Abstract
We examined Babesia bovis sporozoites for the expression of two molecules, merozoite surface antigen 1 (MSA-1) and rhoptry-associated protein 1 (RAP-1), that are postulated to be involved in the invasion of host erythrocytes. Both MSA-1 and RAP-1 were transcribed and expressed in infectious sporozoites. Importantly, monospecific MSA-1 and RAP-1 antisera each inhibited sporozoite invasion of erythrocytes in vitro. This is the first identification of antigens expressed in Babesia sp. sporozoites and establishes that, at least in part, sporozoites and merozoites share common targets of antibody mediated inhibition of erythrocyte invasion.
Parasites in the genus Babesia are transmitted when sporozoites are released with the saliva during tick feeding, and cause disease when merozoites invade and replicate within host erythrocytes (35). Unlike their close relatives Plasmodium sp., in which sporozoites initiate an exo-erythrocytic cycle by invasion of hepatocytes, sporozoites of true babesial species, including Babesia bovis, Babesia bigemina, Babesia divergens, Babesia canis, Babesia caballi, and Babesia ovis, directly invade erythrocytes (7, 14, 15, 24). We hypothesized that surface molecules may be shared by both babesial sporozoites and merozoites and represent a common target for antibody-mediated inhibition of erythrocyte invasion. In this study, we tested this hypothesis by using Boophilus microplus transmission of B. bovis sporozoites.
Babesial merozoites, like other apicomplexan parasites, use a combination of cell surface and apical complex molecules to bind and invade host cells (2, 4, 29). In B. bovis, merozoite surface antigen 1 (MSA-1) and rhoptry-associated protein 1 (RAP-1) are postulated to be involved in the merozoite invasion of erythrocytes. MSA-1 is a 42-kDa glycoprotein that belongs to the family of variable merozoite surface antigens (VMSA) and is uniformly distributed on the surface of B. bovis merozoites (8, 9, 16). MSA-1 is encoded by a single gene, and antibodies against either native or recombinant MSA-1 neutralize merozoite infection in vitro, suggesting a role in the early steps of erythrocyte invasion (10, 11, 30). RAP-1 of B. bovis is a 60-kDa protein localized to the apical surface and within the rhoptries of merozoites (8, 26, 33). RAP-1 is encoded by two identical, tandemly arranged rap-1a genes and is highly conserved among diverse isolates (1, 31-33). Studies using its orthologue in B. bigemina, a 58-kDa RAP-1, show that monoclonal antibodies (MAbs) against RAP-1 are able to inhibit multiplication in vitro (5, 6). Consistent with a role in invasion, calves immunized with native RAP-1 or a recombinant fusion protein develop a significantly reduced mean peak parasitemia upon merozoite challenge (20, 34).
To obtain sporozoites for the examination of msa-1 and rap-1 expression, adult B. microplus ticks were allowed to feed on a splenectomized calf by using skin patches (12). A Babesia-free colony of B. microplus ticks (La Minita strain) was used in all the experiments. Adult female ticks start engorgement approximately 21 days after being placed on the calf (22). Calves were inoculated with 5 ml of the Mexico strain of B. bovis at 13 days postattachment so that parasitemia, determined by microscopic examination of Giemsa-stained blood smears, was maximal during the final stages of female tick engorgement. Engorged ticks were washed and placed in individual vials during ovoposition (13). To obtain a high percentage of infected ticks, only those females replete during the period of highest parasitemia were used. Infection of female ticks was determined on day 10 of ovoposition by the hemolymph test (28), and only eggs from infected females with more than 10 kinetes per hemolymph sample were used. Eggs laid during the first 120 h postengorgement were discarded, and the rest of the eggs were incubated at 27°C and 92% relative humidity for 3 weeks (17). Once the larvae hatched and their cuticles hardened, they were kept at 14°C and 92% relative humidity for an additional 21 days (3). To stimulate the development of B. bovis sporozoites, infected larvae were fed on an uninfected calf for 60 h using skin patches (R. J. Dalgliesh and N. P. Stewart, Letter, Aust. Vet. J. 52:543, 1976). After this period, larvae were removed and incubated at 37°C for an additional 12 h. Uninfected larvae were obtained by using the same procedure, with ticks from the same colony, except that the adult ticks were fed on an uninfected calf. Temperature and humidity conditions were the same for uninfected adult ticks, eggs, and larvae as those used for the infected ticks.
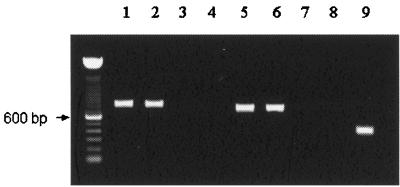
B. bovis migration to salivary glands occurs only after larval feeding commences (23). Inside the salivary gland cells, B. bovis kinetes increase in size and mature into round sporonts from which thousands of sporozoites develop during larval engorgement 3 to 4 days after initial attachment (27). Since maximum sporozoite development occurs at 72 h after larval attachment, infected larvae were examined during this period. To determine if msa-1 and rap-1 transcripts are present in B. bovis stages in fed larvae, transcriptional analysis was carried out using a reverse transcriptase PCR (RT-PCR) on larval extracts. Infected larvae were homogenized in a mortar, and total RNA was extracted using TRIzol Reagent (Gibco BRL). The msa-1 primers (forward and reverse primers for msa-1 were 5′-GCTACGTTTGCTCTTTTCATT and 5′-TTGCAATTCCTTTTCTAATGC, respectively) were predicted to amplify a fragment from nucleotides 4 to 714, and the rap-1 primers (forward and reverse primers for rap-1 were 5′-CTCGCTCCAGCTGAAGTGGTA and 5′-GGAGCTTCAACGTACGAGGTC, respectively) were designed to amplify a fragment from nucleotides 91 to 890. The resulting amplicons from infected larvae had the expected sizes of 711 bp (msa-1) and 800 bp (rap-1) (Fig. 1). The cDNA sequences obtained were 100% identical to the published sequences of both genes (msa-1 accession number, M77192; rap-1 accession number, M38218). Amplicons of similar size to the msa-1 and rap-1 fragments were identified in cDNA from merozoite samples obtained from the in vitro-cultured Mo7 clone, used as a positive control. No amplification was observed when RNA from uninfected larvae was used or when RT was omitted, confirming specificity and purity of RNA (Fig. 1). Presence of cDNA in uninfected larval samples was confirmed by amplification of a 400-bp fragment of Bm86, a B. microplus gene (25).
FIG. 1.
RT-PCR analysis of total RNA extracted from B. bovis-infected erythrocytes (lanes 1 and 5), B. bovis-infected larvae (lanes 2 and 6), B. bovis-infected larvae without RT treatment (lanes 3 and 7), and uninfected larvae (lanes 4, 8, and 9). Amplification used primers specific for rap-1 (lanes 1 to 4, 800 bp), msa-1 (lanes 5 to 8, 711 bp), and Bm86 (lane 9, 400 bp). Amplicons were detected by agarose gel electrophoresis and ethidium bromide staining. Molecular size markers are shown on the left.
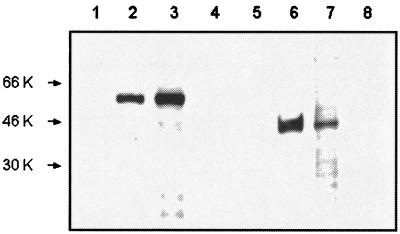
Next, to determine if mRNA was translated, a Western blot analysis was carried out using infected larvae and MAbs specific for MSA-1 and RAP-1. Protein extracts from infected larvae were incubated for 1 h with 2 μg of MAb/ml against MSA-1 (MAb 23/10.36.18; isotype IgG2b) or RAP-1 (MAb BABB75A4; immunoglobulin G2b [IgG2b]). Bound antibody was detected using a peroxidase-labeled goat anti-mouse IgG (Kinkegaard & Barry Laboratories) at a 1:2,500 dilution followed by enhanced chemiluminescence using a Western blotting Detection Reagent (Amersham). MAb 23/10.36.18 bound a protein with the expected size of 42 kDa for MSA-1 in both infected larvae and infected erythrocytes but not from uninfected larvae or uninfected erythrocytes (Fig. 2). MAb BABB75A4 recognized the 60-kDa RAP-1 only in extracts from infected larvae and infected erythrocytes but not in those from uninfected larval or uninfected erythrocytes (Fig. 2).
FIG. 2.
Immunoblot analysis of total protein extracted from normal erythrocytes (lanes 1 and 5), B. bovis-infected erythrocytes (lanes 2 and 6), B. bovis-infected B. microplus larvae (lanes 3 and 7), and uninfected B. microplus larvae (lanes 4 and 8). Protein extracts were electrophoresed on sodium dodecyl sulfate-containing polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were incubated with MAb BABB75A4 against RAP-1 (lanes 1 to 4) or MAb 23/10.36.18 against MSA-1 (lanes 5 to 8). Molecular size markers are on the left.
The identification of msa-1 and rap-1 transcripts and protein expression at 72 h after larval attachment coincided with the development of sporozoites. To confirm that msa-1 and rap-1 are expressed by sporozoites and not only by remaining kinetes or sporonts present in infected larvae tissues at this time, erythrocyte cultures were initiated with infected larvae extracts and examined at various time points prior to merozoite development, which occurs at 8 h postinvasion (18). Expression of MSA-1 and RAP-1 in sporozoites attached to and within erythrocytes was examined using immunocytochemistry. Approximately 300 infected fed larvae in 1.5 ml of M199 complete medium were ground using a mortar. The extracts were centrifuged at 70 × g for 5 min, and the supernatant was collected and then centrifuged again at 400 × g for 8 min to remove tick cells. The supernatant containing B. bovis sporozoites was added to uninfected erythrocytes and cultured in vitro in M199 complete medium for 5 h to allow the detection of binding and invasion prior to development of merozoites. Smears of the culture were made using Probe-On slides (Fisher) and were air-dried for 2 h and fixed in methanol for 5 min. Smears were rinsed in 125 mM Tris buffer containing 0.05% Triton X-100. Smears were blocked with this buffer containing 5% goat serum at 37°C for 10 min. MAbs 23/10.36.18 (anti-MSA-1), BABB75A4 (anti-RAP-1), and ANA22B1 (anti-Anaplasma marginale MSP-1 as a negative control; IgG3 isotype) (21) were used at final concentrations of 10 μg/ml and were incubated at 37°C for 15 min. Biotinylated goat anti-mouse immunoglobulin (USA-HRP Detection System; Signet Laboratories, Inc., Dedham, Mass.) was incubated for 10 min at 37°C followed by addition of streptavidin-horseradish peroxidase complex and incubation for 10 min at 37°C. Slides were blotted and rinsed 10 times between steps. The chromogen AEC (DAKO) was added to develop the reaction, and filtered Mayer's hematoxylin was used as a counterstain. As a positive control, B. bovis Mo7 merozoites in erythrocyte cultures were used. Extracts from uninfected B. microplus larvae cultured in vitro with erythrocytes were used as a negative control.
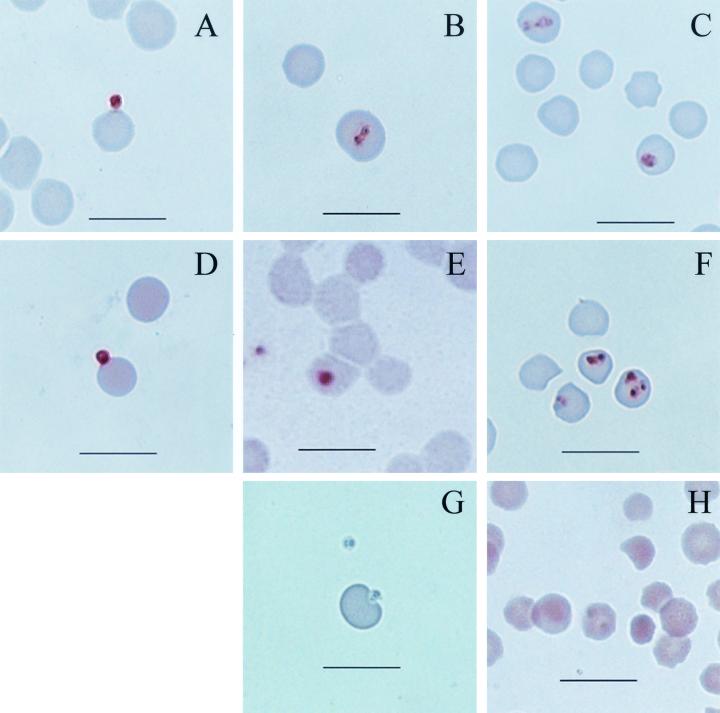
MSA-1-specific MAb 23/10.36.18 recognized both sporozoites bound to erythrocytes (Fig. 3A) and early intra-erythrocytic stages (Fig. 3B). MAb BABB75A4 against RAP-1 also bound to sporozoites attached to the erythrocyte membrane (Fig. 3D) and to early intra-erythrocytic stages (Fig. 3E). Both MAbs reacted with cultured merozoites used as positive controls (Fig. 3C and 3F) but did not bind erythrocytes cultured alone or with extracts from uninfected larvae (data not shown). Neither sporozoites (Fig. 3G) nor merozoites (Fig. 3H) were bound by negative-control MAb ANA22B1. The detection of MSA-1 and RAP-1 proteins both within the infected larvae at 72 h postattachment and in sporozoites attached to erythrocytes in vitro confirms that both of these molecules are expressed in sporozoites.
FIG. 3.
Immunocytochemistry of B. bovis cultured with erythrocytes. Smears of erythrocyte cultures initiated using sporozoites (A, B, D, E, and G) and merozoites (C, F, and H) were incubated with MAb 23/10.36.18 against MSA-1 (A, B, and C), MAb BABB75A4 against RAP-1 (D, E, and F), or negative-control MAb ANA22B1 against A. marginale MSP-1 (G and H). The reaction was visualized with AEC, which results in a brown staining. Bar = 10 μm.
Having demonstrated the presence of MSA-1 and RAP-1 in sporozoites bound to erythrocytes, we determined if antibodies specific for these antigens were able to block sporozoite attachment to erythrocytes. We used an in vitro system previously developed for merozoite inhibition with specific antisera generated against recombinant proteins (10, 33). Antisera specific for MSA-1 or RAP-1 were tested for their ability to block sporozoite binding to erythrocytes. The anti-MSA-1 sera were obtained following the immunization of calves with recombinant MSA-1, and the specificity has been previously shown by immunoblotting and immunofluorescence (10, 30). A bovine anti-ovalbumin serum, obtained following immunization using the same adjuvant and schedule as those for MSA-1, was used as negative control. The anti-RAP-1 sera were obtained by immunizing rabbits with recombinant RAP-1, and the specificity was verified by immunoprecipitation and immunofluorescence (33). Control serum was produced by immunization of a rabbit with recombinant MSP-2 from A. marginale using the same protocol as that for RAP-1. Sporozoites were isolated from approximately 500 infected larvae as described above. The number of live sporozoites was determined using the 6-carboxy fluorescein diacetate staining method (19). Each serum was heat inactivated and diluted 1:5 in complete M199 medium and incubated with 105 live sporozoites at 4°C for 30 min. An equal volume of 1.5% bovine erythrocytes in complete medium was added, and the culture was incubated in 96-well plates at 37°C in a 5% CO2 atmosphere. The number of sporozoites attached to erythrocytes was recorded from a total of 2,000 erythrocytes counted by microscopic examination of Giemsa-stained smears prepared from each well at 5 and 48 h. Results were analyzed by one-way analysis of variance and Fisher's pairwise comparisons using the Minitab 13 software computer program.
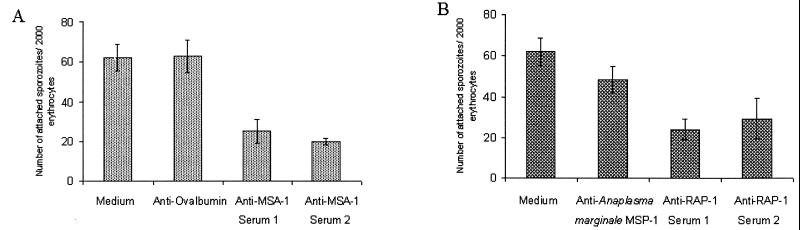
At 5 h, cultures of sporozoites incubated with either of two bovine antisera against MSA-1 showed a significantly lower number of sporozoites attached to the erythrocyte membrane when compared to sporozoites incubated with medium alone or with an unrelated bovine antiserum against ovalbumin (Fig. 4A). Rabbit antisera against RAP-1 also blocked attachment, as indicated by a significantly lower number of sporozoites attached to erythrocytes when compared to medium alone or to control rabbit antiserum against recombinant A. marginale MSP-2 (Fig. 4B). When examined at 48 h, antisera against MSA-1 and RAP-1 still showed significant inhibition of sporozoite attachment compared to the control groups (data not shown), and the percentage of inhibition was similar to that of cultures incubated for 5 h. The level of inhibition of sporozoite attachment at 5 h (59 to 68% in this study) is similar to previous results for MSA-1 antibody inhibition of merozoite invasion (71%) (11). Previously, in vitro inhibition of merozoite multiplication by RAP-1 antibodies has been demonstrated only in B. bigemina using MAbs. A MAb against a surface exposed region of RAP-1 inhibited 62% of merozoite multiplication (5). In the present experiment with B. bovis, the percentage inhibition of sporozoite attachment to erythrocytes at 5 h was up to 61% with the anti-RAP-1 sera, comparable to the previous results with B. bigemina merozoites. This is the first report of inhibition of B. bovis attachment or invasion by any stage using RAP-1 antibodies. Since we tested only sporozoite viability prior to incubation with antibodies, we cannot rule out an effect of antibody on sporozoite survival that subsequently resulted in decreased binding compared with actual neutralization of a receptor-ligand interaction. Nonetheless, these results demonstrate that antibodies against MSA-1 and RAP-1 can inhibit babesial infection initiated by sporozoites, as well as subsequent cycles of merozoite invasion (5, 11).
FIG. 4.
Antibody-mediated inhibition of sporozoite attachment to erythrocytes at 5 h. (A) Total number of parasites attached to 2,000 erythrocytes after incubation with antibodies against MSA-1, control antibody, or culture medium. The percentages of inhibition were 59 and 68% for serum 1 and serum 2, respectively. (B) Total number of parasites attached to 2,000 erythrocytes after incubation with antibodies against RAP-1, control antibody, or culture medium. The percentages of inhibition were 61 and 53% for serum 1 and serum 2, respectively. Error bars indicate standard deviation of the results from triplicate cultures.
The expression of MSA-1 and RAP-1 in both sporozoites and merozoites increases the likelihood of effective vaccination against a tick challenge using these antigens. Importantly, each sporozoite that invades an erythrocyte generates only a pair of merozoites, unlike the thousands generated by Plasmodium sporozoite invasion of a hepatocyte. Thus, inhibition of initial invasion by sporozoites, followed by blocking subsequent rounds of merozoite invasion, may be particularly effective in control of babesiosis.
Acknowledgments
This work was supported by USAID grant PCE-G-0098-00043-00, by U.S. Department of Agriculture grant USDA-ARS-CRIS 5348-32000-014-00D, and by a fellowship from CONACyT (115921).
The technical assistance of Ralph Horn, Beverly Hunter, and Carla Robertson is greatly appreciated, as is the administrative support of Don Knowles.
REFERENCES
- 1.Brown, W. C., T. F. McElwain, B. J. Ruef, C. E. Suarez, V. Shkap, C. G. Chitko-McKown, W. Tuo, A. C. Rice-Ficht, and G. H. Palmer. 1996. Babesia bovis rhoptry-associated protein 1 is immunodominant for T helper cells of immune cattle and contains T-cell epitopes conserved among geographically distant B. bovis strains. Infect. Immun. 64:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chitnis, C. E., and M. J. Blackman. 2000. Host cell invasion by malaria parasites. Parasitol. Today 16:411-415. [DOI] [PubMed] [Google Scholar]
- 3.Dalgliesh, R. J., and N. P. Stewart. 1982. Some effects of time, temperature and feeding on infection rates with Babesia bovis and Babesia bigemina in Boophilus microplus larvae. Int J. Parasitol. 12:323-326. [DOI] [PubMed] [Google Scholar]
- 4.Dubremetz, J. F., N. Garcia-Reguet, V. Conseil, and M. N. Fourmaux. 1998. Apical organelles and host-cell invasion by Apicomplexa. Int. J. Parasitol. 28:1007-1013. [DOI] [PubMed] [Google Scholar]
- 5.Figueroa, J. V., and G. M. Buening. 1991. In vitro inhibition of multiplication of Babesia bigemina by using monoclonal antibodies. J. Clin. Microbiol. 29:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueroa, J. V., G. M. Buening, D. A. Kinden, and T. J. Green. 1990. Identification of common surface antigens among Babesia bigemina isolates by using monoclonal antibodies. Parasitology 100(Pt. 2):161-175. [DOI] [PubMed] [Google Scholar]
- 7.Friedhoff, K. T. 1988. Transmission of Babesia, p. 23-52. In M. Ristic (ed.), Babesiosis of domestic animals and man. CRC Press, Boca Raton. Fla.
- 8.Goff, W. L., W. C. Davis, G. H. Palmer, T. F. McElwain, W. C. Johnson, J. F. Bailey, and T. C. McGuire. 1988. Identification of Babesia bovis merozoite surface antigens by using immune bovine sera and monoclonal antibodies. Infect. Immun. 56:2363-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hines, S. A., T. F. McElwain, G. M. Buening, and G. H. Palmer. 1989. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol. Biochem. Parasitol. 37:1-9. [DOI] [PubMed] [Google Scholar]
- 10.Hines, S. A., G. H. Palmer, D. P. Jasmer, W. L. Goff, and T. F. McElwain. 1995. Immunization of cattle with recombinant Babesia bovis merozoite surface antigen-1. Infect. Immun. 63:349-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hines, S. A., G. H. Palmer, D. P. Jasmer, T. C. McGuire, and T. F. McElwain. 1992. Neutralization-sensitive merozoite surface antigens of Babesia bovis encoded by members of a polymorphic gene family. Mol. Biochem. Parasitol. 55:85-94. [DOI] [PubMed] [Google Scholar]
- 12.Hodgson, J. L. 1991. Ph.D. thesis. Washington State University, Pullman.
- 13.Hodgson, J. L., D. Stiller, D. P. Jasmer, G. M. Buening, G. G. Wagner, and T. C. McGuire. 1992. Babesia bigemina: quantitation of infection in nymphal and adult Boophilus microplus using a DNA probe. Exp. Parasitol. 74:117-126. [DOI] [PubMed] [Google Scholar]
- 14.Hoyte, H. M. D. 1961. Initial development of infections with Babesia bigemina. J. Protozool. 8:462-466. [DOI] [PubMed] [Google Scholar]
- 15.Hoyte, M. H. D. 1965. Further observations on the initial development of infections with Babesia bigemina. J. Protozool. 12:83-85. [DOI] [PubMed] [Google Scholar]
- 16.Jasmer, D. P., D. W. Reduker, S. A. Hines, L. E. Perryman, and T. C. McGuire. 1992. Surface epitope localization and gene structure of a Babesia bovis 44-kilodalton variable merozoite surface antigen. Mol. Biochem. Parasitol. 55:75-83. [DOI] [PubMed] [Google Scholar]
- 17.Mahoney, D. F., and G. B. Mirre. 1977. The selection of larvae of Boophilus microplus infected with Babesia bovis (syn B argentina). Res. Vet. Sci. 23:126-127. [PubMed] [Google Scholar]
- 18.Mahoney, D. F., I. G. Wright, and P. J. Ketterer. 1973. Babesia argentina: the infectivity and immunogenicity of irradiated blood parasites for splenectomized calves. Int J. Parasitol. 3:209-317. [DOI] [PubMed] [Google Scholar]
- 19.McElwain, T. F., L. E. Perryman, W. C. Davis, and T. C. McGuire. 1987. Antibodies define multiple proteins with epitopes exposed on the surface of live Babesia bigemina merozoites. J. Immunol. 138:2298-2304. [PubMed] [Google Scholar]
- 20.McElwain, T. F., L. E. Perryman, A. J. Musoke, and T. C. McGuire. 1991. Molecular characterization and immunogenicity of neutralization-sensitive Babesia bigemina merozoite surface proteins. Mol. Biochem. Parasitol. 47:213-222. [DOI] [PubMed] [Google Scholar]
- 21.McGuire, T. C., G. H. Palmer, W. L. Goff, M. I. Johnson, and W. C. Davis. 1984. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect. Immun. 45:697-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuñez, J. L., M. E. Muñoz-Cobeñas, and H. L. Moltedo. 1985. Boophilus microplus, the common cattle tick. Springer-Verlag, Berlin, Germany.
- 23.Potgieter, F. T., and H. J. Els. 1976. Light and electron microscopic observations on the development of small merozoites of Babesia bovis in Boophilus microplus larvae. Onderstepoort J. Vet. Res. 43:123-128. [PubMed] [Google Scholar]
- 24.Purnell, R. E. 1981. Babesiosis in various hosts, p. 25-63. In M. Ristic, and J. P. Kreier (ed.), Babesiosis. Academic Press, New York, N.Y.
- 25.Rand, K. N., T. Moore, A. Sriskantha, K. Spring, R. Tellam, P. Willadsen, and G. S. Cobon. 1989. Cloning and expression of a protective antigen from the cattle tick Boophilus microplus. Proc. Natl. Acad. Sci. USA 86:9657-9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reduker, D. W., D. P. Jasmer, W. L. Goff, L. E. Perryman, W. C. Davis, and T. C. McGuire. 1989. A recombinant surface protein of Babesia bovis elicits bovine antibodies that react with live merozoites. Mol. Biochem. Parasitol. 35:239-247. [DOI] [PubMed] [Google Scholar]
- 27.Riek, R. F. 1966. The life cycle of Babesia argentina (lignieres, 1903) (Sporozoa: Piroplasmidea) in the tick vector Boophilus microplus (Canestrini). Aust. J. Agric. Res. 17:247-254. [Google Scholar]
- 28.Riek, R. F. 1964. The life cycle of Babesia bigemina (Smith and Kilborne, 1893) in the tick vector Boophilus microplus (Canestrini). Aust. J. Agric. Res. 15:802-821.
- 29.Sam-Yellowe, T. Y. 1996. Rhoptry organelles of Apicomplexa: Their role in host cell invasion and intracellular survival. Parasitol. Today 12:308-316. [DOI] [PubMed] [Google Scholar]
- 30.Suarez, C. E., M. Florin-Christensen, S. A. Hines, G. H. Palmer, W. C. Brown, and T. F. McElwain. 2000. Characterization of allelic variation in the Babesia bovis merozoite surface antigen 1 (MSA-1) locus and identification of a cross-reactive inhibition-sensitive MSA-1 epitope. Infect. Immun. 68:6865-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suarez, C. E., T. F. McElwain, I. Echaide, S. Torioni de Echaide, and G. H. Palmer. 1994. Interstrain conservation of babesial RAP-1 surface-exposed B-cell epitopes despite rap-1 genomic polymorphism. Infect. Immun. 62:3576-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suarez, C. E., G. H. Palmer, I. Hotzel, and T. F. McElwain. 1998. Structure, sequence, and transcriptional analysis of the Babesia bovis rap-1 multigene locus. Mol. Biochem Parasitol. 93:215-224. [DOI] [PubMed] [Google Scholar]
- 33.Suarez, C. E., G. H. Palmer, D. P. Jasmer, S. A. Hines, L. E. Perryman, and T. F. McElwain. 1991. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface exposed epitopes. Mol. Biochem Parasitol. 46:45-52. [DOI] [PubMed] [Google Scholar]
- 34.Wright, I. G., R. Casu, M. A. Commins, B. P. Dalrymple, K. R. Gale, B. V. Goodger, P. W. Riddles, D. J. Waltisbuhl, I. Abetz, D. A. Berrie, et al. 1992. The development of a recombinant Babesia vaccine. Vet. Parasitol. 44:3-13. [DOI] [PubMed] [Google Scholar]
- 35.Wright, I. G., and B. V. Goodger. 1988. Pathogenesis of Babesiosis, p. 100-118. In M. Ristic (ed.), Babesiosis of domestic animals and man. CRC Press, Boca Raton, Fla.