Abstract
We have studied polyacryl starch microparticles as an adjuvant in oral vaccination in mice. Secreted antigens from Salmonella enterica serovar Enteritidis were administered covalently conjugated to microparticles, or as free antigens, orally or intramuscularly and evaluated for their immunogenicity and ability to elicit protective immune response against an oral challenge with live serovar Enteritidis. The highest immunoglobulin M (IgM)-plus-IgG titers were obtained in the groups immunized with antigen-conjugated microparticles. The subclass profile switched to a stronger Th1 influence in the oral groups after booster, while the intramuscular group showed a constant Th1/Th2 profile. A strong specific IgA response was seen in feces in the oral groups, which was further confirmed in an enzyme-linked immunospot assay. The delayed-type hypersensitivity test, as a measure of the cellular response, showed a significant increase in ear thickness in all the immunized groups, except for the group that received free antigen orally, compared to the nonimmunized group. The cytokines released from in vitro-stimulated spleens showed a strong gamma interferon response in all immunized groups. A significant reduction in CFU in liver and spleen was seen in the orally immunized groups compared to the nonimmunized group after oral challenge with serovar Enteritidis. Western blotting analysis with both sera and feces revealed that antibodies against three bands, 53, 56, and 60 kDa, dominated the oral groups, and an electrospray-mass spectroscopy analysis of these bands showed amino acid sequences coinciding with those of phase-1 flagellin and hook-associated protein 2.
Oral administration of vaccines has many advantages over parenteral administration. Oral vaccines normally elicit a stronger mucosal response, and they are more practical in use since their administration does not require professional assistance or sterile preparations. However, a prerequisite for the development of new vaccines is the availability of safe and effective adjuvants to which the individual immunogenic components can be attached. Soluble starch is a biocompatible macromolecule that can easily be formulated into microparticles with stabilizing hydrocarbon chains after acryloylation and radical polymerization in water-in-oil emulsions (5). Such microparticles, with an average diameter of 1 to 3 μm, have previously been used parenterally as carriers in vivo for both small molecular drugs and macromolecules after entrapment or covalent coupling (6, 7, 38). The microparticles stimulate macrophages weakly, resulting in interleukin 1 (IL-1) secretion, but they are not inherently immunogenic (4), not even with homologous proteins (6). However, with heterologous proteins conjugated to the particles a strong immune response can be detected after parenteral administration. Both a cellular and a humoral response were seen in mice with human serum albumin (HSA) as a model antigen covalently conjugated to the microparticles (15). Moreover, recombinant DNA-derived gp63 from Leishmania donovani has been conjugated to starch microparticles and used in parenteral vaccination studies in mice and produced an immune response, which significantly reduced the parasite burden after a challenge with live parasites (L. Degling, R. M. McMaster, and I. Sjöholm, submitted for publication). The starch microparticles are thus an interesting candidate to be used as an adjuvant for component vaccines or for recombinant proteins, which in many cases are only weakly immunogenic.
In this study we examined the effect of the microparticles as an oral adjuvant with covalently conjugated antigens. We have chosen Salmonella enterica serovar Enteritidis as an example of a pathogen that is active in the intestines, and we used the secreted antigens obtained after a short cultivation of the bacteria, whereas most other studies have focused on whole-cell vaccines with attenuated or killed bacteria. Salmonella species are gram-negative, facultative intracellular pathogens known to adhere to and pass through intestinal epithelial cells, primarily the M cells of the follicle-associated epithelium (12, 23). Several studies have been performed that characterize the pathogenicity of Salmonella, and these studies have identified genes or plasmid-derived genes that are essential for the bacteria to be infectious (22). Many of the invasion proteins are related to the type III secretion family proteins, including those involved in the flagellar biosynthesis (27). The components of immunity in salmonellosis have also been widely studied, and it is generally agreed that both the triggering of an antigen-specific cell-mediated immune response and the induction of the immune response at the site of entry of the bacteria are important. With our earlier experience we considered Salmonella to be a good candidate for studying oral immunization in combination with our starch microparticles.
In this study, we found that starch microparticles given orally with both covalently conjugated and free secreted antigens from serovar Enteritidis could induce both a local and a systemic immune response in mice, which was shown to significantly reduce the bacterial burden upon oral challenge with serovar Enteritidis. The flagellin component in the secreted antigens was shown by Western blotting and electrospray-mass spectroscopy to play an important role in the protection against the challenge.
MATERIALS AND METHODS
Preparation of polyacryl starch microparticles.
The microparticles were prepared from acryloylated starch (maltodextrin [MD6]; Stadex AB, Malmö, Sweden) by polymerization in an emulsion, as previously described (5, 25). Briefly, 500 mg of acryloylated starch was dissolved in 5 ml of a 0.2 M sodium phosphate buffer, at pH 7.5, containing 1 mM EDTA. Ammonium peroxodisulfate was added to give a final concentration of 0.8 M in the aqueous phase, which was homogenized in toluene-chloroform (4:1). TEMED (Sigma, St. Louis, Mo.) was used to initiate the polymerization. The D-T-C of the particles obtained was 10-0.5-0 according to the definition given earlier (16, 20).
Preparation of antigens.
Wild-type S. enterica serovar Enteritidis (O-9, 12) phage type 1 with a monophasic distribution of flagella (human isolate strain 225 from the Swedish Institute for Infectious Diseases Control, Stockholm, Sweden) was inoculated in Luria broth (LB) (1% tryptone, 0.5% yeast extract, 1% NaCl [Difco, Detroit, Mich.]) and incubated at 37°C in an orbital shaker incubator overnight. The following day the culture was diluted in LB and grown as described above until an optical density of 1 (at 600 nm) was obtained. After centrifugation, the bacterial pellet was resuspended in RPMI 1640 with 20 mM HEPES and 4 mM glutamine (Gibco BRL, Gaithersburg, Md.). The culture was then incubated at 37°C in an orbital shaker incubator for 2 h. The supernatant, obtained after centrifugation of the culture, was filtered through a 0.22-μm-pore-size express filter (Millipore, Bedford, Mass.). The protein solution was concentrated on an Ultrafilter YM (Millipore) with a cutoff level of 10,000 Da and transferred into the coupling buffer prior to the preparation of the starch microparticle conjugate or to sterile saline. The protein composition was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12% polyacrylamide) and subsequent Coomassie brilliant blue staining. The final protein concentration was determined by amino acid analysis.
Conjugation of Salmonella proteins to polyacryl starch microparticles.
The Salmonella proteins were conjugated to the microparticles using the carbodiimide method of Bethell et al. (10). Microparticles were activated with carbonyldiimidazole (Merck, Darmstadt, Germany) in dry dimethylformamide (DMF) during rotation for 1 h at room temperature. After several centrifugal washings with DMF to remove unreacted carbonyldiimidazole, the microparticles were transferred through several washing steps into saline solution before the immediate transfer into the coupling buffer, 0.25 M boric acid with 0.15 M NaCl, at pH 8.6, containing the Salmonella proteins. The mixture was rotated at 4°C for 72 h end over end. The microparticles were then washed several times with saline, filtered through an 11-μm-pore-size nylon filter, and stored at 4°C. The amount of Salmonella proteins conjugated to the microparticles was determined by amino acid analysis. Normally, a coupling yield of about 28% was reached. The mean diameter of the microparticles, based on the number distribution, was determined in a laser diffractometer (LS 230; Coulter). The mean diameter of the microparticles was consistently <2.8 μm.
Immunization of mice.
Female inbred mice of the BALB/c Abom strain (Bomholtgård, Ry, Denmark), 8 to 12 weeks old, were used. The mice were kept according to good laboratory practice guidelines under the supervision of a licensed veterinarian. The Animal Ethics Committee of Uppsala University approved the experiments. Groups of mice (six mice/group) were either injected intramuscularly in the hind leg with 1 mg of antigen-conjugated microparticles (29 μg of antigen/mg of microparticles), injected intramuscularly with 29 μg of free antigen, or immunized orally with 2 mg of antigen-conjugated microparticles (58 μg of antigen/2 mg of microparticles), orally with 58 μg of free antigen or with 58 μg of free antigen mixed with 2 mg of empty microparticles. All oral groups were given the vaccine on three consecutive days by gastric intubation. Booster doses were given after 3 weeks. Untreated mice were used as a control group. As a positive control mice were injected intraperitoneally with 58 μg of antigen in Freund's complete adjuvant or in Freund's incomplete adjuvant (Difco) in the booster.
Sampling.
Blood samples were taken from the tail artery, and the serum was prepared by centrifugation and stored at −80°C. Six to ten freshly voided fecal samples were collected into Ellerman tubes before and intermittently after immunizations, frozen, and freeze-dried. After the net dry weights were determined, extracts of the feces were prepared, keeping all samples on ice at 0°C. Briefly, phosphate-buffered saline (PBS) with 5% nonfat dry milk, 0.1 mg of soybean trypsin inhibitor/ml, and 2 mM phenylmethylsulfonyl fluoride (Sigma) was added to dry feces (20 μl of solution/mg of dry feces). Solid matter was suspended by rigorous vortexing and separated by centrifugation at 18,000 × g for 10 min at 4°C. The clear supernatants were frozen at −80°C until analyzed for antigen-specific and total immunoglobulin A (IgA).
Splenocyte cultures.
Single-cell suspensions were prepared as described elsewhere. Briefly, the single-cell suspensions were incubated for 10 min in Tris buffer with ammonium chloride. After two washes in RPMI 1640 medium the cells were resuspended in culture medium (RPMI 1640 including 10% fetal calf serum, penicillin [100 U/ml], streptomycin [100 μg/ml], 50 μM 2-mercaptoethanol, and 2 mM l-glutamine [Gibco BRL]) and counted. The cells were plated (3 × 106/well) with a total volume of 1.5 ml/well into sterile 24-well microplates (Costar, Corning Inc., N.Y.) and stimulated with either Salmonella proteins (50 μg/well), lipopolysaccharide (LPS) (40 μg/well), concanavalin A (ConA) (4 μg/well; Sigma), or, as a negative control, sterile PBS. All incubations were carried out in triplicate. Cultures were incubated for 48 and 120 h at 37°C in 5% CO2. The cells were harvested by centrifugation at 2,600 × g for 10 min at 4°C, and the supernatants were collected and frozen at −80°C until analyzed for the amount of cytokines produced.
Proliferation assay.
Spleen cell proliferation was determined according to Gerlier and Thomasset (16a) with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma). The cells were incubated in 96-well microplates (Costar) at concentrations ranging from 100,000 to 400,000 cells/well in a total volume of 0.2 ml/well. The cells were stimulated with either Salmonella proteins (50 μg/well), LPS (10 μg/well), ConA (1 μg/well), or, as a negative control, sterile PBS. At day 3, MTT solution (5 mg of MTT/ml in PBS) was added to the wells and the cells were incubated for 3 h at 37°C in 5% CO2. The reaction was stopped with stop solution (0.2 M SDS dissolved in 0.02 M HCl and isobutanol [1:1]), and the plate was shaken overnight in the dark to release the formazan produced by the proliferated cells. The next day, the absorbance was measured at 590 nm in a microtiter plate spectrophotometer (Titertek Multiscan MC; Flow Laboratories), and a proliferation curve was made for each spleen and type of stimulation. The splenocytes proliferated as expected in the wells stimulated with ConA, LPS, or Salmonella proteins, while no proliferation was seen in the control wells (data not shown).
ELISPOT assay.
Lamina propria lymphocytes (LPL) were analyzed for antigen-specific and total antibody production. Briefly, the intestines from mice were taken out on day 36, rinsed, cut into small pieces, and incubated in Ca2+- and Mg2+-free Hanks balanced salt solution medium (Gibco BRL) containing 5 mM EDTA. The intestinal pieces were then incubated on a shaker at 37°C, in two consecutive rounds with fresh RPMI 1640 containing 0.4 U of collagenase A (Boehringer Mannheim GmbH, Mannheim, Germany), to extract the LPL. The single-cell suspension obtained was washed twice in Ca2+- and Mg2+-free Hanks balanced salt solution, filtered through a nylon filter (pore size, 70 μm), and resuspended in culture medium as described under splenocyte cultures. Cells were incubated for 2 h at 37°C in 5% CO2, before being adjusted to the appropriate cell density and used in the enzyme-linked immunospot (ELISPOT) assay.
To detect anti-Salmonella antibodies, sterile 96-well nitrocellulose-based plates (Multiscreen-HA; Millipore) were prewetted with PBS and coated with Salmonella protein solution (1.5 μg/well) overnight at 4°C. To detect the total IgA-producing cells, wells were coated with goat anti-mouse IgA (0.2 μg/well; Sigma). Control wells were coated with 1% ovalbumin (OVA) solution. The plates were blocked with 2% OVA in PBS and incubated overnight with LPL at 2 × 103 to 2 × 105 cells/well. The plates were incubated with biotinylated anti-IgA and then with avidin-alkaline phosphatase (Sigma). As the substrate solution, 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (BCIP-NTB) (Sigma) was used. After incubation the plates were thoroughly rinsed with water and dried. The number of dark spots representing anti-Salmonella IgA- or total IgA-producing cells were counted in eight wells per cell concentration and the mean was calculated for each cell concentration. The number of antigen-specific IgA-producing cells was compared to the total number of IgA-producing cells and expressed as the percentage of specific IgA-producing cells based on 105 cells plated.
DTH test.
One week after the last immunization, the mice were challenged with intradermal injections (10 μl) of Salmonella proteins (1 mg/ml) in the left ear and, as a control, an injection of sterile physiological saline in the right ear. The thickness of the ears was measured with a dial thickness gauge (Mitutoyo Scandinavia AB, Upplands Väsby, Sweden), before and 24, 48, and 72 h after challenge. The delayed-type hypersensitivity (DTH) response for each mouse was expressed as the percentage of increase of ear thickness of the antigen-challenged ear compared to the increase of the thickness of the ear challenged with physiological saline, i.e., (At − Bt/A0) × 100, where At is the ear thickness of the ear challenged with antigen at time t after challenge, Bt is the ear thickness of the ear challenged with physiological saline at time t after challenge, and A0 is the ear thickness of the ear challenged with antigen before challenge. Thus, each mouse was its own control.
Enzyme-linked immunosorbent assays (ELISA) of antigen-specific antibodies (IgM-IgG, subclasses in serum, and IgA in feces).
Nunc Immunoplate Maxisorb F96 plates (Nalge Nunc International, Rochester, N.Y.) were coated with Salmonella proteins or LPS (O-1, 9, 12) from serovar Enteritidis (L-2012; Sigma) (4.5 μg/well) in 0.05 M sodium bicarbonate buffer at pH 9.6 and incubated overnight in a moist chamber at 4°C. The plates were blocked with 1.5% OVA. The antisera or extracts of feces were diluted to appropriate concentrations in PBS with 0.05% Tween 20 and added to the plates in series of twofold dilutions. The following antibodies were used as secondary antibodies: alkaline phosphatase-conjugated goat anti-mouse IgM-IgG (Biosource, Camarillo, Calif.), goat anti-mouse IgA (Sigma), rat anti-mouse IgG2a, rat anti-mouse IgG2b, or rat anti-IgG1-HRP (Pharmingen, San Diego, Calif.). 4-Nitrophenylphosphate (Merck), 1 mg/ml in 10% diethanolamine buffer, pH 9.6, with 0.5 mM MgCl2 was used as substrate for the alkaline phosphatase-conjugated antibodies and tetramethylbenzidine (0.1 mg/ml; Sigma) in 0.1 M NaAc buffer, pH 6.0, with 0.2% H2O2 for the IgG1-horseradish peroxidase. In the latter case, the reaction was stopped with stop solution, 2 M H2SO4. The absorbance was measured at 405 or 450 nm for IgG1 in a microtiter plate spectrophotometer (Titertek Multiscan MC; Flow Laboratories).
A sandwich-type ELISA was developed to determine IgG1, IgG2a, and IgG2b in sera against relevant subclass standard curves. Nunc Immunoplate Maxisorb F96 plates were coated with rat anti-mouse IgG1, IgG2a, or IgG2b (Pharmingen), respectively, and incubated overnight in a moist chamber at 4°C. The plates were blocked with 1% OVA, and the standard mouse IgG1, IgG2a, and IgG2b were diluted to appropriate concentrations in PBS with 3% OVA to create a standard curve. The subsequent procedures were performed as described above. In each sample, the final concentration of antigen-specific IgG1, IgG2a, or IgG2b was determined from pooled standard curves by calculating a mean concentration value from three different dilutions of a sample. When IgM-IgG antibodies and subclasses were being measured, pooled negative serum was added to each plate as a negative control. The negative serum absorbance values were collected from all the plates and were found to lie below 0.1. Thus, 0.1 was set as the limiting value for a positive result. Titers are given as −log2 (dilution × 10). A positive sample (from hyperimmunized mice) was included as a standard on all plates and treated in the same way as the antisera to ensure consistency between plates.
ELISA of total IgA in fecal samples.
A sandwich-type ELISA was developed to determine the total amount of IgA in fecal samples. It was performed as described above for antigen-specific IgA with the plates coated with goat anti-mouse IgA (0.5 μg/well; Sigma). The standard IgA was diluted to appropriate concentrations in PBS with 1% OVA to create a standard curve, and the extracts from the fecal samples were diluted in PBS-T and added to the plates in series of twofold dilutions. The concentration of specific and total IgA was determined from the standard curve by calculating a mean concentration from three different dilutions of the sample. A positive sample and a negative sample were included on all plates and treated in the same way as the unknown samples. The negative feces absorbance values were collected from all the plates and were found to lie below 0.1. Thus, 0.1 was set as the limiting value for a positive result.
Cytokine assay.
A sandwich-type ELISA was developed to determine levels of gamma interferon (IFN-γ), IL-2, IL-4, and IL-5 produced by the splenocytes on antigen activation. Antibody pairs and recombinant standards were purchased from Pharmingen. Nunc Immunoplate Maxisorb F96 plates were coated in 0.1 M sodium bicarbonate buffer at pH 9.6 with rat anti-mouse IFN-γ, IL-2, IL-4, or IL-5, respectively, and incubated overnight in a moist chamber at 4°C. The plates were blocked with 1% OVA. The standard, IFN-γ, IL-2, IL-4, or IL-5 was diluted to appropriate concentrations in PBS with 1% OVA to create a standard curve. The supernatants from cell medium were added from duplicate wells to the plates in series of twofold dilutions. Biotin-conjugated rat anti-mouse IFN-γ, IL-2, IL-4, or IL-5, diluted in PBS with 1% OVA, was added to the plates, whereupon avidin-peroxidase and, later, 2,2′-azinobis-3-ethylbenzthiazoline-sulfonic acid (ABTS) (Sigma) with 0.3% H2O2 were added to the wells. The reaction was stopped with 0.7 M SDS in DMF and purified water (1:1; Sigma). The absorbance was measured at 405 nm. In each sample, the final concentration of IFN-γ, IL-2, IL-4, or IL-5 was determined from pooled standard curves by calculating a mean concentration value from three different dilutions.
Challenge.
To assess the virulence of the strain we measured the growth of the bacteria in the liver and spleen of mice. Several concentrations of bacteria were inoculated orally in 8- to 12-week-old female BALB/c mice. Mice were sacrificed between days 5 and 7, and livers and spleens were removed and homogenized. Suitable dilutions of the homogenates were plated to determine the number of bacteria present. A concentration of bacteria that gave a clear infection in nonimmunized mice at day 7 was chosen for the subsequent challenge.
Immunized mice were challenged with serovar Enteritidis (3 × 104 CFU/mouse) 4 weeks after the booster was administered. The mice were deprived of food and water 6 h before challenge. The mice were given 50 μl of 1% sodium carbonate and then 30 μl of a bacterial suspension (106 CFU/ml) orally. The liver and spleen were taken out 7 days after challenge and homogenized in 5 ml of sterile PBS using a Stomacher laboratory blender (Seward Medical, London, United Kingdom). The homogenates, diluted to appropriate concentrations, were plated onto LB agar plates in duplicates and incubated at 37°C overnight, after which the number of colonies was counted.
SDS-PAGE and immunoblotting.
SDS-PAGE was performed according to the method of Laemmli (26) with gels composed of 15% acrylamide and 0.09% bisacrylamide containing 0.1% (wt/vol) SDS. The Salmonella protein samples (100 μg) were precipitated in trichloroacetic acid, resuspended in loading buffer, and heated for 5 min at 100°C just before the run. Electrophoresis was carried out at 80 V for 15 h, and the subsequent immunoblotting was performed essentially as described by Andersson and Jörnvall (3). Briefly, the proteins were transferred onto a Hybond enhanced chemiluminescence (ECL) nitrocellulose membrane (Amersham Pharmacia Biotech, Uppsala, Sweden) at 200 mA in a Trans-blot system (Bio-Rad Laboratories, Hercules, Calif.) with blotting buffer (25 mM Tris-HCl, 0.2 M glycine in 20% methanol) for 4 h. After blocking with 5% nonfat milk in PBS, the sera and fecal extracts were diluted 1:50,000 and 1:5, respectively, in blocking solution and incubated with the membranes overnight at 4°C. A negative control from nonimmunized mice (dilution 1:50 and 1:2 for sera and feces, respectively) was run in parallel. After washing in PBS with Tween (0.05%), the membranes were incubated with the secondary antibody and anti-mouse IgG-horseradish peroxidase and developed using an ECL system according to the manufacturer's instructions (Amersham Pharmacia Biotech). ECL-biotinylated molecular mass markers ranging from 97 to 14 kDa were used. No bands were detectable with the negative control samples.
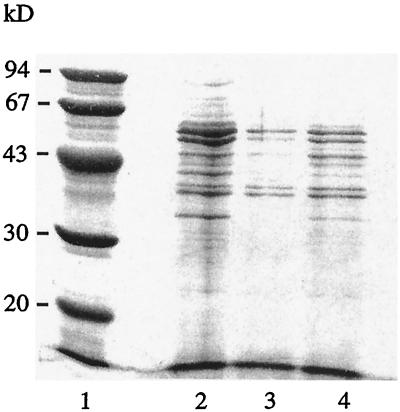
SDS-PAGE with 12% acrylamide from Bio-Rad was used to check the protein composition of each batch of the antigens obtained from the serovar Enteritidis cultures (Fig. 1). In these cases the electrophoresis time was 2.5 h at 200 V. Molecular mass markers from 94 to 30 kDa were used (Pharmacia, Uppsala, Sweden).
FIG. 1.
Electrophoretic analysis on SDS-12% PAGE with Coomassie brilliant blue staining. Shown are molecular mass markers (lane 1), Salmonella antigens from pooled cultures before conjugation to the microparticles (lane 2), Salmonella antigens from the supernatant remaining after the conjugation (lane 3), and Salmonella antigens from a single culture (lane 4).
Silver staining and immunoblotting of LPS.
SDS-PAGE was performed as described above with small modifications. The Salmonella protein samples were digested with proteinase K and analyzed as described by Hitchcock and Brown (19) to visualize LPS present in the protein solution. The gels were either silver-stained or transferred to a Hybond ECL nitrocellulose membrane as described above to detect possible antibodies directed toward LPS. An Escherichia coli LPS 0128:B12 standard was used as a positive control in both the silver-staining and the immunoblotting, with a rabbit anti-LPS E. coli 0128:B12 as primary antibody (Calbiochem-Novabiochem Corp., San Diego, Calif.). Peroxidase-labeled anti-rabbit antibody (Amersham Pharmacia Biotech) was used as secondary antibody. The silver-stained gel with proteinase K-digested Salmonella proteins showed LPS with the typical ladder-like bands. However, no such bands were seen in the Western blot with digested Salmonella proteins, nor were any anti-LPS antibodies detected in the serum or fecal samples.
LAL test.
Limulus amoebocyte lysate (LAL) tests were performed according to the European Pharmacopoeia with method A, the gel-clot, limit test, and showed that the free antigen sample had a high content of LPS, 36,300 IU/μg of protein, while the antigen-conjugated microparticles only contained 28 IU/μg of protein. As a standard, Endosafe Control Standard Endotoxin E. coli 055:B5 was used.
Sequencing of protein bands.
The main bands detected in the Western blot were digested with trypsin, extracted, and desalted essentially as described by Wilm et al. (41). Nano-electrospray was performed on a Q-top mass spectrometer (Micromass, Manchester, United Kingdom).
Statistics.
Data were logarithmically transformed, and statistical analysis (factorial analysis of variance) in conjugation with Fishers's multiple comparison test was performed for the challenge and IFN-γ results. Arithmetic data were used for the IgA quota, the DTH results, and the IgM-IgG titers in repeated-measures analysis of variance in conjugation with Fishers's multiple comparison test. A P of less than 0.05 was considered to be significant. The software Statview 4.0 was used for these calculations.
RESULTS
The Th1 influence on the humoral response increased after the booster in the oral groups.
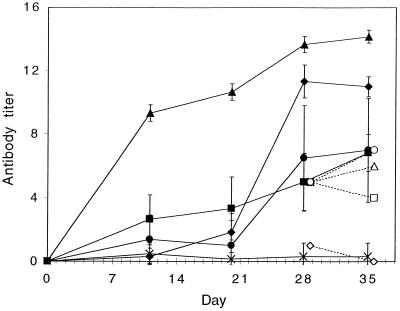
The Salmonella antigens gave rise to a strong systemic IgM-IgG response in all the groups, as shown in Fig. 2. The strongest response was obtained in the groups that received the antigens conjugated to the starch microparticles, but free antigens—even when administered orally—also gave a good IgM-IgG response. The response was found to be mainly directed towards anti-LPS for the groups that received free antigens, where the anti-LPS titer at days 28 and 35 more or less was equal to the total IgM-IgG titer. In contrast, the groups that had received antigen-conjugated microparticles did not have any pronounced LPS response. The anti-LPS response was less than 1% of the total IgM-IgG response for the intramuscular group and was hardly detectable for the oral group. The IgG subclass profile, calculated as the ratio IgG1/(IgG2a + IgG2b), was analyzed at day 20 (before the booster) and at day 36 (2 weeks after booster) (Table 1). The ratio was higher in the groups that received the antigen orally, indicating a larger Th2 dominating response, than in the groups that received the antigens intramuscularly. However, the Th1 influence increased after the booster in the oral groups, while the subclass ratio remained the same after the booster in the intramuscular groups.
FIG. 2.
Serum IgM-IgG before and after booster given at day 21. Mean antibody titers are given as −log2 (dilution × 10) ± standard deviation (error bars) (n = 6). Mice were given antigen-conjugated microparticles orally (⧫), microparticles plus free antigen orally (▪), free antigen orally (•), or antigen-conjugated microparticles intramuscularly (▴). Naive mice were used as a control (×). The group that received antigen-conjugated microparticles orally had significantly higher antibody titers over time compared to both the groups that received the free antigens orally (P < 0.05). The open symbols represent the anti-LPS titers from pooled sera at days 28 and 35.
TABLE 1.
Subclass ratio in serum before and 2 weeks after booster (day 21)a
| Immunization | Mean subclass ratio ± SEM at day:
|
|
|---|---|---|
| 20 | 36 | |
| Antigen-conjugated microparticles orally | 2.4 ± 1.6 | 0.9 ± 0.7 |
| Free antigen orally | 4.1 ± 1.9 | 1.0 ± 0.8 |
| Microparticles + free antigen orally | 6.8 ± 2.2 | 1.2 ± 0.4 |
| Antigen-conjugated microparticles intra-muscularly | 0.3 ± 0.3 | 0.3 ± 0.2 |
| Free antigen intramuscularly | 0.02 ± 0.1 | 0.3 ± 0.1 |
Values represent means ± standard errors of the means (SEM) of the ratio IgG1/(IgG2a + IgG2b) from six mice.
Western blotting revealed that flagellin is the predominant immunogen for the humoral systemic and mucosal immune responses.
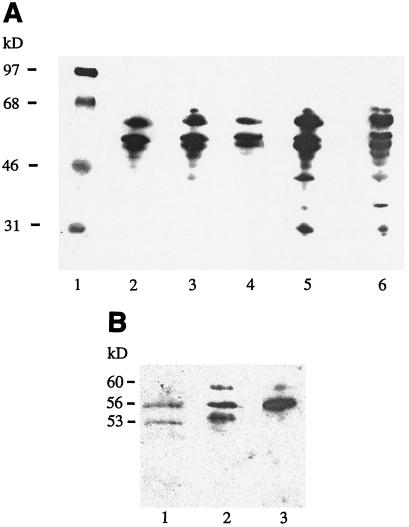
The specificity of the humoral response was analyzed by Western blotting, and the results are shown in Fig. 3. A relatively small number of immunogenic protein bands was detected, particularly in sera from mice that received the Salmonella antigens orally, whereas the intramuscular groups showed antibodies against a larger number of protein bands ranging from approximately 30 to 60 kDa. Three distinct bands, with estimated molecular masses of 53, 56, and 60 kDa, dominated in the oral groups. These three bands were further analyzed with electrospray-mass spectroscopy after trypsin digestion, and the peptide fragments present were searched against the peptide database at Matrixscience (34a). These peptides were consistent with those present in phase-1 flagella. Peptides from both the 53-kDa and the 60-kDa proteins gave a score of 123 against phase-1 flagellin (accession no. U12963) (scores above 69 indicate significance [P < 0.05]). The 56-kDa peptide did not, however, generate a significant match in this database. Nevertheless, a query using this peptide in a BLAST search in the NCBI database (www.ncbi.nlm.nih.gov/blast) revealed that the 56-kDa peptide matched the flagellar hook-associated protein 2 (accession no. P16328) with a high expectation value. The same flagellum-related protein bands as those seen in sera also dominated the mucosal IgA response, as detected in fecal samples and shown in Fig. 3B. In summary, we conclude that the humoral systemic and mucosal immune responses are mainly directed against flagellum-related protein components.
FIG. 3.
Western blot analysis on sera from day 35 and fecal samples from day 28 against extracellular antigens from serovar Enteritidis. (A) The sera were diluted 1:50,000. Sera were obtained from mice immunized with antigen-conjugated microparticles orally (lane 2), free antigen orally (lane 3), microparticles plus free antigen orally (lane 4), antigen-conjugated microparticles intramuscularly (lane 5), or free antigen intramuscularly (lane 6). Lane 1 shows molecular mass markers. (B) The fecal extracts were diluted 1:5. The samples were obtained from mice immunized with antigen-conjugated microparticles orally (lane 1), antigen-conjugated microparticles intramuscularly (lane 2), or free antigen orally (lane 3).
Induction of a strong intestinal mucosal immune response.
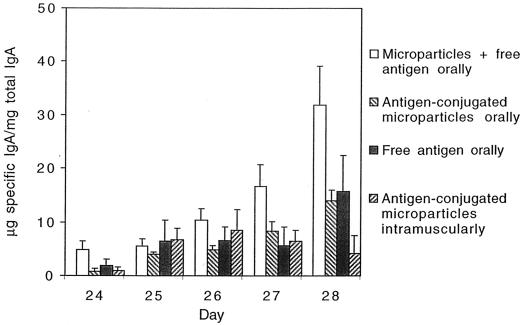
The specific secretory IgA (s-IgA) content in feces increased when the booster vaccination was given orally but not when it was given intramuscularly (Fig. 4). To ensure that the specific IgA measured in the feces originated from the intestine, an ELISPOT assay was performed in another set of experiments. The ELISPOT assay showed that the oral groups had a high percentage of specific IgA-producing cells. Free antigens orally induced the highest percentage of specific antibody-producing cells (1.63% per 105 cells), while the groups that received antigen-conjugated microparticles or free antigens with microparticles had 0.94 or 0.93% specific IgA-producing cells, respectively. The intramuscular group that received antigen-conjugated microparticles did not have any detectable specific IgA-producing cells, whereas the group that received free antigen had 0.52% specific IgA-producing cells in the gut.
FIG. 4.
Fecal samples analyzed for specific IgA and total IgA. The quota (specific IgA/total IgA) is shown before and 5 days after booster. Values are given as arithmetic means + standard errors of the means (error bars) (n = 6). Microparticles with free antigen administered orally had significantly higher quotas compared to the other groups over time (P < 0.05). No specific IgA was detected in the control group.
Very low values of LPS antibodies were found in the fecal extracts even though an extended incubation time with the substrate was used. The groups that had received free antigen orally had the highest value of anti-LPS antibodies with only 1 μg of specific IgA/mg of total IgA at day 27. No anti-LPS antibodies could be detected in feces in the groups that had received antigen-conjugated microparticles.
Induction of strong cellular immune response.
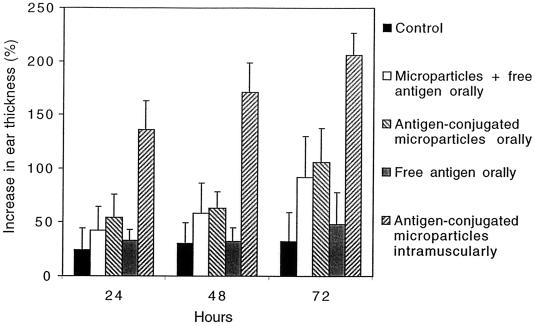
The development of the cellular response was studied by measuring the DTH reaction in the ears of the immunized mice. A strong response was detected at 72 h after challenge of mice with antigens, 3 weeks after booster (Fig. 5), in all groups vaccinated with the starch microparticles. The most marked increase in ear thickness, indicating a Th1 cellular immune response, was seen in the intramuscular group (about 200% after 72 h), while the increase in the oral groups was about 100% after 72 h. These findings support the results obtained from the determination of the subclass profile of the systemic humoral response, where the subclass ratio indicated an increased Th1 response in the oral groups after the booster.
FIG. 5.
DTH test 1 week after the booster. Salmonella antigens (10 μl, 1 mg/ml) were injected intradermally into the left ear and 10 μl of physiological saline was injected intradermally into the right ear of each mouse and the ear thickness was measured before and at 24, 48, and 72 h after injection. Values are given as arithmetic means + standard deviations (error bars) (n = 6). All immunized groups except the group that received free antigen orally induced a significantly higher increase in ear thickness compared to the control group over time (P < 0.05).
Antigen-stimulated primed spleen cells produce IFN-γ.
Splenocytes from mice sacrificed 2 weeks after the booster were stimulated with 50 μg of Salmonella antigens per well (3 × 106 cells). Supernatants were collected on day 2 and day 5 and assayed in duplicate for IL-4, IL-5, IFN-γ, and IL-2. No response was seen for IL-4 or IL-5. However, an increasing IFN-γ response was seen in all groups, peaking on day 5 (Table 2). IL-2 showed a weak response on day 2 for some mice in all groups (data not shown).
TABLE 2.
IFN-γ in supernatants from activated primed splenocytesa
| Immunization | Mean IFN-γ ± SEM (ng/ml) at day:
|
|
|---|---|---|
| 2 | 5 | |
| Antigen-conjugated microparticles orally | 1.2 ± 0.4 | 5.2 ± 1.1 |
| Free antigen orally | 1.7 ± 0.8 | 15 ± 6.1 |
| Microparticles + free antigen orally | 3.6 ± 1.1 | 6.3 ± 0.7 |
| Antigen-conjugated microparticles intra-muscularly | 5.8 ± 0.6 | 13 ± 4.9 |
| Free antigen intramuscularly | 4.7 ± 2.1 | 13 ± 4.6 |
| Control | 1.3 ± 1.3 | 1.7 ± 1.7 |
Mice were sacrificed 2 weeks after booster, and in vitro stimulation of the splenocytes was performed with 50 μg of Salmonella antigens/well (3 × 106 cells). Cytokine assays were performed on duplicate supernatants collected on days 2 and 5. The IFN-γ responses are shown as arithmetic means ± standard errors of the means (SEM) (n = 6). All immunized groups induced a significantly higher IFN-γ response compared to the control group at day 5 (P < 0.05), using the logarithmically transformed values in the statistical analysis.
Protection of immunized mice against oral challenge with serovar Enteritidis.
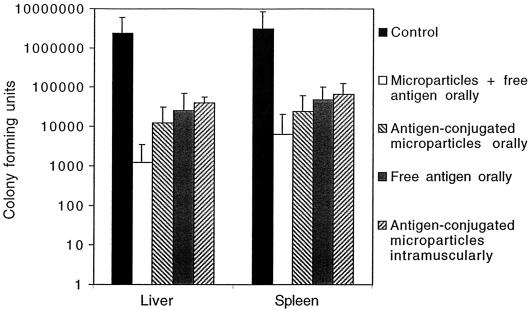
After challenge with S. enteritidis a significant reduction in CFU was seen in all groups immunized orally compared to the control group, and the results were concordant in the liver and the spleen samples from the same mice (Fig. 6). This was also confirmed by studying the weight loss, which showed a 10.3% decrease for the control group, a 4.0% decrease for the group immunized orally with free antigen, a 3.6% decrease for the group immunized orally with microparticles with free antigen, a 4% decrease for the group immunized intramuscularly with antigen-conjugated microparticles, and only a 1.8% decrease for the group immunized orally with antigen-conjugated microparticles.
FIG. 6.
CFU in liver and spleen after oral challenge with serovar Enteritidis (3 × 104/mouse). Individual homogenates were plated onto LB-agar plates in serial dilutions in duplicate 7 days after oral challenge. A mean was calculated for each mouse, and the arithmetic means for each group + standard deviations are presented (n = 6 to 12). All oral groups had significantly lower degrees of infection in both liver and spleen compared to those of the control group (P < 0.05), using the logarithmically transformed values in the statistical analysis.
DISCUSSION
Model studies with HSA as an antigen conjugated to starch microparticles have shown that the starch microparticles can serve as an effective adjuvant in oral vaccination (L. Degling Wikingsson and I. Sjöholm, submitted for publication). The model vaccine induced both a cellular and a humoral systemic immune response in mice as well as a local mucosal immune response, while free HSA given orally did not provoke any immune response. Another study showed that a parenteral vaccine prepared with ribosomal DNA-derived gp63 from L. donovani covalently linked to starch microparticles resulted in a significantly lower parasite burden after challenge than those seen in the group that received free gp63 and in the control group (Degling Wikingsson et al., submitted). Both of these studies showed that the microparticles induced a cellular immune response, which is an important protective component of an effective immunological defense against intracellular microorganisms.
The purpose of this study was to investigate if the starch microparticles could function as an adjuvant in oral vaccination against a bacterium that is known to adhere to and pass through the intestinal epithelium. Thus, we chose S. enterica serovar Enteritidis, which is a gram-negative, facultative intracellular bacterium that proliferates inside macrophages in liver and spleen. In contrast to many other vaccination studies, in which attenuated Salmonella whole-cell vaccines have been used, we used the secreted proteins after short cultivation of the bacteria and conjugated these proteins to the microparticles or used them in free form.
Despite an extensive number of studies on the pathogenicity of Salmonella species and the factors of significance for the development of protection against infection, no conclusive evidence is yet available on the necessary components in a Salmonella vaccine. The investigations have focused on outer membrane components, like motility, adhesion, and invasion proteins, and other virulence factors (for a recent review, see reference 14). Moreover, the characterization of the protective immune responses after vaccination with different types of preparations and administration routes has focused on the importance of the cellular response and the specificity of the humoral response (for a recent review, see reference 32). In our study we have found that all groups that received microparticles with the secreted antigens (conjugated or not conjugated) gave a strong cellular response 72 h after challenge in the DTH test. In particular, the response was pronounced (100 to 200% increase of the ear thickness) in the groups given microparticles with conjugated antigens. Other studies have also shown that a cellular response is obtained when immunizing with attenuated or heat-killed S. enterica serovar Typhimurium (40), or with outer membrane proteins given parenterally (2, 34). However, in this study we show that secreted antigens given orally alone or conjugated to microparticles are capable of giving an appropriate immune response.
The immune response in this study was further characterized in antigen-activated primed spleen cells, and we found that all the groups produced high amounts of IFN-γ and, in some samples, IL-2. This supports the hypothesis that the response was mainly of a Th1 nature. This was further supported by results from the groups vaccinated via the oral route, in which analysis of the subclass profile of the systemic humoral response showed that the ratio IgG1/(IgG2a + IgG2b) decreased after the booster.
The hypothesis that IFN-γ plays a decisive role in inducing protection to infection after Salmonella vaccinations was recently strengthened, when Bao et al. (8) showed that serovar Typhimurium challenge of IFN-γ-deficient mice resulted in widespread septicemia, despite elevated antibody titers in both mucosal and systemic compartments. It is clear that IFN-γ- induced Th1-cells are an essential component of the protective response against Salmonella infections. However, this conclusion does not rule out the possibility that mucosal or systemic humoral responses can effectively prevent the invasion and the spread of Salmonella bacteria. Indeed, specific s-IgA contributes to blocking bacterial adhesion to epithelial cells and to agglutinate bacteria (31). Another possible mechanism may be that s-IgA triggers opsonization, which would induce an efficient uptake and destruction of bacteria by phagocytes in the gut-associated lymphoid tissues. This hypothesis has been presented by Michetti et al., who showed that s-IgA alone can prevent infection by serovar Typhimurium (30). Other studies, however, have questioned the importance of s-IgA. Pier et al. (35) found that circulating antibodies against Pseudomonas aeruginosa O antigen, induced by intraperitoneal immunization with purified antigens, were as efficient in promoting mucosal clearance as was a combination of mucosal and circulating antibodies, induced by oral immunization with recombinant serovar Typhimurium. In our study, we observed a good, specific mucosal IgA response, in addition to the systemic responses. The IgA response was particularly good in the groups that were given the antigens orally. The ELISPOT assay showed that the specific IgA measured in the feces originated from the intestine and confirmed that the oral groups had a high percentage of specific IgA-producing cells. This high mucosal IgA response may seem to contradict the differentiation of the cellular response towards a dominating Th1 influence. However, recent findings (9, 17) indicate that locally produced IL-6 from intestinal epithelial cells strongly enhances the mucosal IgA+ B-cell responses.
We observed an impressive systemic, humoral response in all treated groups. The important role of antibody-producing B cells in protection against salmonellosis has been shown by Mastroeni et al. (28). Moreover, Yokoyama et al. (43) showed that egg yolk antibodies specific for Salmonella flagellin, LPS, and outer membrane proteins such as porin protect mice from salmonellosis when administered orally. This is in line with our study, in which we have shown that oral administration of secreted proteins primarily gives antibodies against flagellin. However, measurable titers of anti-LPS antibodies were also found in the groups immunized with free antigens.
We studied the specificity of the humoral immune response by Western blotting and found that essentially three major antigens had triggered the antibody response. These proteins, with molecular masses of approximately 53, 56, and 60 kDa, were analyzed by electrospray-mass spectroscopy and were found to originate from the flagellum, namely, flagellin and the hook-associated protein 2. Interestingly, the same three protein bands also appeared in the Western blot performed with the fecal samples from both the oral and the intramuscular groups, indicating that the s-IgA is also primarily directed towards flagellar epitopes. The significance of the flagella in an immune response has been highlighted in several studies, in which, e.g., the Salmonella flagellin system based on the parenteral inoculation of live cells or purified flagella or flagellin subunits have been used to induce antigen-specific immune responses (33, 39). Wyant et al. (42) demonstrated that flagella from S. enterica serovar Typhi are powerful monocyte activators in human peripheral blood, inducing proinflammatory cytokines such as tumor necrosis factor alpha and IL-1β, and inducing the production of IL-6, IL-10, and IFN-γ. Another human study with live oral Salmonella-Shigella hybrid vaccines demonstrated that protection was provided by batches of vaccine in which bacteria reacted with antiflagellar serum, while batches of vaccine that did not react with antiflagellar serum failed to provide protection (37). Other studies have also detected an antiflagellar antibody response both in mice inoculated with attenuated serovar Typhimurium intravenously, and in humans diagnosed with typhoid fever (11). Moreover, other studies have indicated that flagellin is a strong T-cell activator. Thus, Cookson and Bevan identified a natural T-cell epitope presented by Salmonella-infected macrophages that was recognized by orally immunized mice (13). This T-cell epitope was shown to be the flagellar filament protein C and the dominant recall antigen for CD4+ T cells from protectively immunized C3H/HeJ mice. The presentation of the epitope was greatly enhanced by pretreatment of macrophages with IFN-γ, which again indicates that IFN-γ-activated macrophages are important in the stimulation of T cells that respond to facultative intracellular pathogens such as serovar Typhimurium. Another recent study showed that CD4+ T cells from mice orally immunized with attenuated serovar Typhimurium respond to an epitope from a constant region of FliC, which enables these cells to cross-react with flagellar proteins expressed by different Salmonella serovars (29). Mice immunized subcutaneously with purified FliC were also protected against infection with a virulent strain.
Several studies have shown that contaminating LPS may also evoke strong antibody responses (24, 36). Hormaeche et al. (21) studied the role of the main LPS O antigen in the specificity of protection in mice, but concluded that LPS contamination alone could not fully account for the protection. In our study, contaminating LPS can only explain the protective response elicited in those groups that received free antigens orally or intramuscularly. The antigens conjugated to the microparticles contained approximately 1,200 times less LPS than the free antigens, and thus the secreted Salmonella proteins present in the starch microparticle formulation are primarily responsible for triggering the immune response. This was further confirmed by the Western blots performed with proteinase K-digested Salmonella proteins, in which the typical ladder-like pattern normally seen with LPS was not seen in the serum or fecal samples. When further analyses of sera with the more sensitive anti-LPS ELISA were performed, the antibodies directed toward LPS were mainly found in the groups that had received free antigens. The fecal samples showed very low values on LPS antibodies and were only detectable in the groups that had received free antigen orally.
It has recently been proposed that LPS, as a complex with an LPS binding protein that exists in serum, binds to the CD14 receptor on macrophages. This complex presumably activates a Toll-like receptor, TLR4 (1). These TLRs are believed to have a central role in triggering the innate immune response and also to send alert signals to the adaptive immune response. TLR5 has recently been shown to be activated by flagellin (18), which in our study has induced a strong antibody response. It is therefore quite possible that LPS and flagellin together in a synergistic way give the protective immune response seen in the groups that received free antigen in our study.
Indeed, the group that received free antigens mixed with microparticles orally had the highest local immune response in the intestines and was also shown to get the highest protection in the challenge. It is known that proteins adsorb to surfaces to lower the surface tension. Therefore, the proteins might be protected against degradation in the intestines and exposed to the immune system together with the starch microparticles just by mixing them with the microparticles. We have also shown that the LPS did not conjugate to the microparticles when covalent conjugation of the proteins was made. Therefore, the group that received free antigen mixed with microparticles got both the proteins and an additional dose of LPS. This may explain the outcome of the response seen as discussed above. However, we can also conclude that starch microparticles with LPS-depleted, conjugated Salmonella antigens are an effective adjuvant in oral vaccination, as seen in the challenge experiments. It is not possible to conclude from the present studies whether the cellular or the humoral responses are essential for the protective effects detected, nor is it possible to conclude whether the antiflagellin response obtained is decisive. However, it is not unlikely that a mucosal antiflagellin response and a Th1-related cellular response together are responsible for the protection against the challenge. However, the role of the antiflagellin response must be further elucidated, and we are currently carrying out experiments with purified flagellar proteins conjugated to the microparticles.
Acknowledgments
We thank Mikael Rehn for providing the bacterial strain; Charlotta Hofstedt, Brittmarie Andersson, Linda Stertman, Per Asimus, and Alexandra Constantinescu for skillful technical assistance; Kristina Erlandsson-Persson for the LAL analyses; the late Johanna Björkman for the introduction to the challenge protocol; Bo Ek for the mass spectroscopy analyses; and Maria Norlin for placing the Western blot equipment at our disposal and providing technical advice.
We gratefully acknowledge the financial support from SBL Vaccine AB, NUTEK (project P 11381), and AstraZeneca.
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed]
- 2.Alurkar, V., and R. Kamat. 1997. Immunomodulatory properties of porins of some members of the family Enterobacteriaceae. Infect. Immun. 65:2382-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, S., and H. Jörnvall. 1986. Sex differences in cytochrome P-450-dependent 25-hydroxylation of C27-steroids and vitamin D3 in rat liver microsomes. J. Biol. Chem. 261:16932-16936. [PubMed] [Google Scholar]
- 4.Artursson, P., E. Arro, P. Edman, J. L. E. Ericsson, and I. Sjöholm. 1987. Biodegradable microspheres. V. Stimulation of macrophages with microparticles made of various polysaccharides. J. Pharm. Sci. 76:127-133. [DOI] [PubMed] [Google Scholar]
- 5.Artursson, P., P. Edman, T. Laakso, and I. Sjöholm. 1984. Characterization of polyacryl starch microparticles as carriers for proteins and drugs. J. Pharm. Sci. 73:1507-1513. [DOI] [PubMed] [Google Scholar]
- 6.Artursson, P., P. Edman, and I. Sjöholm. 1985. Biodegradable microspheres. II. Immune response to a heterologous and an autologous protein entrapped in polyacryl starch microparticles. J. Pharmacol. Exp. Ther. 234:255-260. [PubMed] [Google Scholar]
- 7.Artursson, P., P. Edman, and I. Sjöholm. 1984. Biodegradable microspheres. I. Duration of action of dextranase entrapped in polyacrylstarch microparticles in vivo. J. Pharmacol. Exp. Ther. 231:705-712. [PubMed] [Google Scholar]
- 8.Bao, S., K. W. Beagley, M. P. France, J. Shen, and A. J. Husband. 2000. Interferon-gamma plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology 99:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao, S., K. W. Beagley, A. M. Murray, V. Caristo, K. I. Matthaei, I. G. Young, and A. J. Husband. 1998. Intestinal IgA plasma cells of the B1 lineage are IL-5 dependent. Immunology 94:181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bethell, G. S., J. S. Ayers, W. S. Hancock, and M. T. Hearn. 1979. A novel method of activation of cross-linked agaroses with 1,1′-carbonyldiimidazole which gives a matrix for affinity chromatography devoid of additional charged groups. J. Biol. Chem. 254:2572-2574. [PubMed] [Google Scholar]
- 11.Brown, A., and C. E. Hormaeche. 1989. The antibody response to salmonellae in mice and humans studied by immunoblots and ELISA. Microb. Pathog. 6:445-454. [DOI] [PubMed] [Google Scholar]
- 12.Clark, M. A., M. A. Jepson, N. L. Simmons, and B. H. Hirst. 1994. Preferential interaction of Salmonella typhimurium with mouse Peyer's patch M cells. Res. Microbiol. 145:543-552. [DOI] [PubMed] [Google Scholar]
- 13.Cookson, B. T., and M. J. Bevan. 1997. Identification of a natural T cell epitope presented by Salmonella-infected macrophages and recognized by T cells from orally immunized mice. J. Immunol. 158:4310-4319. [PubMed] [Google Scholar]
- 14.Darwin, K. H., and V. L. Miller. 1999. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 12:405-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degling, L., and P. Stjärnkvist. 1995. Biodegradable microspheres. XVIII. The adjuvant effect of polyacryl starch microparticles with conjugated human serum albumin. Vaccine 13:629-636. [DOI] [PubMed] [Google Scholar]
- 16.Edman, P., B. Ekman, and I. Sjöholm. 1980. Immobilization of proteins in microspheres of biodegradable polyacryldextran. J. Pharm. Sci. 69:838-842. [DOI] [PubMed] [Google Scholar]
- 16a.Gerlier, D., and N. Thomasset. 1986. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 94:57-63. [DOI] [PubMed] [Google Scholar]
- 17.Goodrich, M. E., and D. W. McGee. 1999. Effect of intestinal epithelial cell cytokines on mucosal B-cell IgA secretion: enhancing effect of epithelial-derived IL-6 but not TGF-beta on IgA+ B cells. Immunol. Lett. 67:11-14. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 19.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hjertén, S. 1962. “Molecular sieve” chromatography on polyacrylamide gels, prepared according to a simplified method. Arch. Biochem. Biophys. (Suppl. 1):147-151. [PubMed]
- 21.Hormaeche, C. E., P. Mastroeni, J. A. Harrison, R. Demarco de Hormaeche, S. Svenson, and B. A. Stocker. 1996. Protection against oral challenge three months after i.v. immunization of BALB/c mice with live Aro Salmonella typhimurium and Salmonella enteritidis vaccines is serotype (species)-dependent and only partially determined by the main LPS O antigen. Vaccine 14:251-259. [DOI] [PubMed] [Google Scholar]
- 22.Jones, B. D., and S. Falkow. 1996. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14:533-561. [DOI] [PubMed] [Google Scholar]
- 23.Jones, B. D., N. Ghori, and S. Falkow. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuusi, N., M. Nurminen, H. Saxén, and P. H. Mäkelä. 1981. Immunization with major outer membrane protein (porin) preparations in experimental murine salmonellosis: effect of lipopolysaccharide. Infect. Immun. 34:328-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laakso, T., P. Artursson, and I. Sjöholm. 1986. Biodegradable microspheres. IV: Factors affecting the distribution and degradation of polyacryl starch microparticles. J. Pharm. Sci. 75:962-967. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 27.Lee, V. T., and O. Schneewind. 1999. Type III secretion machines and the pathogenesis of enteric infections caused by Yersinia and Salmonella spp. Immunol. Rev. 168:241-255. [DOI] [PubMed] [Google Scholar]
- 28.Mastroeni, P., C. Simmons, R. Fowler, C. E. Hormaeche, and G. Dougan. 2000. Igh-6−/− (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar Typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect. Immun. 68:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McSorley, S. J., B. T. Cookson, and M. K. Jenkins. 2000. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J. Immunol. 164:986-993. [DOI] [PubMed] [Google Scholar]
- 30.Michetti, P., M. J. Mahan, J. M. Slauch, J. J. Mekalanos, and M. R. Neutra. 1992. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect. Immun. 60:1786-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michetti, P., N. Porta, M. J. Mahan, J. M. Slauch, J. J. Mekalanos, A. L. Blum, J. P. Kraehenbuhl, and M. R. Neutra. 1994. Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology 107:915-923. [DOI] [PubMed] [Google Scholar]
- 32.Mittrucker, H. W., and S. H. Kaufmann. 2000. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 67:457-463. [DOI] [PubMed] [Google Scholar]
- 33.Newton, S. M., C. O. Jacob, and B. A. Stocker. 1989. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science 244:70-72. [DOI] [PubMed] [Google Scholar]
- 34.Ogunniyi, A. D., P. A. Manning, and I. Kotlarski. 1994. A Salmonella enteritidis 11RX pilin induces strong T-lymphocyte responses. Infect. Immun. 62:5376-5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Perkins, D. N., D. J. Pappin, D. M. Creasy, and J. S. Cottrell. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551-3567. [DOI] [PubMed] [Google Scholar]
- 35.Pier, G. B., G. Meluleni, and J. B. Goldberg. 1995. Clearance of Pseudomonas aeruginosa from the murine gastrointestinal tract is effectively mediated by O-antigen-specific circulating antibodies. Infect. Immun. 63:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxén, H., M. Nurminen, N. Kuusi, S. B. Svenson, and P. H. Mäkelä. 1986. Evidence for the importance of O antigen specific antibodies in mouse-protective Salmonella outer membrane protein (porin) antisera. Microb. Pathog. 1:433-441. [DOI] [PubMed] [Google Scholar]
- 37.Schultz, C. L., B. Kaufman, D. Hamilton, A. Hartman, M. Ruiz, C. Powell, and S. Berman. 1990. Cell wall structures which may be important for successful immunization with Salmonella-Shigella hybrid vaccines. Vaccine 8:115-120. [DOI] [PubMed] [Google Scholar]
- 38.Stjärnkvist, P., P. Artursson, T. Brunmark, T. Laakso, and I. Sjöholm. 1987. Biodegradable microspheres VIII. Killing of Leishmania donovani in cultured macrophages by microparticle-bound primaquine. Int. J. Pharm. 40:215-222. [Google Scholar]
- 39.Stocker, B. A., and S. M. Newton. 1994. Immune responses to epitopes inserted in Salmonella flagellin. Int. Rev. Immunol. 11:167-178. [DOI] [PubMed] [Google Scholar]
- 40.Thatte, J., S. Rath, and V. Bal. 1995. Analysis of immunization route-related variation in the immune response to heat-killed Salmonella typhimurium in mice. Infect. Immun. 63:99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilm, M., A. Shevchenko, T. Houthaeve, S. Breit, L. Schweigerer, T. Fotsis, and M. Mann. 1996. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature 379:466-469. [DOI] [PubMed] [Google Scholar]
- 42.Wyant, T. L., M. K. Tanner, and M. B. Sztein. 1999. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect. Immun. 67:3619-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokoyama, H., K. Umeda, R. C. Peralta, T. Hashi, F. C. Icatlo, Jr., M. Kuroki, Y. Ikemori, and Y. Kodama. 1998. Oral passive immunization against experimental salmonellosis in mice using chicken egg yolk antibodies specific for Salmonella enteritidis and S.typhimurium Vaccine 16:388-393. [DOI] [PubMed] [Google Scholar]