One of the causes for the failure of chemotherapy in the treatment of cancer is the emergence of MDR. Once MDR appears, chemotherapy is not effective even when using high doses of drugs enough to overcome resistance, toxic effects are brought about and the resistance mechanism could be further stimulated. These problems could be resolved by the use of the anticancer drugs that could bypass the resistance mechanism. For example, we could use other anticancer drugs such as alkylating drugs (cyclophosphamide), antimetabolites (5-fluorouracil), and the anthracycline modified drugs (annamycin and doxorubicin-peptide) that would not function as the substrates of ABC transporters [183-185]. The final method of overcoming resistance is to administer substances inhibiting ABC transporters with anticancer drugs at the same time. These substances would reverse resistance against anticancer drugs to eventually being sensitized for anticancer drugs so they are called chemosensitizers. They are also called MDR modulators and MDR reverters. Chemosensitizers against each transporter are summarized according to the publishing years (Table 3). Of these, some are chemosensitizers against one transporter and some others against more than two transporters.
Table 3.
Chemosensitizers inhibiting Pgp, MRP and BCRP
| Name | Year | Chemosensitizer |
| Pgp | 2004 | Benzyl-, phenethyl-, and alpha-naphthyl isothiocyanates [16], diallyl sulfide [17], PK11195 [18], small scFv recombinant Pgp antibody fragment [19] |
| 2003 | Amooranin [20], etrandrine, fangchinoline [21], ginsenoside Rg(3) [22], KR30031 [23], methylenedioxyethylamphetamine [24], protopanaxatriol ginsenosides [25], saquinavir [26], siRNA of mdr1 gene [27, 28], tRA 98006* [29] | |
| 2002 | 3,5-dibenzoyl-1,4-dihydropyridines[30], PKC412 [31], pyronaridine [32], sinensetin [33] | |
| 2001 | Agosterol A [34], haloperidol and dihydrohaloperidol [35], SB203580 [36], tropane alkaloid esters [37], SNF4435C and D [38], tea polyphenol [39], trans-N,N'-bis(3,4-dimethoxybenzyl)-N-solanesyl-1,2-diaminocyclo hexane (N-5228) [40] | |
| 2000 | Astemizole [41], atorvastatin [42], 7-O-benzoylpyripyropene A [43], 5-O-benzoylated taxinine k [44], clarithromycin and YM17K (3,4'-dideoxy mycaminosyl tylonolide hydrochloride) [45], cyclopamine and tomatidine[46], 3,5-diacetyl-1,4-dihydropyridines [47], 7, 8-dihydroxy-3-benzazepine [48], doxorubicin-gallium-transferrin conjugate [49], macrolide antibiotics (josamycin, tamolarizine) [50], nelfinavir [51] norverapamil [52], ontogen (ONT-093, formerly OC-144-093) [53], R101933 [54], taxuspine C, 2'-desacetoxyaustrospicatine and 2-desacetoxytaxinine [55], V-104 [56] | |
| 1999 | D-alpha-tocopheryl polyethylene glycol 1000 succinate [57], anti-MDR1 ribozymes [58], AR-2 [59], carvedilol [60], erythromycin [61], ketoconazole [62], kopsiflorine [63], nomegestrol [64], PAK-200S [65], pluronic block copolymer [66], reversin [67], ritonarvir [68], rosemary extract [69], TTD [70], XR9576(2) [71] | |
| 1998 | Ardeemins [72], AV200 [73], 5-O-benzoylated taxuspine C [74], bromocriptine [75], dipyridamole [76], droloxifene [77], imidazothiazole derivatives (N276-12, N276-14, N276-17) [78], oxalyl bis(N-phenyl)hydroxamic acid [79], tetrandine and fangchinoline [21], tiamulin [80], XR9051 [81] | |
| 1997 | Biricodar (VX-710; Incel) [82, 83], cyproheptadine [84] | |
| 1996 | CL 329,753 [85], indole-3-carbinol [86], itraconazole [87], LY335979 [88], medroxyprogesterone [89], mefloquine [90], mifepristone (RU-486) [91], reserpine [92] | |
| 1995 | Azelastine and flezelastine [93], B9209-005 [94], dexniguldipine (B8509-035) [95], dexverapamil [96], epidermal growth factor (EGF), insulin-like growth factor I (IGF-I) [97], quercetin [98] | |
| 1994 | MS-209 [99], pentoxifylline [100], Ro11-2933 (DMDP) [101], RU486 [102] | |
| 1993 | Dilantin [103], GF120918[104], meperidine, pentazocine, and methadone [105], Pgp monoclonal antibodies and antisense oligonucleotide [106], tamoxifen and toremifene [107] | |
| 1992 | Staurosporine and NA-382 [108] | |
| 1991 | Biperidil [109], SDZ PSC 833[110] | |
| 1990 | Cremophor EL [111] | |
| 1989 | Cefoperazone, cetriaxone [112], phenothiazine [113], YM534 [114] | |
| 1987 | Diltiazem[115], cyclosporine A [116] | |
| 1986 | Aamiodarone [117] | |
| 1984 | Quinidine [118] | |
| 1981 | Verapamil [119], | |
| MRP | 2004 | benzyl-, phenethyl-, and alpha-naphthyl isothiocyanates [16] |
| 2003 | tRA 98006 [29] | |
| 2001 | Agosterol A [34] | |
| 2000 | 5-O-benzoylated taxinine k [44], 4-deacetoxyagosterol A [120], doxorubicin-gallium-transferrin conjugate [49], V-104 [56], pluronic block copolymer [66], quinoline-based drugs (chloroquine, quinine, quinidine, and primaquine) [121], | |
| 1999 | dipyridamole [122], erythromycin and ofloxacin [123], mifepristone (RU-486) [124], MS-209 [125], rifampicin [126] | |
| 1998 | Biricodar (VX-710; Incel) [83], imidazothiazole derivatives (N276-12, N276-14, N276-17) [78], NSAIDs (indomethacin, sulindac, tolmetin, acemetacin, zomepirac and mefenamic acid) [127], ONO-1078 [128], quercetin [98] | |
| 1997 | Indomethacin [129], probenecid [130] | |
| 1996 | Acrolein and chloroacetaldehyde [131], d,l-buthionine-(S,R)-sulfoximine [132], itraconazol [87], PAK-104P [133] | |
| 1995 | Difloxacin [134], MK571 [135] | |
| BCRP | 2004 | Chrysin and biochanin A [136], genistein and naligenin [137], Imatinib mesylate (Gleevec, STI571) [138] |
| 2003 | Estrone, diethylstilbestrol and TAG-139 [139], tRA 98006 [29] | |
| 2002 | Ko143 [140] | |
| 1999 | GF120918 [141] | |
| 1998 | fumitremorgin C [142] |
* Boldface compounds indicate chemosensitizers inhibiting more than two transporters
Many drugs such as the calcium channel blocker verapamil and the immunosuppressant cyclosporin A would inhibit resistance by functioning as competitive substrates of Pgp regardless of their innate pharmacological functions. Different clinical studies also showed that these drugs could reverse resistance to anticancer drugs. Verapamil is the first reported chemosensitizer inhibiting MDR [119] and its effect was also proven in the recent clinical study [186]. However, verapamil brings about cardiac toxicity at the concentration inhibiting resistance; thus, in order to resolve this problem, the attempts were made to develop (R)-verapmil [187] and verapamil analogues having lower cardiac toxicity compared with (S)-verapamil [188,189]. The immunosuppressant cyclosporin A was first reported to reverse resistance by acute leukemia against vincristine and daunorubicin [190]. Following cyclosporin A, researchers found that other immunosuppressants including FK506 and rapamycin could inhibit MDR [191]. However, when cyclosporin A is applied clinically, researchers placed efforts to develop cyclosporin analogues having few side effects due to their innate immuno suppressant effects and hepatic and renal toxicity with excellent chemosensitizing effects. As a result, PSC-833 (Valspodar), which is the non-immune suppressant analogue of cyclosporin, was developed [110]. In addition to non-immunosuppressant effect, its chemosensitivity is about 10 times higher than that by cyclosporin in Pgp-mediated MDR, so clinical studies are being performed on this drug [192]. Among those drugs having their innate pharmacological activities such as verapamil and cyclosporin A, those having chemosensitizing effect is called the first-generation chemosensitizers. The problems related with the first-generation chemosensitizers are that they generally show low effects and high toxicity at resistance-inhibiting doses. In order to supplement these problems, the chemosensitizers developed only for chemosensitizing effects are called the second-generation chemosensitizers, which include PSC-833, VX-710, LY335979, XR9051 and XR9576 [193]. Multi-national companies are pursuing the development of second-generation chemosensitizers by overcoming the problems of existing chemosensitizers (low effects, side effects, and drug-drug interaction), and some of these chemosensitizers are in the process of being tested clinically.
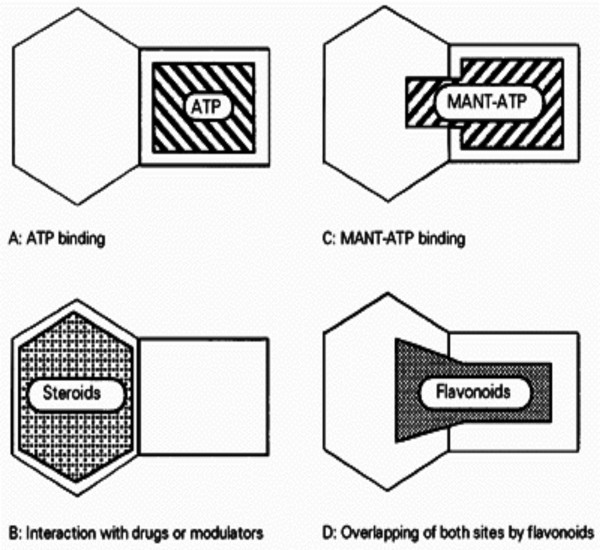
Most chemosensitizers bind with TMD in transporter, but steroid and flavonoid are new recently introduced chemosensitizers, which inhibit transporters by binding with NBD. The binding site of steroid is different from the binding site of ATP but is probably in the vicinity of the ATP binding site [194]. On the other hand, the flavonoid, kaempferide, is bifunctional in that it would partially block the binding of the antiprogestin RU-486 in the cytoplasm domain of Pgp and block ATP binding [195] (Fig. 4). Recently, flavonoid chemosensitizers reversing Pgp-mediated MDR have been screening. It is believed that flavonoid chemosensitizers have a significant advantage with respect with a therapeutic index (Table 4). These may be second-generation flavonoid chemosensitizers [33].
Figure 4.
Proposed schematic model of NBDs showing the relative positions of different nucleotide- and effector-binding sites. MANT-ATP binding is prevented by preincubation with antiprogestin RU-486 and bound MANT-ATP is displaced by Ru-486, suggesting the existence of a cytosolic steroidal-interacting region adjacent to the ATP-binding site. Since the flavonoid binding is prevented by preincubation with ATP and RU-486, bound flavonoids most likely cover both ATP site and the vicinal steroid site. MANT, 2'(3')-N-methylanthraniloyl [3].
Table 4.
Comparison of chemosensitizing effects of flavonoids and verapamil against Pgp
| Chemosensitizer | IC50a (μM) | CIc | |
| (VCRb-) | (VCR+) | ||
| 5,7,3',4',5' – pentamethoxyflavone | > 400 | 0.4 | >1000 |
| 7,3',4' – trimethoxyflavone | > 400 | 1.2 | >333.3 |
| 3',4' – dimethoxyflavone | 386 | 1.2 | 321.7 |
| 3,6,3',4' – tetramethoxyflavone | > 400 | 1.9 | >210.5 |
| Verapamil | 61 | 0.4 | 152.5 |
| 5,6,7,3',4' – pentamethoxyflavone | > 400 | 3.2 | >125 |
Cytotoxic and chemosensitizing effects of chemosensitizers in the presence or absence of vincristine in Pgp-overexpressing AML-2/D100 cells.
a, Drug concentrations with inhibit 50% growth of the cells.
b, Vincristine (100 ng/ml)
c, Chemosensitizing index = IC50 (VCR-)/IC50(VCR+)
The fungal toxin, fumitremorgin C (FTC), is a strong inhibitor of BCRP but its use in vivo has been limited due to its neurotoxicity [196]. It was recently reported that the tetracyclic analogue of FTC, Ko143, is the most strong chemosensitizer aginst BCRP having little toxicity [140].
Since ABC transporters can be coexpressed in some types of cancer cells, the development of chemosensitizers against MRP and/or BCRP as well as Pgp has been highly demanding. These include VX-710 against Pgp and MRP [82,83], GF120918 against Pgp and BCRP [104,141] and tRA98006 against all three transporters [29].
Conclusion
One of the major causes of failure in anticancer chemotherapy is resistance against anticancer drugs. Overexpression of ABC transporters such as Pgp, MRP and BCRP has been shown to be responsible for the major portion of MDR. Therefore elucidation of the structure and the function for each ABC transporter is prerequisite for understanding how these transporters work and for reversing MDR. One strategy for reversal of MDR cells expressing ABC transporters is combined use of anticancer drugs with chemosensitizers. Second-generation chemosensitizers have been developed for the purpose of obtaining higher efficacy and lower toxicity than first-generation chemosensitizers. Inhibitors of ABC transporters can be exploited to enhance the oral bioavailablilty or the brain penetration of various drugs.
Combination of a conventional anticancer chemotherapy with new strategies such as chemosensitizers, receptor-mediated targeting and nanotechnology will shed light on cancer patients in the near future.
Acknowledgments
Acknowledgements
This study was supported by grants from Ministry of Science and Technology, Korea, and from Korea Science and Engineering Foundation through Research Center for Resistant Cells (R13-2003-009). I gratefully thank Dr. Bum-Chae Choi of the CL hospital located in Gwangju (Korea) for critical reading of the manuscript.
References
- Stavrovskaya AA. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Mosc) 2000;65:95–106. [PubMed] [Google Scholar]
- Riordan JR, Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. J Biol Chem. 1979;254:12701–12705. [PubMed] [Google Scholar]
- Di Pietro A, Dayan G, Conseil G, Steinfels E, Krell T, Trompier D, Baubichon-Cortay H, Jault J. P-glycoprotein-mediated resistance to chemotherapy in cancer cells: using recombinant cytosolic domains to establish structure-function relationships. Braz J Med Biol Res. 1999;32:925–939. doi: 10.1590/S0100-879X1999000800001. [DOI] [PubMed] [Google Scholar]
- Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. [PubMed] [Google Scholar]
- Efferth T. The human ATP-binding cassette transporter genes: from the bench to the bedside. Curr Mol Med. 2001;1:45–65. doi: 10.2174/1566524013364194. [DOI] [PubMed] [Google Scholar]
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, Floot BG, Schellens JH. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–4563. [PubMed] [Google Scholar]
- Bell DR, Trent JM, Willard HF, Riordan JR, Ling V. Chromosomal location of human P-glycoprotein gene sequences. Cancer Genet Cytogenet. 1987;25:141–148. doi: 10.1016/0165-4608(87)90169-5. [DOI] [PubMed] [Google Scholar]
- van Kuijck MA, Kool M, Merkx GF, Geurts van Kessel A, Bindels RJ, Deen PM, van Os CH. Assignment of the canalicular multispecific organic anion transporter gene (CMOAT) to human chromosome 10q24 and mouse chromosome 19D2 by fluorescent in situ hybridization. Cytogenet Cell Genet. 1997;77:285–287. doi: 10.1159/000134599. [DOI] [PubMed] [Google Scholar]
- Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, Baas F, Borst P. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- Kool M, van der Linden M, de Haas M, Baas F, Borst P. Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res. 1999;59:175–182. [PubMed] [Google Scholar]
- Hopper E, Belinsky MG, Zeng H, Tosolini A, Testa JR, Kruh GD. Analysis of the structure and expression pattern of MRP7 (ABCC10), a new member of the MRP subfamily. Cancer Lett. 2001;162:181–191. doi: 10.1016/S0304-3835(00)00646-7. [DOI] [PubMed] [Google Scholar]
- Bera TK, Lee S, Salvatore G, Lee B, Pastan I. MRP8, a new member of ABC transporter superfamily, identified by EST database mining and gene prediction program, is highly expressed in breast cancer. Mol Med. 2001;7:509–516. [PMC free article] [PubMed] [Google Scholar]
- Knutsen T, Rao VK, Ried T, Mickley L, Schneider E, Miyake K, Ghadimi BM, Padilla-Nash H, Pack S, Greenberger L, Cowan K, Dean M, Fojo T, Bates S. Amplification of 4q21-q22 and the MXR gene in independently derived mitoxantrone-resistant cell lines. Genes Chromosomes Cancer. 2000;27:110–116. doi: 10.1002/(SICI)1098-2264(200001)27:1<110::AID-GCC14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hu K, Morris ME. Effects of benzyl-, phenethyl-, and alpha-naphthyl isothiocyanates on P-glycoprotein- and MRP1-mediated transport. J Pharm Sci. 2004;93:1901–1911. doi: 10.1002/jps.20101. [DOI] [PubMed] [Google Scholar]
- Arora A, Seth K, Shukla Y. Reversal of P-glycoprotein-mediated multidrug resistance by diallyl sulfide in K562 leukemic cells and in mouse liver. Carcinogenesis. 2004;25:941–949. doi: 10.1093/carcin/bgh060. [DOI] [PubMed] [Google Scholar]
- Walter RB, Raden BW, Cronk MR, Bernstein ID, Appelbaum FR, Banker DE. The peripheral benzodiazepine receptor ligand PK11195 overcomes different resistance mechanisms to sensitize AML cells to gemtuzumab ozogamicin. Blood. 2004;103:4276–4284. doi: 10.1182/blood-2003-11-3825. [DOI] [PubMed] [Google Scholar]
- Haus-Cohen M, Assaraf YG, Binyamin L, Benhar I, Reiter Y. Disruption of P-glycoprotein anticancer drug efflux activity by a small recombinant single-chain Fv antibody fragment targeted to an extracellular epitope. Int J Cancer. 2004;109:750–758. doi: 10.1002/ijc.20037. [DOI] [PubMed] [Google Scholar]
- Ramachandran C, Rabi T, Fonseca HB, Melnick SJ, Escalon EA. Novel plant triterpenoid drug amooranin overcomes multidrug resistance in human leukemia and colon carcinoma cell lines. Int J Cancer. 2003;105:784–789. doi: 10.1002/ijc.11180. [DOI] [PubMed] [Google Scholar]
- Choi SU, Park SH, Kim KH, Choi EJ, Kim S, Park WK, Zhang YH, Kim HS, Jung NP, Lee CO. The bisbenzylisoquinoline alkaloids, tetrandine and fangchinoline, enhance the cytotoxicity of multidrug resistance-related drugs via modulation of P-glycoprotein. Anticancer Drugs. 1998;9:255–261. doi: 10.1097/00001813-199803000-00008. [DOI] [PubMed] [Google Scholar]
- Kim SW, Kwon HY, Chi DW, Shim JH, Park JD, Lee YH, Pyo S, Rhee DK. Reversal of P-glycoprotein-mediated multidrug resistance by ginsenoside Rg(3) Biochem Pharmacol. 2003;65:75–82. doi: 10.1016/S0006-2952(02)01446-6. [DOI] [PubMed] [Google Scholar]
- Lee BH, Lee CO, Kwon MJ, Yi KY, Yoo SE, Choi SU. Differential effects of the optical isomers of KR30031 on cardiotoxicity and on multidrug resistance reversal activity. Anticancer Drugs. 2003;14:175–181. doi: 10.1097/00001813-200302000-00012. [DOI] [PubMed] [Google Scholar]
- Ketabi-Kiyanvash N, Weiss J, Haefeli WE, Mikus G. P-glycoprotein modulation by the designer drugs methylenedioxymethamphetamine, methylenedioxyethylamphetamine and paramethoxyamphetamine. Addict Biol. 2003;8:413–418. doi: 10.1080/13556210310001646475. [DOI] [PubMed] [Google Scholar]
- Choi CH, Kang G, Min YD. Reversal of P-glycoprotein-mediated multidrug resistance by protopanaxatriol ginsenosides from Korean red ginseng. Planta Med. 2003;69:235–240. doi: 10.1055/s-2003-38483. [DOI] [PubMed] [Google Scholar]
- Dupuis ML, Tombesi M, Sabatini M, Cianfriglia M. Differential effect of HIV-1 protease inhibitors on P-glycoprotein function in multidrug-resistant variants of the human CD4+ T lymphoblastoid CEM cell line. Chemotherapy. 2003;49:8–16. doi: 10.1159/000069782. [DOI] [PubMed] [Google Scholar]
- Nieth C, Priebsch A, Stege A, Lage H. Modulation of the classical multidrug resistance (MDR) phenotype by RNA interference (RNAi) FEBS Lett. 2003;545:144–150. doi: 10.1016/S0014-5793(03)00523-4. [DOI] [PubMed] [Google Scholar]
- Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63:1515–1519. [PubMed] [Google Scholar]
- Brooks T, Minderman H, O'Loughlin KL, Pera P, Ojima I, Baer MR, Bernacki RJ. Taxane-based reversal agents modulate drug resistance mediated by P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Mol Cancer Ther. 2003;2:1195–1205. [PubMed] [Google Scholar]
- Kawase M, Shah A, Gaveriya H, Motohashi N, Sakagami H, Varga A, Molnar J. 3,5-dibenzoyl-1,4-dihydropyridines: synthesis and MDR reversal in tumor cells. Bioorg Med Chem. 2002;10:1051–1055. doi: 10.1016/S0968-0896(01)00363-7. [DOI] [PubMed] [Google Scholar]
- Ganeshaguru K, Wickremasinghe RG, Jones DT, Gordon M, Hart SM, Virchis AE, Prentice HG, Hoffbrand AV, Man A, Champain K, Csermak K, Mehta AB. Actions of the selective protein kinase C inhibitor PKC412 on B-chronic lymphocytic leukemia cells in vitro. Haematologica. 2002;87:167–176. [PubMed] [Google Scholar]
- Qi J, Yang CZ, Wang CY, Wang SB, Yang M, Wang JH. Function and mechanism of pyronaridine: a new inhibitor of P-glycoprotein-mediated multidrug resistance. Acta Pharmacol Sin. 2002;23:544–550. [PubMed] [Google Scholar]
- Choi CH, Sun KH, An CS, Yoo JC, Hahm KS, Lee IH, Sohng JK, Kim YC. Reversal of P-glycoprotein-mediated multidrug resistance by 5,6,7,3',4'-pentamethoxyflavone (Sinensetin) Biochem Biophys Res Commun. 2002;295:832–840. doi: 10.1016/S0006-291X(02)00755-6. [DOI] [PubMed] [Google Scholar]
- Murakami N, Sugimoto M, Morita M, Kobayashi M. Total synthesis of agosterol A: an MDR-modulator from a marine sponge. Chemistry. 2001;7:2663–2670. doi: 10.1002/1521-3765(20010618)7:12<2663::AID-CHEM26630>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Ishikawa M, Miura M, Takeshita M, Fujita R, Furusawa S, Takayanagi M, Takayanagi Y, Sasaki K. Reversal of vinblastine resistance in human leukemic cells by haloperidol and dihydrohaloperidol. Biol Pharm Bull. 2001;24:612–617. doi: 10.1248/bpb.24.612. [DOI] [PubMed] [Google Scholar]
- Barancik M, Bohacova V, Kvackajova J, Hudecova S, Krizanova O, Breier A. SB203580, a specific inhibitor of p38-MAPK pathway, is a new reversal agent of P-glycoprotein-mediated multidrug resistance. Eur J Pharm Sci. 2001;14:29–36. doi: 10.1016/S0928-0987(01)00139-7. [DOI] [PubMed] [Google Scholar]
- Silva GL, Cui B, Chavez D, You M, Chai HB, Rasoanaivo P, Lynn SM, O'Neill MJ, Lewis JA, Besterman JM, Monks A, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD. Modulation of the multidrug-resistance phenotype by new tropane alkaloid aromatic esters from Erythroxylum pervillei. J Nat Prod. 2001;64:1514–1520. doi: 10.1021/np010295+. [DOI] [PubMed] [Google Scholar]
- Kurosawa K, Takahashi K, Tsuda E, Tomida A, Tsuruo T. Reversal of multidrug resistance by novel nitrophenyl pyrones, SNF4435C and D. Jpn J Cancer Res. 2001;92:1235–1241. doi: 10.1111/j.1349-7006.2001.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Wang X, Guo Z. Study of tea polyphenol as a reversal agent for carcinoma cell lines' multidrug resistance (study of TP as a MDR reversal agent) Nucl Med Biol. 2001;28:735–740. doi: 10.1016/S0969-8051(00)90202-6. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Koike K, Rho MC, Kuwano M, Kishiye T, Komiyama K. Reversal of P-glycoprotein associated multidrug resistance by new isoprenoid derivatives. Anticancer Drug Des. 2001;16:255–260. [PubMed] [Google Scholar]
- Ishikawa M, Fujita R, Takayanagi M, Takayanagi Y, Sasaki K. Reversal of acquired resistance to doxorubicin in K562 human leukemia cells by astemizole. Biol Pharm Bull. 2000;23:112–115. doi: 10.1248/bpb.23.112. [DOI] [PubMed] [Google Scholar]
- Boyd RA, Stern RH, Stewart BH, Wu X, Reyner EL, Zegarac EA, Randinitis EJ, Whitfield L. Atorvastatin coadministration may increase digoxin concentrations by inhibition of intestinal P-glycoprotein-mediated secretion. J Clin Pharmacol. 2000;40:91–98. doi: 10.1177/00912700022008612. [DOI] [PubMed] [Google Scholar]
- Rho MC, Hayashi M, Fukami A, Obata R, Sunazuka T, Tomoda H, Komiyama K, Omura S. Reversal of multidrug resistance by 7-O-benzoylpyripyropene A in multidrug-resistant tumor cells. J Antibiot (Tokyo) 2000;53:1201–1206. doi: 10.7164/antibiotics.53.1201. [DOI] [PubMed] [Google Scholar]
- Okumura H, Chen ZS, Sakou M, Sumizawa T, Furukawa T, Komatsu M, Ikeda R, Suzuki H, Hirota K, Aikou T, Akiyama SI. Reversal of P-glycoprotein and multidrug-resistance protein-mediated drug resistance in KB cells by 5-O-benzoylated taxinine K. Mol Pharmacol. 2000;58:1563–1569. doi: 10.1124/mol.58.6.1563. [DOI] [PubMed] [Google Scholar]
- Wang L, Kitaichi K, Hui CS, Takagi K, Sakai M, Yokogawa K, Miyamoto KI, Hasegawa T. Reversal of anticancer drug resistance by macrolide antibiotics in vitro and in vivo. Clin Exp Pharmacol Physiol. 2000;27:587–593. doi: 10.1046/j.1440-1681.2000.03308.x. [DOI] [PubMed] [Google Scholar]
- Lavie Y, Harel-Orbital T, Gaffield W, Liscovitch M. Inhibitory effect of steroidal alkaloids on drug transport and multidrug resistance in human cancer cells. Anticancer Res. 2001;21:1189–1194. [PubMed] [Google Scholar]
- Shah A, Gaveriya H, Motohashi N, Kawase M, Saito S, Sakagami H, Satoh K, Tada Y, Solymosi A, Walfard K, Molnar J. 3,5-diacetyl-1,4-dihydropyridines: synthesis and MDR reversal in tumor cells. Anticancer Res. 2000;20:373–377. [PubMed] [Google Scholar]
- Kawase M, Saito S, Motohashi N. Chemistry and biological activity of new 3-benzazepines. Int J Antimicrob Agents. 2000;14:193–201. doi: 10.1016/S0924-8579(99)00155-7. [DOI] [PubMed] [Google Scholar]
- Wang F, Jiang X, Yang DC, Elliott RL, Head JF. Doxorubicin-gallium-transferrin conjugate overcomes multidrug resistance: evidence for drug accumulation in the nucleus of drug resistant MCF-7/ADR cells. Anticancer Res. 2000;20:799–808. [PubMed] [Google Scholar]
- Miyake N, Fujita R, Ishikawa M, Takayanagi M, Takayanagi Y, Sasaki K. Reversal of multidrug resistance in human leukemia K562 by tamolarizine, a novel calcium antagonist. Jpn J Pharmacol. 2000;82:265–268. doi: 10.1254/jjp.82.265. [DOI] [PubMed] [Google Scholar]
- Shiraki N, Hamada A, Yasuda K, Fujii J, Arimori K, Nakano M. Inhibitory effect of human immunodeficiency virus protease inhibitors on multidrug resistance transporter P-glycoproteins. Biol Pharm Bull. 2000;23:1528–1531. doi: 10.1248/bpb.23.1528. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, von Richter O, Burk O, Ziegler A, Mettang T, Eichelbaum M, Fromm MF. Characterization of the major metabolites of verapamil as substrates and inhibitors of P-glycoprotein. J Pharmacol Exp Ther. 2000;293:376–382. [PubMed] [Google Scholar]
- Newman MJ, Rodarte JC, Benbatoul KD, Romano SJ, Zhang C, Krane S, Moran EJ, Uyeda RT, Dixon R, Guns ES, Mayer LD. Discovery and characterization of OC144-093, a novel inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Res. 2000;60:2964–2972. [PubMed] [Google Scholar]
- van Zuylen L, Sparreboom A, van der Gaast A, van der Burg ME, van Beurden V, Bol CJ, Woestenborghs R, Palmer PA, Verweij J. The orally administered P-glycoprotein inhibitor R101933 does not alter the plasma pharmacokinetics of docetaxel. Clin Cancer Res. 2000;6:1365–1371. [PubMed] [Google Scholar]
- Kobayashi J, Shigemori H, Hosoyama H, Chen Z, Akiyama S, Naito M, Tsuruo T. Multidrug resistance reversal activity of taxoids from Taxus cuspidata in KB-C2 and 2780AD cells. Jpn J Cancer Res. 2000;91:638–642. doi: 10.1111/j.1349-7006.2000.tb00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers R, Kool M, Smith AJ, van Deemter L, de Haas M, Borst P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br J Cancer. 2000;83:366–374. doi: 10.1054/bjoc.2000.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintaman JM, Silverman JA. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) Pharm Res. 1999;16:1550–1556. doi: 10.1023/A:1015000503629. [DOI] [PubMed] [Google Scholar]
- Wang FS, Kobayashi H, Liang KW, Holland JF, Ohnuma T. Retrovirus-mediated transfer of anti-MDR1 ribozymes fully restores chemosensitivity of P-glycoprotein-expressing human lymphoma cells. Hum Gene Ther. 1999;10:1185–1195. doi: 10.1089/10430349950018175. [DOI] [PubMed] [Google Scholar]
- Alexanian AR, Arutyunian NS. Reversal of drug resistance in sarcoma-45 by the new calmodulin antagonist--trihydrochloride of [1,2,5-trimethyl-4-phenyl-4-beta-[N-(beta-ethylamino)-N-4'-methoxybe nzy l]-ethylamino] piperidine (AR-2) Invest New Drugs. 1999;17:105–110. doi: 10.1023/A:1006397014409. [DOI] [PubMed] [Google Scholar]
- Jonsson O, Behnam-Motlagh P, Persson M, Henriksson R, Grankvist K. Increase in doxorubicin cytotoxicity by carvedilol inhibition of P-glycoprotein activity. Biochem Pharmacol. 1999;58:1801–1806. doi: 10.1016/S0006-2952(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Siedlik PH, Olson SC, Yang BB, Stern RH. Erythromycin coadministration increases plasma atorvastatin concentrations. J Clin Pharmacol. 1999;39:501–504. [PubMed] [Google Scholar]
- Kim RB, Wandel C, Leake B, Cvetkovic M, Fromm MF, Dempsey PJ, Roden MM, Belas F, Chaudhary AK, Roden DM, Wood AJ, Wilkinson GR. Interrelationship between substrates and inhibitors of human CYP3A and P-glycoprotein. Pharm Res. 1999;16:408–414. doi: 10.1023/A:1018877803319. [DOI] [PubMed] [Google Scholar]
- Rho MC, Toyoshima M, Hayashi M, Koyano T, Subramaniam G, Kam TS, Komiyama K. Reversal of multidrug resistance by kopsiflorine isolated from Kopsia dasyrachis. Planta Med. 1999;65:307–310. doi: 10.1055/s-1999-13991. [DOI] [PubMed] [Google Scholar]
- Li J, Xu L, He K. [Modulation of multiple drug resistance by nomegestrol acetate and droloxifene in K562/A02] Zhonghua Xue Ye Xue Za Zhi. 1999;20:288–291. [PubMed] [Google Scholar]
- Vanhoefer U, Muller MR, Hilger RA, Lindtner B, Klaassen U, Schleucher N, Rustum YM, Seeber S, Harstrick A. Reversal of MDR1-associated resistance to topotecan by PAK-200S, a new dihydropyridine analogue, in human cancer cell lines. Br J Cancer. 1999;81:1304–1310. doi: 10.1038/sj.bjc.6694384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DW, Batrakova EV, Kabanov AV. Inhibition of multidrug resistance-associated protein (MRP) functional activity with pluronic block copolymers. Pharm Res. 1999;16:396–401. doi: 10.1023/A:1018873702411. [DOI] [PubMed] [Google Scholar]
- Sharom FJ, Yu X, Lu P, Liu R, Chu JW, Szabo K, Muller M, Hose CD, Monks A, Varadi A, Seprodi J, Sarkadi B. Interaction of the P-glycoprotein multidrug transporter (MDR1) with high affinity peptide chemosensitizers in isolated membranes, reconstituted systems, and intact cells. Biochem Pharmacol. 1999;58:571–586. doi: 10.1016/S0006-2952(99)00139-2. [DOI] [PubMed] [Google Scholar]
- Drewe J, Gutmann H, Fricker G, Torok M, Beglinger C, Huwyler J. HIV protease inhibitor ritonavir: a more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem Pharmacol. 1999;57:1147–1152. doi: 10.1016/S0006-2952(99)00026-X. [DOI] [PubMed] [Google Scholar]
- Plouzek CA, Ciolino HP, Clarke R, Yeh GC. Inhibition of P-glycoprotein activity and reversal of multidrug resistance in vitro by rosemary extract. Eur J Cancer. 1999;35:1541–1545. doi: 10.1016/S0959-8049(99)00180-X. [DOI] [PubMed] [Google Scholar]
- Xu JY, Zhou Q, Shen P, Tang W. Reversal effect of TTD on human multidrug resistant KBV200 cell line. J Exp Clin Cancer Res. 1999;18:549–552. [PubMed] [Google Scholar]
- Roe M, Folkes A, Ashworth P, Brumwell J, Chima L, Hunjan S, Pretswell I, Dangerfield W, Ryder H, Charlton P. Reversal of P-glycoprotein mediated multidrug resistance by novel anthranilamide derivatives. Bioorg Med Chem Lett. 1999;9:595–600. doi: 10.1016/S0960-894X(99)00030-X. [DOI] [PubMed] [Google Scholar]
- Chou TC, Depew KM, Zheng YH, Safer ML, Chan D, Helfrich B, Zatorska D, Zatorski A, Bornmann W, Danishefsky SJ. Reversal of anticancer multidrug resistance by the ardeemins. Proc Natl Acad Sci U S A. 1998;95:8369–8374. doi: 10.1073/pnas.95.14.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Vidal C, Quesada AR. Reversal of P-glycoprotein-mediated multidrug resistance in vitro by AV200, a new ardeemin derivative. Cancer Lett. 1998;132:45–50. doi: 10.1016/S0304-3835(98)00156-6. [DOI] [PubMed] [Google Scholar]
- Sako M, Suzuki H, Hirota K. Syntheses of taxuspine C derivatives as functional inhibitors of P-glycoprotein, an ATP-associated cell-membrane transporter. Chem Pharm Bull (Tokyo) 1998;46:1135–1139. doi: 10.1248/cpb.46.1135. [DOI] [PubMed] [Google Scholar]
- Orlowski S, Valente D, Garrigos M, Ezan E. Bromocriptine modulates P-glycoprotein function. Biochem Biophys Res Commun. 1998;244:481–488. doi: 10.1006/bbrc.1998.8289. [DOI] [PubMed] [Google Scholar]
- Hosoi E, Hirose M, Hamano S, Morimoto M, Kuroda Y. Effect of MDR antagonists on the cidal activity of vincristine for cells expressing MDR-1 is superior to those expressing MRP. Int J Oncol. 1998;13:343–348. doi: 10.3892/ijo.13.2.343. [DOI] [PubMed] [Google Scholar]
- Nussler V, Pelka-Fleisc R, Gieseler F, Hasmann M, Loser R, Gullis E, Stotzer O, Zwierzina H, Wilmanns W. In vitro efficacy of known P-glycoprotein modulators compared to droloxifene E and Z: studies on a human T-cell leukemia cell line and their resistant variants. Leuk Lymphoma. 1998;31:589–597. doi: 10.3109/10428199809057619. [DOI] [PubMed] [Google Scholar]
- Naito S, Koike K, Ono M, Machida T, Tasaka S, Kiue A, Koga H, Kumazawa J. Development of novel reversal agents, imidazothiazole derivatives, targeting MDR1- and MRP-mediated multidrug resistance. Oncol Res. 1998;10:123–132. [PubMed] [Google Scholar]
- Choudhuri SK, Chatterjee A. Reversal of resistance against doxorubicin by a newly developed compound, oxalyl bis(N-phenyl)hydroxamic acid in vitro. Anticancer Drugs. 1998;9:825–832. doi: 10.1097/00001813-199810000-00013. [DOI] [PubMed] [Google Scholar]
- Baggetto LG, Dong M, Bernaud J, Espinosa L, Rigal D, Bonvallet R, Marthinet E. In vitro and in vivo reversal of cancer cell multidrug resistance by the semi-synthetic antibiotic tiamulin. Biochem Pharmacol. 1998;56:1219–1228. doi: 10.1016/S0006-2952(98)00229-9. [DOI] [PubMed] [Google Scholar]
- Dale IL, Tuffley W, Callaghan R, Holmes JA, Martin K, Luscombe M, Mistry P, Ryder H, Stewart AJ, Charlton P, Twentyman PR, Bevan P. Reversal of P-glycoprotein-mediated multidrug resistance by XR9051, a novel diketopiperazine derivative. Br J Cancer. 1998;78:885–892. doi: 10.1038/bjc.1998.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann UA, Shlyakhter D, Mason VS, Zelle RE, Duffy JP, Galullo V, Armistead DM, Saunders JO, Boger J, Harding MW. Cellular and biochemical characterization of VX-710 as a chemosensitizer: reversal of P-glycoprotein-mediated multidrug resistance in vitro. Anticancer Drugs. 1997;8:125–140. doi: 10.1097/00001813-199702000-00004. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, Smith L, Wang YM, Chaturvedi P, Villalona M, Campbell E, Aylesworth C, Eckhardt SG, Hammond L, Kraynak M, Drengler R, Stephenson J, Jr, Harding MW, Von Hoff DD. Phase I and pharmacokinetic study of paclitaxel in combination with biricodar, a novel agent that reverses multidrug resistance conferred by overexpression of both MDR1 and MRP. J Clin Oncol. 1998;16:2964–2976. doi: 10.1200/JCO.1998.16.9.2964. [DOI] [PubMed] [Google Scholar]
- Miao ZH, Li XT, Zhu YJ. [Reversal of multidrug resistance by cyproheptadine in KBV200 cells] Yao Xue Xue Bao. 1997;32:321–325. [PubMed] [Google Scholar]
- Greenberger LM, Collins KI, Annable T, Boni JP, May MK, Lai FM, Kramer R, Citeralla RV, Hallett WA, Powell D. alpha-(3,4-dimethyoxyphenyl)-3,4-dihydro-6,7-dimethoxy-alpha- [(4-methylphenyl)thio]-2(1H)-isoquinolineheptanenitrile (CL 329,753): a novel chemosensitizing agent for P-glycoprotein-mediated resistance with improved biological properties compared with verapamil and cyclosporine A. Oncol Res. 1996;8:207–218. [PubMed] [Google Scholar]
- Christensen JG, LeBlanc GA. Reversal of multidrug resistance in vivo by dietary administration of the phytochemical indole-3-carbinol. Cancer Res. 1996;56:574–581. [PubMed] [Google Scholar]
- Kurosawa M, Okabe M, Hara N, Kawamura K, Suzuki S, Sakurada K, Asaka M. Reversal effect of itraconazole on adriamycin and etoposide resistance in human leukemia cells. Ann Hematol. 1996;72:17–21. doi: 10.1007/BF00663011. [DOI] [PubMed] [Google Scholar]
- Dantzig AH, Shepard RL, Cao J, Law KL, Ehlhardt WJ, Baughman TM, Bumol TF, Starling JJ. Reversal of P-glycoprotein-mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator, LY335979. Cancer Res. 1996;56:4171–4179. [PubMed] [Google Scholar]
- Claudio JA, Emerman JT. The effects of cyclosporin A, tamoxifen, and medroxyprogesterone acetate on the enhancement of adriamycin cytotoxicity in primary cultures of human breast epithelial cells. Breast Cancer Res Treat. 1996;41:111–122. doi: 10.1007/BF01807156. [DOI] [PubMed] [Google Scholar]
- Riffkin CD, Chung R, Wall DM, Zalcberg JR, Cowman AF, Foley M, Tilley L. Modulation of the function of human MDR1 P-glycoprotein by the antimalarial drug mefloquine. Biochem Pharmacol. 1996;52:1545–1552. doi: 10.1016/S0006-2952(96)00556-4. [DOI] [PubMed] [Google Scholar]
- Fardel O, Courtois A, Drenou B, Lamy T, Lecureur V, le Prise PY, Fauchet R. Inhibition of P-glycoprotein activity in human leukemic cells by mifepristone. Anticancer Drugs. 1996;7:671–677. doi: 10.1097/00001813-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Beck WT, Schuetz JD. Modulators and substrates of P-glycoprotein and cytochrome P4503A coordinately up-regulate these proteins in human colon carcinoma cells. Mol Pharmacol. 1996;49:311–318. [PubMed] [Google Scholar]
- Hu YP, Robert J. Azelastine and flezelastine as reversing agents of multidrug resistance: pharmacological and molecular studies. Biochem Pharmacol. 1995;50:169–175. doi: 10.1016/0006-2952(95)02055-1. [DOI] [PubMed] [Google Scholar]
- Borchers C, Ulrich WR, Klemm K, Ise W, Gekeler V, Haas S, Schodl A, Conrad J, Przybylski M, Boer R. B9209-005, an azido derivative of the chemosensitizer dexniguldipine-HCl, photolabels P-glycoprotein. Mol Pharmacol. 1995;48:21–29. [PubMed] [Google Scholar]
- Hofmann J, Gekeler V, Ise W, Noller A, Mitterdorfer J, Hofer S, Utz I, Gotwald M, Boer R, Glossmann H. Mechanism of action of dexniguldipine-HCl (B8509-035), a new potent modulator of multidrug resistance. Biochem Pharmacol. 1995;49:603–609. doi: 10.1016/0006-2952(94)00479-6. [DOI] [PubMed] [Google Scholar]
- Thurlimann B, Kroger N, Greiner J, Mross K, Schuller J, Schernhammer E, Schumacher K, Gastl G, Hartlapp J, Kupper H. Dexverapamil to overcome epirubicin resistance in advanced breast cancer. J Cancer Res Clin Oncol. 1995;121:R3–6. doi: 10.1007/BF02351063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch-Ernst KI, Ziemann C, Schmitz-Salue C, Foth H, Kahl GF. Modulation of P-glycoprotein and mdr1b mRNA expression by growth factors in primary rat hepatocyte culture. Biochem Biophys Res Commun. 1995;215:179–185. doi: 10.1006/bbrc.1995.2450. [DOI] [PubMed] [Google Scholar]
- Kim SH, Yeo GS, Lim YS, Kang CD, Kim CM, Chung BS. Suppression of multidrug resistance via inhibition of heat shock factor by quercetin in MDR cells. Exp Mol Med. 1998;30:87–92. doi: 10.1038/emm.1998.13. [DOI] [PubMed] [Google Scholar]
- Tsuruo T. [MDR reversing drugs for clinical development] Gan To Kagaku Ryoho. 1994;21:962–967. [PubMed] [Google Scholar]
- Breier A, Barancik M, Stefankova Z, Uhrik B, Tribulova N. Effect of pentoxifylline on P-glycoprotein mediated vincristine resistance of L1210 mouse leukemic cell line. Neoplasma. 1994;41:297–303. [PubMed] [Google Scholar]
- Wigler PW, Patterson FK. Reversal agent inhibition of the multidrug resistance pump in human leukemic lymphoblasts. Biochim Biophys Acta. 1994;1189:1–6. doi: 10.1016/0005-2736(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Gruol DJ, Zee MC, Trotter J, Bourgeois S. Reversal of multidrug resistance by RU 486. Cancer Res. 1994;54:3088–3091. [PubMed] [Google Scholar]
- Ganapathi R, Hercbergs A, Grabowski D, Ford J. Selective enhancement of vincristine cytotoxicity in multidrug-resistant tumor cells by dilantin (phenytoin) Cancer Res. 1993;53:3262–3265. [PubMed] [Google Scholar]
- Hyafil F, Vergely C, Du Vignaud P, Grand-Perret T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993;53:4595–4602. [PubMed] [Google Scholar]
- Callaghan R, Riordan JR. Synthetic and natural opiates interact with P-glycoprotein in multidrug-resistant cells. J Biol Chem. 1993;268:16059–16064. [PubMed] [Google Scholar]
- Efferth T, Volm M. Modulation of P-glycoprotein-mediated multidrug resistance by monoclonal antibodies, immunotoxins or antisense oligodeoxynucleotides in kidney carcinoma and normal kidney cells. Oncology. 1993;50:303–308. doi: 10.1159/000227200. [DOI] [PubMed] [Google Scholar]
- Kirk J, Houlbrook S, Stuart NS, Stratford IJ, Harris AL, Carmichael J. Selective reversal of vinblastine resistance in multidrug-resistant cell lines by tamoxifen, toremifene and their metabolites. Eur J Cancer. 1993;29A:1152–1157. doi: 10.1016/s0959-8049(05)80306-5. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Wakusawa S, Inoko K, Takagi K, Koyama M. Reversal of vinblastine resistance by a new staurosporine derivative, NA-382, in P388/ADR cells. Cancer Lett. 1992;64:177–183. doi: 10.1016/0304-3835(92)90079-B. [DOI] [PubMed] [Google Scholar]
- van Kalken CK, van der Hoeven JJ, de Jong J, Giaccone G, Schuurhuis GJ, Maessen PA, Blokhuis WM, van der Vijgh WJ, Pinedo HM. Bepridil in combination with anthracyclines to reverse anthracycline resistance in cancer patients. Eur J Cancer. 1991;27:739–744. doi: 10.1016/0277-5379(91)90178-g. [DOI] [PubMed] [Google Scholar]
- Boesch D, Gaveriaux C, Jachez B, Pourtier-Manzanedo A, Bollinger P, Loor F. In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res. 1991;51:4226–4233. [PubMed] [Google Scholar]
- Friche E, Jensen PB, Sehested M, Demant EJ, Nissen NN. The solvents cremophor EL and Tween 80 modulate daunorubicin resistance in the multidrug resistant Ehrlich ascites tumor. Cancer Commun. 1990;2:297–303. [PubMed] [Google Scholar]
- Gosland MP, Lum BL, Sikic BI. Reversal by cefoperazone of resistance to etoposide, doxorubicin, and vinblastine in multidrug resistant human sarcoma cells. Cancer Res. 1989;49:6901–6905. [PubMed] [Google Scholar]
- Ford JM, Prozialeck WC, Hait WN. Structural features determining activity of phenothiazines and related drugs for inhibition of cell growth and reversal of multidrug resistance. Mol Pharmacol. 1989;35:105–115. [PubMed] [Google Scholar]
- Sato S, Watanabe Y, Okamura K, Hamanaka R, Mori T, Kohno K, Kuwano M. Potentiation of vincristine and actinomycin D by a new synthetic imidazole anti-tumor agent YM534 active against human cancer cells and multidrug-resistant cells. Anticancer Drug Des. 1989;4:125–135. [PubMed] [Google Scholar]
- Cornwell MM, Pastan I, Gottesman MM. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987;262:2166–2170. [PubMed] [Google Scholar]
- Twentyman PR, Fox NE, White DJ. Cyclosporin A and its analogues as modifiers of adriamycin and vincristine resistance in a multi-drug resistant human lung cancer cell line. Br J Cancer. 1987;56:55–57. doi: 10.1038/bjc.1987.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauffert B, Martin M, Hammann A, Michel MF, Martin F. Amiodarone-induced enhancement of doxorubicin and 4'-deoxydoxorubicin cytotoxicity to rat colon cancer cells in vitro and in vivo. Cancer Res. 1986;46:825–830. [PubMed] [Google Scholar]
- Tsuruo T, Iida H, Kitatani Y, Yokota K, Tsukagoshi S, Sakurai Y. Effects of quinidine and related compounds on cytotoxicity and cellular accumulation of vincristine and adriamycin in drug-resistant tumor cells. Cancer Res. 1984;44:4303–4307. [PubMed] [Google Scholar]
- Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967–1972. [PubMed] [Google Scholar]
- Murakami N, Sugimoto M, Morita M, Akiyama S, Kobayashi M. Synthesis and evaluation of 4-deacetoxyagosterol A as an MDR-modulator. Bioorg Med Chem Lett. 2000;10:2521–2524. doi: 10.1016/S0960-894X(00)00502-3. [DOI] [PubMed] [Google Scholar]
- Vezmar M, Georges E. Reversal of MRP-mediated doxorubicin resistance with quinoline-based drugs. Biochem Pharmacol. 2000;59:1245–1252. doi: 10.1016/S0006-2952(00)00270-7. [DOI] [PubMed] [Google Scholar]
- Curtin NJ, Turner DP. Dipyridamole-mediated reversal of multidrug resistance in MRP over-expressing human lung carcinoma cells in vitro. Eur J Cancer. 1999;35:1020–1026. doi: 10.1016/S0959-8049(99)00038-6. [DOI] [PubMed] [Google Scholar]
- Terashi K, Oka M, Soda H, Fukuda M, Kawabata S, Nakatomi K, Shiozawa K, Nakamura T, Tsukamoto K, Noguchi Y, Suenaga M, Tei C, Kohno S. Interactions of ofloxacin and erythromycin with the multidrug resistance protein (MRP) in MRP-overexpressing human leukemia cells. Antimicrob Agents Chemother. 2000;44:1697–1700. doi: 10.1128/AAC.44.6.1697-1700.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payen L, Delugin L, Courtois A, Trinquart Y, Guillouzo A, Fardel O. Reversal of MRP-mediated multidrug resistance in human lung cancer cells by the antiprogestatin drug RU486. Biochem Biophys Res Commun. 1999;258:513–518. doi: 10.1006/bbrc.1999.0671. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Oka M, Aizawa K, Soda H, Fukuda M, Terashi K, Ikeda K, Mizuta Y, Noguchi Y, Kimura Y, Tsuruo T, Kohno S. Direct interaction between a quinoline derivative, MS-209, and multidrug resistance protein (MRP) in human gastric cancer cells. Biochem Biophys Res Commun. 1999;255:618–624. doi: 10.1006/bbrc.1999.0245. [DOI] [PubMed] [Google Scholar]
- Courtois A, Payen L, Vernhet L, de Vries EG, Guillouzo A, Fardel O. Inhibition of multidrug resistance-associated protein (MRP) activity by rifampicin in human multidrug-resistant lung tumor cells. Cancer Lett. 1999;139:97–104. doi: 10.1016/S0304-3835(99)00024-5. [DOI] [PubMed] [Google Scholar]
- Duffy CP, Elliott CJ, O'Connor RA, Heenan MM, Coyle S, Cleary IM, Kavanagh K, Verhaegen S, O'Loughlin CM, NicAmhlaoibh R, Clynes M. Enhancement of chemotherapeutic drug toxicity to human tumour cells in vitro by a subset of non-steroidal anti-inflammatory drugs (NSAIDs) Eur J Cancer. 1998;34:1250–1259. doi: 10.1016/S0959-8049(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Chen ZS, Kitazono M, Takebayashi Y, Niwa K, Yamada K, Tani A, Haraguchi M, Sumizawa T, Furukawa T, Aikou T, Akiyama S. Increased sensitivity to vincristine of MDR cells by the leukotriene D4 receptor antagonist, ONO-1078. Cancer Lett. 1998;130:175–182. doi: 10.1016/S0304-3835(98)00132-3. [DOI] [PubMed] [Google Scholar]
- Draper MP, Martell RL, Levy SB. Indomethacin-mediated reversal of multidrug resistance and drug efflux in human and murine cell lines overexpressing MRP, but not P-glycoprotein. Br J Cancer. 1997;75:810–815. doi: 10.1038/bjc.1997.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S, Kim CH, Tran BN, Sangha S, Gupta S. Probenecid reverses multidrug resistance in multidrug resistance-associated protein-overexpressing HL60/AR and H69/AR cells but not in P-glycoprotein-overexpressing HL60/Tax and P388/ADR cells. Cancer Chemother Pharmacol. 1997;40:150–158. doi: 10.1007/s002800050640. [DOI] [PubMed] [Google Scholar]
- Manzano RG, Wright KA, Twentyman PR. Modulation by acrolein and chloroacetaldehyde of multidrug resistance mediated by the multidrug resistance-associated protein (MRP) Clin Cancer Res. 1996;2:1321–1326. [PubMed] [Google Scholar]
- Vanhoefer U, Cao S, Minderman H, Toth K, Skenderis BS, 2nd, Slovak ML, Rustum YM. d,l-buthionine-(S,R)-sulfoximine potentiates in vivo the therapeutic efficacy of doxorubicin against multidrug resistance protein-expressing tumors. Clin Cancer Res. 1996;2:1961–1968. [PubMed] [Google Scholar]
- Vanhoefer U, Cao S, Minderman H, Toth K, Scheper RJ, Slovak ML, Rustum YM. PAK-104P, a pyridine analogue, reverses paclitaxel and doxorubicin resistance in cell lines and nude mice bearing xenografts that overexpress the multidrug resistance protein. Clin Cancer Res. 1996;2:369–377. [PubMed] [Google Scholar]
- Gollapudi S, Thadepalli F, Kim CH, Gupta S. Difloxacin reverses multidrug resistance in HL-60/AR cells that overexpress the multidrug resistance-related protein (MRP) gene. Oncol Res. 1995;7:213–225. [PubMed] [Google Scholar]
- Gekeler V, Ise W, Sanders KH, Ulrich WR, Beck J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem Biophys Res Commun. 1995;208:345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004;65:1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/Flavonoids Reverse Breast Cancer Resistance Protein/ABCG2-Mediated Multidrug Resistance. Cancer Res. 2004;64:4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E, Traxler P. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res. 2004;64:2333–2337. doi: 10.1158/0008-5472.CAN-03-3344. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Tsukahara S, Imai Y, Ueda K, Tsuruo T. Reversal of breast cancer resistance protein-mediated drug resistance by estrogen antagonists and agonists. Mol Cancer Ther. 2003;2:105–112. [PubMed] [Google Scholar]
- Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- de Bruin M, Miyake K, Litman T, Robey R, Bates SE. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999;146:117–126. doi: 10.1016/S0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]
- Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T, Greenberger LM. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998;58:5850–5858. [PubMed] [Google Scholar]
- Sharom FJ, Liu R, Qu Q, Romsicki Y. Exploring the structure and function of the P-glycoprotein multidrug transporter using fluorescence spectroscopic tools. Semin Cell Dev Biol. 2001;12:257–265. doi: 10.1006/scdb.2000.0251. [DOI] [PubMed] [Google Scholar]
- Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–242. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- Wolf DC, Horwitz SB. P-glycoprotein transports corticosterone and is photoaffinity-labeled by the steroid. Int J Cancer. 1992;52:141–146. doi: 10.1002/ijc.2910520125. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang L, Hoffman T, Gosland M, Vore M. MDR1 substrates/modulators protect against beta-estradiol-17beta-D-glucuronide cholestasis in rat liver. Cancer Res. 1996;56:4992–4997. [PubMed] [Google Scholar]
- Ernest S, Bello-Reuss E. Secretion of platelet-activating factor is mediated by MDR1 P-glycoprotein in cultured human mesangial cells. J Am Soc Nephrol. 1999;10:2306–2313. doi: 10.1681/ASN.V10112306. [DOI] [PubMed] [Google Scholar]
- Liu XD, Liu GQ. P glycoprotein regulated transport of glutamate at blood brain barrier. Acta Pharmacol Sin. 2001;22:111–116. [PubMed] [Google Scholar]
- King M, Su W, Chang A, Zuckerman A, Pasternak GW. Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nat Neurosci. 2001;4:268–274. doi: 10.1038/85115. [DOI] [PubMed] [Google Scholar]
- Vogelgesang S, Cascorbi I, Schroeder E, Pahnke J, Kroemer HK, Siegmund W, Kunert-Keil C, Walker LC, Warzok RW. Deposition of Alzheimer's beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12:535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- Lam FC, Liu R, Lu P, Shapiro AB, Renoir JM, Sharom FJ, Reiner PB. beta-Amyloid efflux mediated by p-glycoprotein. J Neurochem. 2001;76:1121–1128. doi: 10.1046/j.1471-4159.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- Leier I, Jedlitschky G, Buchholz U, Cole SP, Deeley RG, Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem. 1994;269:27807–27810. [PubMed] [Google Scholar]
- Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D. Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res. 1996;56:988–994. [PubMed] [Google Scholar]
- Loe DW, Stewart RK, Massey TE, Deeley RG, Cole SP. ATP-dependent transport of aflatoxin B1 and its glutathione conjugates by the product of the multidrug resistance protein (MRP) gene. Mol Pharmacol. 1997;51:1034–1041. doi: 10.1124/mol.51.6.1034. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- Tsuruoka S, Sugimoto KI, Fujimura A, Imai M, Asano Y, Muto S. P-glycoprotein-mediated drug secretion in mouse proximal tubule perfused in vitro. J Am Soc Nephrol. 2001;12:177–181. doi: 10.1159/000051255. [DOI] [PubMed] [Google Scholar]
- Malingre MM, Beijnen JH, Schellens JH. Oral delivery of taxanes. Invest New Drugs. 2001;19:155–162. doi: 10.1023/A:1010635000879. [DOI] [PubMed] [Google Scholar]
- Mickley LA, Lee JS, Weng Z, Zhan Z, Alvarez M, Wilson W, Bates SE, Fojo T. Genetic polymorphism in MDR-1: a tool for examining allelic expression in normal cells, unselected and drug-selected cell lines, and human tumors. Blood. 1998;91:1749–1756. [PubMed] [Google Scholar]
- Fromm MF. The influence of MDR1 polymorphisms on P-glycoprotein expression and function in humans. Adv Drug Deliv Rev. 2002;54:1295–1310. doi: 10.1016/S0169-409X(02)00064-9. [DOI] [PubMed] [Google Scholar]
- Brinkmann U, Eichelbaum M. Polymorphisms in the ABC drug transporter gene MDR1. Pharmacogenomics J. 2001;1:59–64. doi: 10.1038/sj.tpj.6500001. [DOI] [PubMed] [Google Scholar]
- Ito S, Ieiri I, Tanabe M, Suzuki A, Higuchi S, Otsubo K. Polymorphism of the ABC transporter genes, MDR1, MRP1 and MRP2/cMOAT, in healthy Japanese subjects. Pharmacogenetics. 2001;11:175–184. doi: 10.1097/00008571-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Kerb R, Hoffmeyer S, Brinkmann U. ABC drug transporters: hereditary polymorphisms and pharmacological impact in MDR1, MRP1 and MRP2. Pharmacogenomics. 2001;2:51–64. doi: 10.1517/14622416.2.1.51. [DOI] [PubMed] [Google Scholar]
- Moriya Y, Nakamura T, Horinouchi M, Sakaeda T, Tamura T, Aoyama N, Shirakawa T, Gotoh A, Fujimoto S, Matsuo M, Kasuga M, Okumura K. Effects of polymorphisms of MDR1, MRP1, and MRP2 genes on their mRNA expression levels in duodenal enterocytes of healthy Japanese subjects. Biol Pharm Bull. 2002;25:1356–1359. doi: 10.1248/bpb.25.1356. [DOI] [PubMed] [Google Scholar]
- Tian R, Koyabu N, Takanaga H, Matsuo H, Ohtani H, Sawada Y. Effects of grapefruit juice and orange juice on the intestinal efflux of P-glycoprotein substrates. Pharm Res. 2002;19:802–809. doi: 10.1023/A:1016100715125. [DOI] [PubMed] [Google Scholar]
- Kurata Y, Ieiri I, Kimura M, Morita T, Irie S, Urae A, Ohdo S, Ohtani H, Sawada Y, Higuchi S, Otsubo K. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin Pharmacol Ther. 2002;72:209–219. doi: 10.1067/mcp.2002.126177. [DOI] [PubMed] [Google Scholar]
- Hennessy M, Kelleher D, Spiers JP, Barry M, Kavanagh P, Back D, Mulcahy F, Feely J. St Johns wort increases expression of P-glycoprotein: implications for drug interactions. Br J Clin Pharmacol. 2002;53:75–82. doi: 10.1046/j.0306-5251.2001.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Fasold MB, Zadina JE. Endomorphins, Met-enkephalin, Tyr-MIF-1, and the P-glycoprotein efflux system. Drug Metab Dispos. 2002;30:231–234. doi: 10.1124/dmd.30.3.231. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Sakata A, Miyashita T, Tamai I, Tsuji A. Physiologically based pharmacokinetics of digoxin in mdr1a knockout mice. J Pharm Sci. 1999;88:1281–1287. doi: 10.1021/js9901763. [DOI] [PubMed] [Google Scholar]
- Miyama T, Takanaga H, Matsuo H, Yamano K, Yamamoto K, Iga T, Naito M, Tsuruo T, Ishizuka H, Kawahara Y, Sawada Y. P-glycoprotein-mediated transport of itraconazole across the blood-brain barrier. Antimicrob Agents Chemother. 1998;42:1738–1744. doi: 10.1128/aac.42.7.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Wagenaar E, Dorobek B, Beijnen JH, Borst P, Schinkel AH. Full blockade of intestinal P-glycoprotein and extensive inhibition of blood-brain barrier P-glycoprotein by oral treatment of mice with PSC833. J Clin Invest. 1997;100:2430–2436. doi: 10.1172/JCI119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, Schinkel AH. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–1656. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, Gallagher MP, Gill DR, Hubbard RE, Higgins CF. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Sonveaux N, Shapiro AB, Goormaghtigh E, Ling V, Ruysschaert JM. Secondary and tertiary structure changes of reconstituted P-glycoprotein. A Fourier transform attenuated total reflection infrared spectroscopy analysis. J Biol Chem. 1996;271:24617–24624. doi: 10.1074/jbc.271.40.24617. [DOI] [PubMed] [Google Scholar]
- Rosenberg MF, Velarde G, Ford RC, Martin C, Berridge G, Kerr ID, Callaghan R, Schmidlin A, Wooding C, Linton KJ, Higgins CF. Repacking of the transmembrane domains of P-glycoprotein during the transport ATPase cycle. Embo J. 2001;20:5615–5625. doi: 10.1093/emboj/20.20.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MF, Kamis AB, Callaghan R, Higgins CF, Ford RC. Three-dimensional structures of the mammalian multidrug resistance P-glycoprotein demonstrate major conformational changes in the transmembrane domains upon nucleotide binding. J Biol Chem. 2003;278:8294–8299. doi: 10.1074/jbc.M211758200. [DOI] [PubMed] [Google Scholar]
- Rosenberg MF, Callaghan R, Ford RC, Higgins CF. Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J Biol Chem. 1997;272:10685–10694. doi: 10.1074/jbc.272.3.1665. [DOI] [PubMed] [Google Scholar]
- Senior AE, Bhagat S. P-glycoprotein shows strong catalytic cooperativity between the two nucleotide sites. Biochemistry. 1998;37:831–836. doi: 10.1021/bi9719962. [DOI] [PubMed] [Google Scholar]
- Loo TW, Bartlett MC, Clarke DM. Drug binding in human P-glycoprotein causes conformational changes in both nucleotide-binding domains. J Biol Chem. 2003;278:1575–1578. doi: 10.1074/jbc.M211307200. [DOI] [PubMed] [Google Scholar]
- Gao M, Cui HR, Loe DW, Grant CE, Almquist KC, Cole SP, Deeley RG. Comparison of the functional characteristics of the nucleotide binding domains of multidrug resistance protein 1. J Biol Chem. 2000;275:13098–13108. doi: 10.1074/jbc.275.17.13098. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Identification of residues within the drug-binding domain of the human multidrug resistance P-glycoprotein by cysteine-scanning mutagenesis and reaction with dibromobimane. J Biol Chem. 2000;275:39272–39278. doi: 10.1074/jbc.M007741200. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. The transmembrane domains of the human multidrug resistance P-glycoprotein are sufficient to mediate drug binding and trafficking to the cell surface. J Biol Chem. 1999;274:24759–24765. doi: 10.1074/jbc.274.35.24759. [DOI] [PubMed] [Google Scholar]
- Consoli U, Priebe W, Ling YH, Mahadevia R, Griffin M, Zhao S, Perez-Soler R, Andreeff M. The novel anthracycline annamycin is not affected by P-glycoprotein-related multidrug resistance: comparison with idarubicin and doxorubicin in HL-60 leukemia cell lines. Blood. 1996;88:633–644. [PubMed] [Google Scholar]
- Perez-Soler R, Neamati N, Zou Y, Schneider E, Doyle LA, Andreeff M, Priebe W, Ling YH. Annamycin circumvents resistance mediated by the multidrug resistance-associated protein (MRP) in breast MCF-7 and small-cell lung UMCC-1 cancer cell lines selected for resistance to etoposide. Int J Cancer. 1997;71:35–41. doi: 10.1002/(SICI)1097-0215(19970328)71:1<35::AID-IJC8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Mazel M, Clair P, Rousselle C, Vidal P, Scherrmann JM, Mathieu D, Temsamani J. Doxorubicin-peptide conjugates overcome multidrug resistance. Anticancer Drugs. 2001;12:107–116. doi: 10.1097/00001813-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Belpomme D, Gauthier S, Pujade-Lauraine E, Facchini T, Goudier MJ, Krakowski I, Netter-Pinon G, Frenay M, Gousset C, Marie FN, Benmiloud M, Sturtz F. Verapamil increases the survival of patients with anthracycline-resistant metastatic breast carcinoma. Ann Oncol. 2000;11:1471–1476. doi: 10.1023/A:1026556119020. [DOI] [PubMed] [Google Scholar]
- Haussermann K, Benz B, Gekeler V, Schumacher K, Eichelbaum M. Effects of verapamil enantiomers and major metabolites on the cytotoxicity of vincristine and daunomycin in human lymphoma cell lines. Eur J Clin Pharmacol. 1991;40:53–59. doi: 10.1007/BF00315139. [DOI] [PubMed] [Google Scholar]
- Teodori E, Dei S, Scapecchi S, Gualtieri F. The medicinal chemistry of multidrug resistance (MDR) reversing drugs. Farmaco. 2002;57:385–415. doi: 10.1016/S0014-827X(02)01229-6. [DOI] [PubMed] [Google Scholar]
- Toffoli G, Simone F, Corona G, Raschack M, Cappelletto B, Gigante M, Boiocchi M. Structure-activity relationship of verapamil analogs and reversal of multidrug resistance. Biochem Pharmacol. 1995;50:1245–1255. doi: 10.1016/0006-2952(95)02003-U. [DOI] [PubMed] [Google Scholar]
- Slater LM, Sweet P, Stupecky M, Gupta S. Cyclosporin A reverses vincristine and daunorubicin resistance in acute lymphatic leukemia in vitro. J Clin Invest. 1986;77:1405–1408. doi: 10.1172/JCI112450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arceci RJ, Stieglitz K, Bierer BE. Immunosuppressants FK506 and rapamycin function as reversal agents of the multidrug resistance phenotype. Blood. 1992;80:1528–1536. [PubMed] [Google Scholar]
- Fracasso PM, Brady MF, Moore DH, Walker JL, Rose PG, Letvak L, Grogan TM, McGuire WP. Phase II study of paclitaxel and valspodar (PSC 833) in refractory ovarian carcinoma: a gynecologic oncology group study. J Clin Oncol. 2001;19:2975–2982. doi: 10.1200/JCO.2001.19.12.2975. [DOI] [PubMed] [Google Scholar]
- Mistry P, Stewart AJ, Dangerfield W, Okiji S, Liddle C, Bootle D, Plumb JA, Templeton D, Charlton P. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001;61:749–758. [PubMed] [Google Scholar]
- Dayan G, Jault JM, Baubichon-Cortay H, Baggetto LG, Renoir JM, Baulieu EE, Gros P, Di Pietro A. Binding of steroid modulators to recombinant cytosolic domain from mouse P-glycoprotein in close proximity to the ATP site. Biochemistry. 1997;36:15208–15215. doi: 10.1021/bi9718696. [DOI] [PubMed] [Google Scholar]
- Conseil G, Baubichon-Cortay H, Dayan G, Jault JM, Barron D, Di Pietro A. Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci U S A. 1998;95:9831–9836. doi: 10.1073/pnas.95.17.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]