Abstract
The protective potential of immunoglobulin A (IgA) monoclonal antibodies (MAbs) directed against O and H antigens of Salmonella enterica serotype Enteritidis to prevent bacterial adhesion to and invasion of HEp-2 cells was evaluated. Although anti-flagellar IgA MAbs showed strong agglutinating capacities, they did not protect cell monolayers. In contrast, IgA MAbs specific for the O:9 epitope of Salmonella lipopolysaccharide antigen alone prevented S. enterica serotype Enteritidis entry and replication within HEp-2 cells, and the protection was not mediated by direct binding of antibodies to bacterial adhesins or by agglutination of microorganisms.
Immunoglobulin A (IgA) is a principal mucosal antibody isotype that can bind to surface bacterial antigens and can prevent bacterial attachment to and penetration of epithelial cells (10). Host defense against Salmonella pathogens at the mucous membranes is mediated by nonspecific and immunologically specific mechanisms. The complexity of the mucosal defense has made it difficult to identify the specific surface epitopes that induce protective immunity and to elucidate the role of IgA in the protection. Mucosal immunization with attenuated Salmonella strains induces not only local but also cell-mediated and systemic humoral immune responses (6, 7, 15, 24). Most studies have focused on Salmonella enterica serotype Typhimurium pathogenesis, while the pathogenesis of Salmonella enterica serotype Enteritidis infection is poorly understood. In recent years, S. enterica serotype Enteritidis has proved to be the major food-borne pathogen, and its incidence has increased dramatically worldwide (12). This necessitates the profound analysis of the virulence characteristics of S. enterica serotype Enteritidis clinical isolates, their pathogenesis, and the immune mechanisms of host defense against infection.
The antibodies specific for surface epitopes of S. enterica serotype Typhi are protective against typhoid fever, and oral immunization of humans with a live S. enterica serotype Typhi vaccinal strain induces protective mucosal and systemic immunity (1, 3, 20, 21). S. enterica serotype Enteritidis, like S. enterica serotype Typhimurium, can cause generalized infection in mice similar to that caused by S. enterica serotype Typhi in humans (2). The abilities of Salmonella strains to invade cell culture monolayers strongly correlate with their virulence and potential to produce disease (4, 5, 9, 16). The HEp-2 invasion assay is a suitable in vitro model to assess the abilities of different bacteria, including Salmonella strains, to enter and replicate within cultured epithelial cells (19). These models make it possible to assess the protective efficacy of IgA directed against the O:9 epitope common to group D Salmonella strains. On the other hand, most S. enterica serotype Enteritidis strains, like most S. enterica serotype Typhi strains, are monophasic and express flagellar antigens in phase 1. This allows evaluation of the protective ability of IgA specific for a single epitope of Salmonella flagellar antigen. In this study, we have used monoclonal antibodies (MAbs) to evaluate the protective potentials of IgA antibodies directed against flagellar and lipopolysaccharide (LPS) antigens of S. enterica serotype Enteritidis.
IgA MAbs (clones 177E6 and 178) directed against LPS epitopes were produced, and their antigen specificities were characterized as described previously (8). IgA MAbs (clones 187g3, 188ND9, and 189C1) against H:g,m Salmonella flagellar antigen were generated after intragastral immunization with live Salmonella enterica serotype Suberu (3,10:g,m:−). All three MAbs were characterized as H:g epitope specific, and the production of monomeric and polymeric IgA forms was confirmed.
MAbs 177E6 and 187g3 were purified by anion-exchange chromatography on a Mono Q column (Pharmacia) as described previously (8). The other three MAbs were partially purified by ammonium sulfate precipitation of ascitic fluids. The antibody concentrations in the preparations were measured by capture enzyme-linked immunosorbent assay using purified mouse IgA MOPC 315 (Cappel) as a standard antibody, and all MAbs were sterilized by filtration through 0.22-μm-pore-size filters (Millipore).
The agglutinating properties of IgA MAbs were tested by slide agglutination tests with the S. enterica serotype Enteritidis type strain (ATCC 13076) and with eight S. enterica serotype Enteritidis clinical isolates from the collection of our diagnostic laboratory (the results are shown in Table 1). IgA MAbs did not reveal in vitro bactericidal activity alone or in the presence of complement (data not shown).
TABLE 1.
Agglutinating properties of IgA MAbs in slide agglutination test
| MAba | Titerb
|
Agglutinating concn (μg/ml)c | |
|---|---|---|---|
| 0.5 μg/ml | 5 μg/ml | ||
| 177E6 | 4-8 | 1.25 | |
| 178H11 | 2-4 | 1.5 | |
| 187g3 | 2-4 | 32-64 | 0.1 |
| 188ND9 | 4-8 | 32-128 | 0.1 |
| 189C1 | 4-8 | 128 | 0.05 |
Purified MAbs were serially diluted in PBS.
Endpoint titer (1/T) in slide agglutination test with S. enterica serotype Enteritidis type strain (ATCC 13076) and eight clinical isolates at indicated MAb concentration.
Antibody concentration sufficient to agglutinate S. enterica serotype Enteritidis clinical isolate 5293 used in protection assays.
HEp-2 cells were cultured in 24-well plates in RPMI 1640 medium (Gibco). Confluent monolayers were infected with 107 exponentially growing bacteria as described previously (19). To evaluate the protective potential of monoclonal IgA, purified MAbs were diluted in fresh RPMI 1640 medium and mixed with the bacterial inoculum. The plates were incubated at 37οC for 3 h, which was sufficient time for bacterial entry into HEp-2 cells. For the last 30 min of incubation, 100 μg of gentamicin (Sigma)/ml was added to kill extracellular bacteria. Then, the monolayers were washed six times with phosphate-buffered saline (PBS) and lysed with 0.5% sodium desoxycholate (Merck) in distilled water. Serial 10-fold dilutions of cell lysates were plated on Trypticase soy agar (Difco), and the number of CFU per well was calculated after overnight cultivation. A highly invasive S. enterica serotype Enteritidis strain (clinical isolate no. 5293) was selected for the antibody protection assays. More than 20% of the initial bacterial inoculum was protected from gentamicin killing after 3 h of infection (Fig. 1). Purified IgA MAb MOPC315 was used as an antibody isotype control. MAb 8aC10 of IgG3 isotype specific for the O:5 LPS antigen of Salmonella was also used as a control antibody, and S. enterica serotype Typhimurium strain C5 was used as a control bacterial strain. All MAbs were applied in two final concentrations—0.5 and 5 μg/ml. To determine whether bacterial LPS takes part in bacterial attachment, cell monolayers were pretreated with purified S. enterica serotype Enteritidis LPS for 1 h before inoculation with bacteria.
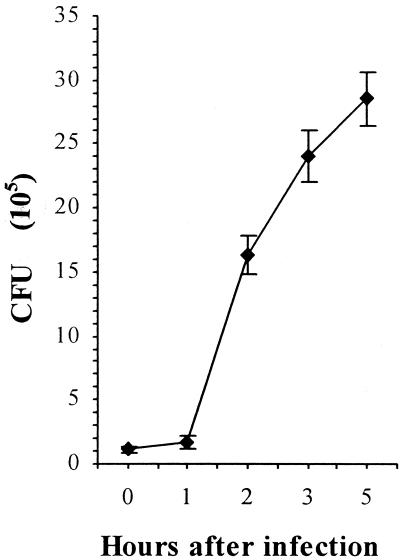
FIG. 1.
Kinetics of S. enterica serotype Enteritidis strain 5293 growth in HEp-2 cells (in CFU per well). The confluent monolayers were inoculated with 10.6 × 106 bacteria per well, and each of the samples was run in six wells.
The number of bacteria recovered after 3 h of incubation with control MAbs (MOPC315 and 8aC10) and with low (0.5-μg/ml) concentrations of specific IgA MAbs was similar to that with the control without antibodies (Table 2). In concentrations of 5 μg/ml, anti-LPS IgA 177E6 and 178H11 considerably inhibited S. enterica serotype Enteritidis entry into HEp-2 cells. For example, the number of viable intracellular bacteria was reduced more than six times in the presence of MAb 177E6. Although the anti-flagellar antibodies possessed greater agglutinating capacity, none of the three H:g,m-specific IgA MAbs (187g3, 188ND9, and 189C1) prevented S. enterica serotype Enteritidis entry into and multiplication in HEp-2 cells. Invasion of monolayers by S. enterica serotype Typhimurium C5 was not affected in the presence of IgA MAbs, indicating that the mechanism of protection was immunologically specific for S. enterica serotype Enteritidis and directed against a single O:9 LPS epitope (data not shown).
TABLE 2.
Effect of IgA MAbs on bacterial attachment and entry of S. enterica serotype Enteritidis into HEp-2 cells
| Assay | No. of bacteriaa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | MOPC315 | 8aC10 | 177E6 | 178H11 | 187g3 | 188ND9 | 189C1 | LPS | |
| Invasionb | 26.0 ± 3.5 | 25.3 ± 2.9 | 26.6 ± 3.1 | 3.9 ± 1.1 | 8.1 ± 3.3 | 22.8 ± 2.6 | 23.8 ± 2.7 | 27.3 ± 5.7 | 28.2 ± 3.5 |
| Adhesionc | 4.2 ± 0.6 | 4.1 ± 0.9 | 4.3 ± 1.2 | 1.5 ± 0.3 | 1.5 ± 0.6 | 4.2 ± 1.1 | 3.9 ± 1.3 | 4.1 ± 1.2 | ND |
| Adhesiond | 19.7 ± 1.9 | 20.4 ± 2.0 | ND | 8.5 ± 2.1 | 8.6 ± 2.8 | 20.4 ± 3.7 | 22.1 ± 4.4 | ND | 20.6 ± 1.5 |
Mean (106 CFU) per well (with standard deviation) with indicated MAb; initial inoculum, 9.2 × 106 CFU of S. enterica serotype Enteritidis clinical isolate 5293 per well; control, bacteria without antibodies; ND, not defined.
Invasion of bacteria into cell monolayers after centrifugation of the inoculum and 3-h incubation.
Adhesion assay performed like invasion assay after 1 h of incubation but without centrifugation and gentamicin treatment.
Adhesion to glutaraldehyde-fixed monolayers after centrifugation of the inoculum and 3-h incubation.
Recent data suggest that centrifugation of the initial inoculum onto HEp-2 monolayers might bypass the important role of bacterial motility (23). To avoid that and to assess the abilities of IgA MAbs to prevent the initial attachment of bacteria to the cells, the assay was performed as described above but without the steps of centrifugation and gentamicin killing. The plates were incubated for 1 h at 37οC and, after six washes with PBS, were lysed with 0.5% sodium desoxycholate. A bacterial adhesion assay with centrifugation onto glutaraldehyde-fixed monolayers was performed as described previously (14).
Both anti-LPS MAbs significantly inhibited the initial adhesion of S. enterica serotype Enteritidis strain 5293 to HEp-2 cells (Table 2). The results were similar to those obtained with glutaraldehyde-fixed monolayers. The anti-flagellar MAbs did not prevent initial bacterial attachment to the cell monolayers. In the protective concentration of 5 μg/ml, anti-LPS MAbs agglutinated bacteria in a 1:4 dilution in a slide agglutination test (Table 1). The anti-flagellar IgA in the same concentration possessed more than 10-fold-greater agglutinating capacity (up to 1:128 for clone 189C1). Thus, the results demonstrated the absence of correlation between IgA agglutination capacity and protection of cell monolayers against bacterial entry.
In mucosal secretions, IgA directed against surface epitopes can prevent the attachment and penetration of microorganisms. The antibacterial effect may be potentiated by nonspecific humoral factors of mucosal defense, such as lysozyme, lactoferrin, and lactoperoxidase (10). IgA antibodies alone can protect epithelial cells against bacterial adherence and invasion in the absence of other immune and nonimmune protective mechanisms. IgA binding can be directed to the adhesion molecules, thus inhibiting the initial step of attachment to a surface receptor on epithelial cells. IgA binding to surface epitopes may also interfere with the adhesins for steric reasons, preventing adherence by an indirect mechanism (10). In our study, anti-LPS IgA in an agglutinating concentration of 5 μg/ml prevented invasion of HEp-2 cell monolayers by a large initial S. enterica serotype Enteritidis inoculum (107 CFU per well). Pretreatment of HEp-2 monolayers with 10 μg of purified S. enterica serotype Enteritidis LPS/ml for 1 h prior to inoculation had no effect on bacterial entry, indicating that LPS did not take part in the initial attachment as an adhesion molecule (Table 2). Thus, one possible explanation of protection is that binding of IgA antibodies to LPS may interfere with bacterial adhesins for steric reasons.
Secretory dimeric and polymeric IgA forms are effective agglutinators. It has been reported that monoclonal IgA antibody specific for LPS antigen protected mice against oral challenge with a lethal dose of a virulent S. enterica serotype Typhimurium strain (13) and, in agglutinating concentrations, prevented bacterial entry into polarized MDCK cell monolayers (14). Although the reported results demonstrate a correlation between agglutinating ability and protection, there is no direct evidence for the suggested important role of IgA-mediated agglutination. The molecular structure of mouse IgA shows some differences from the conventional immunoglobulin structure (22), and generation of stabile Fab fragments is not possible (14). In our investigation, we demonstrated that strong agglutinating anti-flagellar IgA alone is not protective against S. enterica serotype Enteritidis invasion. Bacterial motility enables pathogens to penetrate the mucous layer and attach to host epithelial cells even when fimbriae are not present (11, 17). Very recently, it was reported that flagella are necessary for full S. enterica serotype Typhimurium pathogenicity (18). However, the mucosal defense is complex, and IgA specific for Salmonella flagellin in vivo may contribute to protection in concert with other mechanisms. Agglutination of the pathogens and inhibition of their motility within mucous secretions in cooperation with ciliary action and peristaltic clearance prevent their attachment and promote their removal.
In conclusion, IgA antibodies directed against the O:9 epitope of Salmonella LPS alone can prevent bacterial adhesion to and entry into epithelial cells. In contrast, IgA specific for H:g,m Salmonella flagellin in high agglutinating concentration is not protective in HEp-2 cell monolayers in an in vitro model, suggesting the absence of correlation between the agglutinating abilities of antibodies and protection. S. enterica serotype Enteritidis LPS does not mediate attachment to HEp-2 cells. Thus, the mechanism of anti-LPS IgA protection is not mediated by direct binding to adhesion molecules or by bacterial agglutination.
Acknowledgments
This work is supported by grant 12-2000 from the Medical University of Sofia, Sofia, Bulgaria.
We thank Vesela Paskaleva for additional technical support and colleagues V. Paskova and A. Cherneva for excellent assistance. We also thank C. Galanos (Max Planck Institute of Immunobiology, Freiburg, Germany) for kindly providing purified S. enterica serotype Enteritidis LPS and the S. enterica serotype Typhimurium C5 strain.
REFERENCES
- 1.Cao, Y., Z. Wen, and D. Lu. 1992. Construction of a recombinant oral vaccine against Salmonella typhi and Salmonella typhimurium. Infect. Immun. 60:2823-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter, P. B., J. B. Woolcock, and F. M. Collins. 1975. Involvement of the upper respiratory tract in orally induced salmonellosis in mice. J. Infect. Dis. 131:570-574. [DOI] [PubMed] [Google Scholar]
- 3.Forrest, B. D. 1992. Indirect measurement of intestinal immune responses to an orally administered attenuated bacterial vaccine. Infect. Immun. 60:2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gahring, L. C., F. Heffron, B. B. Finlay, and S. Falkow. 1990. Invasion and replication of Salmonella typhimurium in animal cells. Infect. Immun. 58:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannella, R. A., O. Washington, P. Gemski, and S. B. Formal. 1973. Invasion of HeLa cells by Salmonella typhimurium: a model for study of invasiveness of Salmonella. J. Infect. Dis. 128:69-75. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins, S., J. P. Kraehenbuhl, F. Schodel, A. Potts, D. Peterson, P. de Grandi, and D. Nardelli-Haefliger. 1995. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect. Immun. 63:3279-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hormaeche, C. E., H. S. Joysey, L. Desilva, M. Izhar, and B. A. Stocker. 1990. Immunity induced by live attenuated Salmonella vaccines. Res. Microbiol. 141:757-764. [DOI] [PubMed] [Google Scholar]
- 8.Iankov, I. D., D. P. Petrov, I. V. Mladenov, I. H. Haralambieva, R. Ivanova, L. Sechanova, and I. G. Mitov. 2001. Monoclonal antibodies of IgA isotype specific for lipopolysaccharide of Salmonella enteritidis: production, purification, characterization and application as serotyping reagents. FEMS Microbiol. Lett. 196:215-221. [DOI] [PubMed] [Google Scholar]
- 9.Kops, S. K., D. K. Lowe, W. M. Bement, and A. B. West. 1996. Migration of Salmonella typhi through intestinal epithelial monolayers: an in vitro study. Microbiol. Immunol. 40:799-811. [DOI] [PubMed] [Google Scholar]
- 10.Lamm, M. E. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51:311-340. [DOI] [PubMed] [Google Scholar]
- 11.Lockman, H. A., and R. Curtiss. 1992. Virulence of non-type 1-fimbriated and nonfimbriated nonflagellated Salmonella typhimurium mutants in murine typhoid fever. Infect. Immun. 60:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu, S., A. R. Manges, Y. Xu, F. C. Fang, and L. W. Riley. 1999. Analysis of virulence of clinical isolates of Salmonella enteritidis in vivo and in vitro. Infect. Immun. 67:5651-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michetti, P., M. J. Mahan, J. M. Slauch, J. J. Mekalanos, and M. R. Neutra. 1992. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect. Immun. 60:1786-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michetti, P., N. Porta, M. J. Mahan, J. M. Slauch, J. J. Mecalanos, A. L. Blum, J. Kraehenbuhl, and M. R. Neutra. 1994. Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology 107:915-923. [DOI] [PubMed] [Google Scholar]
- 15.Mitov, I., V. Denchev, and K. Linde. 1992. Humoral and cell-mediated immunity in mice after immunization with live oral vaccines of Salmonella typhimurium: auxotrophic mutants with two attenuating markers. Vaccine 10:61-66. [DOI] [PubMed] [Google Scholar]
- 16.Niesel, D. W., C. E. Chambers, and S. L. Stockman. 1985. Quantitation of HeLa cell monolayer invasion by Shigella and Salmonella species. J. Clin. Microbiol. 22:897-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson, J. M., G. Grant, E. Allen-Vercoe, M. J. Woodward, A. Pusztai, and H. J. Flint. 2000. Adhesion of Salmonella enterica var. Enteritidis strains lacking fimbriae and flagella to rat ileal explants cultured at the air interface or submerged in tissue culture medium. J. Med. Microbiol. 49:691-696. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt, C. K., J. S. Ikeda, S. C. Darnell, P. R. Watson, J. Bispham, T. S. Wallis, D. L. Weinstein, E. S. Metcalf, and A. D. O'Brien. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect. Immun. 69:5619-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small, P. L., R. R. Isberg, and S. Falkow. 1987. Comparison of the ability of enteroinvasive Escherichia coli, Salmonella typhimurium, Yersinia pseudotuberculosis, and Yersinia enterocolitica to enter and replicate within HEp-2 cells. Infect. Immun. 55:1674-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagliabue, A., L. Nencioni, A. Caffarena, L. Villa, D. Boraschi, G. Cazzola, and S. Cavalieri. 1985. Cellular immunity against Salmonella typhi after live oral vaccine. Clin. Exp. Immunol. 62:242-247. [PMC free article] [PubMed] [Google Scholar]
- 21.Viret, J. F., D. Favre, B. Wegmuller, C. Herzog, J. U. Que, S. J. Cryz, and A. B. Lang. 1999. Mucosal and systemic immune responses in humans after primary and booster immunizations with orally administered invasive and noninvasive live attenuated bacteria. Infect. Immun. 67:3680-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner, N. L., and J. J. Marchalonis. 1972. Structural differences in IgA myeloma proteins of different allotypes. J. Immunol. 109:657-661. [PubMed] [Google Scholar]
- 23.Young, G. M., J. L. Badger, and V. L. Miller. 2000. Motility is required to initiate host cell invasion by Yersinia enterocolitica. Infect. Immun. 68:4323-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, X., S. M. Kelly, W. Bollen, and R. Curtiss. 1999. Protection and immune responses induced by attenuated Salmonella typhimurium UK-1 strains. Microb. Pathog. 26:121-130. [DOI] [PubMed] [Google Scholar]