Abstract
Bordetella avium causes bordetellosis, an upper respiratory disease of birds. Commercially raised turkeys are particularly susceptible. We report here on the use of a recently described B. avium bacteriophage, Ba1, as a tool for investigating the effects of lysogeny and phage resistance on virulence. We found that lysogeny had no effect on any of the in vivo or in vitro measurements of virulence we employed. However, two-thirds (six of nine) spontaneous phage-resistant mutants of our virulent laboratory strain, 197N, were attenuated. Phage resistance was associated, in all cases, with an inability of the mutants to bind phage. Further tests of the mutants revealed that all had increased sensitivities to surfactants, and increased amounts of incomplete (O-antigen-deficient) lipopolysaccharide (LPS) compared to 197N. Hot phenol-water-extracted 197N LPS inactivated phage in a specific and dose-dependent manner. Acid hydrolysis and removal of lipid A had little effect upon the ability of isolated LPS to inactivate Ba1, suggesting that the core region and possibly the O antigen were required for phage binding. All of the mutants, with one exception, were significantly more sensitive to naive turkey serum and, without exception, significantly less able to bind to tracheal rings in vitro than 197N. Interestingly, the three phage-resistant mutants that remained virulent appeared to be O antigen deficient and were among the mutants that were the most serum sensitive and least able to bind turkey tracheal rings in vitro. This observation allowed us to conclude that even severe defects in tracheal ring binding and serum resistance manifested in vitro were not necessarily indicative of attenuation and that complete LPS may not be required for virulence.
Bordetella avium causes bordetellosis, an upper respiratory tract disease of birds. Commercially raised turkeys are particularly susceptible (24). In diseased turkeys, B. avium is typically found bound to the ciliated cells of tracheal epithelium (24). During the infection, the ciliated epithelium is lost and replaced by nonciliated cuboidal epithelium (2). Experimentally infected turkey poults normally recover after several weeks (26). However, in instances where the infection is severe, malformation of tracheal rings can lead to tracheal collapse and suffocation (2). Also, naturally infected birds are subject to a variety of secondary infections that result in severe economic losses in all poultry-producing regions of the world (24).
As with all of the medically important species of Bordetella, B. avium has factors associated with virulence. These include dermonecrotic toxin, tracheal cytotoxin (10, 27), at least one hemagglutinin (3, 27), lipopolysaccharide (LPS) (25), and fimbriae (1). In order to discover additional factors that may influence B. avium virulence, we have utilized several strategies for isolating attenuated mutants (25, 27).
We describe here the use of a recently isolated B. avium temperate bacteriophage, Ba1 (22), as a tool for investigating B. avium virulence. Both lysogeny (5, 6) and phage resistance (7, 21) have been shown to affect the interaction of bacteria with their hosts. Consequently, we examined both of these paramaters. We found that lysogeny had no discernible effect upon virulence. However, isolation and characterization of a collection of nine spontaneous phage-resistant mutants revealed that all of the mutants had changes in their cell surfaces that prevented phage binding, and two-thirds of these mutants were avirulent. Biochemical evidence supported a direct role for the LPS core and/or O antigen in Ba1 binding. In the mutants, lack of O antigen was associated with the most severe reduction in serum resistance and tracheal ring binding in vitro. Interestingly, the three phage-resistant mutants that remained virulent appeared to lack O antigen.
MATERIALS AND METHODS
Strains and growth conditions.
B. avium 197N (27) was used exclusively as the parental strain in these studies. Strain 197N is sensitive to bacteriophage Ba1 and nonlysogenic for Ba1 (22). Strain AP21 is the same as strain 197N except that it is lysogenic for Ba1 (22). Strain AP82 is the same as 197N, except that it has a mini-Tn5 insertion in the wbl gene cluster and is thus defective in proper LPS core and O-antigen assembly, and is Ba1 resistant (25). B. avium broth cultures were grown at 37°C with shaking in brain heart infusion (BHI) medium (Difco) as described previously (27). BHI agar plates consisted of BHI broth with 1.5% agar added. Soft agar consisted of BHI broth with 0.7% agar added. Bacteriophage Ba1 c1 (22), a clear plaque mutant of the normally temperate Ba1, was propagated as described by Shelton et al. (22).
Genetic techniques.
Phage-resistant mutants were isolated by spotting a high-titer lysate of Ba1 c1 (>5 × 109 PFU) onto a lawn of strain 197N. The lawn was formed by suspending ca. 5 × 109 CFU (0.2 ml) of an overnight culture of strain 197N in 3 ml of BHI soft agar. After overnight incubation at 37°C, individual colonies present in the cleared area of the phage spot were purified twice through single-colony isolation. In order to promote the isolation of independently derived phage-resistant mutants, independent cultures of B. avium were employed, and only one mutant was saved from each spot. The mutants, once purified, were screened for phage sensitivity by cross-streaking (23, 25). To ensure that the mutants were not lysogenized (e.g., by a Ba1 c1 mutant that had reverted to temperate growth), culture supernatants of the resistant mutants were screened for plaque-forming phage; none were detected (22). Also, DNA hybridization studies with whole-phage DNA failed to detect any difference between the resistant mutants and the nonlysogenic parental strain (22). Finally, plaquing efficiency tests revealed that all mutant strains were completely unable to support Ba1 c1 plaque formation even at phage/bacterium ratios of >10:1 (a level of resistance inconsistent with the immunity conferred by lysogeny [22; unpublished observations]).
Tests for in vivo attenuation.
Phage-resistant mutants were initially screened for attenuation by comparing infectivity rates by a single-dose test that employed 10 birds dosed at ca. 107 CFU/bird. This test revealed that a high proportion of phage-resistant mutants were likely reduced in virulence. A more precise measurement of attenuation was accomplished by determining the 50% infectious dose (ID50) for each mutant as previously described (27).
Phage binding and inactivation assays.
Bacteria for phage-binding assays were obtained after overnight growth at 37°C, collection (centrifugation for 10 min at 5,000 × g), and concentration in phosphate-buffered saline (PBS). Phage were obtained from high-titer lysates diluted with PBS. Unless otherwise noted, the phage-neutralizing capacity of whole bacteria was determined by incubating ca. 5 × 107 PFU of Ba1 c1 with ca. 1010 CFU of B. avium for 1 h in a total volume of 250 μl. After incubation, the bacteria were pelleted by centrifugation, and the titer of a 100-μl sample of the supernatant was determined (22) to enumerate the unbound PFU. The same assay was used to test the neutralizing capacity of LPS preparations except that no centrifugation was employed at the end of the incubation. Numbers of phage bound to bacteria or inactivated by LPS were determined from control incubations that had no bacteria (or LPS) added but were otherwise treated identically. The percent phage inactivated (%PI) was calculated from the control PFU (PFUc) and the PFU remaining (PFUr) after incubation with bacteria or LPS as follows: %PI = [(PFUc − PFUr)/PFUc] × 100. The percentage of phage inactivating ability retained in a subfraction (subscript “sf”) of equal volume to an initial fraction (subscript “if”) was calculated as follows: {(100 − [%PI]if)/(100 − [%PI]sf)} × 100.
Sensitivity of Ba1 c1-resistant mutants to cell surface reactive agents (surfactants).
The sensitivity of the mutants to EDTA (0.1%) or Triton X-100 (1.0%) was determined from their plating efficiency (PE) on medium containing the additives to medium without additions. Decreased resistance was defined as a minus value (−) when the PE was lowered by a factor of ≥104 when normalized to the PE change of 197N (the agents employed had essentially no effect on the PE of 197N).
LPS solubilization and gel electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on whole-cell LPS solubilized as described by Spears et al. (25) and separated on an 18% polyacrylamide gel (16). Gels were stained by the silver-staining technique of Tsai and Frasch (28).
LPS isolation.
LPS was isolated by the hot phenol-water method as described by Inzana (12). Briefly, ca. 1011 CFU of B. avium from an overnight broth culture, were collected by centrifugation (5,000 × g for 8 min at 4°C). The bacterial pellet was resuspended in 10 ml of PBS, and the bacteria were reisolated and washed once in 40 ml of PBS containing 0.15 mM CaCl2 and 0.5 mM MgCl2. The resulting pellet was resuspended in 4.5 ml of distilled H2O, and proteinase K was added to a final concentration of 0.25 μg/μl. The suspension was then incubated for 30 min at room temperature. Hot (65 to 70°C) phenol (5 ml) was added, and the suspension was stirred vigorously for 30 min while a constant temperature of 65 to 70°C was maintained. The suspension was chilled on ice for 20 min and then transferred to a 15-ml tube, and phases were separated by centrifugation (7,000 × g for 15 min at 4°C). The aqueous phase was removed and transferred to a separate tube. The remaining phenol phase was washed with 5 ml of distilled H2O. The aqueous phases were pooled and adjusted to 0.05 M sodium acetate by the addition of concentrated (3 M) stock. To this solution, 10 volumes of 95% ethanol were added, and the sample was stored at −20°C overnight. The resulting precipitate was collected by centrifugation (2,000 × g for 10 min at 4°C). The supernatant was discarded, and the pellet resuspended in 2.0 ml of distilled H2O and reprecipitated with ethanol. The final precipitate was collected as described above, dried under a vacuum, resuspended in distilled H2O, and stored at 4°C. LPS was quantitated by measuring the amount of 2-keto-3-deoxyoctonic acid (KDO), an eight-carbon sugar peculiar to LPS (4) in whole cells and isolated LPS preparations. KDO measurements indicated that B. avium had 21 ± 4 μg (ca. 82 nmol) of KDO per 1010 cells and that the extraction efficiency was 69% ± 5%.
LPS fractionation.
LPS extracted from ca. 1011 cells was hydrolyzed in 5% acetic acid at 100°C for 3 h (8, 15). After hydrolysis, the material was lyophilized to remove the acetic acid and resuspended in 750 μl of distilled H2O. The phage-inactivating activity of the resuspended material was measured, and the insoluble material (presumed to include the lipid A fraction) was removed by centrifugation. The soluble saccharide-containing fraction was concentrated by lyophilization, resuspended in 40 μl of 50 mM pyridine-acetate buffer (pH 5.0), and applied to a Sephadex G-50 column (5-ml bed volume) that had been equilibrated with sample buffer (14). Fractions (0.1 ml) were collected, and a portion of each fraction was assayed for neutral sugars by the phenol-sulfuric acid method (9) and for KDO.
Serum resistance and tracheal ring adherence assays.
The sensitivity of the phage-resistant mutants to naive turkey serum and the adherence to turkey tracheal rings in vitro were tested as described by Temple et al. (27).
Statistical analysis.
The standard deviation of the mean was calculated with the aid of the Microsoft Excel STDEV function. The standard error was calculated as the standard deviation divided by the square root of the number of experiments. The statistical significance of the mean differences was determined by using Student's t test with the aid of Microsoft Excel statistical analysis software (version 4.0). In some cases, significant differences were determined by the Z-Test (Microsoft Excel version 4.0). The Z-test was employed to examine the probability of a single mutant ID50 value falling within two standard deviations of the mean ID50 of the parental strain. The mean parental ID50 was calculated by using 23 independent determinations.
RESULTS
Effect of Ba1 lysogeny on B. avium virulence.
Lysogeny had no detectable effect on the ability of B. avium to colonize the turkey tracheas, as indicated in experiments in which a Ba1 lysogen, strain AP21, was compared to the parental (nonlysogenic) strain (197N) for its ability to colonize 1-week-old turkey poults. ID50 determinations revealed that there was no significant change in the average ID50 of the lysogen (Table 1). Similarly, in vitro measurements of the ability of the lysogen and the parental strain to bind to tracheal rings and to withstand the bactericidal effect of normal turkey poult serum revealed no significant differences (Table 1). Further, monitoring of clinical signs of infection (e.g., ocular-nasal discharge, snicking [coughing], and anorexia) and colonization levels over a 6-week period in one group of turkeys revealed no striking differences in the length or the severity of the disease between the lysogen and nonlysogens (unpublished observations).
TABLE 1.
Comparison of the parental strain (197N) and a Ba1 lysogenic strain (AP21) with respect to turkey colonization, in vitro tracheal ring binding, and serum resistance
| Strain | Mean ID50 (106)a ± SD | Mean CFU/ring (104)b ± SD | Mean % serum resistancec |
|---|---|---|---|
| 197N | 6.7 ± 7.0 | 3.7 ± 1.5 | (100) |
| AP21 | 9.8 ± 9.3 | 2.8 ± 1.6 | 134 ± 25 |
ID50 determinations in 1-week-old turkey poults were performed as previously described (27). Isolates recovered from poults infected with strain AP21 were tested for lysogeny, and all were found to be lysogenic. There was no significant difference (P > 0.05) in the colonization rates between strains AP21 and 197N in an unpaired Student t test.
Bacteria were incubated with tracheal rings for 1 h and treated as described by Temple et al. (27). There was no significant difference (P > 0.05) in the attachment levels between strains AP21 and 197N in an unpaired Student’s t test. A minimum of three independent assays, performed in duplicate, were averaged.
The percentage of bacteria that survived in 50% naive turkey serum normalized to the percentage of 197N that survived (set at 100%) (25). Three separate measurements were averaged. There was no significant difference (P > 0.05) in serum resistance between the parent and lysogen in an unpaired, two-tailed Student t test.
Attenuation of phage-resistant mutants.
In contrast to the results with the lysogenic strain, a screening of nine spontaneous phage-resistant mutants revealed that six were attenuated, as defined by their statistically different ID50 values compared to the parent (Table 2). The degree of attenuation varied: the least-attenuated mutant of the six mutants had an ID50 ca. 10-fold higher than that of the parental strain, and most attenuated mutants did not infect turkeys at any dose tested. For these latter mutants, an exact ID50 could not be calculated (this is denoted by a “greater-than” sign in the table). Such mutants were tabulated in order of the highest unsuccessful dose tested (Table 2). Routine screening of the mutants revealed no noticeable differences in growth rate or hemagglutination properties of any of the mutants compared to the parental (197N) strain (hemagglutination is a phenotypic trait closely associated with virulence [27]).
TABLE 2.
Virulence of B. avium phage-resistant mutants
| Strain | Ba1 c1 sensitivity | ID50 (106)a | Statistical difference from 197Nb |
|---|---|---|---|
| 197N | + | 8 ± 2 | NA |
| AP104 | − | 3 | − |
| AP99 | − | 9 | − |
| AP108 | − | 12 | − |
| AP103 | − | 81 | + |
| AP101 | − | 150 | + |
| AP107c | − | >900 | + |
| AP105 | − | >3,000 | + |
| AP106 | − | >4,000 | + |
| AP102 | − | >5,000 | + |
ID50 measurements were determined as described in the text. The “greater-than” sign indicates that no turkeys were colonized at any dose given. The numeric value shown after the greater-than sign indicates the lowest possible ID50 achievable (i.e., it represents the ID50 value generated if all birds were colonized at a dose 1 order of magnitude higher than the highest dose employed). The standard deviation of the mean ID50 is indicated for the parental strain (197N) in which the ID50 values for 23 independent and identical experiments were averaged.
The statistical analysis was performed with the log10 ID50 values shown in the table. A plus sign indicates that there was a significant difference; a minus sign indicates there was no significant difference (P > 0.05). The Z-test was employed as described in the text. NA, not applicable.
Strain AP107 was recovered from turkeys at a rate similar to that for the parent. However, all isolates examined were Ba1-sensitive revertants. None of the other strains exhibited reversion to phage sensitivity in vivo.
Mechanism of phage resistance.
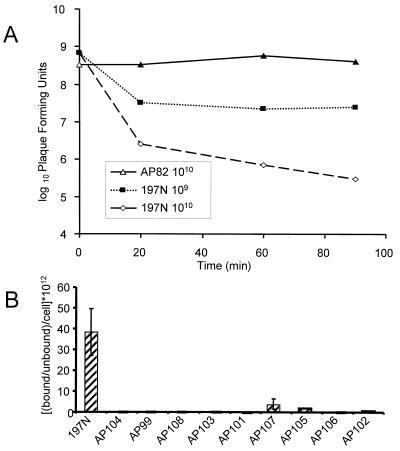
In order to determine the properties responsible for phage resistance and the high frequency of attenuation, we developed a phage-binding assay that measured the degree to which the phage-resistant mutants could adsorb and inactivate phage. In this assay, we found an excellent correlation between the number of phage-sensitive bacteria added and the decrease in PFU over time (Fig. 1A). From the results of this test, we established a fixed concentration of phage and bacteria (ca. 5 × 107 phage/1010 bacteria) and time (1 h) to use in subsequent screening tests. (Incubations of >90 min resulted in phage production from infected cells.) With this assay, we found that none of the phage-resistant mutants bound significant numbers of phage compared to the parental strain (Fig. 1B).
FIG. 1.
(A) Adsorption of Ba1 c1 to strain 197N and the LPS-defective insertion mutant strain AP82. Approximately 108 PFU of Ba1 c1 phage were mixed with 1010 CFU of strain AP82 and 109 or 1010 CFU of the Ba1-sensitive strain 197N. At the times indicated, a portion of the mixture was withdrawn; the bacteria and bacterium-bound phage were removed by centrifugation, and the titer of the supernatant was determined for unadsorbed phage as described in the text. The graph illustrates the results from a single representative experiment. (B) Adsorption of Ba1 c1 to strain 197N and to each of the phage-resistant mutants according to the protocol described in the text. Values on the y axis represent the proportion of the phage removed by the parent and each mutant after 1 h, normalized to the number of cells added to each assay. The multiplication factor (1012) is present to adjust the scale for legibility. The mean error bars are shown for the results of two independent experiments.
Sensitivity of the phage-resistant mutants to surfactants and alterations in LPS SDS-PAGE profile.
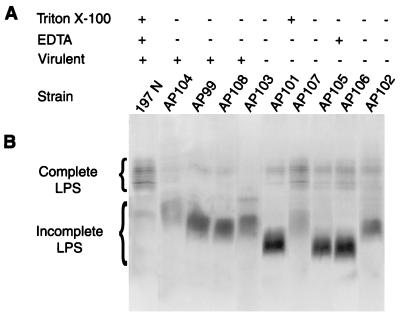
Since all of the mutants failed to bind phage, we examined the mutants for alterations in their cell surface properties. Alterations in sensitivity to at least one of the two surfactants tested were noted with each of the mutants (Fig. 2A). Additionally, all mutants showed a change in their SDS-PAGE LPS banding patterns (Fig. 2B). An increased amount of incomplete LPS (LPS lacking the O-antigen extension possessed by complete LPS and having a faster migration rate) was common to all of the mutants, and in some of the mutants only incomplete LPS was detected (e.g., strain AP104). Faster- and slower-migrating versions of incomplete LPS were noted in B. avium and likely correspond to molecules that contain (i) lipid A plus core or (ii) lipid A-core plus a distal trisaccharide added to the core, respectively (25). Although it is possible to distinguish mutant classes based upon these criteria, only the more obvious criteria of complete and incomplete LPS were used here.
FIG. 2.
(A) The resistance (+) or sensitivity (−) of strain 197N and the nine phage-resistant mutants to cell surface active agents was determined by the PE on medium containing each of the agents as described in the text. For the virulence scores, a plus indicates that that mutant showed no significant difference from the parent in terms of virulence (ID50) and a minus indicates a statistically significant decrease in virulence (refer to Table 2 for additional information). (B) Silver-stained SDS-PAGE analysis of LPS solubilized from equivalent numbers of the parent (197N) and phage-resistant mutant cells. Complete (O-antigen-containing) LPS and incomplete LPS (O antigen absent) are denoted by the brackets.
Phage inactivation by B. avium LPS.
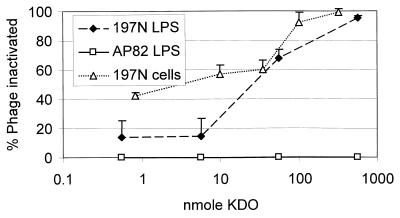
The foregoing results suggested that LPS was the receptor for Ba1 c1. Also suggestive was our finding that 197N cells, solubilized by boiling and treatment with enzymes that digest protein, RNA and DNA, retained ca. 40% (38% ± 25%) of the phage-inactivating activity of whole cells. No phage-inactivating activity could be detected in solubilized material from the nine mutants. For a definitive result, we initiated phage-binding assays that employed hot phenol-water-extracted LPS. The results indicated that LPS extracted from strain 197N inactivated phage in a dose-dependent manner that was not seen with LPS from the phage-resistant control strain AP82 (Fig. 3). Interpolation of the data represented in Fig. 3 revealed that ca. 26 nmol of extracted LPS (ca. 3 × 109 cell equivalents) was required to inactivate 50% (ca. 2.5 × 107) of Ba1 c1. Under similar assay conditions, ca. 5.2 × 108 197N whole cells were required for 50% phage inactivation (Fig. 3).
FIG. 3.
Phage inactivation by isolated LPS from strain 197N and the LPS-defective, phage-resistant insertion mutant AP82. Whole cells of strain 197N, assayed under similar conditions, are shown for comparison. LPS preparations or whole cells were diluted to give the amounts of KDO indicated and incubated with ca. 5 × 107 PFU of Ba1c1, and the %PI was calculated after a 1 h of incubation (volumes were constant in all experiments). Vertical bars denote the standard error from a minimum of two separate experiments.
Approximately 60% (56% ± 7.1%) of the phage-inactivating activity of isolated LPS was retained after acid hydrolysis. Subsequent removal of the insoluble lipid A from the hydrolysate by centrifugation had no significant effect upon the phage-inactivating ability. Size fractionation of the soluble portion of the hydrolysate by exclusion chromatography failed to produce distinct neutral saccharide-containing peaks (data not shown).
Phage resistance and in vitro indicators of virulence.
The phage-resistant mutants were subjected to two in vitro assays that measured (i) the ability of the mutants to bind to isolated tracheal rings from turkey embryos and (ii) the resistance of the mutants to the bactericidal effects of normal turkey poult serum. All but one of the mutants (strain AP106) exhibited a significant decrease in tracheal ring binding compared to the parental strain, and all of the mutants had a significant decrease in serum resistance compared to the parental strain (Table 3). However, mutants in which some complete LPS could be detected (refer to Fig. 2B) were more likely to have higher levels of serum resistance. A similar trend was evident with tracheal ring binding (Table 3).
TABLE 3.
Tracheal adherence and serum sensitivity of the parental (197N) strain and the phage-resistant mutants
| Strain | Virulencea | % Bound to tracheal ringb | % Survival in 50% serumc | Complete LPS detectedd |
|---|---|---|---|---|
| 197N | + | 100 | 100 | +++ |
| AP104 | + | 18.1 ± 2.1 | <0.01 | − |
| AP99 | + | 12.1 ± 2.2 | 0.30 ± 0.18 | − |
| AP108 | + | 14.0 ± 2.3 | <0.01 | − |
| AP103 | − | 6.2 ± 1.2 | <0.01 | − |
| AP101 | − | 39.5 ± 7.6 | <0.01 | + |
| AP107 | − | 41.7 ± 7.6 | 29.9 ± 14.4 | ++ |
| AP105 | − | 46.2 ± 9.6 | 0.58 ± 0.27 | ++ |
| AP106 | − | 65.4 ± 14.0e | 22.7 ± 18.6 | ++ |
| AP102 | − | 16.0 ± 4.3 | 0.08 ± 0.07 | + |
For virulence, a plus sign indicates that the mutant showed no significant difference in ID50 from the parent in terms of turkey infectivity and a minus sign indicates a significant decrease in ID50.
Tracheal ring binding was determined as described by Temple et al. (27). The percentage of bacteria bound to rings was normalized to that of the parental strain (197N) (set to 100%). Values represent averages from two separate experiments performed in triplicate. With one exception (noted below), all mutants were significantly different (P < 0.05) from the parent in their binding ability as determined by Student's t test in a paired two-tailed test.
The percentage of bacteria that survived in 50% naive turkey serum was normalized to the percentage of 197N that survived (set to 100%) (25). All mutants were significantly different (P < 0.05) from the parent in their sensitivity, as determined by Student's t test in a paired two-tailed test.
The presence of complete LPS was determined by examination of the data presented in Fig. 2B. +++, Parental amounts; ++, slightly less than parental amounts; +, less than parental amounts but still detectable; −, not detectable.
Strain AP106 was not significantly different from 197N in its ability to bind tracheal rings.
DISCUSSION
We employed a recently discovered B. avium temperate bacteriophage, Ba1, as a tool to better understand the mechanism by which B. avium causes disease. We found that, unlike many other temperate phage that infect medically important bacteria (18), lysogeny did not affect virulence by any measure we applied. However, we found that a significant fraction of the phage-resistant mutants we isolated were attenuated.
Lysogeny can affect virulence by altering the cell surface (20) or causing the bacterium to elaborate a factor (e.g., toxin) that alters its ability to cause disease (6). Also, one preliminary report indicates that genes similar to those encoding pertussis toxin reside on Ba1 (L. M. Temple, C. B. Shelton, D. R. Crosslin, R. J. Fraytic, and P. E. Orndorff, Abstr. 98th Gen. Meet. Am. Soc. Microbiol., abstr. B-66, 1998). There was thus ample reason to suspect that Ba1 lysogeny could influence virulence. However, we found no evidence that our lysogenic strain was more or less virulent than the parental (nonlysogenic) strain. Also, lysogeny is clearly not required for virulence because most clinical B. avium isolates are Ba1 sensitive (22). In spite of the above, unappreciated differences in the pathogenesis of the disease produced by lysogens and nonlysogens may exist (e.g., at the histopathological or immunological level).
We were more successful in obtaining attenuated mutants by isolating and examining spontaneous phage-resistant mutants. This strategy produced an assortment of mutants, two-thirds of which were attenuated to various degrees. Marginally attenuated mutants had ID50 values ca. 10-fold higher than that of the parental strain, and the most attenuated were at least 1,000-fold higher.
In an effort to better understand the relationship between phage resistance and virulence, we examined the phage-resistant mutants further. All of the phage-resistant mutants were unable to bind phage. Also, all of the mutants were more sensitive to surfactants, and all had differences in the pattern that their solubilized LPS made upon gel electrophoresis. In some mutants, the difference in pattern represented an increase in the ratio of complete LPS (i.e., LPS containing an O-antigen extension) to incomplete LPS. In other mutants, essentially no complete LPS could be detected. These results prompted experiments to determine whether LPS was directly responsible for phage binding.
Virtually all of the biochemical and genetic information collected from phage inactivation experiments supported the conclusion that LPS was directly required for Ba1 binding. Initial experiments in which soluble LPS was obtained from cells that had been boiled and treated with enzymes that digest protein, RNA, and DNA revealed that almost half of the phage-inactivating ability was retained in the case of the parental strain (197N) but was not detected in the phage-resistant mutants. Subsequent experiments revealed the similar shapes of the inactivation curves produced by 197N whole cells and hot phenol-water-extracted LPS and indicated the specificity of the phage inactivation effected by extracted LPS. However, our observation that 197N whole cells were ∼6-fold more efficient at inactivating phage than extracted LPS (when normalized to nanomoles of KDO) leaves open the possibility that a hot phenol-sensitive component of whole cells participates in phage inactivation. Nevertheless, it may be equally likely that extraction with the highly reactive phenol and the attendant solubilization of the LPS reduce the specific activity compared to LPS presented on an intact cell.
Phage inactivation studies performed by others indicate that all regions of LPS (O antigen, core, and lipid A) can serve as receptors for one type of phage or another (13, 17, 19). Consequently, we attempted to identify the portion of LPS required for Ba1 inactivation. Both prior genetic evidence (25) and the biochemical evidence presented here tend to rule out lipid A as the receptor. Regarding the prior genetic evidence, Spears et al. (25) showed that mutations precluding proper core assembly and (consequently) O-antigen addition render such mutants phage resistant--a finding inconsistent with lipid A being the phage receptor. In support, our biochemical data indicated that acid hydrolysis and removal of the lipid A-containing precipitate produced only a modest decrease in the phage-inactivating ability of isolated LPS. Procedures to further separate the soluble portion of the hydrolysate into core fractions and core-O-antigen fractions based upon molecular size were unsuccessful. B. avium appears to contain naturally small amounts of incomplete LPS and has a short O antigen compared to members of the family Enterobacteriaceae (25); these factors may have been in part responsible. We conclude that portions of the core and/or O antigen are required for phage inactivation. Since many of the phage resistant mutants produced some complete LPS (see Fig. 2B), a region of the core not specifically required for O-antigen addition may be required for phage binding, although other interpretations are also possible.
All of the phage-resistant mutants were more sensitive to the bactericidal effects of normal (nonimmune) turkey serum, and all (with one exception) were less able to bind turkey tracheal rings. Serum sensitivity and failure to bind tracheal rings have been noted as characteristics of attenuated B. avium mutants (25, 27). The presence of O antigen is the characteristic most clearly associated with serum resistance in all species of the Bordetella (11, 25). Also, O antigen is associated with tracheal ring binding ability in B. avium (25). Consistent with all of these observations, we found a positive correlation between serum resistance, tracheal ring binding, and the presence of detectable amounts of complete LPS. Surprisingly, however, the mutants that were among the most defective in complete LPS, tracheal ring binding, and serum resistance were the ones that remained virulent in vivo. This result indicated that even severe defects in tracheal ring binding and serum resistance were not necessarily indicative of in vivo attenuation and suggested that complete LPS was not required for virulence. The latter conclusion is qualified because of our detection limits and because we could not rule out the possibility that the absence of O antigen was conditional (e.g., the mutants, under in vivo conditions, might have produced complete LPS).
Earlier studies by Spears et al. (25) examined the role of LPS and B. avium virulence. The results of these studies indicated that elimination of gene products involved in core assembly produces mutants that are phage resistant, lack O antigen, and are avirulent. Such mutants are also defective in tracheal ring binding and are serum sensitive. However, it was unclear from this work whether LPS acted directly as the phage receptor and whether the phenotypic characteristics of the mutants were due to the absence of O antigen or to the core defect precluding O-antigen addition. Our present results provide biochemical evidence for the direct participation of LPS in phage binding and support the correlation of O antigen with serum resistance and tracheal ring binding. However, our work also indicates that O antigen may not be required for virulence. This raises the possibility that the avirulence observed by Spears et al. in O-antigen-deficient mutants was due to the core defect precluding O-antigen addition rather than the absence of O antigen per se.
We do not as yet know why only a subset of the phage-resistant mutants were attenuated. One explanation is that only certain types of LPS alterations contribute (directly or indirectly) to cell surface changes that fundamentally alter the normal host-pathogen relationship. Alternatively, the attenuating lesions may reside in genes whose products control the expression of multiple traits. More precise genetic and biochemical studies will be required to ascertain the molecular mechanisms involved.
Acknowledgments
We thank Craig Altier for critical reading of the manuscript. The expert technical assistance of J. R. Horton and Denarra Nevels is also greatly appreciated.
This work was supported in part by grants from the U.S. Department of Agriculture, the National Institutes of Health, and the State of North Carolina.
REFERENCES
- 1.Akeila, M. A., and Y. M. Saif. 1988. Protection of turkey poults from Bordetella avium infection and disease by pili and bacterins. Avian Dis. 32:641-649. [PubMed] [Google Scholar]
- 2.Arp, L. H., and N. F. Cheville. 1984. Tracheal lesions in young turkeys infected with Bordetella avium. Am. J. Vet. Res. 45:2196-2200. [PubMed] [Google Scholar]
- 3.Arp, L. H., R. D. Leah, and R. W. Griffith. 1988. Adherence of Bordetella avium to tracheal mucosa of turkeys: correlation with hemagglutination. Am. J. Vet. Res. 49:693-696. [PubMed] [Google Scholar]
- 4.Ashwell, G. 1966. New colorimetric methods of sugar analysis. Methods Enzymol. 8:85-95.
- 5.Barksdale, L., and S. B. Arden. 1974. Persisting bacteriophage infections, lysogeny and phage conversions. Annu. Rev. Microbiol. 28:265-299. [DOI] [PubMed] [Google Scholar]
- 6.Bishai, W. R., and J. R. Murphy. 1988. Bacteriophage gene products that cause human disease, p. 683-724. In R. Calender (ed.), The bacteriophages, vol. 1. Plenum Press, Inc., New York, N.Y. [Google Scholar]
- 7.Cleary, P. P., and L. Johnson. 1977. Possible dual function of M protein: resistance to bacteriophage A25 and resistance to phagocytosis by human leukocytes. Infect. Immun. 16:280-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Fabio, J. L., M. Caroff, D. Karibian, J. C. Richards, and M. B. Perry. 1992. Characterization of the common antigenic lipopolysaccharide O-chains produced by Bordetella bronchiseptica and Bordetella parapertussis. FEMS Microbiol. Lett. 97:275-282. [DOI] [PubMed] [Google Scholar]
- 9.Dubois, M. K., A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 10.Gentry-Weeks, C. R., B. T. Cookson, W. E. Goldman, R. B. Rimler, S. B. Porter, and R. Curtiss III. 1988. Dermonecrotic toxin and tracheal cytotoxin, putative virulence factors of Bordetella avium. Infect. Immun. 56:1698-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvill, E. T., A. Preston, P. A. Cotter, A. G. Allen, D. J. Maskell, and J. F. Miller. 2000. Multiple roles for Bordetella lipopolysaccharide molecules during respiratory tract infection. Infect. Immun. 58:6720-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inzana, T. J. 1983. Electrophoretic heterogeneity and inter-strain variation of the lipopolysaccharide of Haemophilus influenzae. J. Infect. Dis. 148:492-499. [DOI] [PubMed] [Google Scholar]
- 13.Ishiguro, E. E., T. Ainsworth, D. H. Shaw, W. W. Kay, and T. J. Trust. 1983. A lipopolysaccharide-specific bacteriophage for Aeromonas salmonicida. Can. J. Microbiol. 29:1458-1461. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, K. G., and M. B. Perry. 1976. Improved techniques for the preparation of bacterial lipopolysaccharides. Can. J. Microbiol. 22:29-34. [DOI] [PubMed] [Google Scholar]
- 15.Koval, S. F., and P. M. Meadow. 1977. The isolation and characterization of lipopolysaccharide-defective mutants of Pseudomonas aeruginosa PAC1. J. Gen. Microbiol. 98:387-397. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 29:122-126. [DOI] [PubMed] [Google Scholar]
- 17.Merino, S., S. Camprubi, and J. M. Tomas. 1990. Identification of the cell surface receptor for bacteriophage 18 from Aeromonas hydrophila. Res. Microbiol. 141:173-180. [DOI] [PubMed] [Google Scholar]
- 18.Miao, E. A., and S. I. Miller. 1999. Bacteriophages in the evolution of pathogen-host interactions. Proc. Natl. Acad. Sci. USA 96:9452-9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nesper, J., D. Kapfhammer, K. E. Klose, H. Merkert, and J. Reidl. 2000. Characterization of Vibrio cholerae O1 antigen as the bacteriophage K139 receptor and identification of IS1004 insertions aborting O1 antigen biosynthesis. J. Bacteriol. 182:5097-5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nnalue, N. A., S. Newton, and B. A. D. Stocker. 1990. Lysogenization of Salmonella choleraesuis by phage 14 increases average length of O-antigen chains, serum resistance and intraperitoneal mouse virulence. Microb. Pathog. 8:393-402. [DOI] [PubMed] [Google Scholar]
- 21.Raleigh, E. A., and E. R. Signer. 1982. Positive selection of nodulation-deficient Rizobium phaseoli. J. Bacteriol. 151:83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shelton, C. B., L. M. Temple, and P. E. Orndorff. 2000. Discovery, purification, and characterization of a temperate transducing bacteriophage for Bordetella avium. J. Bacteriol. 182:6130-6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silhavey, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 24.Skeeles, J. K., and L. H. Arp. 1997. Bordetellosis (turkey coryza), p. 275-288. In B. W. Calnek, H. J. Barnes, C. W. Beard, L. R. McDougal, and Y. M. Saif (ed.), Diseases of poultry. Iowa State University Press, Ames.
- 25.Spears, P. A., L. M. Temple, and P. E. Orndorff. 2000. A role for lipopolysaccharide in turkey tracheal colonization by Bordetella avium as demonstrated in vivo and in vitro. Mol. Microbiol. 36:1425-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suresh, L. H. Arp, and E. L. Huffman. 1994. Mucosal and systemic humoral and immune response to Bordetella avium in experimentally infected turkeys. Avian Dis. 38:225-230. [PubMed] [Google Scholar]
- 27.Temple, L. M., A. A. Weiss, K. E. Walker, H. J. Barnes, V. L. Christensen, D. M. Miyamoto, C. B. Shelton, and P. E. Orndorff. 1998. Bordetella avium virulence measured in vivo and in vitro. Infect. Immun. 66:5244-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]