Abstract
The human granulocytic ehrlichiosis agent, Anaplasma phagocytophila, resides and multiplies exclusively in cytoplasmic vacuoles of granulocytes. A. phagocytophila rapidly inhibits the superoxide anion (O2−) generation by human neutrophils in response to various stimuli. To determine the inhibitory mechanism, the influence of A. phagocytophila on protein levels and localization of components of the NADPH oxidase were examined. A. phagocytophila decreased levels of p22phox, but not gp91phox, p47phox, p67phox, or P40phox reactive with each component-specific antibody in human peripheral blood neutrophils and HL-60 cells. Double immunofluorescence labeling revealed that p47phox, p67phox, Rac2, and p22phox did not colocalize with A. phagocytophila inclusions in neutrophils or HL-60 cells, and p22phox levels were also reduced. A. phagocytophila did not prevent either membrane translocation of cytoplasmic p47phox and p67phox or phosphorylation of p47phox upon stimulation by phorbol myristate acetate. The inhibitory signals for O2− generation was independent of several signals required for A. phagocytophila internalization. These results suggest that rapid alteration in p22phox induced by binding of A. phagocytophila to neutrophils is involved in the inhibition of O2− generation. Absence of colocalization of NADPH oxidase components with the inclusion further protects A. phagocytophila from oxidative damage.
Human granulocytic ehrlichiosis (HGE) is an emerging tick-borne zoonosis characterized by systemic signs such as fever, chills, malaise, headache, and/or myalgia (3, 32). Laboratory tests may reveal thrombocytopenia, elevated levels of C-reactive protein, leukopenia, and elevated liver enzyme levels. Severity may range from asymptomatic infections to severe morbidity or mortality. HGE, first described in 1994 (3, 7), has become increasingly recognized in both the United States and Europe. The etiologic HGE agent, Anaplasma phagocytophila, is a unique obligatory intracellular bacterium that cannot survives or replicate anywhere other than in cytoplasmic inclusions in neutrophils and other granulocytes, once transmitted from ticks to mammals. Upon exposure to microbial pathogens, neutrophils are the first cells recruited to the site, where their most powerful oxygen-dependent and -independent defense systems kill invading pathogens. One of the oxygen-dependent systems generates the superoxide anion (O2−) directly from molecular oxygen by the NADPH oxidase enzyme, which is converted to more potent toxic species, such as hydrogen peroxide, hydroxyl radical, and hypochlorous acid (2).
We have previously demonstrated that A. phagocytophila not only does not induce O2− generation by human peripheral blood neutrophils but also rapidly (within 30 min) deprives neutrophils of the ability to generate O2− in response to a variety of potent stimuli, such as phorbol myristic acetate (PMA), formylmethionyl-leucyl-phenylalanine (fMLP), or Escherichia coli as determined by both ferricytochrome c reduction and luminol-dependent chemiluminescent assays (22). The inhibition requires the carbohydrate rather than the protein residue of A. phagocytophila as well as A. phagocytophila and host cell contact (22). The inhibition is specific to neutrophils and does not occur with human monocytes (only a few minutes' delay in O2− release), and it requires at least 30 min of preincubation of A. phagocytophila and neutrophils, as well as host cell protein synthesis, for the complete inhibition (22). The inhibition is not caused by direct interaction of components of A. phagocytophila with O2− or ferrocytochrome c (or reduced luminol). Instead, the results suggest that A. phagocytophila inhibits the NADPH oxidase.
The NADPH oxidase consists of a cytochrome b558 (heterodimer of integral membrane proteins gp91phox and p22phox) and several cytosolic components (p67phox, p47phox, and p40phox) (2). In resting neutrophils, the inactive oxidase components remain unassembled and segregated into membranes of secretory vesicles, specific granules, and a cytosolic complex (2). Upon activation, secretory vesicles and specific granules rapidly fuse with plasma or the phagosomal membrane, and a complex of p47phox, p67phox, and possibly p40phox translocates and associates with cytochrome b558 (2, 11). A functional NADPH oxidase enzyme is assembled at the phagosomes and/or the plasma membrane, allowing exertion of the lethal effects of O2− and its derivatives on extracellular or ingested bacteria in close proximity. The small GTP-binding protein, Rac2, a major Rac protein expressed in neutrophils, is required for oxidase activity through direct interaction with p67phox and cytochrome b558 (14). The cytosolic component p47phox becomes phosphorylated on the C-terminal eight to nine serine residues, some of which are required for unmasking the SH3 domain for binding to Pro-rich regions of p22phox, while phosphorylation of p67phox and p40phox also occurs; however, whether it is required for NADPH oxidase activation is unknown (2, 28).
Banerjee et al. (4) reported the down-regulation of gp91phox mRNA levels in A. phagocytophila-infected HL-60 cells (a human promyelocytic leukemia cell line) at 5 day postinfection and splenic neutrophils from mice at 2 to 8 days postinfection; however, earlier time points critical for A. phagocytophila survival or human peripheral blood neutrophils were not examined in the study. Furthermore, there has been no study examining the effects of A. phagocytophila on protein levels, phosphorylation, translocation, or localization of the NADPH oxidase enzyme components. The objectives of the present study were the following: (i) to examine the influences of A. phagocytophila on protein levels, phosphorylation, translocation, and/or localization of NADPH oxidase components in human peripheral blood neutrophils and HL-60 cells, and (ii) to examine whether signals required for internalization of A. phagocytophila are involved in the rapid inhibition of O2− production induced by A. phagocytophila.
MATERIALS AND METHODS
A. phagocytophila culture.
The A. phagocytophila HZ strain isolated from an HGE patient was cultured in the HL-60 cell line in RPMI 1640 medium as previously described (22). When approximately 75% of the HL-60 cells were infected as determined by Diff-Quik staining (Baxter Scientific Products, Obetz, Ohio), cells were suspended in Hanks' balanced salt solution (HBSS) without phenol red and sodium bicarbonate (Sigma Chemical Co., St. Louis, Mo.) and mildly sonicated on ice at predetermined conditions to release intact organisms from infected HL-60 cells (22).
Preparation of human neutrophils.
Buffy coat (∼50 ml) from healthy donors was centrifuged at 1,500 × g for 5 min. Following centrifugation, the plasma was removed, and 10 ml of buffy coat was placed in a 50-ml centrifuge tube containing 10 ml of Histopaque 1077 overlaying 15 ml of Histopaque 1119 (Sigma). Following centrifugation at 2,200 × g for 25 min, the interface between Histopaque 1077 and Histopaque 1119 was collected and added to 0.83% NH4Cl for 5 min at room temperature to lyse any remaining red blood cells. Neutrophils were centrifuged at 1,500 × g for 5 min and washed twice with HBSS. By Diff-Quik staining, cells were determined to be ∼97% neutrophils. The viability of the neutrophil preparations was determined during each experiment by a trypan blue exclusion test and was found to be ∼99%. To consider individual human variations, all experiments were independently repeated more than three times on different days using neutrophils derived from different donors and freshly prepared host-cell-free A. phagocytophila. Donor cells were never mixed, and each donor's neutrophil assay included positive and negative controls to ensure the quality of each neutrophil and A. phagocytophila preparation.
Effects of various compounds on A. phagocytophila-induced inhibition of O2− release and internalization.
H89 (10 μM), genistein (50 μM), neomycin (10 μM), monodansylcadaverine (MDC) (250 μM), or monoclonal antibody against P selectin glycoprotein ligand (PSGL-1) clone PL1 (final concentration, 2.5 μg/ml; Ancell Immunology Research Products, Bayport, Minn.) was added to 2 × 106 neutrophils in wells of a 24-well plate at 37°C for 30 min prior to addition of host-cell-free A. phagocytophila derived from 2 × 106 infected HL-60 cells. The O2− released in response to PMA was measured for 2 h at 37°C using the ferricytochrome c reduction assay as previously described (22).
To determine the internalization, host-cell-free A. phagocytophila in RPMI medium was incubated in the presence of each inhibitor with neutrophils pretreated with each compound, as described above, in RPMI medium for 2 h at 37°C. The cells were centrifuged at 500 × g for 5 min to remove the inhibitor and host cell nonassociated A. phagocytophila. Cells were further treated with 2 mg of pronase/ml in phosphate-buffered saline (PBS) for 5 min at 37°C to remove surface-bound uninternalized A. phagocytophila, washed twice with RPMI medium, and incubated at 37°C to allow internalized A. phagocytophila growth in the absence of any inhibitor. At 16 h, cells were cytocentrifuged (Cytospin2; Shandon, Inc., Pittsburgh, Pa.) and Diff-Quik stained to determine the percentage of infected neutrophils. Data were compared with controls using the Student t test. P values of <0.05 were considered significant.
Preparation of NADPH oxidase-rich membrane fractions.
The membrane fraction was prepared by the method of Clark et al. (9). Fresh human neutrophils were plated in wells of a 24-well plate at 107 cells/well in HBSS containing 2 mg of dextrose per ml (HBSSd). HL-60 cell lysate or host-cell-free A. phagocytophila derived from sonication of 107 uninfected or infected cells was added to the appropriate wells and incubated for 30 min at 37°C in 5% CO2-95% air. Following incubation, PMA (0.5 μg/ml) was added to the appropriate wells and incubated for 5 min at 37°C. Reactions were stopped by adding a 10-fold excess of ice-cold HBSSd containing 1 μM okadaic acid (Sigma), 1 mM phenylmethanesulfonyl fluoride) (Sigma), 2 mM sodium orthovanadate (Sigma), 10 mM sodium fluoride (Sigma), and 10 μM phenylarsine oxide (Sigma). Cells were pelleted by centrifugation for 5 min at 500 × g before resuspension in 1 ml of ice-cold relaxation buffer [10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (pH 7.3), 3.5 mM MgCl2, 100 mM KCl, 3 mM NaCl, 200 μM phenylmethanesulfonyl fluoride, 10 μg of leupeptin/ml, and 10 μg of pepstatin/ml]. Cells were then disrupted by sonication at power setting 2 at 20 kHz for 7 s by a W-380 ultrasonic processor (Heat Systems, Farmingdale, N.Y.) and centrifuged at 500 × g for 5 min at 4°C to pellet nuclei and unbroken cells. Supernatants were removed to new tubes and centrifuged at 4°C for 5 min at 3,000 × g. The resulting supernatants were centrifuged at 100,000 × g at 4°C for 10 min to pellet the membrane fraction. The pellet was washed twice with ice-cold relaxation buffer containing inhibitors before the final suspension in relaxation buffer. Protein concentrations were determined by a bicinchoninic acid assay (Pierce, Rockford, Ill.).
Effects of chloroquine, concanamycin A, or MG-132 on A. phagocytophila-induced alteration of NADPH components.
Neutrophils at 5 × 106 cells/well in a 24-well plate containing 300 μM chloroquine (Sigma), 100 nM concanamycin A (Sigma), or 100 μM MG-132 (BIOMOL Research Laboratories, Plymouth Meeting, Pa.) were incubated for 1 h at 37°C followed by incubation for 1 h with host-cell-free A. phagocytophila. Cells were lysed in ice-cold NP-40 lysis buffer. An equal volume of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer was added, and the lysates were boiled for 5 min prior to SDS-PAGE and Western blot analysis. We previously found that MG-132 accelerates the apoptosis of human neutrophils in vitro (33). Therefore, to confirm biological activity of MG-132 in each assay, fresh human neutrophils (106) were incubated in triplicate wells with or without 100 μM MG-132 for 16 h to evaluate the percentage of apoptotic cells under a microscope, as previously described (33). To confirm biological activities of lysosomal acidification inhibitors, human neutrophils (106) were added to a well of a 12-well plate in the presence or absence of 300 μM chloroquine or 100 nM concanamycin A for 1 h at 37°C. Acridine orange (Sigma) at a final concentration of 2 μg/ml was added to neutrophils, and the mixture was incubated for 1 h at 37°C before cytocentrifugation for 2 min at 450 × g to wash away unincorporated dye. Cytospin preparations were mounted, and the cells were viewed by epifluorescence microscopy.
SDS-PAGE and Western blot analysis.
Twenty-five micrograms of protein from each sample (membrane fraction or whole-cell lysate) was dissolved in sample buffer (5% 2-mercaptoethanol, 10% glycerol, 2% SDS, and 0.08% bromophenol blue in 62.5 mM Tris buffer [pH 6.8]). Samples were boiled for 5 min. SDS-PAGE and Western blotting were performed as described elsewhere (24). Protein blots were immersed in blocking buffer (5% nonfat dry milk in 1× PBS) at 4°C overnight with primary antibodies (Table 1). After three rinses with TBT buffer (50 mM Tris-HCl, 150 mM NaCl, and 0.02% Tween-20), the blots were incubated at room temperature for 2 h with peroxidase-conjugated anti-rabbit or anti-goat immunoglobulin G (IgG) (ICN pharmaceuticals, Aurora, Ohio) at a 1:1,000 dilution in blocking buffer. The blots were washed three times in TBT buffer for 15 min each. Peroxidase-positive bands were detected by immersing the blots in DAB developing solution (73 mM sodium acetate [pH 6.2], 0.3% diaminobenzidine tetrahydrochloride [Nacalai Tesque, Inc., Kyoto, Japan], and 0.04% hydrogen peroxide) at room temperature. Washing with 0.1 M H2SO4 terminated the enzyme reaction.
TABLE 1.
Primary antibodies used to label A. phagocytophila, NADPH oxidase components, or α-tubulin in human peripheral blood neutrophils and HL-60 cells
| Antigen | Origin | Dilution | Source |
|---|---|---|---|
| A. phagocytophila (BDS strain) | Horse | 1:100a (serum) | J. E. Madigan, University of California, Davis |
| gp91phox (C-15) | Goat | 1:100,b 1:1,000b (purified goat IgG in PBS) | Santa Cruz Biotechnology, Inc., Santa Cruz, Calif. |
| gp91phox C-terminal aa 536-555 | Rabbit | 1:1,000b (serum) | S. Tsunawaki, National Children's Medical Research Center, Tokyo, Japan |
| p22phox C-terminal aa 177-195 | Rabbit | 1:100,a 1:1,000b (serum) | S. Tsunawaki |
| p47phox C-terminal aa 376-390 | Rabbit | 1:100,a 1:1,000b (serum) | S. Tsunawaki |
| p40phox N-terminal aa 1-15 | Rabbit | 1:1,000b (serum) | S. Tsunawaki |
| p67 phax C-terminal aa 317-469 | Mouse | 1:100,a 1:1000b (IgG2b in PBS) | Transduction Laboratories, Lexington, Ky. |
| Rac2 (C-11) | Rabbit | 1:100a (purified rabbit IgG in PBS) | Santa Cruz Biotechnology, Inc. |
| α-tubulin | Mouse | 1:1,000b (ascitic fluid in PBS) | Cedarlane, Hornby, Ontario, Canada |
Dilution used for double immunofluorescence labeling.
Dilution used for Western immunoblotting.
Phosphorylation and immunoprecipitation of p47phox.
The assay was performed as described by DeLeo et al. (11). Neutrophils at a concentration of 3.2 × 107 cells/ml in loading buffer (10 mM Na-HEPES, 138 mM NaCl, 2.7 mM KCl, and 7.5 mM d-glucose [pH 7.5]) containing 0.4 mCi of [32P]orthophosphoric acid (NEN Life Science Products, Inc., Boston, Mass.) were incubated for 1 h at 25°C. 32P-labeled neutrophils (0.8 × 107 cells/ml) in loading buffer containing 1 mM MgCl2 and 0.5 mM CaCl2 were incubated in the presence of either HL-60 cell lysate or host-cell-free A. phagocytophila derived from 0.8 × 107 uninfected or infected cells for 30 min at 25°C and stimulated by addition of PMA (0.8 μg/0.8 × 107 neutrophils) for 5 min at 37°C before termination by addition of 10 volumes of ice-cold loading buffer supplemented with a phosphatase inhibitor mixture (5 mM EDTA, 1 mM sodium orthovanadate, 5 mM sodium fluoride, 6.25 μM okadaic acid, and 1 mM p-nitrophenol phosphate). The neutrophils were lysed in ice-cold NP-40 lysis buffer (150 mM NaCl, 1.2% [wt/vol] Nonidet P-40, 1% Triton X-100, 2.5% [wt/vol] glycerol, 1 mM EGTA, 5 mM MgCl2, and 50 mM Tris-HCl [pH 7.4]) supplemented with the phosphatase inhibitor cocktail and incubated with 5 μl of anti-p47phox rabbit polyclonal antibody for 1 h at 4°C. To each sample, 20 μl of protein G-agarose (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) in NP-40 lysis buffer was added and incubated for 1 h at 4°C with constant gentle rotation. Beads were boiled in SDS-PAGE sample buffer for 5 min, and solubilized proteins were subjected to SDS-PAGE on 10% polyacrylamide gels. Gels were autoradiographed with Hyperfilm-enhanced chemiluminescence film (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom).
Double immunofluorescence labeling.
Neutrophils in RPMI 1640 medium at a concentration of 5 × 106/ml and host-cell-free A. phagocytophila from an equal amount of infected HL-60 cells were added to each well of a six-well plate in a total volume of 2 ml and incubated for 16 h at 37°C. Uninfected or A. phagocytophila-infected HL-60 cells or neutrophils were placed into microcentrifuge tubes at a concentration of 106 cells/tube. For PMA treatment, PMA (0.5 μg/ml) was added to appropriate wells and incubated for 5 min at 37°C. Cells were fixed in 200 μl of 1% paraformaldehyde in PBS (0.009 M Na2HPO4, 0.005 M NaH2PO4, 0.15 M NaCl) at room temperature for 30 min. Fixed cells were incubated with primary (Table 1) antibodies in PBS supplemented with 0.1% gelatin and 0.3% saponin for 30 min with constant rotation at room temperature followed by three washes with PBS supplemented with gelatin and saponin as previously described (21). Lissamine rhodamine-conjugated anti-mouse, -rabbit, or -goat IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) at a concentration of 7.5 μg/ml was used to label the respective primary antibodies. Fluorescein isothiocyanate-conjugated anti-horse IgG (Jackson) at 7.5 μg/ml was used to label horse anti-A. phagocytophila serum. Cells were incubated for 30 min at room temperature with constant rotation with secondary antibodies. Cells were washed three times in PBS prior to a final resuspension in PBS. Cytocentrifuged preparations of A. phagocytophila-infected or uninfected HL-60 cells or neutrophils were mounted with a semipermanent Mowiol mounting medium containing polyvinyl alcohol (Mowiol 4-88; Calbiochem, La Jolla, Calif.) and viewed by epifluorescence microscopy.
RESULTS
Effects of A. phagocytophila on the levels of NADPH oxidase components.
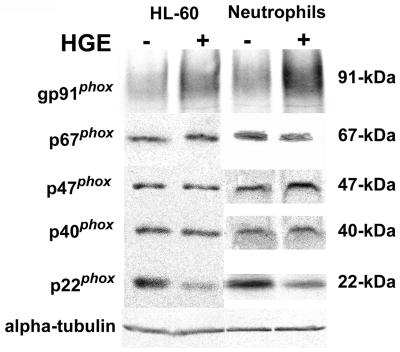
The p22phox level but not levels of gp91phox, p47phox, p40phox, or p67phox in human peripheral blood neutrophils decreased within 30 min after addition of A. phagocytophila and in HL-60 cells at 7 days postinfection, as revealed by Western blot analysis (Fig. 1). The housekeeping protein α-tubulin was used as a control for neutrophil and HL-60 cells protein input across the lanes. Antibody to p22phox was specific to its C terminus (Table 1), suggesting modification of C-terminal p22phox or loss of this protein.
FIG. 1.
Analysis of cellular levels of NADPH oxidase components following exposure to A. phagocytophila. HL-60 culture cells or human neutrophils were infected with A. phagocytophila (HGE) for 7 days or 30 min, respectively. Cell lysates (25 μg) of infected and uninfected cells were subjected to SDS-PAGE and Western immunoblotting and probed with antibodies against gp91phox (rabbit), p67phox, p47phox, p40phox, p22phox, and α-tubulin. Results shown are representative of three independent experiments.
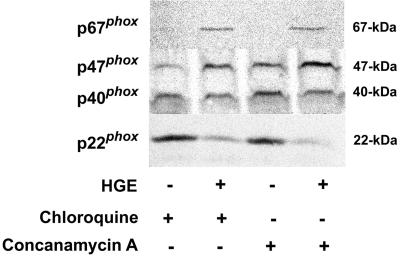
MG-132, a peptide-aldehyde and potent reversible inhibitor of the chymotryptic-like activity of the proteasome (16), did not have any effect on levels of p47phox, p22phox, and p67phox with or without A. phagocytophila in the neutrophil (data not shown). MG-132 used in this assay was confirmed to be biologically active, since it induced significant apoptosis in neutrophils after 16 h of treatment: 99.6% cells had apoptotic nuclei (condensed nuclei which lost all lobular connections) as determined by microscopic examination of Diff Quik-stained neutrophils as previously described (33), in contrast with untreated neutrophils (43.0% of cells had apoptotic nuclei). The result suggests that A. phagocytophila-induced change in p22phox is independent of proteasome activity. Lysosomal acidification inhibitors, chloroquine (a membrane-permeative lysosomotropic reagent that becomes protonated in an increasing vacuolar pH) (23) and concanamycin A (a highly sensitive and specific inhibitor of vacuolar-type H+-ATPase) (12) did not prevent the reduction of levels of whole-cell p22phox following incubation with host-cell-free A. phagocytophila. On the contrary, levels of p47phox and p67phox were greater in A. phagocytophila-infected neutrophils than in uninfected neutrophils in the presence of inhibitors (Fig. 2). Control neutrophils incubated with concanamycin A or chloroquine showed decreased accumulation of the acridine orange vital stain, indicating the effectiveness of both acidification inhibitors (data not shown). These results suggest that A. phagocytophila-induced loss of the protein recognizable with antibody to p22phox does not occur through trafficking of p22phox to lysosomes and subsequent digestion by lysosomal enzymes. The results also suggest that the A. phagocytophila either enhanced synthesis of p47phox and p67phox or slowed down their degradation in the presence of lysosomal acidification inhibitors (Fig. 2).
FIG. 2.
Effects of lysosomal acidification inhibitors on A. phagocytophila-induced p22phox removal. Human neutrophils were incubated for 30 min at 37°C in HBSSd containing either 300 μM chloroquine or 100 nM concanamycin A prior to 30 min of incubation with host-cell-free A. phagocytophila (HGE). Cell lysates (25 μg/sample) were separated on a polyacrylamide gel, transferred to a nitrocellulose membrane, and probed with anti-p67phox, -p47phox, -p40phox, or -p22phox antibody. Results are representative of two independent experiments, each with neutrophils derived from different donors and freshly prepared host-cell-free A. phagocytophila.
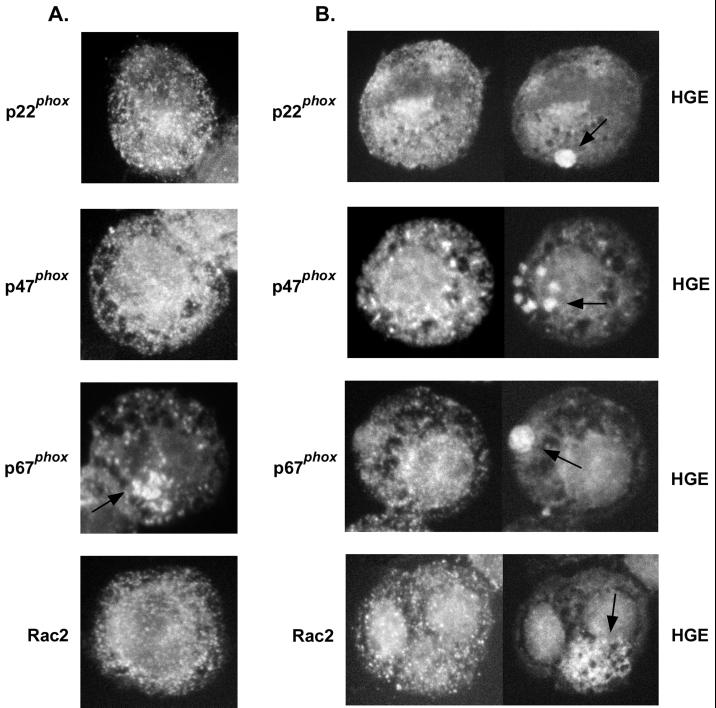
Double immunofluorescence labeling of infected HL-60 cells and neutrophils.
Overall intensity of the fluorescence labeling of p47phox, p67phox, and Rac2, which appeared as white dots, did not change between uninfected and infected HL-60 cells or neutrophils, whereas the intensity of p22phox labeling, which also appeared as white dots throughout the cells, was reduced in infected cells from that in uninfected HL-60 cells and neutrophils (Fig. 3, data of neutrophils not shown). p47phox, p67phox, p22phox, and Rac2 did not colocalize with A. phagocytophila inclusions (Fig. 3), and the PMA treatment did not induce colocalization of these components (data not shown). The results of immunofluorescence labeling, along with Western blot results (Fig. 1 and 2), indicate that A. phagocytophila reduced the levels of the protein recognizable with antibody to p22phox in the host cell and the loss was not reversed by adding PMA. The results also suggest that cytoplasmic inclusions occupied by A. phagocytophila are able to exclude most of the NADPH oxidase enzyme components.
FIG. 3.
Double immunofluorescence labeling of the NADPH oxidase enzyme components p22phox, p47phox, p67phox, and Rac2, which appear as white dots in uninfected (A) or A. phagocytophila-infected (B) HL-60 cells. Note reduced amounts of p22phox in infected cells compared to those in uninfected cells. An arrow in the panel showing p67phox labeling in uninfected HL-60 cells (A) indicates an area of intense fluorescent labeling near the Golgi apparatus. Arrows in the infected HL-60 cells (B) indicate the locations of A. phagocytophila (HGE) morulae. Magnification, ×750. Results are representative of three independent labeling experiments.
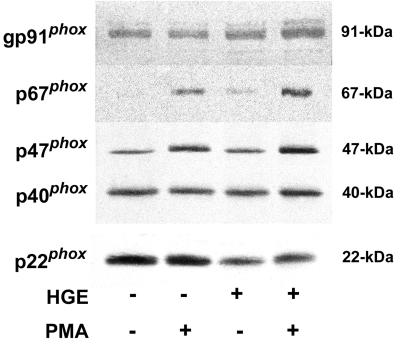
Effects of A. phagocytophila on translocation of p47phox and p67phox to the neutrophil membrane in response to PMA.
To determine whether A. phagocytophila inhibits the translocation of cytosolic complex p47phox and p67phox to the membrane, the neutrophil membrane fraction was isolated following incubation with or without host-cell-free A. phagocytophila and PMA stimulation. A. phagocytophila did not induce translocation of p47phox, p67phox, and p40phox to the membrane fraction (Fig. 4). Following stimulation of neutrophils by PMA, levels of p47phoxand p67phox in the membrane fraction increased in both control neutrophils and neutrophils preincubated with host-cell-free A. phagocytophila (Fig. 4). However, membrane fractions from neutrophils preincubated with host-cell-free A. phagocytophila consistently showed decreased levels of p22phox with or without PMA stimulation compared with uninfected unstimulated neutrophils (Fig. 4). gp91phox levels in the membrane fraction did not decrease with infection. These results indicate that A. phagocytophila did not inhibit membrane translocation of cytosolic components in response to PMA.
FIG. 4.
Analysis of neutrophil membrane-associated NADPH oxidase components after incubation with A. phagocytophila and PMA stimulation. Human neutrophils were incubated in the presence or absence of host-cell-free A. phagocytophila (HGE) for 30 min prior to stimulation with PMA (5 min with 0.5 μg of PMA/ml). Cells were lysed, and membrane fractions were obtained by high-speed centrifugation. Membrane fractions (25 μg) were separated by SDS-PAGE and transferred to a nitrocellulose membrane prior to being probed with antibodies against gp91phox (goat), p67phox, p47phox, p40phox, and p22phox. Western blot results are representative of more than three independent experiments, each performed with neutrophils derived from different donors and freshly prepared host-cell-free A. phagocytophila.
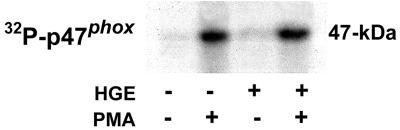
Phosphorylation of p47phox in the presence of A. phagocytophila.
To examine whether the phosphorylation of p47phox is inhibited in the presence of A. phagocytophila, 32P-orthophosphate-preloaded neutrophils were incubated with A. phagocytophila for 30 min prior to stimulation with PMA. The p47phox was then immunoprecipitated and autoradiographed. A. phagocytophila did not induce p47phox phosphorylation in neutrophils (Fig. 5). In contrast, neutrophils stimulated with PMA showed increased levels of p47phox phosphorylation in both infected and uninfected neutrophils (Fig. 5). This result indicates that A. phagocytophila does not inhibit the phosphorylation of p47phox in response to PMA.
FIG. 5.
Phosphorylation of p47phox in PMA-stimulated neutrophils in the presence of A. phagocytophila. 32P-preloaded neutrophils were incubated in the presence or absence of host-cell-free A. phagocytophila (HGE) for 30 min at 37°C. PMA (0.5 μg/ml) was added to stimulate neutrophils for 5 min at 37°C. Cells were lysed and immunoprecipitated with anti-p47phox antibody and protein G-agarose. The precipitated proteins were subjected to SDS-PAGE and identified by autoradiography. Data shown are representative of three independent experiments using neutrophils from three separate donors.
Signals required for internalization of A. phagocytophila are not required for inhibition of O2− release by A. phagocytophila.
Previous results in our laboratory (33) have shown that internalization of A. phagocytophila in human neutrophils was inhibited by the transglutaminase inhibitor, MDC (18). In the present study, H89, a cell-permeative, selective protein kinase A (PKA) inhibitor (8), neomycin, phospholipase C (PLC) inhibitor (5), and genistein, a PTK inhibitor (1), were found to inhibit internalization of A. phagocytophila bacteria into neutrophils (Table 2). Herron et al. (13) reported that monoclonal antibody PL1 directed against PSGL-1 significantly prevented A. phagocytophila binding and infection in HL-60 cells. None of these inhibitors or the antibody abrogated A. phagocytophila inhibition of O2− generation in response to PMA (Table 3). Altogether, these results indicate that A. phagocytophila inhibition of the NADPH oxidase is independent of signals required for internalization of A. phagocytophila.
TABLE 2.
Effects of MDC, genistein, neomycin, and H89 treatment on A. phagocytophila internalization in human peripheral blood neutrophils
| Treatmenta | % Infected cellsb |
|---|---|
| None | 7.0 ± 1.7 |
| MDC | 0.9 ± 0.4 |
| Genistein | 1.6 ± 0.6 |
| Neomycin | 0.3 ± 0.6 |
| H89 | 0.7 ± 1.2 |
Neutrophils preincubated for 30 min with inhibitors were further incubated with host cell-free A. phagocytophila in the presence of each inhibitor for 2 h at 37°C. The cells were treated with 2 mg of pronase/ml to remove uninternalized A. phagocytophila and cultured in the absence of inhibitors for 16 h at 37°C, and infection rates were determined.
Means ± standard deviations (n = 3). Results are representative of three independent experiments.
TABLE 3.
Inhibition of O2− release by human peripheral blood neutrophils in response to PMA by A. phagocytophila in the presence of MDC, neomycin, genistein, H89, and anti-PSGL-1
| Treatmenta | Reduction of ferricytochrome c (nmol of O2−/106 neutrophils)b |
|---|---|
| Experiment 1 | |
| Medium control | 1.8 ± 0.2 |
| PMA | 21.1 ± 3.4∗ |
| MDC | 4.5 ± 0.4 |
| MDC + PMA | 18.7 ± 0.3∗ |
| MDC + A. phagocytophila + PMA | 4.7 ± 0.3 |
| Neomycin | 2.3 ± 0.4 |
| Neomycin + PMA | 14.7 ± 0.2∗ |
| Neomycin + A. phagocytophila + PMA | 2.8 ± 0.5 |
| Genistein | 3.7 ± 2.2 |
| Genistein + PMA | 15.4 ± 1.2∗ |
| Genistein + A. phagocytophila + PMA | 4 ± 3.1 |
| H89 | 3.8 ± 1.4 |
| H89 + PMA | 16.4 ± 0.3∗ |
| H89 + A. phagocytophila + PMA | 2.4 ± 0.1 |
| Experiment 2 | |
| Medium control | 0.4 ± 0.6 |
| PMA | 8.5 ± 0.6∗ |
| A. phagocytophila | 1.7 ± 0.2 |
| A. phagocytophila + PMA | 1.9 ± 1.0 |
| PL1 + PMA | 6.3 ± 0.6∗ |
| A. phagocytophila + PL1 + PMA | 1.8 ± 0.5 |
Neutrophils were pretreated with or without reagents (250 μM MDC, 10 μM neomycin, 50 μM genistein, 10 μM H89, or 2.5 pg of anti-PSGL-1) for 30 min prior to addition of A. phagocytophila and PMA stimulation. Reagents were present throughout assays.
Mean ± standard deviation (n = 3). Results are representative of more than three independent experiments. ∗, P < 0.01 compared to neutrophil-alone (no PMA) control (Student's t test).
DISCUSSION
Our previous study showed active inhibition of O2− generation in response to divergent stimuli (PMA, fMLP, and E. coli) by A. phagocytophila, suggesting that the site of inhibition is a converged step downstream to divergent signaling pathways induced by all these stimuli (22). The present results indicate reduction in p22phox protein levels in neutrophils within 30 min and in HL-60 cells within 7 days after incubation with A. phagocytophila by both Western blotting and immunofluorescence labeling, suggesting that this reduction is responsible for the inhibition of O2− generation by A. phagocytophila. Protein levels of gp91phox and other components, as well as membrane translocation of p47phox or p67phox and phosphorylation of p47phox in response to PMA, were not reduced by A. phagocytophila infection. It remains to be studied why the inhibition of O2− generation by A. phagocytophila is 80 to 100% (22), while the reduction of p22phox was partial, if the reduction of p22phox is solely responsible for the inhibition. According to the 35S-methionine pulse-chase experiment, both p22phox and gp91phox are produced in excess and their rapid degradation is evident within 1 h (10). Therefore, there seems to be a pool of biologically inactive but antibody-reactive p22phox, which may not be affected by A. phagocytophila. Both gp91phox and p22phox were still visible by Western immunoblot analysis when NADPH enzyme activity was reduced by 95% compared with controls by the treatment with an inhibitor of heme synthesis (34), suggesting that a complete loss of antibody-reactive p22phox is not required for ∼95% inhibition of superoxide generation. In our previous study we demonstrated that the host protein synthesis is required for the complete inhibition of O2− generation by A. phagocytophila (22); preliminary data of members of our group (M. Lin and Y. Rikihisa, unpublished data) revealed that cycloheximide prevents p22phox reduction, supporting our hypothesis that the reduction of p22phox is responsible for the inhibition.
The p22phox antibody used in the present study targets C-terminal amino acids (aa) 177 to 195, which includes aa 181 to 188 exposed to the cytoplasmic side (6) but does not include the Pro-rich sequence of aa 149 to 162 that is critical for p22phox interaction with the SH3 domains of p47phox to translocate to the membrane (17, 28). We don't know whether the whole p22phox molecule was lost or modified by A. phagocytophila so that the C-terminal cytoplasmic domain was no longer recognizable with this antibody. Since membrane translocation of p47phox or p67phox in response to PMA was not impaired by A. phagocytophila infection, the p47phox binding site on p22phox appears still to be available. Thus, p22phox may be modified rather than completely lost by A. phagocytophila infection. The fact that A. phagocytophila-induced inhibition was reversible upon its removal from the surface of neutrophils (22) further supports the possibility of p22phox modification. Proteolysis through proteasomes is suggested to be involved in part in the normal turnover process of gp91phox and p22phox in human neutrophils (10). However, neither neutrophil proteasomes nor lysosomes alone appear to be responsible for the decreased p22phox antigen levels. Either modification or degradation must have made the proper assembly of NADPH oxidase subunits impossible, resulting in no or minimum enzyme activity. The reason for apparent discrepancy between Banerjee et al. (4) and the present studies is unknown. Since we found a reduction in p22phox antigen levels but not in gp91phox antigen levels using both human peripheral blood neutrophils at 30 min postinfection and HL-60 cells at 7 days postinfection, differences in host cell types or in postinfection time points of examination do not seem to be the reason. Since there are several posttranscriptional and -translational modifications on p22phox and gp91phox, the discrepancy may be due to different detection targets: mRNA in the study by Banerjee et al. (4) versus antigenically recognizable proteins in the present paper.
The replicative inclusion of A. phagocytophila that excludes the NADPH components partially resembles the compartment occupied by Salmonella spp. In the case of Salmonella enterica serovar Typhimurium, the localization of the NADPH oxidase components to the salmonella phagosome in murine macrophages was prevented by a cluster of genes encoded by the salmonella pathogenicity island 2; however, the overall production of O2− in response to salmonella was not inhibited (30). The inhibition of O2− generation induced by A. phagocytophila does not seem to involve any previously reported mechanisms. It has been suggested that elevated levels of cAMP and activation of PKA lead to activation of a downstream phosphatase responsible for dephosphorylation and inactivation of the NADPH oxidase enzyme (2, 25). However, PKA is not required for the inhibition by A. phagocytophila of production of O2− by neutrophils. Protein-tyrosine phosphatase activity of Yersinia enterocolitica and Coxiella burnetti is responsible for inhibition of the fMLP-stimulated O2− generation (19, 31). However, since the PTK inhibitor genistein had no influence on PMA-induced O2− release, as shown in the present study and by Mocsai et al. (20), and A. phagocytophila can inhibit PMA-induced O2− release, a protein-tyrosine phosphatase is not the inhibitory mechanism of O2− release by A. phagocytophila. Interleukin 13 (IL-13) inhibition of O2− production by human monocytes following PMA stimulation is mediated by intracellular Ca2+ mobilization induced by PLC activation (26, 27). Pseudomonas aeruginosa hemolytic PLC suppresses PMA- but not fMLP-stimulated NADPH oxidase activity in human neutrophils (29). The O2− inhibition by A. phagocytophila did not require the PLC activity. Legionella pneumophila was also shown to inhibit the PMA-induced O2− generation in human monocytes by down-modulation of PKC isozymes α and β (15). A. phagocytophila did not interfere with the phosphorylation of 47phox in response to PMA. Altogether, lack of involvement of PKA, PTK, or PLC in the inhibition of O2− production induced by A. phagocytophila represents a novel mechanism.
Our previous study using Transwell showed that A. phagocytophila binding is required (22). Although we found that several signals are required for A. phagocytophila internalization, for the inhibition of O2− production binding alone is sufficient, and signals required for internalization of A. phagocytophila are not involved in the inhibition. In the present study we found that the previously described antibody to PSGL-1 (14) did not have any effect the inhibition of O2− production. A. phagocytophila did not induce fusion of secretory vesicles containing cytochrome b558 with the plasma membrane. Therefore, engagement of A. phagocytophila to an undetermined neutrophil-specific receptor on the plasma membrane induces the synthesis of a host protein which facilitates generalized (not limited to the plasma membrane) reduction of p22phox in the neutrophils, leading to the inhibition of O2− production.
Acknowledgments
This work was supported by grant R01 AI30010 from the National Institutes of Health.
REFERENCES
- 1.Akiyama, T., J. Ishida, S. Nakagawa, H. Ogawara, S. Watanabe, N. Itoh, M. Shibuya, and Y. Fukamki. 1987. Genistein, a specific inhibitor of tyrosine kinases. J. Biol. Chem. 262:5592-5595. [PubMed] [Google Scholar]
- 2.Babior, B. 1999. NADPH oxidase: an update. Blood 93:1464-1476. [PubMed] [Google Scholar]
- 3.Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. Van Etta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272:212-218. [PubMed] [Google Scholar]
- 4.Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J. Immunol. 164:3946-3949. [DOI] [PubMed] [Google Scholar]
- 5.Berridge, M. J. 1993. Inositol triphosphate and calcium signaling. Nature 361:315-325. [DOI] [PubMed] [Google Scholar]
- 6.Burritt, J. B., M. T. Quinn, M. A. Jutila, C. W. Bond, and A. J. Jesaitis. 1995. Topological mapping of neutrophil cytochrome b epitopes with phage-display libraries. J. Biol. Chem. 270:16974-16980. [DOI] [PubMed] [Google Scholar]
- 7.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chijiwa, T., A. Mishima, M. Hagiwara, M. Sano, K. Hayashi, T. Inoue, K. Naito, T. Toshioka, and H. Hidaka. 1990. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 265:5267-5272. [PubMed] [Google Scholar]
- 9.Clark, R. A., B. D. Volpp, K. G. Leidal, and W. M. Nauseef. 1990. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J. Clin. Investig. 85:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLeo, F. R., J. B. Burritt, L. Yu, A. J. Jesaitis, M. C. Dinauer, and W. M. Nauseef. 2000. Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J. Biol. Chem. 275:13986-13993. [DOI] [PubMed] [Google Scholar]
- 11.DeLeo, F. R., L.-A. H. Allen, M. Apicella, and W. N. Nauseef. 1999. NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 163:6732-6740. [PubMed] [Google Scholar]
- 12.Drose, S., and K. Altendorf. 1997. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200:1-8. [DOI] [PubMed] [Google Scholar]
- 13.Herron, M. J., C. M. Nelson, J. Larson, K. R. Snapp, G. S. Kansas, and J. L. Goodman. 2000. Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science 288:1653-1656. [DOI] [PubMed] [Google Scholar]
- 14.Heyworth, P. G., B. P. Bohl, G. M. Bokoch, and J. T. Curnutte. 1994. Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. Evidence for its interaction with flavocytochrome b 558. J. Biol. Chem. 269:30749-30752. [PubMed] [Google Scholar]
- 15.Jacob, T., J. C. Escallier, M. V. Sanguedolce, C. Chicheportiche, P. Bongrand, C. Capo, and J. L. Mege. 1994. Legionella pneumophila inhibits superoxide generation in human monocytes via the down-modulation of α and β protein kinase C isotypes. J. Leukoc. Biol. 55:310-312. [DOI] [PubMed] [Google Scholar]
- 16.Jensen, T. J., M. A. Loo, S. Pind, D. B. Williams, A. L. Goldberg, and J. R. Riordan. 1995. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83:129-135. [DOI] [PubMed] [Google Scholar]
- 17.Leto, T. L., A. G. Adams, and I. de Mendez. 1994. Assembly of the phagocyte NADPH oxidase: Binding of Src homology 3 domains to proline-rich targets. Proc. Natl. Acad, Sci. USA 91:10650-10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitzki, A., M. Willingham, and I. Pastan. 1980. Evidence for participation of transglutaminase in receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 77:2706-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Y. P., G. Curley, M. Lopez, M. Chavez, R. Glew, A. Aragon, H. Kumar, and O. G. Baca. 1996. Protein-tyrosine phosphatase activity of Coxiella burnetti that inhibits human neutrophils. Acta Virol. 40:263-272. [PubMed] [Google Scholar]
- 20.Mocsai, A., B. Banfi, A. Kapus, G. Farkas, M. Geist, L. Buday, A. Farago, and E. Ligeti. 1997. Differential effects of tyrosine kinase inhibitors and an inhibitor of the mitogen-activated protein kinase cascade on degranulation and superoxide production of human neutrophil granulocytes. Biochem. Pharmacol. 54:781-789. [DOI] [PubMed] [Google Scholar]
- 21.Mott, J., R. Barnewall, and Y. Rikihisa. 1999. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect. Immun. 67:1368-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mott, J., and Y. Rikihisa. 2000. Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils. Infect. Immun. 68:6697-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Neill, P. M., P. G. Bray, S. R. Hawley, S. A. Ward, and B. K. Park. 1998. 4-Aminoquinolines--past, present, and future: a chemical perspective. Pharmacol. Ther. 77:29-58. [DOI] [PubMed] [Google Scholar]
- 24.Rikihisa, Y. 1991. Cross-reacting antigens between Neorickettsia helminthoeca and Ehrlichia species, shown by immunofluorescence and Western immunoblotting. J. Clin. Microbiol. 29:2024-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savitha, G., and B. P. Salimath. 1993. Cross-talk between protein kinase C and protein kinase A down-regulates the respiratory burst in polymorphonuclear leukocytes. Cell. Signal. 5:107-117. [DOI] [PubMed] [Google Scholar]
- 26.Sozzani, P., C. Cambon, N. Vita, M. Seguelas, D. Caput, P. Ferrara, and B. Pipy. 1995. Interleukin-13 inhibits protein kinase C-triggered respiratory burst in human monocytes. J. Biol. Chem. 270:5084-5088. [DOI] [PubMed] [Google Scholar]
- 27.Sozzani, P., L. Hasan, M. Seguelas, D. Caput, P. Ferrara, B. Pipy, and C. Cambon. 1998. IL-13 induces tyrosine phosphorylation of phospholipase Cγ-1 following IRS-2 association in human monocytes: relationship with the inhibitory effect of IL-13 on ROI production. Biochem. Biophys. Res. Commun. 244:665-670. [DOI] [PubMed] [Google Scholar]
- 28.Sumimoto, H., Y. Kage, H. Nunoi, H. Sasaki, T. Nose, Y. Fukumaki, M. Ohno, S. Minakami, and K. Takeshige. 1994. Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc. Natl. Acad. Sci. USA 91:5345-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terada, L. S., K. A. Johansen, S. Nowbar, A. I. Vasil, and M. L. Vasil. 1999. Pseudomonas aeruginosa hemolytic phospholipase C suppresses neutrophil respiratory burst activity. Infect. Immun. 67:2371-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 31.Visser, L. G., E. Seijmonsbergen, P. H. Nibbering, P. J. van den Broek, and R. van Furth. 1999. Yops of Yersinia enterocolitica inhibit receptor-dependent superoxide anion production by human granulocytes. Infect. Immun. 67:1245-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wormser, G., D. Mckenna, M. Aguero-Rosenfeld, et al. 1995. Human granulocytic ehrlichiosis-New York. Morb. Mortal. Wkly. Rep. 44:593-595. [PubMed] [Google Scholar]
- 33.Yoshiie, K., Y.-H. Kim, J. Mott, and Y. Rikihisa. 2000. Intracellular infection by human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect. Immun. 68:1125-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu, L., L. Zhen, and M. C. Dinnauer. 1997. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. J. Biol. Chem. 272:27288-27294. [DOI] [PubMed] [Google Scholar]