Abstract
Previous research and theory suggest that two stable personality dimensions, extroversion and neuroticism, differentially influence emotional reactivity to a variety of pleasurable phenomena. Here, we use event-related functional MRI to address the putative neural and behavioral associations between humor appreciation and the personality dimensions of introversion-extroversion and emotional stability-neuroticism. Our analysis showed extroversion to positively correlate with humor-driven blood oxygenation level-dependent signal in discrete regions of the right orbital frontal cortex, ventrolateral prefrontal cortex, and bilateral temporal cortices. Introversion correlated with increased activation in several regions, most prominently the bilateral amygdala. Although neuroticism did not positively correlate with any whole-brain activation, emotional stability (i.e., the inverse of neuroticism) correlated with increased activation in the mesocortical-mesolimbic reward circuitry encompassing the right orbital frontal cortex, caudate, and nucleus accumbens. Our findings tie together existing neurobiological studies of humor appreciation and are compatible with the notion that personality style plays a fundamental role in the neurobiological systems subserving humor appreciation.
Keywords: laughter, emotion, extroversion, neuroticism, functional MRI
Since Eysenck and Jung (1-3) described their pioneering and highly influential views of personality, it has been widely acknowledged that laughter and merriment are common characteristics of the extroverted individual. Both empirical and anecdotal observations point to extroverts as having a higher frequency of laughter, smiling, feelings of subjective well-being, and an increased propensity to tell jokes (4, 5). The opposite is thought to be true of neurotics, who are epitomized by decreased feelings of subjective well-being, expressive laughter, smiling, and increased negative emotionality (4, 6-8). Although these stable individual differences in personality are posited to be deeply rooted in the brain's functional (9-11) and structural architecture (12), little is known of how they are associated with the underlying neurobiological systems responsible for the emotive and hedonic regulation associated with humor appreciation.
Historically, a considerable body of clinicopathological literature, most famously Harlow's (13-16) lucid depiction of Phineas Gage, point to the prefrontal cortex (PFC) as a pivotal player in the maintenance of personality. With the recent emergence of functional MRI (fMRI), coupled with well validated measures of personality (17), the neuroscience community has witnessed a rekindled interest in the neural systems mediated by personality (11). For example, two recent fMRI studies by Canli and colleagues (9, 10) showed that extroversion positively correlates with phasic blood oxygenation level-dependent (BOLD) activity in the PFC and amygdala during the presentation of positively valanced faces, whereas neuroticism increases in association with middle temporal and frontal cortical activation during the presentation of negatively valanced faces. Collectively, these observations have led to the hypothesis that extroverts and neurotics are phenomenologically attuned to stimuli of positive and negative emotional significance, respectively (4, 7, 18).
Paralleling these studies is clinical evidence demonstrating that similar neurobiological systems orchestrate the comprehension and appreciation of humor (19-24). In the most recent study of its kind, Shammi and Stuss (24) reported that damage to the right PFC profoundly disrupted both the ability to appreciate and react emotionally to jokes. In line with these observations is preliminary brain imaging evidence showing the right PFC activity to parametrically increase with the explicit funniness of a joke (25). Despite this complementary overlap, subsequent fMRI investigations of humor have failed to replicate these findings, instead hinting at amygdala and mesolimbic dopaminergic structures as being as equally critical in humor appreciation (26-29). To date, attempts to understand the nature of these divergent findings remain speculative and unresolved (30, 31). Accordingly, the rationale for the present study was to further advance our knowledge of neural systems underlying humor appreciation by examining whether broadly accepted dimensions of personality are significantly associated with the cognitive, affective, and hedonic regulation of humor appreciation.
Using fMRI, in conjunction with a correlational analytical approach, we hypothesized that individual variations in introversion-extroversion and emotional stability-neuroticism dimensionality would differentially elicit activation in key cortical and subcortical regions associated with humor appreciation, including the nucleus accumbens (NAcc), right PFC, and amygdalar nuclei. The findings reported herein strongly favor the hypothesis that personality style is a mediating factor in the neurobiological regions known to be involved in humor appreciation and help to resolve inconsistent results from previous neuroimaging studies of this important human phenomenon.
Materials and Methods
Subjects. We scanned 17 healthy volunteers (8 females, 9 males; mean age and SD = 22.8 ± 1.9) All subjects were native English speaking, right-handed (32), and screened for psychiatric or neurological problems by using the Symptom Checklist-90-R (33). Informed consent was obtained from each participant. All protocols were approved by the human subjects committee at Stanford University School of Medicine.
Personality Measures. Personality was indexed by using the NEO Five-Factor Inventory (NEO-FFI) (17), a 60-item, self-report questionnaire that assesses the five personality dimensions of neuroticism, extroversion, openness to experience, agreeableness, and conscientiousness. Based on the theory and research mentioned above (4, 7, 18), we restricted our analysis to extroversion and neuroticism. Resultant T scores were correlated with behavioral data, regions of interest, and whole-brain BOLD signal.
Sense of Humor Measures. We used the revised Sense of Humor Questionnaire (SHQr) to evaluate several dimensions of humor (34). The SHQr is a 21-item, self-report questionnaire that measures three dimensions of humor on a four-point scale, including (i) the habitual sensitivity to humorous messages (Mp), (ii) the habitual tendency to enjoy or dislike comical situations (Lp), and (iii) the habitual tendency to permit or suppress emotional impulses of joy (Ep).
Postscan Ratings. After the scan, each subject was asked to rate each cartoon for humor intensity (i.e., degree of funniness) on a 1-to-10 scale, with 1 being least humorous and 10 being most humorous. Cartoons that were considered nonhumorous were rated zero.
Experimental Stimuli and Design. A more detailed account of stimuli and design can be viewed elsewhere (26, 27). Briefly, 70 cartoons (30 funny) were used based on the ratings of subjects similar in background and age. Subjects were told to respond with a press of a button if they found the cartoon humorous or not. Stimuli were presented by using psyscope (35) in an event-related fMRI paradigm, with each cartoon being presented in random order for 6 s. A jittered interstimulus interval was used, varying between 2, 4, and 6 s, and counterbalanced across humorous and nonhumorous events. The scan took 15 min and 4 s.
fMRI Acquisition. Images were acquired on a 3-T scanner (Signa, General Electric) by using a standard GE whole-head coil. The scanner ran on a LX platform, with gradients in “MiniCRM” configuration (35 mT/m, slew rate 190 mT per m/s), and has a 3-T 80-cm magnet (Magnex). A custom-built head holder was used to prevent head movement associated with laughter. Twenty-eight axial slices (4-mm thick, 0.5-mm skip) parallel to the anterior-posterior commissure covering the whole brain were imaged with a temporal resolution of 2 s by using a T2*-weighted gradient echo spiral pulse sequence (repetition time = 2,000 ms, echo time = 30 ms, flip angle = 80°, and 1 interleave) (36). The field of view was 200 × 200 mm2, and the matrix size was 64 × 64, giving an in-plane spatial resolution of 3.125 mm. To maximize magnetic-field homogeneity, a high-order shimming method based on spiral acquisitions was used to reduce B0 heterogeneity.∥
Statistical Analysis. Statistical parametric mapping (spm99; www.fil.ion.ucl.ac.uk/spm/spm99.html) was used to preprocess all fMRI data and included realignment, normalization, and smoothing. These methods are described in more detail in ref. 27. Statistical parametric maps were first generated for the humorous, compared with nonhumorous, stimuli, for each subject by using a general linear model. In the second level of analysis, random-effects analysis was performed to determine each subject's voxel-wise activation during humorous events, compared with nonhumorous events. For the entire group of 17 subjects, significant clusters of humor-related activation were determined by using height (P < 0.05) and extent (P < 0.05) thresholds corrected for multiple comparison (37).
Clusters of Interest Analysis. To address the nature of the association between personality and brain activation, we examined the correlation among NEO-FFI, SHQr scores, and BOLD response by using the group-wise activation clusters as regions of interest generated at the whole-brain level (as described above). The percentage of voxels in each cluster of interest, with z > 1.96 (P < 0.05), was determined for each contrast. An α level for significance of P < 0.05 (two-tailed) was used.
Results
Behavioral Scores. Examination of behavioral data in the subject group showed the mean response time (RT) for humorous stimuli to be 3,818.4 ± 461.7 ms and the percentage of total cartoons found humorous to be 84 ± 17.8%. The correlation between RT and extroversion was not significant (Spearman's r = 0.340, P < 0.182). Likewise, extroversion did not significantly increase with the number of cartoon jokes found humorous (r = -0.289, P < 0.261). No significant correlations were detected between RT (r = -0.432, P < 0.083) or the number of stimuli found humorous (r = -0.077, P < 0.770) with neuroticism. Together, these results suggest, at least behaviorally, that the extroversion and neuroticism dimensions are not mediating factors in the comprehension and ability to appreciate humorous material.
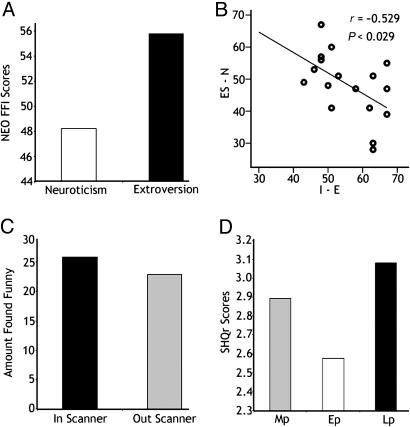
Personality Scores. NEO-FFI T scores ranged from 43 to 67 for extroversion (mean ± standard deviation: 55.7 ± 8.3) and from 28 to 67 for neuroticism (48.2 ± 10.1; Fig. 1A). Extroversion negatively correlated with neuroticism (Spearman's r = -0.529, P < 0.029; Fig. 1B).
Fig. 1.
Comparison of behavioral, personality and SHQr scores. (A) T scores for the NEO-FFI traits of neuroticism and extroversion. Note the higher extroversion scores. (B) Significant negative correlation between emotional stability-neuroticism (ES-N) and introversion-extroversion (I-E). (C) Proportion of cartoons subjectively found humorous in and out of the scanner. (Cartoons found funny only outside the scanner were discarded from analysis.) (D) SHQr scores; Mp, the habitual sensitivity to humorous messages; Lp, the habitual tendency to enjoy or dislike comical situations; Ep, the habitual tendency to permit or suppress emotional impulses of joy.
Sense of Humor Scores. The SHQr was available from 16 of the 17 subjects (one subject was missing data). Scores on the Mp dimension ranged from 2.14 to 3.28 (mean and SD, 2.89 ± 0.26), Ep ranged from 2.14 to 3.14 (2.57 ± 0.30), and Lp ranged from 2.42 to 3.85 (3.08 ± 0.32; Fig. 1D). No significant correlations between SHQr dimensions and NEO-FFI scores were found (P < 0.05).
Postscan Ratings. The individual means (for all humorous jokes) ranged from 3.70 to 7.53, with a group mean of 6.26 ± 0.93. No correlations were found among the individual mean ratings and extroversion (r = 0.242 P < 0.349), neuroticism (r = -0.014 P < 0.959), or dimensions of the SHQr (P < 0.05) (Fig. 1D).
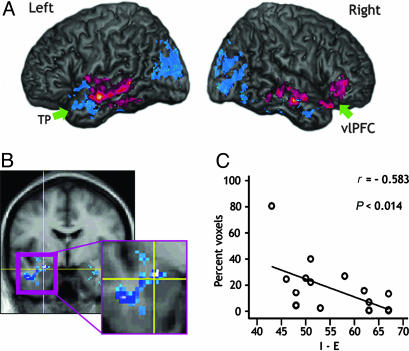
fMRI Correlations. Extroversion positively correlated with humor-related activation in the left middle temporal gyrus (MTG), the superior temporal gyrus (STG) encroaching upon the posterior insula, the anterior fusiform gyrus (FuG), and the parahippocampal gyrus (PHG) [Brodmann's area (BA) 20/21/37]. Another cluster was observed in the homologous right MTG region, extending dorsally to the STG (BA 21/22). A third cluster was seen in the right pars orbitalis of the inferior frontal gyrus extending subjacent to the orbital frontal cortex (OFC) and ventrolateral prefrontal cortex (vlPFC; BA 47/11; see Table 1, Figs. 2A and 3A, and Fig. 4, which is published as supporting information on the PNAS web site).
Table 1. Summary of humor-driven brain activation that correlated with selected dimensions of personality.
| Talairach coordinates |
||||
|---|---|---|---|---|
| Regions | z score | x | y | z |
| Extroversion | ||||
| Left MTG, STG, INS, FuG, PHG | 3.78 | −55 | −12 | −11 |
| Right MTG, STG | 3.58 | 53 | −14 | −13 |
| Right IFG vlPFC, VMPFC, OFC | 3.54 | 28 | 31 | −10 |
| Introversion | ||||
| Left AMYG, PHG, MTG, INS | 3.66 | −26 | −4 | 12 |
| Right STG, MTG, GP, HIPP, AMYG, IFG | 2.97 | 36 | 3 | −25 |
| Left Calcarine, MOG, cuneus | 3.68 | −18 | −87 | 15 |
| Right cuneus, LG, cerebellum | 3.53 | 16 | −86 | 34 |
| Neuroticism | ||||
| No significant correlations | — | — | ||
| Emotional stability | ||||
| Right BG, NAcc, IFG, OFC, vlPFC | 4.19 | 14 | 10 | 7 |
Only clusters with a significance value of P < 0.05 corrected for multiple comparisons are reported. AMYG, amygdala; IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; LG, lingual gyrus; PCu, precuneus; MOG, middle occipital gyrus; STG, superior temporal gyrus; MTG, inferior temporal gyrus; FuG, fusiform gyrus; vlPFC, ventrolateral prefrontal cortex; GP, globus pallidus; OFC, orbital frontal cortex; NAcc, nucleus accumbens; BG, basil ganglia; INS, insula; HIPP, hippocampus; VMPFC, ventromedial PFC.
Fig. 2.
BOLD activity correlation with extroversion and introversion to humor appreciation. (A) Whole-brain BOLD signal correlations with extroversion (red) and introversion (blue) when subjectively chosen humorous cartoons were subtracted from cartoons determined nonhumorous (P < 0.05). Note the right vlPFC activation with extroversion (more orbital portions can be viewed in Fig. 4). TP, temporal pole. (B) Amygdala BOLD signal correlation with introversion. (C) Scatter plot illustrating the increased amygdala activation with introversion.
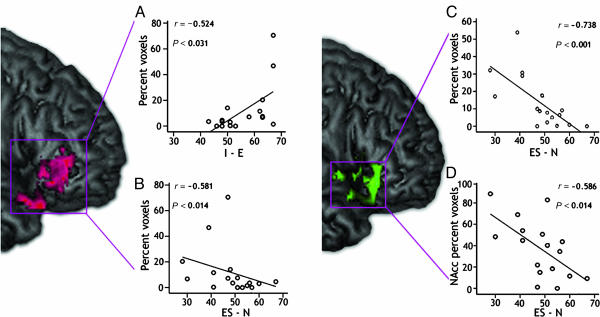
Fig. 3.
Areas concurrently activated with personality. (A) Positive correlation between extroversion and right PFC activation. (B) Negative correlation between right PFC activation and neuroticism. (C) Negative correlation between BOLD activity and neuroticism. (D) Increased activation between emotional stability and BOLD signal. More detailed images, including subcortical and OFC clusters, can be viewed in Figs. 4 and 5.
Introversion (decreased extroversion) correlated with increased BOLD response in the left amygdala, extending to the PHG, and MTG (BA 47/20; see Fig. 2 A and B). A similar cluster also was observed in the right hemisphere that included the STG, MTG, and several subcortical structures, including the globus pallidus, the anterior tip of the hippocampus, and the amygdala (BA 20/38). Finally, two clusters were observed in the visual cortex; one peaking in the left calcarine sulcus, left middle occipital gyrus (MOG), and extending to the cuneus and precuneus (BA 18/19). The second cluster peaked in the right cuneus, extending to the lingual gyrus and cerebellum (BA 18/19; Table 1 and Fig. 2 A and B).
No significant positive correlations were found between BOLD signal and neuroticism at the whole-brain level (P < 0.05, corrected). However, a negative correlation (herein defined as emotional stability) was found in the right caudate, putamen, NAcc, extending anterior and lateral to the inferior frontal gyrus, OFC, and vlPFC (BA 11/47) (Fig. 3 B-D and Fig. 5, which is published as supporting information on the PNAS web site).
Based on our a priori predictions, we isolated several clusters of interest (i.e., the right PFC, right NAcc, and left amygdala) to examine correlations with our traits of interest (see Fig. 2). The right PFC (peak Talairach cluster 28, 31, -10) positively correlated with extroversion (r = 0.524, P < 0.031). In contrast, this region negatively correlated with neuroticism (r = -0.581, P < 0.014). The right NAcc (peak 6, 2, -4) increased with emotional stability (r = -0.586, P < 0.014), whereas the left amygdala (peak, -26, -4, 12) correlated with introversion (r = -0.583, P < 0.014) (Fig. 2B). None of these clusters correlated with SHQr scores or percentage of cartoons that were found humorous (P < 0.05, corrected).
Discussion
Understanding the specific manner in which individual differences mediate cognition and emotion presents one of the formidable challenges of contemporary cognitive neuroscience. Despite the absence of correlations between NEO-FFI scores and behavioral measures, our preliminary findings revealed marked correlational differences in BOLD activity associated with variations in personality. The right OFC and adjacent vlPFC concurrently increased with extroversion during the appreciation of humorous cartoons. Increases in introversion led to elevated bilateral amygdala nuclei and anterior temporal lobe activation. Neuroticism did not positively correlate with whole-brain activation, a finding probably due to the unforeseen high extroversion scores in our sample (cf. ref. 10). Nonetheless, emotional stability correlated with BOLD activity in the right vlPFC, OFC, extending to the right caudate and NAcc.
As previously noted, the right PFC plays an important role in the neural circuitry underlying humor appreciation (19, 20, 25, 31). The right OFC is known to play an extended role in reward, receiving ascending dopamine (DA) projections from the ventral tegmental area, and is critical in representing stimulus reward value (38, 39), including hedonic aspects of humor appreciation (25). In the context of the personality literature, it is intriguing to note that extroverts are particularly sensitive to signals of incentive-reward (40), a finding possibly linked to differences in DA functioning (40, 41). This hypothesis is intimately allied with Eysenck's (42) original notion that extroverts are chronically underaroused, more easily bored, and tend to engage in more sensation-seeking behavior. Importantly, the OFC has high DA density and is activated during the administration of DA agonists (43) and sensation-seeking behaviors (44, 45). Therefore, one conclusion is that the increased modulation of the OFC results in heightened reward during humor appreciation in extroverts.
A significant correlation also was found between the right PFC activity and emotional stability. Extant research has demonstrated that increased emotional stability results in decreased temperamental sensitivity to negative information (4). Moreover, it is widely demonstrated that emotional stability is one of the most reliable personality variables for predicting life-satisfaction (7), self-esteem (46), happiness (4), and susceptibility to episodic depression (47). Fitting with this hypothesis, the right PFC may be overactive during manic episodes in patients with bipolar disorder (48) but decreased during episodes of depression (49). Intuitively, the overlapping modulation of the right PFC for both extroversion and emotional stability suggest the use of similar salutatory neural systems.
The right PFC cluster associated with emotional stability also extended into the mesolimbic region, encompassing several DA-enriched “reward” structures, including the NAcc and caudate nucleus. Notably, a similar pattern of mesocortical-mesolimbic activation has been observed during immediate monetary and aesthetic rewards (50-53), self-reported happiness (54), and the subjective funniness of cartoons (26, 27). Electrical stimulation of the NAcc also results in laughter and giddiness (55). With regard to personality research, emotional stability is strongly correlated with laughter, elation, and perception of everyday reward (46). Although the spatial resolution of fMRI makes it difficult to examine subcortical nuclei more precisely, it has been suggested that the NAcc shell is involved in affect perception, whereas the NAcc core is involved in emotional behavior (56). Thus, the NAcc is a convincing candidate for the elative, or positive emotional, feeling accompanying humor appreciation (27, 31).
Another possibility is that the right PFC is involved in the emotional expressiveness associated with humor (29). Although not conclusive, extroversion has been shown to be positively correlated with laughter (57), smiling (5), and affective expression (58). Clinical evidence also has demonstrated that damage to the right PFC causes hypoaffectivity (17) and depressed laughter and mirth (24). Although we were not able to vigorously probe the relationship between laughter and personality, our results do not support this conclusion, because no correlations were found between Ep scores (i.e., tendency to express or suppress laughter) and personality. Future studies should further explore this association by using, for example, real-time recordings of face expression to humorous material (see ref. 29). Alternatively, activation in the right PFC, particularly the cyto-architectonically distinct lateral portions, may reflect individual differences in cognitive aspects of the humor appreciation (i.e., episodic memory and laughter inhibition). Contrary to prior reports by Canli and colleagues (9-11), the amygdala increasingly activated with the dimension of introversion (Fig. 2 B and C). One explanation for this difference is that the recognition of static facial emotion is very different from the actual feeling of elation that typically accompanies laughter and other arousing stimuli (59, 60). Amygdala activity has been a particularly prevalent finding in fMRI studies of humor (27-29). We have previously suggested that the amygdala is involved in the rewarding aspects of finding a cartoon humorous (27), a view supported by lesion and fMRI studies using monetary and aesthetic rewards (61, 62). Although the functions of the amygdala are complex, one possible explanation is that its modulation is related, in part, to increased pleasure of affective origin.
Given that no significant correlations were observed between personality and either SHQr scores or proportion of cartoons found funny, it is difficult to definitively state whether variations in neural processing translate into overt behavioral differences. Possible explanations for the absence of significant brain-behavior correlations could range from the utility and validity of the behavioral measures to the sample size used in this experiment. However, other large-scale behavioral studies also have failed to detect significant correlations between personality and several dimensions of humor, such as the propensity to smile (63, 64) and the perceived funniness of jokes (65). Finally, the role of learned social and cultural-based behavior also should not be underestimated in attempting to understand individual differences in the neural basis and behavioral manifestations of humor appreciation (66).
In summary, the data presented here support the notion that key neurobiological structures in humor circuitry are mediated by personality. Despite the lack of observed behavioral differences, one could speculate how variation in neural processes associated with personality result in qualitatively different rewards or emotions (e.g., emotionally based rewards vs. sensation-seeking rewards). Humor may tap into several salutary systems, each more or less driven by individual differences. In the broader theoretical framework, an important focus of future research will be to consider the intriguing interaction between personality and humor and how this interaction exerts influence over physiological and psychological functioning.
Supplementary Material
Acknowledgments
We thank Drs. Michael D. Greicius and Amy S. Garrett for their helpful comments. This work was supported by National Institutes of Health Grant MH01142 (to A.L.R.).
Author contributions: D.M., V.M., and A.L.R. designed research; D.M. and E.A. performed research; V.M. contributed new reagents/analytic tools; D.M., C.C.H., and E.A. analyzed data; and D.M., C.C.H., and A.L.R. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BA, Brodmann's area; fMRI, functional MRI; PFC, prefrontal cortex; vlPFC, ventrolateral PFC; BOLD, blood oxygenation level-dependent; NAcc, nucleus accumbens; NEO-FFI, NEO Five-Factor Inventory; SHQr, revised Sense of Humor Questionnaire; MTG, middle temporal gyrus; STG, superior temporal gyrus; OFC, orbital frontal cortex; DA, dopamine.
Footnotes
Kim, D., Adalsteinsson, E., Glover, G. & Spielman, S., Eighth Meeting of the International Society for Magnetic Resonance in Medicine, April 3-7, 2000, Denver, CO, p. 1685 (abstr.).
References
- 1.Eysenck, H. J. & Eysenck, S. B. G. (1975) Manual of the Eysenck Personality Questionnaire (Hodder & Stoughton, London).
- 2.Eysenck, H. J. (1943) Educ. Psychol. Measurement 3 191-214. [Google Scholar]
- 3.Jung, C. (1928) L'Inconscient dans la Vie Psychique Normale et Anormale (Payot, Paris).
- 4.DeNeve, K. M. & Cooper, H. (1998) Psych. Bull. 124 197-229. [DOI] [PubMed] [Google Scholar]
- 5.Ruch, W. & Deckers, L. (1993) Eur. J. Pers. 7 211-220. [Google Scholar]
- 6.Procyk, E., Tanaka, Y. L. & Joseph, J. P. (2000) Nat. Neurosci. 3 502-508. [DOI] [PubMed] [Google Scholar]
- 7.Costa, P. T. & McCrae, R. R. (1980) J. Pers. Soc. Psychol. 38 668-678. [DOI] [PubMed] [Google Scholar]
- 8.Cann, A. & Calhoun, L. G. (2001) Humor 14 117-130. [Google Scholar]
- 9.Canli, T., Sivers, H., Whitfield, S. L., Gotlib, I. H. & Gabrieli, J. D. E. (2002) Science 296 2191. [DOI] [PubMed] [Google Scholar]
- 10.Canli, T., Zhao, Z., Desmond, J. E., Kang, E., Gross, J. & Gabrieli, J. D. E. (2001) Behav. Neurosci. 115 33-42. [DOI] [PubMed] [Google Scholar]
- 11.Canli, T. & Amin, Z. (2002) Brain Cognit. 50 414-431. [DOI] [PubMed] [Google Scholar]
- 12.Knutson, B., Momenan, R., Rawlings, R. R., Fong, G. W. & Hommer, D. (2001) Biol. Psychiatry 50 685-690. [DOI] [PubMed] [Google Scholar]
- 13.Blair, R. J. & Cipolotti, L. (2000) Brain 123 1122-1141. [DOI] [PubMed] [Google Scholar]
- 14.Damasio, H., Grabowski, T., Frank, R., Galaburda, A. M. & Damasio, A. R. (1994) Science 264 1102-1105. [DOI] [PubMed] [Google Scholar]
- 15.Harlow, J. (1848) Boston Med. Surg. J. 13 389-393. [Google Scholar]
- 16.Goldstein, K. (1952) Psychiatry 15 245-260. [DOI] [PubMed] [Google Scholar]
- 17.Costa, P. T. & McCrae, R. R. (1991) NEO Five-Factor Inventory (NEO-FFI) Professional Manual (Psychological Assessment Resources, Odessa, FL).
- 18.Lucas, R. E. & Baird, B. M. (2004) J. Pers. Soc. Psychol. 86 473-485. [DOI] [PubMed] [Google Scholar]
- 19.Brownell, H. H., Michel, D., Powelson, J. & Gardner, H. (1983) Brain Lang. 18 20-27. [DOI] [PubMed] [Google Scholar]
- 20.Bihrle, A. M., Brownell, H. H., Powelson, J. A. & Gardner, H. (1986) Brain Cognit. 5 399-411. [DOI] [PubMed] [Google Scholar]
- 21.Gardner, H., Ling, P. K., Flamm, L. & Silverman, J. (1975) Brain 98 399-412. [DOI] [PubMed] [Google Scholar]
- 22.Gardner, H. (1994) in Integrative Views of Motivation, Cognition, and Emotion, ed. Spaulding, W. D. (Univ. Nebraska Press, Lincoln), Vol. 16, pp. 57-69. [Google Scholar]
- 23.Lehman-Blake, M. (2003) Semin. Speech Lang. 24 107-120. [DOI] [PubMed] [Google Scholar]
- 24.Shammi, P. & Stuss, D. T. (1999) Brain 122 657-666. [DOI] [PubMed] [Google Scholar]
- 25.Goel, V. & Dolan, R. J. (2001) Nat. Neurosci. 4 237-238. [DOI] [PubMed] [Google Scholar]
- 26.Azim, E., Mobbs, D., Jo, B., Menon, V. & Reiss, A. L. (2005) Proc. Natl. Acad. Sci. USA 102 16496-16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mobbs, D., Greicius, M. D., Abdel-Azim, E., Menon, V. & Reiss, A. L. (2003) Neuron 40 1041-1048. [DOI] [PubMed] [Google Scholar]
- 28.Moran, J. M., Wig, G. S., Adam, R. B., Janata, P. & Kelley, W. M. (2004) NeuroImage 3 1055-1060. [DOI] [PubMed] [Google Scholar]
- 29.Iwase, M., Ouchi, Y., Okada, H., Yokoyama, C., Nobezawa, S., Yoshikawa, E., Tsukada, H., Yamashita, K., Takeda, M., et al. (2002) NeuroImage 17 758-768. [PubMed] [Google Scholar]
- 30.Berns, G. (2004) Trends Neurosci. 8 193-194. [Google Scholar]
- 31.Wild, B., Rodden, F. A., Grodd, W. & Ruch, W. (2003) Brain 126 1-18. [DOI] [PubMed] [Google Scholar]
- 32.Oldfield, R. C. (1971) Neuropsychologia 9 97-113. [DOI] [PubMed] [Google Scholar]
- 33.Derogatis, L. R. (1977) SCL-90: Administration, Scoring, and Procedures Manual (Johns Hopkins University, Clinical Psychometrics Research Unit, Baltimore).
- 34.Svebak, S. (1974) Scand. J. Psychol. 15 328-331. [DOI] [PubMed] [Google Scholar]
- 35.Cohen, J. D., MacWhinney, B., Flatt, M. & Provost, J. (1993) Behav. Res. Methods Instrum. Comput. 25 257-271. [Google Scholar]
- 36.Glover, G. H. & Lai, S. (1998) Magn. Reson. Imag. 39 361-368. [DOI] [PubMed] [Google Scholar]
- 37.Poline, J. B., Worsley, K. J., Evans, A. C. & Friston, K. J. (1997) NeuroImage 5 83-96. [DOI] [PubMed] [Google Scholar]
- 38.Schoenbaum, G., Chiba, A. A. & Gallagher, M. (1998) Nat. Neurosci. 1 155-159. [DOI] [PubMed] [Google Scholar]
- 39.O'Doherty, J., Kringelbach, M. L., Rolls, E. T., Hornak, J. & Andrews, C. (2001) Nat. Neurosci. 1 95-102. [DOI] [PubMed] [Google Scholar]
- 40.Depue, R. A. & Collins, P. F. (1999) Behav. Brain Sci. 22 491-517. [DOI] [PubMed] [Google Scholar]
- 41.Depue, R. A., Luciana, M., Arbisi, P., Collins, P. & Leon, A. (1994) J. Pers. Soc. Psychol. 67 485-498. [DOI] [PubMed] [Google Scholar]
- 42.Zuckerman, M., Eysenck, S. & Eysenck, H. J. (1978) J. Consult. Clin. Psychol. 46 139-149. [DOI] [PubMed] [Google Scholar]
- 43.Völlm, B. A., de Araujo, I. E., Cowen, P. J., Rolls, E. T., Kringelbach, M. L., Smith, K. A., Jezzard, P., Heal, R. J. & Matthews, P. M. (2003) Neuropharmacology 29 1715-1722. [DOI] [PubMed] [Google Scholar]
- 44.Potenza, M. N., Leung, H. C., Blumberg, H. P., Peterson, B. S., Fulbright, R. K., Lacadie, C. M., Skudlarski, P. & Gore, J. C. (2003) Am. J. Psychiatry 11 1990-1994. [DOI] [PubMed] [Google Scholar]
- 45.Potenza, M. N., Steinberg, M. A., Skudlarski, P., Fulbright, R. K., Lacadie, C. M., Wilber, M. K., Rounsaville, B. J., Gore, J. C. & Wexler, B. E. (2003) Arch. Gen. Psychiatry 60 828-836. [DOI] [PubMed] [Google Scholar]
- 46.Hills, P. & Argyle, M. (2001) Pers. Indiv. Diff. 31 1357-1364. [Google Scholar]
- 47.Lu, L. & Shih, J. B. (1997) Pers. Indiv. Diff. 22 249-256. [Google Scholar]
- 48.Elliott, R., Ogilvie, A., Rubinsztein, J. S., Calderon, G., Dolan, R. J. & Sahakian, B. J. (2004) Biol. Psychiatry 55 1163-1170. [DOI] [PubMed] [Google Scholar]
- 49.Davidson, R. J., Lewis, D. A., Alloy, L. B., Amaral, D. G., Bush, G., Cohen, J. D., Drevets, W. C., Farah, M. J., Kagan, J., McClelland, J. L., et al. (2002) Biol. Psychiatry 52 478-502. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka, S. C., Doya, K., Okada, G., Ueda, K., Okamoto, Y. & Yamawaki, S. (2004) Nat. Neurosci. 7 887-893. [DOI] [PubMed] [Google Scholar]
- 51.Zink, C. F., Pagnoni, G., Martin-Skurski, M. E., Chappelow, J. C. & Berns, G. S. (2004) Neuron 42 509-517. [DOI] [PubMed] [Google Scholar]
- 52.Blood, A. J. & Zatorre, R. J. (2001) Proc. Natl. Acad. Sci. USA 98 11818-11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aharon, I., Etcoff, N., Ariely, D., Chabris, C. F., O'Connor, E. & Breiter, H. C. (2001) Neuron 32 537-551. [DOI] [PubMed] [Google Scholar]
- 54.Knutson, B., Adams, C. M., Fong, G. W. & Hommer, D. (2001) J. Neurosci. 21, RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okun, M. S., Bowers, D., Springer, U., Shapira, N. A., Malone, D., Rezai, A. R., Nuttin, B., Heilman, K. M., Morecraft, R. J., Rasmussen, S. A., et al. (2004) Neurocase 10 271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Louilot, A. & Besson, C. (2000) Neuroscience 96 73-82. [DOI] [PubMed] [Google Scholar]
- 57.Riggio, R. E. & Friedman, H. S. (1986) J. Pers. Soc. Psychol. 50 421-427. [DOI] [PubMed] [Google Scholar]
- 58.Friedman, H. S., Prince, L. M., Riggio, R. E. & DiMatteo, M. R. (1980) J. Pers. Soc. Psychol. 39 333-351. [Google Scholar]
- 59.Gray, J. A. (1970) Behav. Res. Ther. 8 249-266. [DOI] [PubMed] [Google Scholar]
- 60.Reuter, M., Stark, R., Hennig, J., Walter, B., Kirsch, P., Schienle, A. & Vaitl, D. (2004) Behav. Neurosci. 118 462-469. [DOI] [PubMed] [Google Scholar]
- 61.Bechara, A., Damasio, H., Damasio, A. R. & Lee, G. P. (1999) J. Neurosci. 19 5473-5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zalla, T., Koechlin, E., Pietrini, P., Basso, G., Aquino, P., Sirigu, A. & Grafman, J. (2000) Eur. J. Neurosci. 12 1764-1770. [DOI] [PubMed] [Google Scholar]
- 63.Riggio, R. E. Lippa, R. & Salina, C. (1990) J. Res. Pers. 24 16-31. [Google Scholar]
- 64.Shimizu, A. K., T. Azuma, T. Kawasaki, M. & Nishimur, T. (1982) in Psychobiology of Schizophrenia, eds. Namba, N. & Kaiya, H. (Pergamon, Oxford), pp. 115-120.
- 65.Ruch, W. (1992) in Advances in Personality Assessment, eds. Butcher, J. N. & Spielberger, C. D. (Erlbaum, Hillsdale, NJ), Vol. 9, pp. 27-75. [Google Scholar]
- 66.Deckers, L. & Ruch, W. (1992) Pers. Indiv. Diff. 13 1149-1152. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.