Abstract
We previously demonstrated that genes encoding a putative two-component histidine kinase (CHK1) or a response regulator (CSSK1) are each required for virulence in a murine model of hematogenously disseminated candidiasis and that strains with each gene deleted are also defective in morphogenesis under certain growth conditions. In the present study, the role of these two genes in the adherence to and colonization of reconstituted human esophageal tissue (RHE) is described. We compared strains of Candida albicans with deletions of chk1 (strain CHK21) and cssk1 (strain CSSK21) to wild-type cells (CAF2), as well as strains with CHK1 and CSSK1 reconstituted (strains CHK23 and CSSK23, respectively). Adherence and colonization of RHE were evaluated in periodic acid-Schiff-stained sections, as well as by SEM. We observed that both deletion-containing strains colonized the RHE to a lesser extent than did CAF2 and that the percent germination by both strains was reduced in comparison to that of control strains at 1 h postinfection. Expression of CHK1 or CSSK1 was quantitated by reverse transcription (RT)-PCR from RHE tissues infected with wild-type C. albicans yeast cells. Expression of both CHK1 and CSSK1 increased over the 48-h period following infection of the tissue, although expression of CHK1 was greater than that of CSSK1. By RT-PCR, we have also shown that expression of CHK1 and CSSK1 in the strains with cssk1 and chk1 deleted, respectively, was similar to that of CAF2, indicating that CHK1 and CSSK1 do not regulate each other but probably encode signal proteins of different pathways. Our observations indicate that CHK1 and CSSK1 are each partially required for colonization and conversion to filamentous growth on RHE tissue.
Candida albicans accounts for the largest number of nosocomial fungal infections, and mortality due to this form of candidiasis is believed to be approximately 35% (32). Immune protection against candidiasis seems to be site specific, emphasizing the complex nature of the disease (4, 5). As an endogenous commensal of human mucosal epithelial tissue, C. albicans probably exists predominantly in the yeast form. During disease development, the organism utilizes the yeast-hyphal transition (morphogenesis) to carry out tissue invasion, although direct persorption of yeast cells by mucosal cells has also been observed (6, 14, 17, 20). Another critical event in the disease process that precedes morphogenesis is adherence of the organism to host cells, and several cell surface adhesins have been demonstrated to promote the virulence of the organism (6). Subsequent to adherence and morphogenesis is invasion of mucosal epithelia and dissemination via the bloodstream (6).
Morphogenesis of C. albicans cells in vitro is dependent upon the integration of a variety of environmental signals. Likewise, the organism is able to adapt its growth to a variety of sites in the human host (ecological niches), each with very different environmental stress conditions. Therefore, it is likely that C. albicans utilizes several parallel and cross-talking signal transduction pathways to integrate environmental signals (6, 7, 20, 22, 23). These signal pathways, in turn, regulate the expression of growth phase-specific proteins, cell wall proteins, and, most likely, cell wall biosynthesis (18, 22, 23). For instance, in Saccharomyces cerevisiae, the Hog1 (hyperosmotic glycerol) mitogen-activated protein kinase signal transduction pathway has been shown to adapt cells to changes in osmotic growth conditions (25). In C. albicans, strains with deletions in genes comprising the Hog1 pathway (SLN1, SSK1, and HOG1) are defective in morphogenesis (12, 21). Further, other histidine kinases typical of two-component signaling proteins (Chk1p and Nik1p/Cos1p) likewise seem to be required for morphogenesis or phenotypic switching (1, 6-9, 12, 21, 31, 33).
Strains of C. albicans with deletions in genes that encode signal transduction pathway proteins or transcriptional activators of morphogenesis have reduced virulence (6, 7,20, 30, 33). For example, we have previously demonstrated that mutants lacking either CHK1 or CSSK1 are avirulent in the hematogenously disseminated murine candidiasis model (10, 12). In the present study, we have examined the role of CHK1 and CSSK1 in the adherence and germination of cells on reconstituted human esophageal (RHE) tissues grown in vitro. This model allowed us to measure and correlate the temporal expression of these genes with events such as adherence and morphogenesis. Similarly, Schaller et al., in a series of papers, determined the temporal expression of individual secretory aspartyl proteinase (SAP) genes by reverse transcription (RT)-PCR in vitro using models of reconstituted human and rat oral epithelia, as well as samples from patients with oral candidiasis (27-29); these events were correlated with invasion. In our study, we chose human esophageal tissue for study since it constitutes a target site for the organism during disease in AIDS patients. Temporal studies of expression are reported, along with an analysis of the ability of strains with deletions in CHK1 (CHK21) and CSSK1 (CSSK21) to colonize and germinate on esophageal tissue. In a previous report, we showed that human blood and esophageal isolates of C. albicans, the latter of which cause extensive host inflammation, are more adherent to human esophageal tissue than are commensal type collection cultures or esophageal isolates that do not induce a severe inflammatory response (3). This observation indicates that the RHE model is a reasonable way of studying the pathogenesis of esophageal candidiasis. An additional reason to utilize this in vitro system is that what constitutes virulence of C. albicans may be tissue specific. For example, the chk1 mutant strain (CHK21) of C. albicans is avirulent in the invasive murine model but is virulent in a rat vaginitis model (10). Therefore, the role of CHK1 and CSSK1 in the colonization and germination of human esophageal tissue was explored to understand their role at another tissue site. Our analysis of phenotypic traits was done by using both histopathological sections and scanning electron microscopy (SEM) of infected RHE.
MATERIALS AND METHODS
Strains, media, culture conditions, and preparation of esophageal tissue.
The C. albicans strains used in this study are listed in Table 1. Mutant strains were constructed by the “urablaster” procedure (16) and have been described previously (9, 12). All strains were maintained as frozen stocks and then cultured on YPD (1% yeast extract, 2% glucose, 2% peptone) agar. For inoculation of human esophageal tissue, C. albicans CAF2 (parental strain), CHK21 (chk1/chk1 mutant), and CSSK21 (cssk1/cssk1 mutant), as well as strains reconstituted with either CHK1 or CSSK1 (revertants CHK23 and CSSK23, respectively), were routinely grown in 10 ml of YPD broth at 30°C with shaking at 250 rpm for 14 h. Cells were harvested by centrifugation at 2,000 × g for 15 min at 4°C. The cell pellets were washed twice with 20 ml of Hanks balanced salt solution (HBSS; Gibco BRL) and then suspended in 20 ml of HBSS for inoculation of esophageal tissue.
TABLE 1.
C. albicans strains used in this study
| Strain | Relevant genotype |
|---|---|
| CAF2 | ura3::imm434/URA3 |
| CHK21 | ura3::imm434/ura3::imm434/ura3 cahk1::hisG/cahk1::hisG-URA3-hisG |
| CHK23 | ura3::imm434/ura3::imm434/cahk1::hisG/CaCHK1::URA3-hisG |
| CSSK21 | ura3::imm434/ura3::imm434/ura3 cassk1::hisG/cassk1::hisG-URA3-hisG |
| CSSK23 | ura3::imm434/ura3::imm434/cssk1::hisG/CaSSK1::URA3-hisG |
RHE was supplied by SkinEthic Tissue Culture Laboratories (Nice, France). The tissue was prepared by culturing the Kyse-510 cell line (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany), which was derived from a human esophageal squamous cell carcinoma on an inert polycarbonate membrane (3). Within 3 to 5 days, a 0.5-cm2 tissue consisting of several cell layers formed on the polycarbonate membrane when cells were grown in medium MCDB153 containing insulin at 5 μg/ml (3). Antibiotics were not included in any growth medium. At 24 h prior to infection studies, the growth medium was replaced with a maintenance medium (supplied by SkinEthic Laboratories). The maintenance medium was changed every 24 h if the tissues were not used. The RHE tissues were washed, and the maintenance medium was replaced with 1 ml of HBSS containing 5 × 106 C. albicans yeast cells. Similarly prepared, uninfected RHE tissues were used as controls for the RT-PCR studies described below. All issues were incubated at 37°C, 5% CO2, and saturated humidity and sampled at the times designated below.
Histopathology.
At 1 and 4 h postinfection, all RHE tissues were fixed with 10% formaldehyde at room temperature, washed several times with HBSS, dehydrated, and embedded in paraffin. Semithin sections were prepared and examined by light microscopy for qualitative determination of adherence and germination of strains on esophageal tissues (magnification, ×400) following staining with periodic acid-Schiff (PAS) reagent.
SEM.
For SEM, each sample was fixed in 2.5% glutaraldehyde and 2.0% formaldehyde in phosphate-buffered saline buffer (pH 7.4), washed with phosphate-buffered saline buffer three times, and then postfixed in 1% osmium tetroxide in water for 20 min. All samples were washed three times with water, dehydrated with a series of graded ethanol solutions, and dried with a critical-point dryer. Gold coating of samples was performed with a Hummer I apparatus (Technics Inc.), and specimens were examined with a scanning electron microscope (Hitachi SuperScan Elite 751). Student's t test was used to evaluate differences among strains with regard to germination, length of germ tubes, and gene expression.
RNA extraction.
All of the experiments described below were performed three times with sets of RHE tissues. At designated times following infection with parental (CAF2), CSSK21, or CHK21 cells, the esophageal tissues were immediately frozen at −80°C. Total RNA was prepared by a modified version of the procedure described by Collart et al. (15). Frozen esophageal tissue samples were transferred into Eppendorf tubes containing 0.6 ml of TES solution (10 mM Tris-HCl, 10 mM EDTA, 0.5% sodium dodecyl sulfate, pH 7.5) and centrifuged at 12,000 × g and 4°C for 10 min. The pellets were then treated with 0.2 ml of a digestion solution (0.5% Tween 20, 0.5% Nonidet P-40, 0.5% Triton X-100) at 37°C for 20 min and centrifuged. The supernatant was discarded, and the pellets were suspended in 0.5 ml of TES-0.5 ml of acid phenol (pH 4.6)-0.5 ml of glass beads (400 to 600 μm; Sigma). Samples were vortexed three times (for 2 min each time) with a TurboMix (Fisher Scientific, Inc.) attached to a Fisher vortex. The samples were kept on ice between vortexing and then incubated at 65°C for 1 h with an occasional, brief vortexing. After a 10-min centrifugation at 12,000 × g (4°C), the aqueous phase was extracted with phenol and chloroform. Total RNA was precipitated with ethanol and sodium acetate and then treated with 140 μl of DNase (Gibco BRL) per sample at 30°C for 1 h. DNA-free RNAs were extracted twice with phenol, phenol-chloroform, and chloroform and finally precipitated with 2.5 volumes of ethanol. The concentration and purity of the RNA preparations were determined by measuring the A260 and A280 on a DU-6 spectrophotometer (Beckman). Purified RNA from each sample was confirmed to be DNA free by the absence of amplified products when specific primer sets for CHK1 and CSSK1 (mentioned below) were used in PCRs.
RT-PCR.
In order to quantitate expression of CHK1 and CSSK1 during the infection of human esophageal tissue, RT-PCR was performed for each gene at different times postinfection. As an internal control, a primer set for the C. albicans actin-encoding gene (ACT1) was designed on the basis of the variable region of the actin-encoding gene that was specific for C. albicans ACT1 (ACT1 [5′-GACGGTGAAGAAGTTGCTGC-3′] and ACT2 [5′-CAAACCTAAATCAGCTGGTC-3′]). This primer set amplified an 800-bp fragment in all of the RHE tissue samples infected with C. albicans but failed to amplify uninfected RHE, thus confirming that human actin was not amplified.
The RT-PCR primer set for CHK1 gene expression (CHK1 [5′-GAGCTACAAACTAGACAGGGG-3′] and CHK2 [5′-GTCCGACCGATAATCCACAAC-3′]) amplified a 506-bp region of C. albicans CHK1 from infected tissues. Similarly, a primer set for CSSK1 gene expression (SSK1 [5′-TCACGCCCAGCAATTCGATC-3′] and SSK2 [5′-GAATTTGGTGAAGAAACTGG-3′]) was designed to amplify a 744-bp region of CSSK1 from infected RHE samples.
Each RT-PCR for ACT1, CHK1, and CSSK1 gene expression was performed in triplicate on a PTC-100 thermal controller (MJ Research, Inc., Waltham, Mass.) with total RNA from RHE infected with C. albicans. The One-Step RT-PCR kit (Qiagen, Valencia, Calif.) was used in this study with all samples in a 50-μl reaction mixture containing 10 μl of buffer, 10 μl of Q solution, 2.0 μl of 10 mM deoxynucleoside triphosphate, 1.0 μM each primer for CHK1 or CSSK1 (0.6 μM each primer for ACT1), 1.0 μl of OneStep RT-PCR Enzyme Mix, and 10 U of RNase inhibitor. Template total RNA (900 ng for CHK1 or CSSK1 gene expression or 300 ng for ACT1 gene expression) was added to each mixture. The RT reactions were initiated immediately for 30 min at 54°C for CSSK1 and ACT1 or 52°C for CHK1. All reactions were inactivated by heating the samples to 95°C for 15 min, followed by activation of the Hot Start Taq DNA polymerase. cDNA was amplified for 30 cycles for ACT1 and 35 cycles for CHK1 and CSSK1 for the following cycling times: denaturation at 94°C for 1 min, annealing at 55°C for 1 min for CSSK1 and ACT1 or 56°C for CHK1, extension at 72°C for 1 min, and a final extension of 72°C for 10 min. Five microliters of each PCR amplification reaction from ACT1, CHK1, and CSSK1 was separated on a 1.2% agarose gel and stained with ethidium bromide. The integrated density value (IDV) of each band on the gel was obtained with an Alphaimager 2000 (Alpha Innotech Co., San Leandro, Calif.). All RT-PCR experiments were repeated three times.
PCR amplicons of the same size were obtained with specific PCR primer sets for CHK1 and CSSK1 by using p CHK1 and plasmid pBR34, which includes the entire encoding region of CHK1 and CSSK1, respectively. A negative control in each RT-PCR assay was used that omitted the RNA template in RT-PCRs, as well as the RNA sample from intact but noninfected esophageal tissue.
RESULTS
Adherence and morphogenesis of C. albicans strains on RHE tissue.
As an opportunistic pathogen, C. albicans is a commensal of mucosal surfaces, where it most likely survives as a budding yeast. The organism can become invasive when a human host is debilitated, and associated with this invasiveness is a change in its morphology to a filamentous growth as it integrates a wide variety of environmental stimuli through signal transduction pathways. In order to correlate the early events of pathogenesis (adherence and morphogenesis) with CHK1 and CSSK1 gene expression, we used an in vitro model of RHE tissue to mimic the disease process. Uninfected esophageal tissue, derived from a human esophageal squamous cell cancer cell line, maintained its structural integrity even after incubation in HBSS for up to 4 days (data not shown). Dyskeratotic cells (hyperchromatic nuclei or irregular chromatin) were rarely seen so that the tissue can be used as a reasonable model for the study of host-pathogen relationships. Other investigators have also shown that RHE tissues maintain an array of keratins typical of stratified epithelia (24).
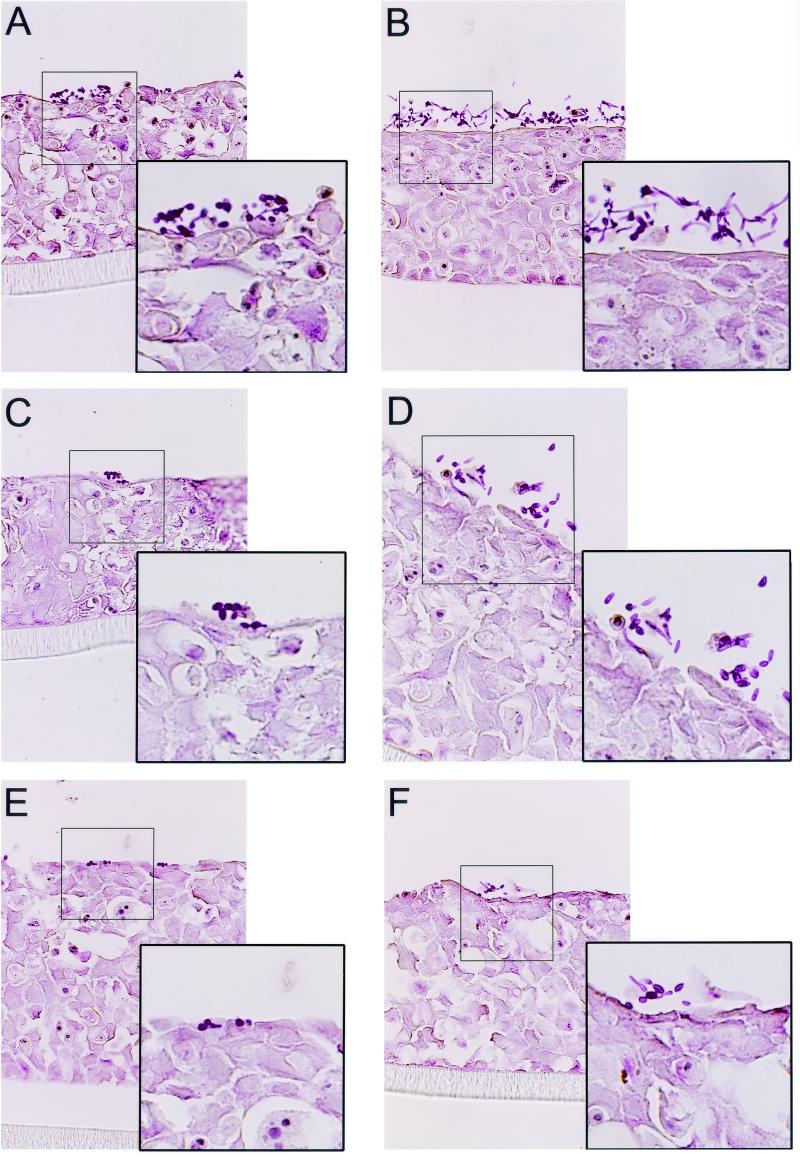
The interactions of C. albicans CAF2, CHK21 (chk1/chk1), and CSSK21 (cssk1/cssk1) with RHE tissue were observed by light microscopy (Fig. 1) and SEM (Fig. 2) at various time points following infection. As germination of yeast cells usually occurs in vitro after 1 to 4 h at 37°C, we chose to make visual observations in this time interval. SEM was especially useful for also obtaining measurements of germ tubes on the esophageal tissue. Compared to uninfected RHE tissue, there were no obvious morphologic alterations in the esophageal tissues during the early stages of infection. C. albicans CAF2 adhered to the outer layer of the esophageal tissue as early as 1 h after inoculation, and short germ tubes were also observed at this same time point (Fig. 1A and insert). After 4 h of incubation, adhering CAF2 cells were still visible and a greater percentage of cells had germinated; as expected, the germ tubes of many cells were considerably longer than at 1 h post infection (Fig. 1B and insert). At 4 h, penetration of the RHE was not observed by light microscopy of PAS-stained tissues; in previous studies, we found that invasion of esophageal tissue did not begin until 8 h postinfection with C. albicans SC5314 (3).
FIG. 1.
PAS-stained sections of RHE infected with CAF2 (A and B), CHK21 (C and D), or CSSK21 (E and F). The sections were taken from tissues infected with these strains for 1 h (A, C, and E) or 4 h (B, D, and F).
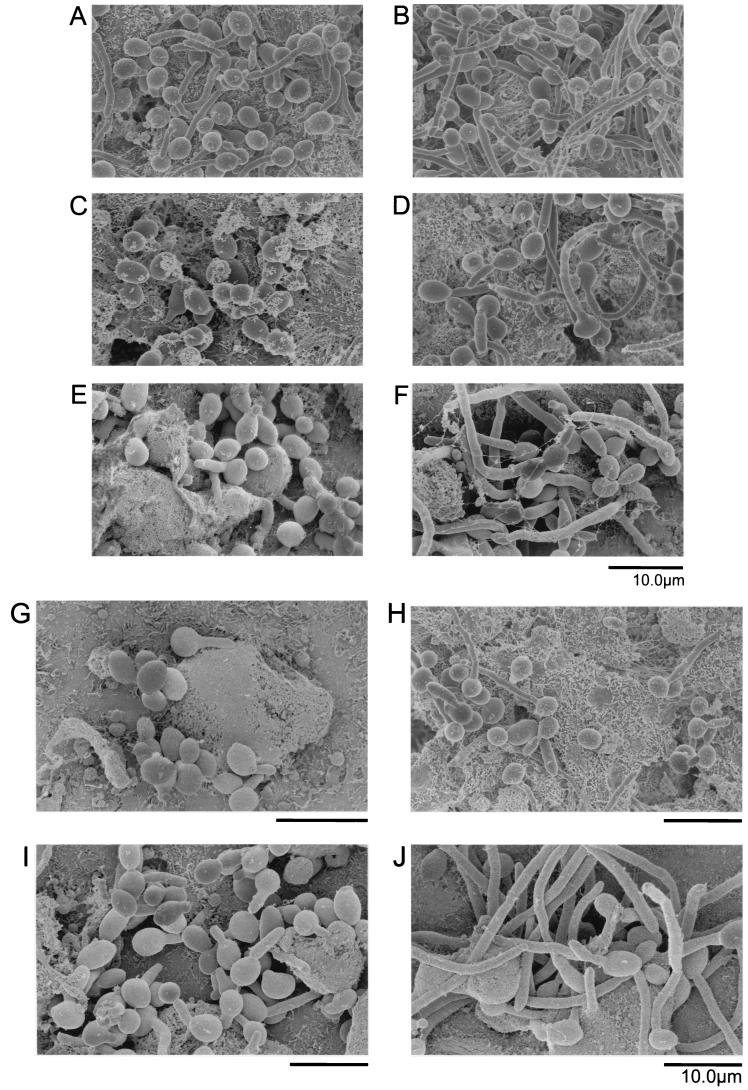
FIG. 2.
SEM of RHE infected with CAF2 (A and B), CHK21 (C and D), CHK23 (E and F), CSSK21 (G and H), or CSSK23 (I and J) for either 1 h (A, C, E, G, and I) or 4 h (B, D, F, H, and J).
As stated above, strains CHK21 and CSSK21 are avirulent in a hematogenously disseminated model of murine candidiasis (10, 12) and both mutants also have defects in morphogenesis under certain growth conditions (9, 12). Therefore, we measured the ability of these strains to adhere to and form hyphae on RHE tissue. By the first hour of infection, the adherence of both CHK21 (Fig. 1C and insert) and CSSK21 (Fig. 1E and insert) to the RHE was lower than that of strain CAF2 or their respective revertants, CHK23 and CSSK23. Adherence of CHK21 and CSSK21 was about 25 and 38%, respectively, in contrast to the 81% adherence of CAF2. The adherence of the revertant strain (CHK23) was approximately 53.3%, but revertant CSSK23 was similar to CSSK21 in its adherence to RHE. Compared to that of CAF2, the germination of CHK21 and CSSK21 was greatly reduced after 1 h of infection (Fig. 1C and E and inserts). Adherence was difficult to measure at 4 h postinfection because of the apparent growth of the strains on the RHE, but germination appeared to be less frequently observed with both mutants (Fig. 1 D and F and inserts).
The early events (adherence and germination) were also studied by SEM (Fig. 2). We again included strains with CHK1 (CHK23) and CSSK1 (CSSK23) reconstituted in measurements of adherence and germination. It should be stated that when examining specimens by SEM, it was difficult to locate the mutant strains on tissues, especially at 1 h postinfection, probably because their adherence was reduced compared to that of CAF2. Similar to the observations made by light microscopy, the adherence of CAF2 to RHE tissue was readily visualized and by 1 h, yeast cells had begun to germinate (Fig. 2A). By 4 h, most of the cells had germinated and extensive hyphal development had occurred (Fig. 2B). In contrast, strain CHK21 had not germinated by 1 h (Fig. 2C), while the strain with the reconstituted gene (CHK23) had initiated germination at 1 h postinfection (Fig. 2E), albeit less than CAF2. By 4 h, both CHK21 and CHK23, like CAF2, had germinated (Fig. 2D and F). Similar observations were noted for CSSK21 and the corresponding strain with the reconstituted gene (CSSK23) at 1 and 4 h (Fig. 2G to J). The germination of CSSK21 was much less than that of CAF2 or CSSK23 at 1 h postinfection (compare Fig. 2G to Fig. 2A and I) but similar to that of CSSK23 at 4 h (Fig. 2H and J).
Using SEM, we calculated the percentage of germinating cells of all of the strains and the lengths of the germ tubes (micrometers) at 1 and 4 h postinfection (Table 2). The data in Table 2 indicate that at 1 h postinfection, the percent germination, as well as the length of those cells that did germinate, was significantly reduced in the mutant strains (CHK21 and CSSK21; P < 0.005) and the strains with the reconstituted genes (CHK23 [P < 0.005] and CSSK23 [P < 0.05]) compared to that of CAF2. The differences in germ tube length were also apparent at 4 h postinfection for both null strains (P < 0.005), but no difference in germ tube length was observed for CSSK23 and CHK23 (Table 2). On the other hand, the germination percentages of all of the strains were similar at 4 h (Table 2). The results of the SEM study thus support our observations with light microscopy in regard to adherence.
TABLE 2.
Germination of wild-type C. albicans and CHK and CSSK mutants on RHE tissue as determined by SEMa
| Strains or comparison | 1 h |
4 h |
||
|---|---|---|---|---|
| Avg germ tube length (μm) ± SD | % of germ tubes | Avg germ tube length (μm) ± SD | % of germ tubes | |
| CAF2 | 6.6 ± 1.5 | 83 | 19 ± 4.0 | 90 |
| CHK21 | 3.5 ± 0.66** | 5** | 13 ± 2.9** | 88 |
| CHK23 | 4.6 ± 0.98* | 18** | 15 ± 3.5 | 88 |
| CSSK21 | 3.6 ± 0.94** | 13** | 9.7 ± 1.9** | 80 |
| CSSK23 | 5.0 ± 1.0 | 25** | 19 ± 3.1 | 82 |
| Among all groups | ** | ** | ** | |
| CHK21 vs CSSK23 | * | * | ||
| CSSK21 vs CSSK23 | * | ** | ||
Ten cells of each strain were measured for germ tube length. Approximately 100 cells were counted for germination. The five genotypes were tested by one-way analysis of variance (germ tube length) and chi square tests (percentage of germ tubes). The four mutations were compared against the wild type and null mutants were compared to revertants by Bonferroni-corrected post-hoc tests (*, corrected P < 0.05; **, corrected P < 0.01).
Temporal CHK1 and CSSK1 gene expression during colonization of RHE.
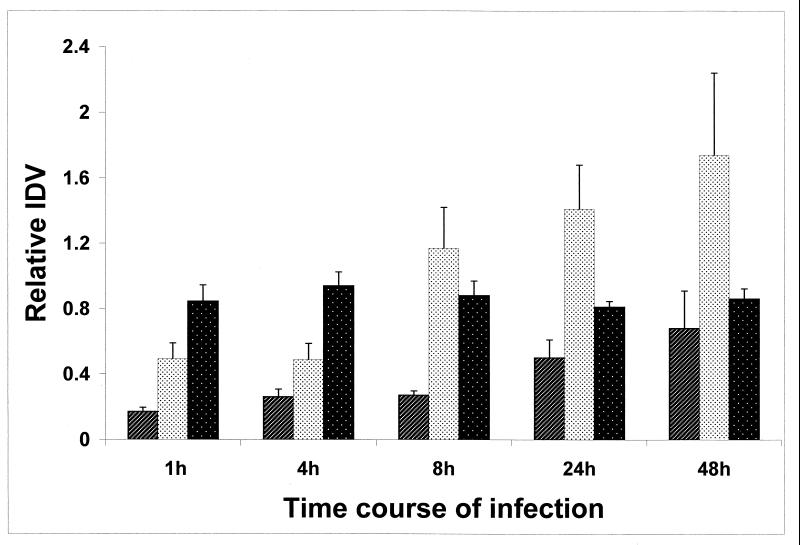
We investigated the temporal expression of CHK1 and CSSK1. At 1, 4, 8, 24, and 48 h postinfection, total RNA was extracted from uninfected RHE, as well as RHE infected with C. albicans CAF2 (Fig. 3). As a control, amplification was not observed by RT-PCR of ACT1, CHK1, or CSSK1 from uninfected RHE tissue (data not shown). In order to quantitate expression of CHK1 and CSSK1 by RT-PCR, ACT1 gene expression was used as an internal control. The linear range of amplification (30 amplification cycles with 300 ng of total ACT1 RNA and 35 cycles with 900 ng each of total CHK1 and CSSK1 RNAs) was chosen based upon the data curves determined from IDV using different cycles. Under these conditions, ACT1 was constitutively and equally expressed in all of the C. albicans strains tested (Fig. 3). Therefore, the density signal of ACT1 was used to normalize the data from each sample for CHK1 and CSSK1 expression in order to minimize any difference in the quality of the RNA for each extraction. Extractions of infected and noninfected tissues were done on three separate occasions, and all RT-PCRs were performed in triplicate. Amplification of ACT1, CHK1, and CSSK1 from infected tissue was not observed in the absence of reverse transcriptase, verifying the absence of genomic DNA contamination. Relative IDVs from different experiments were adjusted to the IDV of a common band from each gel so as to account for any differences that might occur during electrophoresis.
FIG. 3.
Expression of CHK1, CSSK1, and ACT1 at 1 to 48 h from RHE infected with C. albicans strain CAF2. The IDVs were deduced from 300 ng of total RNA for CHK1 (░⃞) and CSSK1(▨) and 10 ng of total RNA for ACT1(▪).
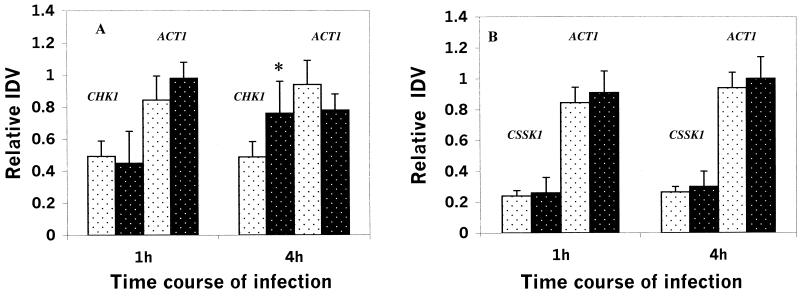
The expression profiles of CHK1 and CSSK1 correlated with adherence, germination, and hyphal growth on RHE tissue. ACT1 was amplified as an 800-bp fragment, and CHK1 was amplified as a 506-bp fragment, while the CSSK1 amplicon was 744 bp in size (data not shown). ACT1 expression was almost the same in samples from each time point and was about 30 times higher than that of either CHK1 or CSSK1, even with fewer PCR cycles (Fig. 3). The expression of both CHK1 and CSSK1 as measured by RT-PCR was detected as early as 1 h after infection and increased thereafter up to 48 h postinfection, although expression of CHK1 was greater than with CSSK1 (Fig. 3). In fact, expression of CHK1 was 2 to 2.5 times higher than that of CSSK1 with the same amount of total RNA extracted during infection of RHE. We also measured the expression of CHK1 in the CSSK21 mutant and CSSK1 expression in the CHK21 mutant (Fig. 4A and B). The reason for doing these experiments was to establish if CHK1 regulates the expression of CSSK1 (or if the opposite is true). We did not observe any statistically significant difference in the expression of CHK1 in the CSSK21 (cssk1/cssk1) mutant and in CAF2 at 4 h (Fig. 4A). Likewise, deletion of CHK1 did not affect the expression of CSSK1 (Fig. 4B).
FIG. 4.
(A) Expression of CHK1 and ACT1 at 1 and 4 h postinfection of RHE in the ssk1/ssk1 strain of C. albicans (wild type) (░⃞) and CSSK1 (▪) (∗, P = 0.051). (B) Expression of CSSK1 and ACT1 at 1 and 4 h postinfection of RHE in the chk1/chk1 strain of C. albicans (wild type) (░⃞) and CHK21 (▪).
DISCUSSION
Among the virulence attributes of C. albicans, host cell recognition by cell surface biomolecules (adhesins), morphogenesis, extracellular protease and lipase production, and phenotypic switching have been studied more extensively (6). Several signal pathways that regulate morphogenesis have been identified, as well as transcription factors that either activate or repress hypha-specific genes (6, 20, 22, 23). The Hog1 pathway is among those that may regulate morphogenesis (6, 7). In S. cerevisiae, the Hog1 pathway is regulated by a two-component signal transduction system. Cells use this pathway to adapt their growth to changes in osmolarity (25). While this pathway appears to have a secondary role in adapting C. albicans cells to osmotic stress, the functional activity of homologues of the Hog1 pathway is also associated with regulation of morphogenesis (7, 12, 21). For example, C. albicans SSK1 (the response regulator gene of the Hog1 pathway) can complement the ssk1 mutation of S. cerevisiae but ssk1 mutant strains of C. albicans, while not osmosensitive, are defective in hyphal formation under certain conditions (12).
Two-component signal transduction has been identified in bacteria, archaea, lower eukaryotes, and higher plants but is not found in humans (2, 19). In C. albicans, three hybrid histidine kinase genes and a single response regulator gene have been isolated and strains with a deletion in each were constructed (1, 7-9, 11-13, 21, 30, 31, 33). Mutant strains have defects in morphogenesis, switch phenotypes, and are either attenuated or avirulent in a murine model of hematogenously disseminated candidiasis. While each strain with a single gene deleted is still viable, deletions in both sln1 and cos1/nik1 are lethal (33). Thus, two-component signal proteins may represent useful targets for drug discovery (2) since they are specific to the pathogen and provide important functions for the organism (7). Further, a histidine kinase has also been identified in Aspergillus fumigatus, an important pathogen of immunocompromised patients (26), and any new antifungal drug should be able to target a broad range of fungal pathogens.
CHK1 of C. albicans encodes a putative hybrid histidine kinase (8) that is required for disease development in a murine model of hematogenously disseminated candidiasis, similar to CSSK1 (putative response regulator) (10, 12). CHK1 is apparently not required for vaginal infections, since rats infected with a strain with this gene deleted still develop a disease similar to that caused by the wild-type strain (10). This observation implies that site specificity may indeed be operative in determining the requirements for disease development.
The intent of this study was to evaluate the role of these two genes in the colonization of human esophageal tissue. To accomplish this objective, we utilized RHE tissue to examine the early events of gene expression and colonization/morphogenesis on RHE tissues. In previous studies, this model seemed appropriate for evaluation of the virulence of strains of C. albicans since we observed that blood isolates and esophageal isolates from patients (the latter with a high degree of tissue inflammation), but not commensal isolates or those from patients with esophagitis with reduced tissue inflammation, were able to colonize and invade RHE tissue and adhered better to a human esophageal cell monolayer (3). Thus, disease severity correlated directly with the pathogenic potential of C. albicans strains. We conducted studies on gene (CHK1 and CSSK1) expression and the role of these genes in adherence and morphogenesis by infecting RHE with strains with CHK1 and CSSK1 deleted or reconstituted. Among our observations were that expression of CHK1 and CSSK1 increased during the course of infection of the RHE and that these events correlated temporally with the adherence and germination of cells. Of the two, expression of CHK1 appeared to be greater than that of CSSK1. The expression of CSSK1 was not affected in the chk1 null strain, and likewise, the expression of CHK1 was not reduced in the cssk1 mutant (CSSK21) compared to that in CAF2.
The microscopic studies (light microscopy and SEM) revealed that strains with deletions in either chk1 or ssk1 colonized the RHE less than did the parental strain or a strain with the reconstituted gene. Likewise, at a similar time point (1 h), germination of both strains was also reduced by each deletion. It is important to note the difficulty of finding mutant strains on RHE tissue by using SEM at 1 h. At 4 h postinfection, both mutant strains had initiated germination but the hyphae of germinating cells were shorter than those of the parental strain or that with the reconstituted gene. Thus, our data indicate that CHK1 and CSSK1 are expressed during the colonization of RHE tissue and that strains with deletions in each gene are less able to establish themselves on the tissue.
The downstream structural proteins regulated by CHK1 or CSSK1 are being identified. In preliminary observations, it appears that both the mannan and glucan profiles of the CHK1 null strain (CHK21) are abnormal compared to those of CAF2. These differences are not apparent in the cssk1 null strain (CSSK21). This observation, along with the fact that CHK1 does not regulate expression of CSSK1 (and vice versa), may indicate that each gene encodes proteins of different signal pathways.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIAID AI47047 and NIAID AI 43465) to R.C.
We acknowledge the support of Tim Maugel of the University of Maryland Microscopy Center, M.-Z. Dai of the Microscopy Core Facility at the Lombardi Cancer Center, Georgetown University, and John Pezzullo, Department of Pharmacology, for his statistical analysis.
REFERENCES
- 1.Alex, L. A., C. Korch, C. P. Selitrennikof, and M. I. Simon. 1998. COS1, a two-component histidine kinase that is involved in hyphal development in the opportunistic pathogen Candida albicans. Proc. Natl. Acad. Sci. USA 95:7069-7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett, J. F., and J. A. Hoch. 1998. Two-component signal transduction as a target for microbial anti-infective therapy. Antimicrob. Agents Chemother. 42:1529-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhardt, J., D. Herman, M. Sheridan, and R. Calderone. Adherence and invasion studies of Candida albicans strains utilizing in vitro models of esophageal candidiasis. J. Infect. Dis., in press. [DOI] [PubMed]
- 4.Black, C. A., F. M. Eyers, A. Russel, M. L. Dunkley, R. L. Clancy, and K. W. Beagley. 1998. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect. Immun. 66:1273-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodey, C. A., M. Buckley, Y. S. Sathe, and E. J. Freirich. 1986. Quantitative relationship between circulating leukocytes and infections in patients with acute leukemia. Ann. Intern. Med. 64:328-340. [DOI] [PubMed] [Google Scholar]
- 6.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 7.Calera, J. A., and R. A. Calderone. 1999. Histidine kinase, two-component signal transduction proteins of Candidia albicans and the pathogenesis of candidosis. Mycoses 42(Suppl.):49-53. [PubMed] [Google Scholar]
- 8.Calera, J. A., G. Cho, and R. A. Calderone. 1998. Identification of a putative histidine kinase two-component phosphorelay gene (CaCHK1) in Candida albicans. Yeast 14:665-674. [DOI] [PubMed] [Google Scholar]
- 9.Calera, J. A., and R. A. Calderone. 1999. Flocculation of hyphae is associated with a deletion in the putative CaHK1 two-component histidine kinase gene from Candida albicans. Microbiology 145:1431-1442. [DOI] [PubMed] [Google Scholar]
- 10.Calera, J.A. X.-J. Zhao, M. Sheridan, and R. A. Calderone. 1999. Avirulence of Candida albicans CaHK1 mutants in a murine model of hematogenously disseminated candidiasis. Infect. Immun. 67:4280-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calera, J. A., and R. A Calderone. 1999. Identification of a putative response regulator, two-component phosphorelay gene (CaSSK1) from Candida albicans. Yeast 15:1243-1254. [DOI] [PubMed] [Google Scholar]
- 12.Calera, J. A., X.-J. Zhao, and R. A. Calderone. 2000. Defective hyphal formation and avirulence caused by a deletion of the CSSK1 response regulator gene in Candida albicans. Infect. Immun. 68:518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calera, J. A., D. Herman, and R. A. Calderone. 2000. Identification of YPD1, a gene of Candida albicans which encodes a two-component phospho-histidine intermediate protein. Yeast 16:1053-1059. [DOI] [PubMed] [Google Scholar]
- 14.Cole, G. T., K. R. Seshan, K. T. Lynn, and M. Franco. 1993. Gastrointestinal candidiasis: histopathology of Candida-host interactions in a murine model. Mycol. Res. 97:385-408. [Google Scholar]
- 15.Collart, M. A., and S. Oliviero. 1993. Preparation of yeast RNA, p. 13.12.1-13.12.5. In F. M. Ausubel (ed.), Current protocols in molecular biology, vol. 2. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 16.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gow, N. A. 1997. Germ tube growth of Candida albicans. Curr. Top. Med. Mycol. 8:43-55. [PubMed] [Google Scholar]
- 18.Kapteyn, J. C., L. L. Hoyer, J. E. Hecht, W. H. Muller, A. Andel, A. J. Verkleij, M. Makarow, H. Van. Den Ende, and F. M. Klis. 2000. The cell wall architecture of Candida albicans wild-type cells and cell wall-deficient mutants. Mol. Microbiol. 35:601-611. [DOI] [PubMed]
- 19.Koretke, K. K. A. N. Lupas, P. V. Warren, M. Rosenberg, and J. R. Brown. 2000. Evolution of two-component signal transduction. Mol. Biol. Evol. 17:1956-1970. [DOI] [PubMed] [Google Scholar]
- 20.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W.-C. Shen, P. Wang, X. Pan, M. Waugh, and J Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagahashi, S., T. Mio, N. Ono, T. Yamada-Okabe, M. Arisawa, H. Bussey, and H. Yamada-Okabe. 1998. Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology 144:425-432. [DOI] [PubMed] [Google Scholar]
- 22.Navarro-Garcia, F., R. Alonso-Monge, H. Rico, J. Pla, R. Sentandreu, and C. Nombela. 1998. A role for the MAP kinase gene MKC1 in cell wall construction and morphological transitions in Candida albicans. Microbiology 144:411-424. [DOI] [PubMed] [Google Scholar]
- 23.Navarro-Garcia, F., M. Sanchez, J. Pla, and C. Nombela. 1995. Functional characteristics of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol. Cell. Biol. 15:2197-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oda, D., C. E. Savard, L. Eng, J. Sekijima, G. Haigh, and S. P. Lee. 1998. Reconstituted human oral and esophageal mucosa in culture. In Vitro Cell Dev. Biol. 34:46-52. [DOI] [PubMed] [Google Scholar]
- 25.Posas, F., S. M. Wurgler-Murphy, T. Maeda, E. A. Witten, T. C. Thai, and H. Saito. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 two-component osmosensor. Cell 86:865-875. [DOI] [PubMed] [Google Scholar]
- 26.Pott, G. B., T. K. Miller, J. A. Bartlett, J. S. Palas, and C. P. Selitrennikoff. 2000. The isolation of FOS-1, a gene encoding a putative two-component histidine kinase from Aspergillus fumigatus. Fungal Genet. Biol. 31:55-67. [DOI] [PubMed] [Google Scholar]
- 27.Schaller, M., W. Schafer, H. C. Korting, and B. Hube. 1998. Differential expression of secreted aspartyl proteinases in a model of human oral candidosis and in patient samples from the oral cavity. Mol. Microbiol. 29:605-615. [DOI] [PubMed] [Google Scholar]
- 28.Schaller, M., H. C. Korting, W. Shafer, D. Sanglard, and B. Hube. 1998. Investigations on the regulation of secreted aspartyl proteases in a rat model of oral candidiasis in vivo. Mycoses 41S:69-73./ [DOI] [PubMed]
- 29.Schaller, M., H. C. Korting, W. Schafer, J. Bastert, W. Chen, and B. Hube. 1999. Secreted aspartyl proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol. Microbiol. 34:169-180. [DOI] [PubMed] [Google Scholar]
- 30.Selitrennikoff, C. P., L. Alex, T. K. Miller, K. V. Clemons, M. I. Simon, and D. A. Stevens. 2001. COS-1, a putative two-component histidine kinase of Candida albicans, is an in vivo virulence factor. Med. Mycol. 39:69-75. [DOI] [PubMed] [Google Scholar]
- 31.Srikantha, T., L. Tsai, K. Daniels, L. Enger, K. Highley, and D. R. Soll. 1998. The two-component hybrid kinase regulator CaNIK1 of Candida albicans. Microbiology 144:2715-2729. [DOI] [PubMed] [Google Scholar]
- 32.Wenzel, R. P. 1995. Nosocomial candidiasis: risk factors and attributable mortality. Clin. Infect. Dis. 20:1531-1534. [DOI] [PubMed] [Google Scholar]
- 33.Yamada-Okabe, T., T. Mio, T. Ono, Y. Kashima, M. Arisawa, and Y. Yamada-Okabe. 1999. Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J. Bacteriol. 181:7243-7247. [DOI] [PMC free article] [PubMed] [Google Scholar]