Abstract
Trypanosome infections are marked by severe pathological features, including anemia, splenomegaly, and suppression of T-cell proliferation. We have used lymphotoxin-α-deficient (LT-α−/−) mice, as well as LT-α-tumor necrosis factor-double-deficient (LT-α−/− TNF−/−) mice, to analyze the contributions of these related cytokines in both induction of trypanosomosis-associated immunopathology and infection control. Moreover, as the cytokine-deficient mice used have no detectable lymph nodes and lack germinal-center formation upon immune stimulation, we have analyzed the functional importance of both the lymph nodes and spleen during experimental Trypanosoma brucei infections. First, we show that the absence of LT-α does not significantly alter early trypanosomosis development or pathology but does result in better control of late-stage parasitemia levels and slightly prolonged survival. This increased survival of infected LT-α−/− mice coincides with the appearance of increased chronic-stage anti-trypanosome immunoglobulin M (IgM)-IgG2a serum titers that are generated in the absence of functional peripheral lymphoid tissue and do not require germinal-center formation. Second, we show that splenectomized mice control their parasitemia to the same extent as fully immune-competent littermates. Finally, using LT-α−/− TNF−/− double-deficient mice, we show that in these mice T. brucei infections are very well controlled during the chronic infection stage and that infection-induced pathology is minimized. Together, these findings indicate that while increased IgM-IgG2a anti-trypanosome antibody titers (generated in the absence of LT-α, peripheral lymph nodes, and germinal-center formation) coincide with improved parasitemia control, it is TNF that has a major impact on trypanosomosis-associated immunopathology.
African trypanosomes are extracellular parasitic protozoa transmitted by the bite of the tsetse fly (38). In order to complete their life cycle, trypanosomes require an obligatory developmental step in a mammalian host. Consequently, these parasites have to cope with the host immune system and establish a state of equilibrated growth regulation ensuring optimal survival and effective transmission. First, African trypanosomes escape from immune recognition through the mechanism of antigenic variation of their variant-specific surface glycoprotein (VSG), the major surface antigen that acts as an ever-changing protective coat for the parasite (4). Besides this active VSG-based defense system, trypanosomes also may utilize host immunomodulatory molecules and networks to regulate their development. Indeed, in different experimental trypanosome infections, modifications of T-cell and macrophage responsiveness were reported to occur in lymph nodes, spleen, and bone marrow (5, 16, 34). In addition, impairment of the antigen presentation capacity of macrophages (27) and the induction of polyclonal B-cell stimulation (2) occurs. At the level of infection-associated cytokine secretion, induction of gamma interferon (IFN-γ) and tumor necrosis factor (TNF) has been extensively documented as well (7, 13, 21). These type 1 cytokines have been described as playing an important dual role in trypanosomosis, being involved both in the induction of host pathology and in parasite control. Indeed, while IFN-γ has been accepted as playing a major role in infection-associated T-cell suppression in the lymph nodes (3), IFN-γ-deficient mice suffer from accelerated parasite growth and exhibit a significantly reduced survival time when infected with African trypanosomes (13). In the case of TNF, a role in trypanosomosis-associated immunopathology has been suggested in several studies, showing (i) the enhanced expression of TNF in the brains of Trypanosoma brucei-infected mice (14), (ii) the association between TNF production by monocytes and the severity of disease-associated anemia in trypanosome-infected cattle (36), (iii) the correlation between serum TNF levels and neuropathological symptoms in human sleeping sickness patients (30), and (iv) the involvement of TNF in trypanosome-elicited immunosuppression and overall morbidity (20). On the other hand, TNF also exerts a direct influence on the control of parasite survival through its trypanolytic effect (6, 22). The overall influence of TNF on the outcome of experimental trypanosome infections has been studied, using TNF-deficient mice (20). As expected, these mice exhibited significantly increased parasitemia but showed at the same time strongly reduced infection-associated pathology. In addition, the capacity to clear successive parasitemia peaks was found to be unaltered and corresponded to an unaltered overall anti-VSG antibody induction pattern in infected TNF−/− mice. It is interesting, with respect to the biological functions of TNF, that the TNF-related cytokine lymphotoxin-α (LT-α) was found to have no direct antitrypanosomal activity at all (19). Although TNF and LT-α have high amino acid sequence homology (28) and both bind to the TNF p55 and p75 receptors as soluble homotrimers (37), alterations in the TIP sequences of both molecules result in TNF exerting a lectin-like affinity for several carbohydrate sequences while LT-α does not (19). Therefore, in the present study we have extended the analysis of the role of the TNF family cytokines by investigating trypanosomosis development and the occurrence of infection-associated pathology in both LT-α-deficient and LT-α-TNF-double-deficient mice. It is important to note here that LT-α is crucial for peripheral lymphoid organ development and segregation of B and T cells in the spleen. As a result, LT-α-deficient mice lack peripheral lymph nodes as well as germinal-center formation upon stimulation with T-cell-dependent antigens (9). In the study presented here, our data show that in the absence of LT-α, increased serum titers of anti-VSG immunoglobulin M (IgM) and IgG2a antibodies occurred during the chronic phase of experimental trypanosome infections, coinciding with an improved parasitemia control and slightly but significantly prolonged survival. In addition, our results show that LT-α-TNF-double-deficient mice also control the chronic infection phase very efficiently and have an even more prolonged survival time than the LT-α-deficient mice, due to the virtual absence of infection-induced immunopathology. Thus, together these results suggest that while LT-α-independent and germinal-center-independent generation of anti-VSG IgM-IgG2a antibodies coincides with improved parasitemia control, excessive TNF production is the main cause of trypnosomosis-associated immunopathology and early death of infected wild-type (WT) mice.
MATERIALS AND METHODS
Mice.
Homozygous B6,129S-Ltatm1Dch (B6,129S) female LT-α-deficient mice (9), 8 weeks of age, as well as 8-week-old female control hybrid F2 C57BL/6Jx129S3/SvImJ (B6129SF2) mice and control inbred C57BL/6J and 129SvImJ mice, were obtained from the Jackson Laboratories. The homozygous 8-week-old B6,129S LT-α-TNF-double-deficient mice (10) came from a laboratory breeding colony.
Parasites and analysis of parasitemia.
All experimental trypanosome infections were performed by intraperitoneal inoculation of 5,000 living pleomorphic Trypanosoma brucei brucei AnTat 1.1E parasites and were repeated at least two times. Using 10 mice per experimental group, we followed parasitemia development in all mice individually by regular microscopic analysis of a 1/100-diluted blood sample taken from the tip of the tail. Occurrence of anemia was followed by red blood cell (RBC) counts of the same sample. For the analysis of infection-induced splenomegaly, three additional mice were infected and individually sacrificed on days 7, 14, and 28 for spleen weight analysis, as was one noninfected control mouse on day 0. Spleen weights from animals that were killed just prior to the lethal end of the infection were recorded as well.
Serum cytokine analysis.
Sera from infected animals were collected on days 0, 7, 14, and 28 and just prior to the lethal end of the infection of the mice used for parasitemia analysis.The sera were first stored at −80°C and later screened in batches for their TNF and IFN-γ contents, using commercially available CytoSets enzyme-linked immunosorbent assay (ELISA) systems from Biosource. The ELISAs were performed according to the producer's guidelines, and a streptavidin-horseradish peroxidase detection system was used to reveal the results. Detection limits for both systems ranged from 15 to 2,000 pg/ml, using serum samples that were diluted 1/4 in sample solution.
Anti-VSG and anti-trypanosome serum antibody titer determination.
For the determination of anti-VSG and anti-trypanosome serum antibody titers, first, purified soluble VSG (sVSG) and total soluble trypanosome extracts were prepared. For preparation of VSG, pleomorphic T. b. brucei AnTat 1.1E parasites were isolated at the peak of their parasitemia and separated from RBCs on a DEAE52 cation-exchange column equilibrated at pH 8.0 with phosphate-buffered saline supplemented with 16 g of glucose/liter (18). sVSG was obtained through osmotic lysis of trypanosomes by 30 min of incubation at 4°C in 125 mM NaH2PO4 (pH 5.5) (109 cells/ml) followed by a 5-min incubation at 37°C in 10 mM sodium phosphate (pH 8.0). The supernatant was passed through a column of DEAE52 equilibrated in 10 mM sodium phosphate (pH 8.0). Following a change of buffer to 10 mM Tris (pH 8.1) on a G25 desalting column (Pharmacia), sVSG was further purified by high-performance liquid chromatography (Akta; Pharmacia) using a Resource Q anion-exchange column (Pharmacia) equilibrated with 10 mM Tris (pH 8.1). A linear NaCl gradient reaching 100 mM was used to elute VSG. The protein concentrations of the VSG were estimated with a detergent-compatible protein assay kit (Bio-Rad Laboratories), using bovine serum albumin as a standard. Total soluble extract of T. b. brucei trypanosomes was obtained by three rounds of sonication of DEAE52-purified parasites, followed by 10 min of centrifugation at 13,000 rpm in an Eppendorf centrifuge. The supernatants were collected, and the total protein concentration was estimated as for VSG.
For the determination of serum antibody titers, VSG and total soluble trypanosome protein extract was used at concentrations of 2 and 5 μg/ml, respectively, to coat ELISA plates (Nunc). Sera from infected mice that were collected on days 0, 7, 14, and 28 and just prior to the lethal end of the infection for cytokine analysis were also used in these experiments. Serial 1/2 dilutions of all sera were prepared starting from a concentration of 1/20. Endpoint titers in serum were determined using the isotype-specific Cloningtype System-HRP from Southern Biotechnology Associates, Inc.
RESULTS
T. b. brucei parasitemia development in LT-α-deficient mice.
We have previously reported that during experimental trypanosome infections TNF plays a crucial role in both control of parasitemia and induction of infection-associated pathology (20). We have now extended our study by analyzing the role of the TNF-related cytokine LT-α in T. brucei infections.
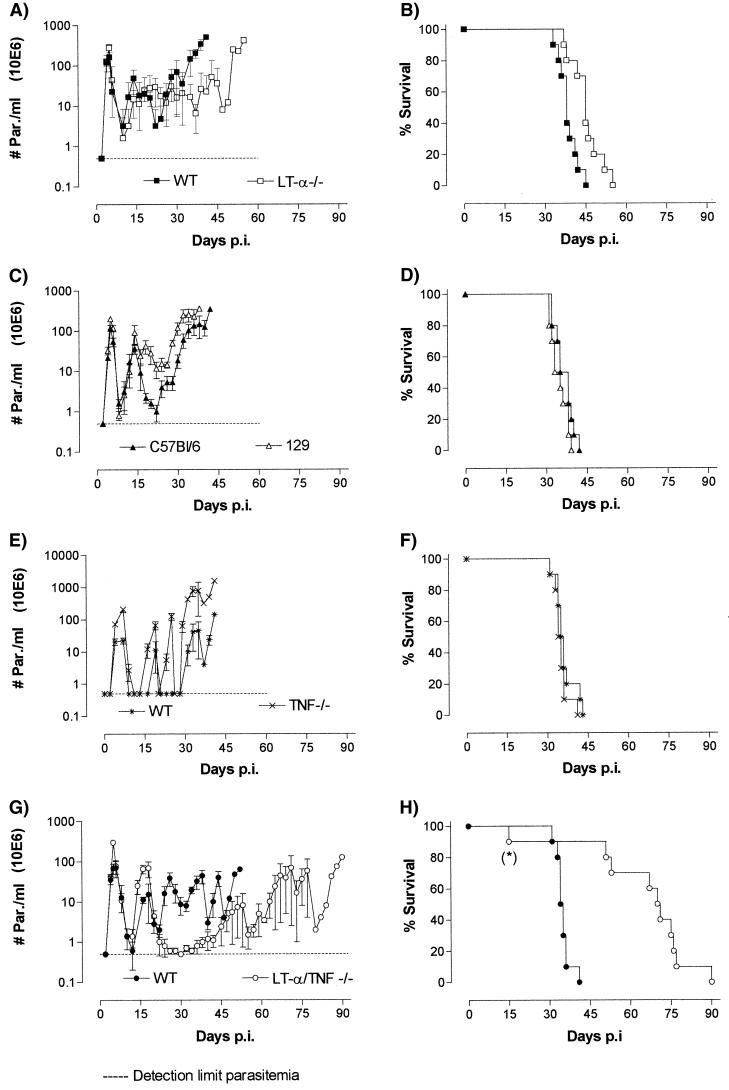
Both B6,129S LT-α-deficient (LT-α−/−) mice and B6129SF2 (WT) control mice were infected with the pleomorphic T. b. brucei AnTat 1.1E strain by intraperitoneal inoculation with 5,000 living parasites. The results presented in Fig. 1A show that both LT-α−/− and WT mice develop their first parasitemia peak around day 7 and that the average peak parasitemia level in both strains of mice reaches 2 × 108 parasites/ml of blood. Following the peak, efficient parasite elimination occurs in both strains, and the infection patterns remain similar and controlled till about the fourth week of infection. During this infection period, parasitemia levels remain below 108 parasites/ml of blood. During the fifth week of infection, parasitemia control is lost in the WT mice and parasite levels in the blood increase exponentially. WT mice succumb to the infection with a median survival time of 38 days and with a parasite load of more than 5 × 108/ml of blood. In contrast, parasitemia levels in the LT-α−/− mice remain controlled for an increased period of time, and median survival is slightly but significantly extended to 45 days (P = 0.003) (Fig. 1B).
FIG. 1.
(A and B) Parasitemia development in LT-α-deficient mice compared to parasitemia development in control WT mice (A), as well as survival of infected individual mice in both groups (B). p.i., postinfection. (C and D) For reference, parasitemia development in parental C56BL/6J and 129SvImJ (129) mice is also shown (C), as well as the individual survival of these mice (D). (E to H) Parasitemia development (E) and survival (F) of T. brucei-infected TNF-deficient and control (WT) mice (data from reference 20), as well as parasitemia development (G) and survival (H) of infected LT-α-TNF-double-deficient and WT mice, are shown. All results were obtained with experimental groups of 10 mice, and individual experiments were repeated at least two times with similar results. Parasitemia data are presented as mean parasite counts (# Par.) per milliliter of blood ± standard deviations. In the experiment represented in panel H, one LT-α-TNF-double-deficient mouse (∗) died early on day 15 for unknown reasons. This mouse was not taken into account for the calculation of the mean survival time of the whole group.
As the LT-α−/− mice were generated on a mixed B6,129S background, T. b. brucei AnTat 1.1E parasitemia development was also analyzed in the C57BL/6J and 129SvImJ strains separately. Neither the pattern of infection nor the survival time of infected mice from either strain was found to show any significant difference from that recorded in the B6129SF2 mice (Fig. 1C and D), the median survival time being 38 and 35 days for the C57BL/6J and the 129SvImJ strains, respectively. This result could be important for future experimental T. brucei infection studies, as a large collection of cytokine-deficient mouse strains is available on a mixed B6,129S background.
T. b. brucei parasitemia development in TNF-LT-α-double-deficient mice.
TNF and LT-α are cytokines that belong to the same family, show high sequence homology, and can both bind as soluble trimers to the same receptors (37). However, the outcome of T. brucei infections differs in some major aspects in LT-α−/− mice compared to those in TNF−/− mice. Indeed, in T. b. brucei-infected TNF−/− mice, no significant alteration in survival time was recorded compared to WT mice, while a significant effect on peak parasitemia control was observed (Fig. 1E and F) (20). To further evaluate the relative contributions of both cytokine genes, T. b. brucei infections were analyzed in TNF−/− LT-α−/− mice as well. As shown in Fig. 1G, parasitemia in the TNF−/− LT-α−/− mice differs significantly from that recorded in the control B6129SF2 WT mice. During the first 2 weeks of the infection, two parasitemia peaks occur in both strains, but parasite levels are higher in the double-deficient mice than in the WT mice. After this acute phase, TNF−/− LT-α−/− mice efficiently control their infection till day 62 of the infection, exhibiting mean parasitemia levels that do not exceed 107 parasites/ml of blood. During the final stage of the infection, parasitemia control is suddenly lost, and the mice succumb to their infection with a mean survival time of 71 days (Fig. 1H) and with an extremely high number of parasites in the circulation. In contrast to the situation recorded in the TNF−/− LT-α−/− double-deficient mice, parasitemia in the WT mice is not controlled after the second peak. Mean parasitemia levels in these mice remain high and oscillate around 5 × 107. The mean survival time of infected WT mice is only 39 days, i.e., 32 days shorter than the mean survival time of mice that lack the LT-α gene.
Trypanosomosis-associated immunopathology in LT-α-deficient mice.
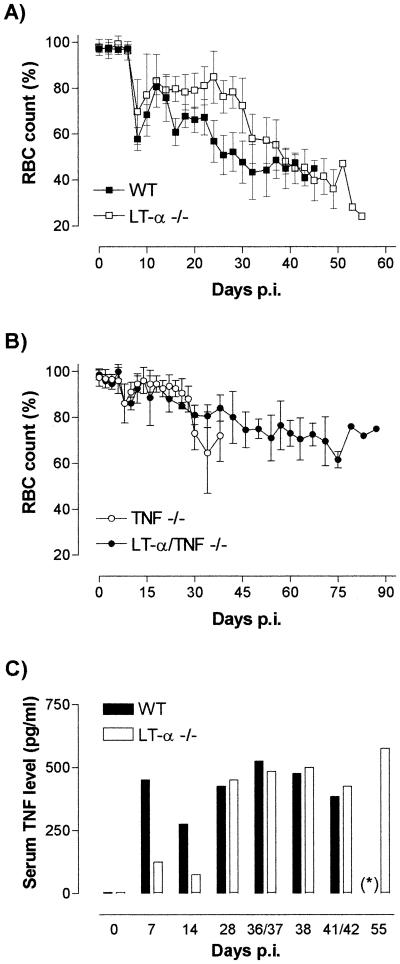
Mice experimentally infected with African trypanosomes manifest severe infection-associated anemia and splenomegaly that most often coincides with a lack of locomotor activity and deterioration of fur quality, finally resulting in high overall morbidity. These pathological features were analyzed in trypanosome-infected LT-α−/−, TNF−/− LT-α−/−, and B6129SF2 WT mice. Induction of anemia was followed by hematocytometric analysis of the blood samples isolated for the parasitemia determination. As shown in Fig. 2A, in mice that suffer from severe infection-associated immunopathology, the occurrence of anemia becomes significant at day 8 of the infection, coinciding with the parasite elimination that follows the control of the first parasitemia peak. Within a 48-h period, mean RBC counts drop to 34 and 45% for the LT-α−/− and WT mice, respectively. From day 10 on, the RBC counts recover slightly in both strains. In the case of the infection of WT mice, however, severe anemia recurs from day 15 of the infection onwards, and RBC counts keep steadily dropping till the end of the infection, resulting in an overall RBC count reduction of between 60 and 75% at the time of death. In contrast, in the infected LT-α−/− mice, the RBC counts remain approximately constant between day 10 and day 28 of the infection, representing only moderate anemia. Afterwards, RBC counts start to drop, finally reaching an average 52% reduction level by the end of the infection.
FIG. 2.
Development of trypanosomosis-associated anemia in LT-α-deficient mice and control WT mice (A) and in LT-α-TNF-double-deficient as well as TNF-deficient mice (B). The values presented are mean RBC counts ± standard deviations, obtained from the same mice as those used for the data presented in Fig. 1 and expressed as percentages compared to mice before infection. p.i., postinfection. Trypanosomosis-induced serum TNF levels (C) in both LT-α-deficient mice and control WT mice were measured. Sera were collected on days 7, 14, and 28 and just prior to the lethal end of the infection. (∗), no WT mice survived the infection as long as 55 days.
In contrast to WT and LT-α−/− mice, TNF−/− and TNF−/− LT-α−/− mice suffer from only very mild trypanosomosis-associated pathology. The alterations in RBC counts in these mice are presented in Fig. 2B. While mice from both strains showed a slight reduction of RBCs during the 2 days that follow clearance of the first parasitemia peak, all of the mice recovered quickly from infection-associated anemia. Besides the reduced anemia, the T. b. brucei-infected TNF−/− LT-α−/− mice also lacked any other signs of immunopathology and resembled the phenotype of T. brucei-infected TNF−/− mice that was reported previously (20). Just prior to the deaths of the TNF−/− LT-α−/− mice, infection-induced reduction of locomotor activity was the only pathological feature that started to become apparent. As mentioned above, death itself occurred due to a so-far-unexplained sudden loss of parasite growth control that resulted in exponential parasite proliferation. This feature is, however, characteristic of T. brucei parasitemia in all strains of mice studied and consequently cannot be linked to the presence or absence of TNF or LT-α during the experimental infection.
Apart from the occurrence of infection-associated anemia, the occurrence of splenomegaly was also followed in all groups of T. b. brucei-infected mice (results not shown). For this purpose, additional infected mice were sacrificed on days 0, 7, 14, and 28 of the infection. In order to measure splenomegaly at later time points, mice that were used for parasitemia determination were sacrificed when they reached the terminal stage of the infection. The results obtained showed that splenomegaly in all of the mice was already significant at day 7 of the infection. A maximal spleen enlargement was observed around day 14, with spleen weights showing up to a 15-fold increase compared to the spleens of noninfected mice. These results indicate that splenomegaly develops similarly in all strains and consequently that neither infection-associated anemia nor parasitemia control is affected by the alterations in the spleen.
Trypanosomosis-associated induction of serum TNF and IFN-γ in LT-α-deficient mice.
Experimental T. b. brucei infections have been reported to be affected by both TNF and IFN-γ (13, 19). Furthermore, it has been documented that LT-α−/− mice are characterized by partially deficient TNF production upon specific stimulation (1). Hence, we compared circulating TNF and IFN-γ levels in T. b. brucei-infected LT-α-deficient and WT mice at different time points of the infection. The results presented in Fig. 2C show that upon infection with African trypanosomes, both strains of mice respond with increased serum TNF levels. However, in particular during the first stage of the infection, serum TNF levels measured in the LT-α−/− mice remained significantly below those of infected WT mice. On the other hand, towards the end of the infection, serum TNF reached the same elevated levels in both strains. As such, the combined results presented in Fig. 2A and C indicate that in LT-α−/− mice infection-associated anemia, as well as the coinciding signs of morbidity, seems to be correlated with the levels of circulating TNF.
In contrast to the differential regulation of TNF production during the early stage of infection in LT-α−/− and WT mice, no differences were found in serum IFN-γ levels (results not shown). Indeed, increased serum IFN-γ levels, reaching about 8,000 pg/ml, were already apparent on day 7 (i.e., during peak parasitemia). A gradual reduction of these levels to about 500 pg/ml at day 28 was recorded during the chronic phase of the infection, followed by a slight increase to about 1,500 pg/ml towards the lethal endpoint of the infection. Most important, however, was the finding that this serum IFN-γ pattern was not affected by the absence or presence of LT-α.
Anti-trypanosome immunoglobulin induction in the absence of LT-α.
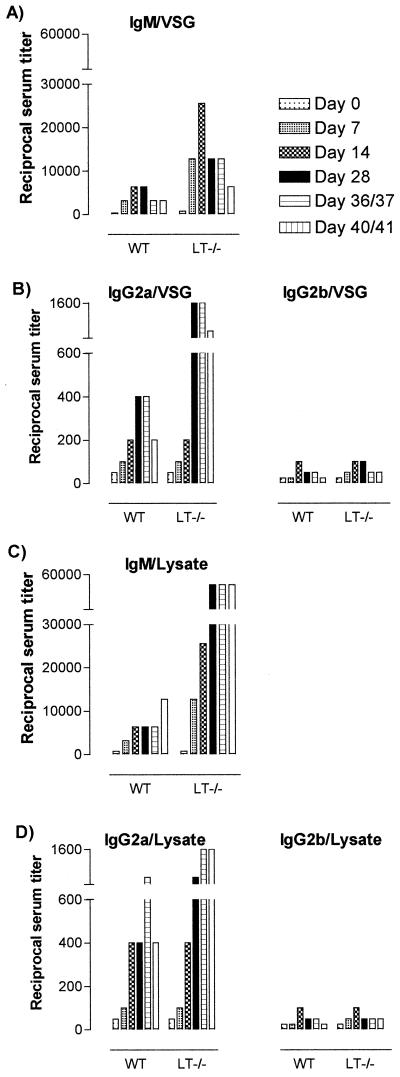
Antigenic variation of the major surface glycoprotein VSG has been developed by trypanosomes as the main defense strategy against the immune system of the mammalian host. However, as VSG is a very immunogenic molecule, anti-VSG antibodies are generated in both a T-cell-dependent and T-cell-independent manner (33). Here, we have analyzed to what extent mice that lack the LT-α gene are capable of generating an infection-induced anti-VSG response as well as a general anti-trypanosome response as shown by endpoint titer determination. Figure 3 shows that in the sera of all infected mice, significant titers of IgM, IgG2a, and IgG2b anti-trypanosome antibodies could be detected. In contrast, IgG1 and IgG3 isotype antibody titers were found to be low and nonspecific in general, showing endpoint titers between 1/10 and 1/20. In Fig. 3A, results of trypanosomosis-induced IgM endpoint titer determinations on a VSG solid-phase coating are shown. From these data, it is clear that specific antibodies are also generated in the absence of LT-α, following LT-α-independent kinetics and reaching peak titers by day 14. However, the results also show that the magnitude of the induced response is significantly increased in the absence of LT-α, reaching endpoint titers of 1/25,600 compared to only 1/6,400 in WT mice. Figure 3B shows that the same trend is observed when measuring the induction of specific anti-VSG IgG2a isotype titers. In this case, maximum specific anti-VSG endpoint titers were measured by day 28, reaching 1/1,600 in the LT-α−/− mice compared to 1/400 in the WT mice. Figure 3B also shows that, in contrast to the IgM-IgG2a titers, the induction of anti-VSG IgG2b isotype titers showed no difference between the two strains and was significantly lower. In this case, maximum endpoint titers were measured by day 14 but only reached 1/100.
FIG. 3.
Infection-induced anti-VSG (A and B) and anti-trypanosome (C and D) responses. Specific IgM (A) and IgG2a and -2b (B) isotype serum endpoint titers were determined in a VSG solid-phase ELISA system (0.2 μg/well), using sera collected on days 7, 14, and 28 and just prior to the lethal end of the infection. The same sera were used to determine specific IgM (C) and IgG2a and -2b (D) isotype serum endpoint titers in an ELISA system that used total soluble trypanosome extracts as a solid-phase coating (0.5 μg/well).
In addition to the determination of specific anti-VSG titers, antibody induction against total trypanosome soluble-protein extracts was analyzed as well. Figure 3C shows that, like the VSG-specific titers, anti-trypanosome antibody titers also reached significantly higher levels in the LT-α−/− mice than in the WT mice. However, in contrast to the VSG-specific titers, the titers shown kept rising during the course of infection, reaching endpoint titers of 1/51,200 and 1/12,800 for the LT-α−/− and WT mice, respectively. Figure 3D shows the same trend for the anti-trypanosome IgG2a titers. While endpoint titers reached 1/1,600 towards the end of the infection in the LT-α−/− mice, these titers remained between 1/400 and 1/800 during the chronic infection stage in WT mice. Again, no difference in infection-induced IgG2b titers was observed between the LT-α−/− and WT mice, and titers of this antibody isotype remained low throughout the infection.
Characteristics of experimental trypanosomosis development in splenectomized mice.
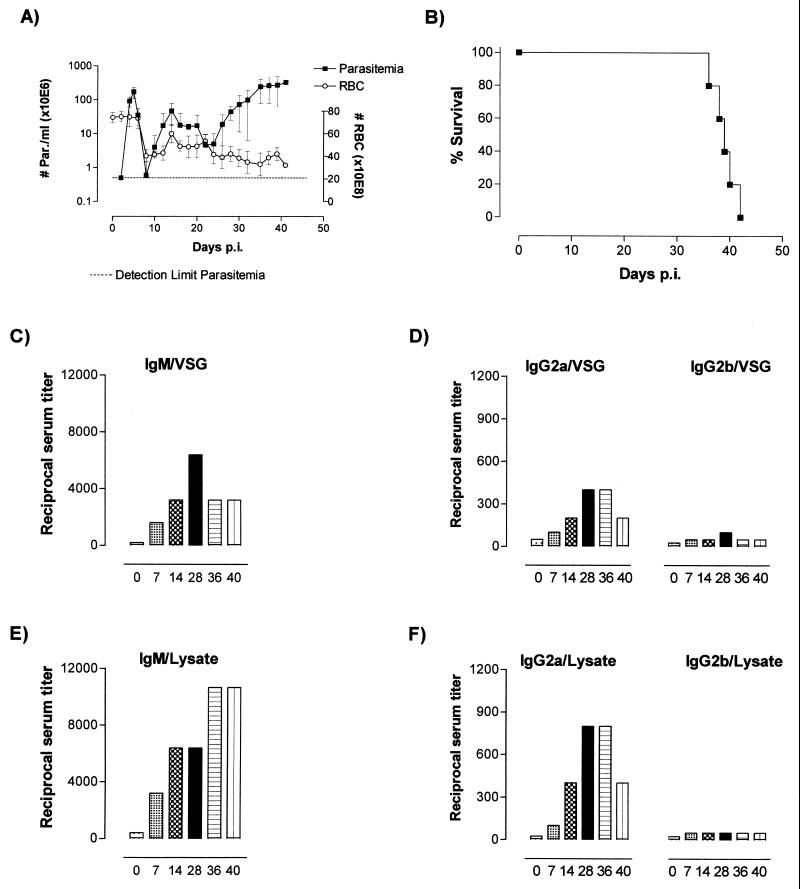
From the results discussed above, it is clear that the capacity of LT-α−/− mice to control trypanosomes is not hampered and that induction of anti-VSG and anti-trypanosome antibodies is even more efficient than in WT mice. Taking into account that LT-α−/− mice not only lack peripheral lymph nodes but also lack splenic germinal centers, the role of the spleen in trypanosomosis was further analyzed in WT mice. To this end, 10 B6129SF2 WT mice were splenectomized 6 weeks prior to infection with the pleomorphic T. b. brucei AnTat 1.1E parasite. After infection, parasitemia, survival, and development of anemia were recorded, as well as the production of anti-VSG and anti-trypanosome antibodies. As shown in Fig. 4A, no difference in the level of parasitemia development or the induction of anemia occurred in the splenectomized mice compared to the fully immune-competent control mice (compare to Fig. 1A and 2A). Also, at the level of survival, splenectomized mice did not show any accelerated mortality, reaching a median survival time of 39 days compared to 38 for the immune-competent WT mice (Fig. 1B). Finally, also at the level of IgM and IgG2a and -2b antibody induction, no alterations appeared to occur in the splenectomized mice compared to control WT mice. This holds true both for anti-VSG induction patterns (compare Fig. 4C and D and WT data in Fig. 3A and B) and for general anti-trypanosome antibody production (compare Fig. 4E and F and WT data in Fig. 3C and D). Hence, during experimental T. b. brucei infections, the efficient induction of anti-trypanosome antibodies occurs in the absence of lymph nodes and splenic-germinal-center formation and also in the complete absence of the spleen.
FIG. 4.
(A) Parasitemia development (left axis) and occurrence of trypanosomosis-associated anemia (RBC; right axis) in splenectomized B6129SF2 mice. The parasitemia values presented are mean parasite counts (# Par.)/ml of blood ± standard deviations. (B) Survival was followed for five individual mice, and five other mice were sacrificed for serum collection on days 7, 14, 28, 36, and 40, respectively. (C and D) Infection-induced specific anti-VSG IgM (C) and IgG2a and -2b (D) isotype serum endpoint titers were determined in a VSG solid-phase ELISA system (0.2 μg/well), using sera collected on days 7, 14, and 28 and just prior to the lethal end of the infection. (E and F) The same sera were used to determine specific IgM (E) and IgG2a and -2b (F) isotype serum endpoint titers in an ELISA system that used total soluble trypanosome extracts as a solid-phase coating (0.5 μg/well).
DISCUSSION
Though it is widely accepted that the main immune escape mechanisms of African trypanosomes rely on antigenic variation of the variant-specific surface glycoprotein VSG (4), it is known that the intensity of the trypanosomosis-induced antibody production by the host does not directly correlate with its relative resistance or susceptibility. Indeed, when F1 mice are generated using one resistant and one susceptible parental mouse, it has been reported that while the antibody-inducing capacity is inherited as a dominant trait, the survival capacity is inherited from the susceptible mouse strain (8). As such, it is clear that besides anti-trypanosome antibody induction, other factors contribute to resistance against trypanosome infections. Most likely a complex cross talk between the host and the parasite takes place in order to ensure optimal host survival while allowing optimal parasite transmission. In this context, it has been shown that apart from triggering antibody production, trypanosomes cause several other immunological activities in their mammalian host, leading to the production of specific cytokines. Indeed, there is accumulating evidence that rapid induction of a type 1 cytokine environment (IFN-γ and TNF) is crucial for initial parasitemia control. It has been reported that while resistant mice express a Th1 cell cytokine response to VSG stimulation, susceptible mice do not (35). Furthermore, using cytokine-deficient mouse models, it has also been suggested that a type 1 cytokine response contributes to trypanosomosis control. First, in IFN-γ-deficient mice, accelerated parasite growth and the lack of proper peak parasitemia control results in early death of the mice (13). Second, in TNF-deficient mice, efficient control of peak parasitemia levels is impaired, although survival time is not shortened (20). In this case, we and others have reported that the effect of TNF on peak parasitemia development is mediated by the direct trypanolytic effect of the cytokine on the trypanosomes (6, 22). The fact that this activity is mediated via the TIP sequence of TNF, the domain that is responsible for the lectin-like activity of the cytokine and that is able to specifically recognize the conserved N-linked high-mannose moiety of the trypanosome VSG, is important (19, 23). Interestingly, LT-α, which is a homologue of TNF with high amino acid sequence homology (28), lacks trypanolytic activity and also lacks the TNF TIP domain (19). Therefore, by using LT-α-deficient mice, we have extended our study to the analysis of the role of LT-α in the control of experimental trypanosomosis and the induced immune pathology. The results presented here show that, in contrast to TNF, LT-α has no direct influence on parasite control. It is interesting, however, that LT-α−/− mice show increased anti-trypanosome IgM-IgG2a serum titers, coinciding with an improved late-stage parasitemia control, while they lack significant IgG1, IgG2b, and IgG3 anti-trypanosome responses. It should be emphasized that LT-α−/− mice do not have peripheral lymph nodes, do not generate splenic germinal centers upon immune stimulation, and also lack the recruitment and development of follicular dendritic cell networks (12) and natural killer cells in the spleen (39). It is important to stress that it has never before been shown that efficient trypanosome control can occur in the absence of lymph nodes. Thus, the findings reported here are of particular interest, as in the past a great deal of effort has gone into trying to unravel lymph node-specific T. brucei-induced immunosuppression, as it was thought that this phenomenon was important for the lack of proper parasitemia control in mice and cattle (3). The results presented here contradict this hypothesis, and as such, call for renewed efforts to unravel host immune responses that are crucial in the control of trypanosomosis. The limited importance of the spleen and splenic-germinal-center formation, on the other hand, has been suggested by previous findings. Indeed, in TNF−/− mice that also lack splenic-germinal-center formation, anti-trypanosome antibodies are formed as efficiently as in WT mice (20). Furthermore, the data presented here indicate that splenectomized mice also control experimental African trypanosomosis in a way similar to that of immune-competent littermates, further suggesting that the spleen plays only a marginal role in control of the infection. Finally, a case report of a splenectomized human trypanosomosis patient also indicated that the lack of a functional spleen does not alter disease progression (24). Hence, neither secondary lymphoid tissue nor germinal-center formation seems to be required for the efficient generation of anti-trypanosome antibodies in an infected host. This may be explained by previous observations indicating that a large portion of the anti-trypanosome IgM response, as well as the anti-VSG IgG2a antibody response, is generated in a T-cell-independent manner (32). Furthermore, it has been suggested that cross activation of “self-reactive” responses (25, 26) together with polyclonal B-cell activation (2) might trigger the development of humoral anti-trypanosome responses. In this context, the fact that LT-α−/− mice are characterized by a fourfold increase in circulating B cells should be taken into account as well (9). One important feature of cross activation of B cells in human trypanosomosis is the possible induction of autoimmune responses (11). However, the lack of increased pathology in the LT-α−/− mice that do show increased levels of anti-trypanosome antibody responses argues against the hypothesis that this type of autoimmune complication is implemented in the infection-associated pathology observed in mice. In fact, our previous work and the results obtained here with TNF-LT-α-double-deficient mice favors the suggested direct role of TNF as a main mediator of trypanosomosis-associated pathology. Indeed, it is only in mice that lack TNF that infection-associated anemia is controlled and the mice do not suffer from severe overall morbidity. Interestingly, during the middle stage of the infection in LT-α−/− mice (day 10 to day 28), anemia is significantly less severe than in WT mice, a finding that can be explained by the reduced TNF induction in LT-α−/− mice during this stage. Moreover, it is clear from the results presented here that during the final stage of infection serum TNF reaches the same levels in both LT-α−/− mice and WT mice, and concomitantly, anemia occurs in the LT-α−/− mice as well. In contrast to the lowered chronic-stage serum TNF levels in the LT-α−/− mice, infection-induced IFN-γ levels appeared to be normal throughout the infection. This finding could be supported by previous reports that attribute both direct and indirect trypanosomosis-associated IFN-γ secretion to CD8+-T-cell activation (31) and to the fact that circulating CD8+-T-cell numbers have been shown to be normal in LT-α−/− mice (9).
In conclusion, the results presented in this study show that the genetic deficiency of LT-α that results in the lack of peripheral lymphoid tissue and germinal-center formation does not hamper the control of experimental trypanosomosis. These results certainly do not reflect a general infection resistance phenotype characteristic of mice carrying an LT deficiency, as it has been shown before that such mice have increased susceptibility to viral infections (17), as well as Mycobacterium and Candida infections (15, 29). In the case of experimental African trypanosomosis, improved infection resistance in the absence of LT-α coincides with improved anti-trypanosome IgM and IgG2a antibody responses, lower chronic-stage parasitemia levels, and prolonged survival. Although the increased antibody titers themselves could be accounted for by a combined effect of increased presence of circulating B cells in these mice and a mechanism of T-cell-independent antibody generation and polyclonal B-cell activation, the results obtained raise the intriguing and still-unanswered question of what the mechanism is that results in the final parasitemia-controlling potential of IgM and/or IgG2a isotype antibodies. At the level of trypanosomosis-associated immune pathology, the results presented confirm the key role of TNF. Indeed, while general pathology, and more particular anemia, is virtually absent in both TNF−/− and TNF−/− LT-α−/− mice, it is also significantly reduced in LT-α−/− mice during the middle infection stage that is characterized by lowered circulating serum TNF levels. However, the exact role of TNF in the functional cascade that leads to trypanosomosis-induced RBC lysis remains to be elucidated. Finally, data presented here show that in experimental African trypanosome infections the combined genetic deficiency of TNF−/− and LT-α−/− gives rise to a phenotype that is characterized by improved antibody-mediated chronic-stage parasitemia control and the virtual absence of infection-associated anemia and immunopathology, together resulting in a significantly improved resistance to T. b. brucei infections. However, even in TNF−/− LT-α−/− mice, trypanosome growth becomes uncontrolled after about 8 to 9 weeks of infection. So far, nothing is known about the signals that trigger the induction of this final exponential growth phase of the parasite, and further research will be needed to fully understand the immunological interactions that mediate both host and parasite survival during African trypanosome infections.
Acknowledgments
This project was partially funded by the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases, The Belgian National Fund for Scientific Research (NFWO no. 6.0325.95), and an Interuniversity Attraction Pole Program financed by the Belgian state, Diensten van de Eerste Minister-Federale diensten voor wetenschappelijke, technische en culturele aangelegenheden. S.M. is a Postdoctoral Fellow of the Foundation of Scientific Research-Flanders (FWO).
REFERENCES
- 1.Alexpoulou, L., M. Pasparakis, and G. Kollias. 1998. Complementation of lymphotoxin alpha knockout mice with tumor necrosis factor-expressing transgene rectifies defective splenic structure and function. J. Exp. Med. 188:745-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assoku, R., I. Tizard, and K. Neilsen. 1977. Free fatty acids, complement activation, and polyclonal B-cell stimulation as factors in the immunopathogenesis of African trypanosomiasis. Lancet ii:956-959. [DOI] [PubMed]
- 3.Beschin, A., L. Brys, S. Magez, M. Radwanska, and P. De Baetselier. 1998. Trypanosoma brucei infection elicits nitric oxide-dependent and nitric oxide-independent suppressive mechanisms. J. Leukoc. Biol. 63:429-439. [DOI] [PubMed] [Google Scholar]
- 4.Cross, G. 1990. Cellular and genetic aspects on antigenic variation in trypanosomes. Annu. Rev. Immunol. 8:83-110. [DOI] [PubMed] [Google Scholar]
- 5.Darji, A., M. Sileghem, H. Heremans, L. Brys, and P. De Baetselier. 1993. Inhibition of T-cell responsiveness during experimental infections with Trypanosoma brucei: active involvement of endogenous gamma interferon. Infect. Immun. 61:3098-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daulouéde, S., B. Bouteille, D. Moynet, P. De Baetselier, P. Courtous, J.-L. Lemesre, A. Buguet, R. Cespuglio, and P. Vincendeau. 2001. Human macrophage tumor necrosis factor (TNF)-alpha production induced by Trypanosoma brucei gambiense and the role of TNF-alpha in parasite control. J. Infect. Dis. 183:988-991. [DOI] [PubMed] [Google Scholar]
- 7.De Gee, A., G. Sonnenfeld, and J. Mansfield. 1985. Genetics of resistance to the African trypanosomes. V. Qualitative and quantitative differences in interferon production among susceptible and resistant mouse strains. J. Immunol. 134:2723-2726. [PubMed] [Google Scholar]
- 8.De Gee, A. L. W., R. F. Levine, and J. M. Mansfield. 1988. Genetics of resistance to the African trypanosomes. VI. Heredity of resistance and variable surface glycoprotein-specific immune responses. J. Immunol. 140:283-288. [PubMed] [Google Scholar]
- 9.De Tongi, P., J. Goellner, N. Ruddle, P. Streeter, A. Fick, S. Mariathasan, S. Smith, R. Carlson, L. Shornick, J. Strauss-Schoenberger, J. Russle, R. Karr, and D. Chaplin. 1994. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264:703-707. [DOI] [PubMed] [Google Scholar]
- 10.Eugster, H. P., M. Muller, U. Karrer, B. Car, B. Schnyder, V. Eng, G. Woerly, M. Le Hir, F. di Padova, M. Aguet, R. Zinkernagel, H. Bluethmann, and B. Ryffel. 1996. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-alpha double-deficient mice. Int. Immunol. 8:23-36. [DOI] [PubMed] [Google Scholar]
- 11.Girard, M., S. Bisser, P. Büscher, B. Bouteille, J. Preud'homme, and M. Jauberteau. 2000. Cross-reactivity of anti-galactocerebroside autoantibodies with a Trypanosoma brucei proteolipidic epitope. Clin. Exp. Immunol. 119:516-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez, M., F. Mackay, J. Browning, M. Kosco-Vilbois, and R. Noelle. 1998. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J. Exp. Med. 187:997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertz, C. J., H. Filutowicz, and J. Mansfield. 1998. Resistance to the African trypanosomes is IFN-gamma dependent. J. Immunol. 161:6775-6783. [PubMed] [Google Scholar]
- 14.Hunter, C., J. Gow, P. Kennedy, F. Jennings, and M. Murray. 1991. Immunopathology of experimental African sleeping sickness: detection of cytokine mRNA in the brains of Trypanosoma brucei brucei-infected mice. Infect. Immun. 59:4636-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, M., N. Brown, N. Allia, and B. Ryffel. 2000. Fatal Mycobacterium bovis infection in TNF-LT-alpha-deficient mice. Clin. Immunol. 94:192-199. [DOI] [PubMed] [Google Scholar]
- 16.Kaushik, R. S., J. Uzonna, Y. Zhang, J. Gordon, and H. Tabel. 2000. Innate resistance to experimental African trypanosomiasis: differences in cytokine (TNF-alpha, IL-6, IL-10 and IL-12) production by bone marrow-derived macrophages from resistant and susceptible mice. Cytokine 12:1024-1034. [DOI] [PubMed] [Google Scholar]
- 17.Kumaragiru, U., I. Davis, S. Desphande, S. Tevethia, and B. Rousse. 2001. Lymphotoxin alpha−/− mice develop functionally impaired CD8+ T cell responses and fail to contain virus infection of the central nervous system. J. Immunol. 166:1066-1074. [DOI] [PubMed] [Google Scholar]
- 18.Lanham, S. 1968. Separation of trypanosomes from the blood of infected rats and mice by anion-exchangers. Nature 218:1273-1274. [DOI] [PubMed] [Google Scholar]
- 19.Lucas, R., S. Magez, R. De Leys, L. Fransen, J.-P. Scheerlinck, M. Rampelberg, E. Sablon, and P. De Baetselier. 1994. Mapping the lectin-like activity of tumor necrosis factor. Science 263:814-817. [DOI] [PubMed] [Google Scholar]
- 20.Magez, S., M. Radwanska, A. Beschin, K. Sekikawa, and P. De Baetselier. 1999. Tumor necrosis factor alpha is a key mediator in the regulation of experimental Trypanosoma brucei infections. Infect. Immun. 67:3128-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magez, S., R. Lucas, A. Darji, E. Songa, R. Hamers, and P. De Baetselier. 1993. Murine tumour necrosis factor plays a protective role during the initial phase of the experimental infection with Trypanosoma brucei brucei. Parasite Immunol. 15:635-641. [DOI] [PubMed] [Google Scholar]
- 22.Magez, S., M. Geuskens, A. Beschin, H. Del Favero, R. Verscheuren, R. Lucas, E. Pays, and P. De Baetselier. 1997. Specific uptake of tumor necrosis factor-α is involved in growth control of Trypanosoma brucei. J. Cell Biol. 137:715-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magez, S., M. Radwanska, B. Stijlemans, H. Xong, E. Pays, and P. De Baetselier. 2001. A conserved flagellar pocket exposed high mannose moiety is used by African trypanosomes as a host cytokine binding molecule. J. Biol. Chem. 276:33458-33464. [DOI] [PubMed] [Google Scholar]
- 24.Malesker, M. A., D. Boken, T. Ruma, P. Vuchetich, P. Murphy, and P. Smith. 1999. Rhodesian trypanosomiasis in a splenectomized patient. Am. J. Trop. Med. Hyg. 61:428-430. [DOI] [PubMed] [Google Scholar]
- 25.Muller, N., J. Mansfield, and T. Seebeck. 1996. Trypanosome variant glycoproteins are recognized by self-reactive antibodies in uninfected hosts. Infect. Immun. 64:4593-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller, N., M. Imbodem, E. Detmer, J. Mansfield, and T. Seebeck. 1993. Cytoskeleton-associated antigens from African trypanosomes are recognized by self-reactive antibodies of uninfected mice. Parasitology 107:411-417. [DOI] [PubMed] [Google Scholar]
- 27.Namangala, B., L. Brys, S. Magez, P. De Baetselier, and A. Beschin. 2000. T. b. brucei infection impairs MHC class II antigen presentation capacity of macrophages. Parasite Immunol. 22:361-370. [DOI] [PubMed] [Google Scholar]
- 28.Nedwin, G., S. Naylor, A. Sakaguchi, D. Smith, J. Jarrett-Nedwin, D. Pennica, D. Goeddel, and P. Gray. 1985. Human lymphotoxin and tumor necrosis factor genes: structure, homology and chromosomal localization. Nucleic Acids Res. 13:6361-6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Netea, M., L. van Tits, J. Curfs, F. Amoit, J. Mais, J. van der Meer, and B. Kullberg. 1999. Increased susceptibility of TNF-alpha lymphotoxin-alpha double knockout mice to systemic candidiasis through impaired recruitment of neutrophils and phagocytosis of Candida albicans. J. Immunol. 163:1498-1505. [PubMed] [Google Scholar]
- 30.Okomo-Assoumou, M. C., S. Daulouede, J. L. Lemesre, A. N'Zila-Mouanda, and P. Vincendeau. 1995. Correlation of high serum levels of tumor necrosis factor-alpha with disease severity in human African trypanosomiasis. Am. J. Trop. Med. Hyg. 53:539-543. [DOI] [PubMed] [Google Scholar]
- 31.Olson, T., M. Bakhiet, C. Edlund, B. Höjeberg, P. H. Van der Meide, and K. Kristensson. 1991. Bidirectional activity signals between Trypanosoma brucei and CD8+ T cells: a trypanosome-released factor triggers interferon-γ production that stimulates parasite growth. Eur. J. Immunol. 21:2447-2454. [DOI] [PubMed] [Google Scholar]
- 32.Radwanska, M., S. Magez, A. Michel, B. Stijlemans, M. Geuskens, and E. Pays. 2000. Comparative analysis of antibody responses against HSP60, ISG70 and VSG reveals a complex antigen-specific pattern of immunoglobulin isotype switching during infections by T. brucei. Infect. Immun. 68:848-860. [DOI] [PMC free article] [PubMed]
- 33.Reintiz, D., and J. Mansfield. 1991. T-cell-independent and T-cell dependent variant surface glycoprotein epitopes in Trypanosoma-infected mice. Infect. Immun. 58:2337-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheifer, K. W., and J. M. Mansfield. 1993. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J. Immunol. 151:5492-5503. [PubMed] [Google Scholar]
- 35.Schopf, L., H. Filotowicz, X. Bi, and J. Mansfield. 1998. Interleukin-4-dependent immunoglobulin G1 isotype switch in the presence of a polarized antigen-specific Th1-cell response to the trypanosome variant surface glycoprotein. Infect. Immun. 66:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sileghem, M., J. Flynn, L. Logan-Henfrey, and J. Ellis. 1994. Tumor necrosis factor production by monocytes from cattle infected with Trypanosoma (Duttonella) vivax and Trypanosoma (Nannomonas) congolense: possible association with severity of anaemia associated with the disease. Parasite Immunol. 16:51-54. [DOI] [PubMed] [Google Scholar]
- 37.Tartaglia, L., and D. Goeddel. 1992. Two TNF receptors. Immunol. Today 13:151-153. [DOI] [PubMed] [Google Scholar]
- 38.Vickerman, K., L. Tetley, K. Hendry, and C. Turner. 1988. Biology of African trypanosomes in the tsetse fly. Biol. Cell 64:109-119. [DOI] [PubMed] [Google Scholar]
- 39.Wu, Q., Y. Sun, J. Wang, X. Lin, Y. Wang, L. Pegg, A. Fütterer, K. Pfeffer, and Y.-X. Fu. 2001. Signal via lymphotoxin-βR on bone marrow stromal cells is required for an early checkpoint of NK cell development. J. Immunol. 166:1684-1689. [DOI] [PubMed] [Google Scholar]