Abstract
During the involution of mammary glands, epithelial cells undergo apoptosis and are cleared for the next cycle of lactation. The clearance of apoptotic epithelial cells is mediated by neighboring epithelial cells and by macrophages that migrate into the mammary glands. Here, we report that milk fat globule EGF factor 8 (MFG-E8), a secreted glycoprotein that binds to apoptotic cells by recognizing phosphatidylserine, was expressed by epithelial cells and macrophages in mammary glands and was involved in engulfment of apoptotic cells. A deficiency of MFG-E8 caused the accumulation of a large number of milk fat globules (MFGs) in the mammary ducts during involution, indicating that the excess MFGs were cleared by an MFG-E8-dependent mechanism. The MFG-E8-/- mice developed mammary duct ectasia with periductal mastitis, and the redevelopment of the mammary gland for their second litter was impaired. These results demonstrate that MFG-E8-mediated phagocytosis of apoptotic epithelial cells and MFGs is important for efficient involution of mammary glands.
Keywords: exosomes, mastitis, phosphatidylserine
Mammary glands are unique mammalian organs that are developed to nurse offspring. For each pregnancy, the glands undergo a cycle of development, lactation, and involution. In each cycle, mammalian epithelial cells proliferate, differentiate, and die. The involution of mammary glands is triggered when the suckling stimulus is lost, usually upon weaning, and milk accumulates in the glands. The involution takes ≈10 days in mice and can be divided into two phases (1-3). In the first phase, which is reversible and lasts 48 h after weaning, mammary epithelial cells lose their differentiated function. In the second phase, the basement membranes and extracellular matrix in the mammary glands are degraded by proteases, leading to destruction of the lobular-alveolar architecture of the mammary glands. As involution progresses, mammary epithelial cells are removed and adipocytes concomitantly reappear, and the lobular-alveolar structure is reorganized to approach that of virgin glands (4).
During the involution process, epithelial cells undergo apoptosis and are shed into the lumen (5). At the initial stage of involution, these apoptotic cells are thought to be engulfed mainly by neighboring epithelial cells; in the later stage, they seem to be cleared by macrophages that migrate into the gland (3, 6). Milk fat globules (MFGs) are minute globules carrying fat that are secreted from the epithelial cells into the lumen during lactation (7). Upon weaning, many MFGs remain in the lumen, and they seem to be engulfed by epithelial cells lining the lobules in the mammary gland (8). How these epithelial cells and macrophages recognize and engulf apoptotic cells and MFGs has not been well elucidated.
Many molecules expressed on the surface of apoptotic cells or phagocytes have been proposed as ligands and receptors for the engulfment of apoptotic cells (9-11). Mouse MFG EGF factor 8 (MFG-E8), called lactadherin in humans, is a 72-kDa glycoprotein secreted from mammary epithelial cells, macrophages, and immature dendritic cells (12-15). We recently showed that MFG-E8 specifically binds to apoptotic cells by recognizing phosphatidylserine (PS) exposed on the surface of the apoptotic cells and promotes the engulfment of apoptotic cells by phagocytes (12). MFG-E8-deficient mice are incapable of removing the apoptotic cells in the germinal centers of the secondary lymphoid tissues and develop lupus-like autoimmune diseases (16).
In this report, we show that the expression of MFG-E8 was strongly up-regulated when mammary glands underwent involution. Primary epithelial cells prepared from involuting mammary glands expressed MFG-E8, and they engulfed apoptotic cells in an MFG-E8-dependent manner. Macrophages present in the mammary gland at the late stage of involution also expressed MFG-E8 and engulfed apoptotic cells. The mammary glands of MFG-E8-deficient mice developed normally in the first pregnancy and could provide sufficient milk for pups. However, the involution of the MFG-E8-/- glands was impaired, and a large quantity of MFG was left uncleared in the mammary ducts. These glands showed ectasia with periductal mastitis, and their redevelopment for the second litter was poor. These results indicate that MFG-E8 plays an important role in removing apoptotic epithelial cells and MFGs during mammary gland involution and that this process is essential for the redevelopment of mammary glands.
Materials and Methods
Mice. MFG-E8-/- mice (16) and caspase-activated DNase null mice (17) were described previously. The MFG-E8-/- mice were backcrossed 10 times to C57BL/6 mice and used in this study, except in Fig. 4 and also in Fig. 5, which is published as supporting information on the PNAS web site, in which the littermates between MFG-E8+/- and MFG-E8-/- mice carrying the mixed background of C57BL/6 and 129/Sv were used. All mice were housed in a specific pathogen-free facility at Osaka University Medical School, and all animal experiments were carried out in accordance with protocols approved by the Osaka University Medical School Animal Care and Use Committee.
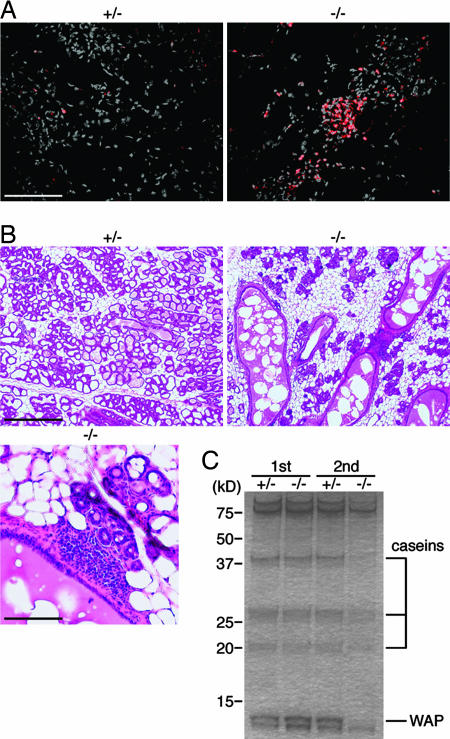
Fig. 4.
Mastitis in mammary glands in MFG-E8-deficient mice and abnormal mammary gland redevelopment. (A) Inflammation in the MFG-E8-/- mammary gland at late involution. MFG-E8+/- or MFG-E8-/- dams were allowed to lactate pups for 5 days and then forced to wean. At day 6 after weaning, sections of mammary glands were stained with anti-CD45 (red) and counter-stained with DAPI (gray). (Scale bar, 0.1 mm.) (B) Abnormal redevelopment of the MFG-E8-/- mammary gland. MFG-E8+/- or MFG-E8-/- mice underwent a second pregnancy and were allowed to nurse the pups. At day 5 of lactation, sections of the mammary glands were stained with H&E. In Lower, the sections from a MFG-E8-/- mammary gland were enlarged. (Scale bars: Upper, 0.5 mm; Lower, 0.1 mm.) (C) Reduced synthesis of milk proteins in MFG-E8-/- mice at the second lactation. MFG-E8+/- and MFG-E8-/- mice underwent one (1st) or two (2nd) cycles of pregnancy and lactation. On day 5 of the first or second lactation, the pups were removed. Twelve hours later, protein extracts were prepared from the mammary glands, and 2-μg aliquots were separated by SDS/PAGE and stained with Coomassie brilliant blue. Molecular mass standard proteins were run in parallel, and their molecular masses are indicated at the left. The proteins seen in the mammary gland extracts were α-casein (43 kDa), β-casein (29 kDa), γ-casein (21 kDa), and whey acidic protein (WAP, 14 kDa). The total protein obtained from each gland was 7.0 and 7.2 mg for MFG-E8+/- at the first and second lactation and 8.2 and 2.9 mg for MFG-E8-/- at the first and second lactation, respectively.
Induction of Involution. Pregnant mice delivered their young at 12-16 weeks of age, and their litter sizes were normalized to 8-10 pups. After full lactation was established (5 days of nursing), dams were separated from their pups to initiate involution. The glands were harvested at 0- to 10-day time points after forced weaning. For a second pregnancy, the dams were separated from pups 21 days after parturition and immediately mated for the next pregnancy. The pregnancy was confirmed by observing the vaginal plug, and the mothers who mated within 7 days after weaning were further analyzed.
Histochemical Analysis. For hematoxylin/eosin (H&E) staining, mammary glands were fixed in 4% paraformaldehyde/4% sucrose in 0.1 M phosphate buffer (pH 7.2), embedded in paraffin, and sectioned at 4 μm. For immunohistochemical analysis, glands were embedded in OCT compound and frozen in liquid nitrogen. Frozen sections (4 μm) were fixed in cold acetone and incubated with an avidin/biotin blocking kit (Vector Laboratories), followed by incubation with 5% goat serum/1% BSA. The sections were stained in PBS containing 5% goat serum/1% BSA with biotinylated anti-CD68 (Serotec) or anti-CD45.2 (BD Biosciences), which was followed by staining with Alexa Fluor 488-conjugated (Molecular Probes) or Cy3-conjugated (Sigma) streptavidin. The sections were also stained with Cy3-conjugated anti-MFG-E8 (clone 18A2) (15). TUNEL staining was performed by using an Apoptag kit (Chemicon). After staining, the sections were mounted with FluorSave mounting reagent (Calbiochem) containing 1 μg/ml DAPI (Dojindo Laboratories, Kumamoto, Japan) and observed by fluorescence microscopy (IX-70, Olympus, Melville, NY).
The whole-mount analysis of mammary glands was carried out according to the method of Thompson et al. (18) (http://labs.amc.org/learn/nec). In brief, glands were excised on day 10 of involution, dehydrated, stained with alum carmine, and stored in methyl salicylate (Sigma). The glands were then observed with a stereomicroscope (Stemi DV4, Zeiss) and photographed with a digital camera (DSC-F505V, Sony, Tokyo).
Cell Culture. Primary mammary epithelial cells were prepared according to the method described by Ehmann et al. (19), with some modifications. Briefly, mice at the age of 12 weeks were subjected to normal parturition and allowed to lactate for 5 days. Two days after removal of the pups, the mammary glands were excised, minced into small pieces, and treated at 37°C for 2 h with liver digest medium (Invitrogen). The suspension was poured through a 133-mm nylon mesh (NBC, Tokyo) to capture the epithelial clumps. The clumps were placed on a 10-cm culture dish in a 1:1 mixture of DMEM and F12 medium containing 10% FBS and 1% insulin-transferrin-selenium-X supplement (Invitrogen), 10 ng/ml TGF-α (Sigma), and 50 ng/ml basic FGF (Sigma) and cultured for 3 days. Cells were harvested with trypsin/EDTA, stained with phycoerythrin-conjugated anti-Mac-1 (BD Biosciences), and subjected to cell sorting (FAC-SAria, BD Biosciences). Mac-1-negative cells grown on eight-well Lab-tek II chamber slides (Nalge Nunc) showed an epithelium-like morphology and produced β-casein. Mac-1-positive cells were cultured for 1 week in DMEM containing 10% FBS and macrophage colony-stimulating factor (20) and used as mammary gland macrophages.
In Vitro Phagocytosis Assay. Phagocytosis of apoptotic cells was assayed as described in ref. 12. In brief, thymocytes from 6-week-old caspase-activated DNase null mice (17) were treated with 10 μM dexamethasone to induce apoptosis. Apoptotic thymocytes (1 × 106 cells) were added to 2.0 × 104 epithelial cells or macrophages that were cultured in eight-well Lab-tek II chamber slides, and phagocytosis was allowed to proceed for 1.5 h. The cells were fixed with 1% paraformaldehyde, subjected to TUNEL, and observed by light microscopy. The TUNEL-positive thymocytes were counted, and the number of TUNEL-positive apoptotic cells per phagocyte (phagocytosis index) in a total of 150 phagocytes was determined.
Analysis of Milk and MFGs. After parturition, dams at the age of 12 weeks for the first pregnancy or 20 weeks for the second pregnancy were allowed to lactate for 5 days, and the pups were removed. To analyze milk, the mammary glands were excised 12 h after weaning and homogenized by using a Polytron in 50 mM Tris·HCl buffer (pH 8.0) containing 150 mM NaCl, 1% Nonidet P-40, 1 mM (p-amidinophenyl) methanesulfonyl fluoride hydrochloride, 1 μg/ml leupeptin, and 1 μg/ml pepstatin. The cell lysates were cleared by centrifugation at 30,000 × g for 15 min, and aliquots (2 μg of protein) were subjected to 10-20% gradient SDS/PAGE.
To analyze MFGs, the mammary glands were excised 2 days after weaning and minced in PBS. Suspension of the minced glands was centrifuged at 3,000 × g for 15 min, and the floating MFGs were collected. After washing four times with PBS, MFGs were suspended in PBS, stained with biotinylated anti-MFG-E8 (clone 18A2) and then phycoerythrin (PE)-conjugated streptavidin (BD Biosciences), and analyzed by flow cytometry (FAC-SCalibur, BD Biosciences). MFGs were also stained with PE-conjugated annexin V (BD Biosciences) in 10 mM Hepes buffer (pH 7.4) containing 140 mM NaCl and 2.5 mM CaCl2.
Results
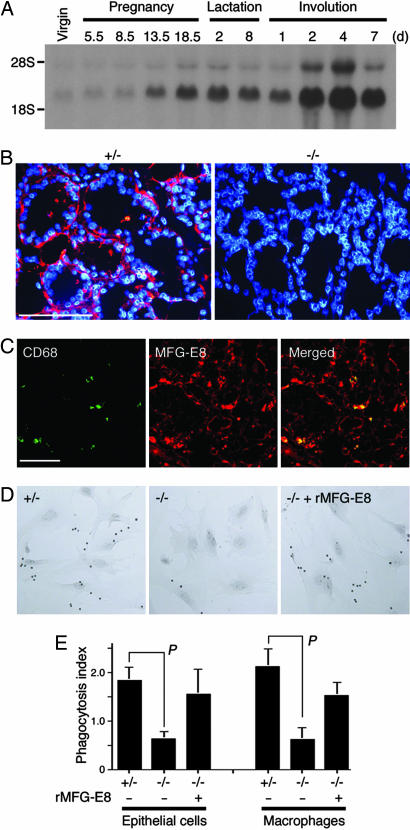
Expression of MFG-E8 in Mammary Epithelial Cells During Involution. MFG-E8, which is expressed in macrophages for the engulfment of apoptotic cells (12), is expressed in mammary glands (21). To investigate the role of MFG-E8 in the development and involution of mammary glands, the expression of MFG-E8 in the gland at various developmental stages was examined by Northern hybridization. As shown in Fig. 1A, MFG-E8 was weakly expressed in the mammary glands of virgin animals and in early pregnancy. Its expression was up-regulated in late pregnancy and increased further soon after the glands underwent involution. Real-time PCR analysis with MFG-E8-specific primers (15) indicated that the expression levels of MFG-E8 mRNA in the mammary gland on day 5 of lactation and days 4, 7, and 10 of involution were 16.5, 30.5, 13.4, and 4.8 times higher, respectively, than the MFG-E8 mRNA level in virgin glands. Staining with an anti-MFG-E8 mAb showed that MFG-E8 was expressed by epithelial cells of the involuting mammary glands (Fig. 1B). There were no CD68-positive cells in the lactating mammary glands. However, they could be detected on day 2 of involution, and their number increased as involution progressed, up to 14.2% of the total cell number in the mammary glands on day 4 of involution. These CD68-positive cells expressed MFG-E8 (Fig. 1C) as well as other macrophage antigens such as Mac-1 and F4/80 (data not shown).
Fig. 1.
MFG-E8-dependent engulfment of apoptotic cells by mammary epithelial cells and macrophages. (A) Expression of MFG-E8 in involuting mammary glands. A mouse mammary aging blot (Seegene, Seoul, Korea) on which total RNA (20 μg) from mouse mammary glands at the indicated pregnant, lactating, and involution stages was loaded was subjected to Northern hybridization using 32P-labeled murine MFG-E8 cDNA. Bands around the position of 28S rRNA are probably precursor RNA for MFG-E8 mRNA. (B) Expression of MFG-E8 in mammary epithelial cells. Mammary gland sections from MFG-E8+/- or MFG-E8-/- mice on the second day of involution were stained with anti-MFG-E8 (red) and counterstained with DAPI (blue). (Scale bar, 100 μm.) (C) Expression of MFG-E8 in the macrophages present in involuting mammary glands. Sections of mammary glands from WT mice on day 4 of involution were doubly stained with mAb against CD68 (green) and MFG-E8 (red); the staining profiles are merged in Right. (Scale bar, 50 μm.) (D) MFG-E8-dependent engulfment of apoptotic cells. Apoptotic thymocytes from caspase-activated DNase null mice were added to mammary epithelial cells prepared from MFG-E8+/- (+/-) or MFG-E8-/- (-/-) mice in the absence or presence of 0.1 μg/ml MFG-E8. After incubation at 37°C for 1.5 h, the cells were stained for TUNEL and observed with a light microscope. Original magnification, ×100. (E) MFG-E8-dependent engulfment of apoptotic cells. Engulfment of apoptotic cells by epithelial cells (Left) or macrophages (Right) from MFG-E8+/- or MFG-E8-/- mice was carried out in the presence or absence of 0.1 μg/ml recombinant MFG-E8. The experiments were performed three times in duplicate, and the average value for the phagocytosis index is shown with SD. The probability of statistical difference was determined by Student's t test. P < 0.02.
Epithelial cells prepared from mammary glands on day 2 of involution efficiently engulfed apoptotic cells (Fig. 1D), and the phagocytosis index (the number of engulfed apoptotic cells per epithelial cell) was 1.83 (Fig. 1E). The ability of MFG-E8-/- epithelial cells to engulf apoptotic cells was significantly reduced, and their phagocytosis index was 0.63. Adding recombinant MFG-E8 (0.1 μg/ml) to MFG-E8-/- epithelial cells caused significant recovery, to 1.55 (Fig. 1E), indicating that the mammary epithelial cells could engulf apoptotic cells by means of an MFG-E8-dependent mechanism. Similarly, the CD68-positive macrophages from the involuting mammary glands efficiently engulfed apoptotic cells in an MFG-E8-dependent manner (Fig. 1E). The phagocytosis index of the macrophages was 2.1, which was similar to that of thioglycollate-elicited peritoneal macrophages (12).
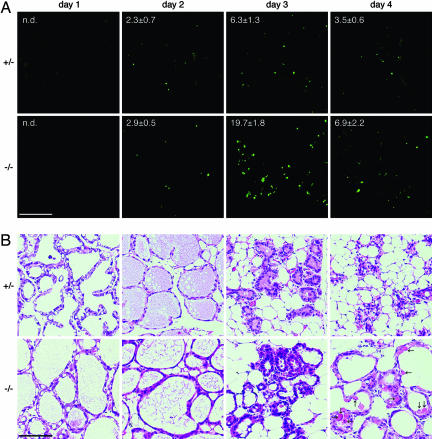
Requirement for MFG-E8 in the Clearance of Apoptotic Cells and MFGs. Apoptotic cells can be recognized in situ by TUNEL staining. When apoptotic cells are engulfed, the DNA of the dying cells is further digested and cannot be stained by TUNEL (22). Using this technique, we examined the effect of MFG-E8 on the clearance of apoptotic cells during involution of the mammary gland. As shown in Fig. 2A, ≈2.3% of the epithelial cells in WT mammary glands were TUNEL-positive on day 2 of involution and increased to 6.3% on day 3 of involution. These TUNEL-positive cells could be stained with mAb against the activated caspase 3 (data not shown), confirming that these cells were apoptotic cells. The number of TUNEL-positive cells in the MFG-E8-/- mammary gland on day 2 of involution was similar to that observed in WT mice (Fig. 2 A). However, as involution progressed, MFG-E8-/- mammary glands showed two to three times more apoptotic cells than the WT glands. That is, ≈20% and 7% of epithelial cells of MFG-E8-/- mammary glands were TUNEL-positive on days 3 and 4 of involution, respectively. These results indicated that the apoptotic cells generated during the involution of the mammary gland were not efficiently cleared in the MFG-E8-/- mice. MFG-E8-/- mammary glands did not show an apparent abnormality in their alveolus structure during the first 3 days of involution (Fig. 2B). But the alveolar lumens of MFG-E8-/- mammary glands became enlarged on day 4 of involution, and some amorphous materials, which could have been debris of dead cells, were found in the lumens.
Fig. 2.
Impaired clearance of apoptotic mammary epithelial cells in MFG-E8-deficient mice. (A) TUNEL staining. The mammary glands were prepared from MFG-E8+/- or MFG-E8-/- mice on the indicated days of involution and stained for TUNEL. The number of TUNEL-positive cells in the mammary glands on the indicated days of involution was determined and is shown as the percentage of DAPI-positive cells. At least 1,800 cells for each gland (300 cells per field) were analyzed. The analyses were performed with three mice for each genotype, and the average values are shown with SD. n.d., not detected. (Scale bar, 100 μm.) (B) Staining with H&E. Sections of mammary glands from MFG-E8+/- or MFG-E8-/- mice at the indicated day of involution were stained with H&E. Amorphous materials, which could be debris of dead cells, are indicated by arrows. (Scale bar, 100 μm.)
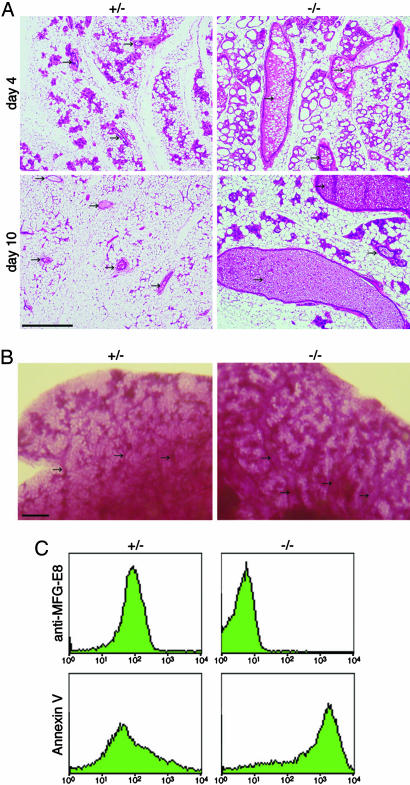
At the late stage of involution, the duct structure showed a large difference in the WT and MFG-E8-/- mammary glands. On day 4 of involution, the alveolus structures of the WT mammary gland had disintegrated, and the gland was filled with fat pads (Fig. 3A). In contrast, the ducts of the MFG-E8-/- mammary gland were enlarged at this stage of involution, and they were filled with a large number of membranous materials that could have been MFGs. Enlarged ducts with uncleared MFGs were still observed in the MFG-E8-/- mammary glands even after 10 days of involution, suggesting that the involution process was severely impaired by the lack of MFG-E8. A whole-mount staining of the mammary glands with alum carmine confirmed the extended ducts in MFG-E8-/- glands on day 10 of involution (Fig. 3B).
Fig. 3.
Accumulation of MFGs in MFG-E8-deficient mice. (A) Accumulation of MFGs in MFG-E8-/- mammary glands. MFG-E8+/- or MFG-E8-/- dams were allowed to nurse pups for 5 days and were separated from the pups. The mammary glands were excised 4 and 10 days after weaning. The paraffin sections of the mammary glands were stained with H&E. Mammary ducts are indicated by arrows. (Scale bar, 0.5 mm.) (B) Whole-mount staining of mammary glands. Mammary glands of MFG-E8+/- or MFG-E8-/- mice were excised 10 days after weaning and stained with alum carmine. Arrows indicate mammary ducts. (Scale bar, 1 mm.) (C) Staining of MFGs for MFG-E8 and PS. MFGs prepared from involuting mammary glands of MFG-E8+/- or MFG-E8-/- mice were stained with anti-MFG-E8 antibody (Upper) or annexin V (Lower).
We postulated that the membranous materials accumulated in the MFG-E8-/- glands were MFGs that escaped from being cleared due to the lack of MFG-E8. To examine this possibility, MFGs were prepared from the mammary glands on day 2 after weaning and analyzed by flow cytometry. As shown in Fig. 3C, MFGs from the WT mammary glands strongly expressed MFG-E8 on their surface and weakly exposed PS that was detected by binding of annexin V. In contrast, MFGs from MFG-E8-/- mammary glands were not associated with MFG-E8 but strongly exposed PS on their surface. These results indicated that, like apoptotic cells (23) and nuclei expelled from erythroid cell precursors (24), MFGs exposed PS on their surface, and MFG-E8 bound to MFGs by recognizing PS.
Inefficient Redevelopment of Mammary Glands in MFG-E8-/- Mice. Clusters of CD45+ cells were often present in the involuting mammary glands of MFG-E8-/- mice (Fig. 4A), indicating that the abnormal involution in the MFG-E8-/- gland induced inflammation. To examine the effect of MFG-E8 deficiency on the redevelopment of the mammary glands after involution, female mice were forced to wean from their pups and were mated within 7 days. As shown in Fig. 4B, the WT mammary glands on day 5 of the second lactation were well developed and were similar to the gland at the first lactation. In contrast, in the mammary glands of MFG-E8-/- mice, the development of epithelial cells was delayed, and a large amount of fat pads were still present on day 5 of lactation. The alveolar structure of the MFG-E8-/- glands was also different from that of WT. That is, the mammary ducts were still enlarged and filled with membranous materials that could have been fused MFGs. In addition, many cells with a plasma cell-like appearance invaded the mammary tissues of the MFG-E8-/- dams.
The aberrant redevelopment of the mammary glands caused a decrease in milk production. As shown in Fig. 4C, extracts of mammary glands from MFG-E8-/- mice at the first lactation contained the major milk proteins (α-, β-, and γ-caseins and whey acidic protein) at a level comparable to that of WT mammary glands. At the second lactation, WT mammary glands expressed a similar level of the major milk proteins, as at the first lactation. In contrast, the expression level of these proteins in MFG-E8-/- glands was significantly reduced at the second lactation. MFG-E8-/- dams could feed their first litter normally (Fig. 5), but the rate of weight gain of the second litter nursed by the MFG-E8-/- dams was significantly reduced, and the average weight of the second litter at age 20 days was ≈20% less than that of the second litter nursed by the WT dams. In addition, ≈20-30% of the pups of the second and third litters of the MFG-E8-/- dams died within 10 days of birth. When these pups were nursed by WT dams, they grew normally, indicating that the ateliosis of the pups nursed by MFG-E8-/- dams was caused by the difficulty in lactation.
Discussion
Unlike most organs, the development and involution of mammary glands occurs postnatally, providing an excellent system for studying the molecular mechanisms of organ development. The development of the mammary gland is hormonally regulated by pregnancy, during which the gland grows greatly and differentiates in preparation for secreting milk (25). Upon weaning, the entire alveolar epithelium involutes, accompanied by the apoptotic cell death of epithelial cells (5). In this process, the remaining MFGs are also cleared from the gland (8). The involution process is regulated at the transcriptional level and is associated with the up- or down-regulation of a set of genes (26, 27). In this report, we showed that the MFG-E8 gene was strongly expressed in the mammary gland during the lactation and involution periods. The epithelial cells that comprise the gland expressed MFG-E8. In addition, macrophages that migrated into the mammary gland during the late stage of involution also expressed MFG-E8. It will be interesting to study whether similar transcription factors are involved in the MFG-E8 gene expression in mammary epithelial cells and macrophages.
As with professional phagocytes of thioglycollate-elicited peritoneal macrophages and immature dendritic cells (12, 15), the engulfment of apoptotic cells by mammary epithelial cells, which are nonprofessional phagocytes, depended on MFG-E8. It is likely that MFG-E8 secreted from epithelial cells binds to PS exposed on apoptotic cells and promotes their engulfment by means of integrins on epithelial cells (28). In addition, we found that epithelial cells cleared MFGs in an MFG-E8-dependent manner. In fact, uncleared MFGs were left in the MFG-E8-/- mammary glands in longer periods than apoptotic cells. It is likely that the unengulfed apoptotic cells undergo the secondary necrosis and that cellular contents are released. However, MFGs are rather stable and left in the mammary ducts.
MFGs are a type of exosome carrying fat and are secreted from mammary epithelial cells by budding (7). They are produced by the lactating epithelial cells that line the lumens of the mammary gland. In this process, fat droplets formed inside the cells move to the apical region, where they become enveloped in plasma membrane and are expelled from the cell into the lumen (7). MFGs prepared from the milk of MFG-E8-/- mice exposed PS on their surface. PS is usually localized to the inner surface of plasma membranes; this localization is maintained by a putative ATP-dependent translocase (29). We recently found that the nuclei expelled from erythroid precursor cells are deprived of ATP and expose PS on their surface (24). Similarly, MFGs carry few organelles such as mitochondria; therefore, ATP would be depleted in MFGs, thus inactivating the translocase and leading to the exposure of PS on the outer surface of the globules. MFGs prepared from WT mice carried MFG-E8 on their surface, suggesting that as soon as MFG-E8 is secreted from mammary epithelial cells, it binds to MFGs. Thus, similar to apoptotic cells, MFG-E8-bound MFGs would be efficiently engulfed by epithelial cells, leading to the clearance of MFGs from the lumen during mammary gland involution. MFG-E8 was strongly expressed in the mammary gland also during the lactation period. The quantity of milk produced by a mother often exceeds that necessary for nursing babies. Thus, during the lactating period, the excess MFGs may be absorbed by epithelial cells in an MFG-E8-dependent manner, to be reused.
The involution of the mammary gland was severely impaired in MFG-E8-/- mice. Even after the mice started to nurse the second litter, the lobular-alveolar structure was not properly developed in the MFG-E8-/- mammary glands. This defect is partly because the mammary gland was largely occupied by the enlarged ducts that were filled with MFGs. Accordingly, when pups started to suckle, the number of MFGs left in the glands was reduced, and the mammary gland started to redevelop (unpublished data). Another possible reason for the poor development of the MFG-E8-/- mammary gland is the mastitis that was observed in almost all MFG-E8-/- mice soon after the first involution. Mastitis is often caused by bacterial infection, which occurs most frequently during early involution (30). However, our mice were maintained in specific pathogen-free conditions, and mastitis was never found in the WT mice, suggesting that the mastitis in the MFG-E8-/- mice was induced without bacterial infection. The involution of the mammary gland is regulated by the STAT3 transcription factor (31, 32) and is accompanied by the activation of various inflammatory genes such as CXCL1 and CXCL10 (27), which recruit inflammatory cells into the mammary gland. It is possible that the prolonged involution process in MFG-E8-/- mice causes the extended activation of inflammatory genes, leading to severe inflammation. The engulfment of apoptotic cells by macrophages and epithelial cells is reported to inhibit inflammation by producing antiinflammatory factors such as TGF-β (33). Moreover, the dead cells that were not efficiently engulfed by phagocytes may undergo necrotic cell death, which is believed to induce inflammation (34). Thus, it is also possible that the dead epithelial cells that escaped from being engulfed may activate inflammation to induce the mastitis. Neutrophils and lymphocytes that were recruited in mastitis will produce cytotoxic substances, which could block the development of the mammary gland (35). In any case, our results indicate that if the mammary glands do not properly undergo involution, they will have problems in redeveloping for the second litter.
Supplementary Material
Acknowledgments
We are grateful to Dr. M. Tanaka for help at the initial stage of this work, Dr. K. Aozasa for advice on histochemical analysis of the mammary gland, and Dr. T. Matsuda for critical reading of the manuscript. We thank M. Fujii for secretarial assistance. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture in Japan.
Author contributions: S.N. designed research; R.H. performed research; R.H. and S.N. analyzed data; and R.H. and S.N. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: MFG, milk fat globule; MFG-E8, MFG EGF factor 8; PS, phosphatidylserine; H&E, hematoxylin/eosin.
References
- 1.Lund, L. R., Romer, J., Thomasset, N., Solberg, H., Pyke, C., Bissell, M. J., Dano, K. & Werb, Z. (1996) Development (Cambridge, U.K.) 122, 181-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furth, P. A., Bar-Peled, U. & Li, M. (1997) Apoptosis 2, 19-24. [DOI] [PubMed] [Google Scholar]
- 3.Monks, J., Geske, F. J., Lehman, L. & Fadok, V. A. (2002) J. Mammary Gland Biol. Neoplasia 7, 163-176. [DOI] [PubMed] [Google Scholar]
- 4.Alexander, C. M., Selvarajan, S., Mudgett, J. & Werb, Z. (2001) J. Cell Biol. 152, 693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker, N. I., Bennett, R. E. & Kerr, J. F. (1989) Am. J. Anat. 185, 19-32. [DOI] [PubMed] [Google Scholar]
- 6.Abrahams, V. M., Kim, Y. M., Straszewski, S. L., Romero, R. & Mor, G. (2004) Am. J. Reprod. Immunol. 51, 275-282. [DOI] [PubMed] [Google Scholar]
- 7.Patton, S. & Keenan, T. W. (1975) Biochim. Biophys. Acta 415, 273-309. [DOI] [PubMed] [Google Scholar]
- 8.Brooker, B. E. (1983) Cell Tissue Res. 229, 639-650. [DOI] [PubMed] [Google Scholar]
- 9.Gregory, C. D. & Devitt, A. (2004) Immunology 113, 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauber, K., Blumenthal, S. G., Waibel, M. & Wesselborg, S. (2004) Mol. Cell 14, 277-287. [DOI] [PubMed] [Google Scholar]
- 11.Savill, J. & Fadok, V. (2000) Nature 407, 784-788. [DOI] [PubMed] [Google Scholar]
- 12.Hanayama, R., Tanaka, M., Miwa, K., Shinohara, A., Iwamatsu, A. & Nagata, S. (2002) Nature 417, 182-187. [DOI] [PubMed] [Google Scholar]
- 13.Stubbs, J. D., Lekutis, C., Singer, K. L., Bui, A., Yuzuki, D., Srinivasan, U. & Parry, G. (1990) Proc. Natl. Acad. Sci. USA 87, 8417-8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanayama, R., Tanaka, M., Miwa, K. & Nagata, S. (2004) J. Immunol. 172, 3876-3882. [DOI] [PubMed] [Google Scholar]
- 15.Miyasaka, K., Hanayama, R., Tanaka, M. & Nagata, S. (2004) Eur. J. Immunol. 34, 1414-1422. [DOI] [PubMed] [Google Scholar]
- 16.Hanayama, R., Tanaka, M., Miyasaka, K., Aozasa, K., Koike, M., Uchiyama, Y. & Nagata, S. (2004) Science 304, 1147-1150. [DOI] [PubMed] [Google Scholar]
- 17.Kawane, K., Fukuyama, H., Yoshida, H., Nagase, H., Ohsawa, Y., Uchiyama, Y., Iida, T., Okada, K. & Nagata, S. (2003) Nat. Immunol. 4, 138-144. [DOI] [PubMed] [Google Scholar]
- 18.Thompson, H. J., McGinley, J. N., Rothhammer, K. & Singh, M. (1995) Carcinogenesis 16, 2407-2411. [DOI] [PubMed] [Google Scholar]
- 19.Ehmann, U. K., DeVries, J. T., Chen, M. S., Adamos, A. A., Guzman, R. C. & Omary, M. B. (2003) Cell Prolif. 36, 177-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshita, S., Kaji, K. & Kudo, A. (2000) J. Bone Miner. Res. 15, 1477-1488. [DOI] [PubMed] [Google Scholar]
- 21.Oshima, K., Aoki, N., Negi, M., Kishi, M., Kitajima, K. & Matsuda, T. (1999) Biochem. Biophys. Res. Commun. 254, 522-528. [DOI] [PubMed] [Google Scholar]
- 22.Wu, Y. C., Stanfield, G. M. & Horvitz, H. R. (2000) Genes Dev. 14, 536-548. [PMC free article] [PubMed] [Google Scholar]
- 23.Fadok, V. A., Voelker, D. R., Campbell, P. A., Cohen, J. J., Bratton, D. L. & Henson, P. M. (1992) J. Immunol. 148, 2207-2216. [PubMed] [Google Scholar]
- 24.Yoshida, H., Kawane, K., Koike, M., Mori, Y., Uchiyama, Y. & Nagata, S. (2005) Nature 437, 754-758. [DOI] [PubMed] [Google Scholar]
- 25.Hovey, R. C. & Trott, J. F. (2004) Adv. Exp. Med. Biol. 554, 219-228. [DOI] [PubMed] [Google Scholar]
- 26.Clarkson, R. W., Wayland, M. T., Lee, J., Freeman, T. & Watson, C. J. (2004) Breast Cancer Res. 6, R92-R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein, T., Morris, J. S., Davies, C. R., Weber-Hall, S. J., Duffy, M. A., Heath, V. J., Bell, A. K., Ferrier, R. K., Sandilands, G. P. & Gusterson, B. A. (2004) Breast Cancer Res. 6, R75-R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taddei, I., Faraldo, M. M., Teuliere, J., Deugnier, M. A., Thiery, J. P. & Glukhova, M. A. (2003) J. Mammary Gland Biol. Neoplasia 8, 383-394. [DOI] [PubMed] [Google Scholar]
- 29.Balasubramanian, K. & Schroit, A. J. (2003) Annu. Rev. Physiol. 65, 701-734. [DOI] [PubMed] [Google Scholar]
- 30.Nickerson, S. C. (1989) J. Dairy Sci. 72, 1665-1678. [DOI] [PubMed] [Google Scholar]
- 31.Chapman, R. S., Lourenco, P. C., Tonner, E., Flint, D. J., Selbert, S., Takeda, K., Akira, S., Clarke, A. R. & Watson, C. J. (1999) Genes Dev. 13, 2604-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humphreys, R. C., Bierie, B., Zhao, L., Raz, R., Levy, D. & Hennighausen, L. (2002) Endocrinology 143, 3641-3650. [DOI] [PubMed] [Google Scholar]
- 33.Monks, J., Rosner, D., Geske, F. J., Lehman, L., Hanson, L., Neville, M. C. & Fadok, V. A. (2005) Cell Death Differ. 12, 107-114. [DOI] [PubMed] [Google Scholar]
- 34.Fadok, V. A., Bratton, D. L., Guthrie, L. & Henson, P. M. (2001) J. Immunol. 166, 6847-6854. [DOI] [PubMed] [Google Scholar]
- 35.Riollet, C., Rainard, P. & Poutrel, B. (2000) Adv. Exp. Med. Biol. 480, 247-258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.