Abstract
An early step governing Shigella flexneri pathogenesis is the invasion of the colonic epithelium from the basolateral surface followed by disruption of the colonic epithelial barrier. Despite recent insight into S. flexneri-host interactions, much remains to be determined regarding the nature of the initial contact between S. flexneri and the host epithelial basolateral membrane domain. Since the lipopolysaccharide (LPS) is located at the outermost part of the bacterial membrane, we considered that this component might be used by S. flexneri to attach to the basolateral surface of the intestinal epithelium and promote a proinflammatory response. Therefore, polarized human T84 intestinal epithelial cells were infected from the basolateral surface with either wild-type S. flexneri or one of its isogenic LPS-defective strains with mutations in either rfc, rfaL, or galU. We found that both adherence to and internalization into the basolateral surface of a polarized intestinal epithelium with S. flexneri were highly dependent on the length of the LPS (i.e., rfc > rfaL > galU). Furthermore, the addition of the anti-inflammatory LPS (RsDPLA) considerably decreased the invasion profile of wild-type S. flexneri by nearly 50%. Since LPS is associated with host inflammation, we further examined whether this molecule was involved in Shigella-induced inflammatory events. We found that S. flexneri LPS plays an important role in mediating epithelial-derived signaling, which leads to directed migration of polymorphonuclear leukocytes across model intestinal epithelium. This signaling most likely involves the activation of the mitogen-activated protein kinase extracellular regulated kinase. Thus, our findings have important implications on the understanding of the mechanisms by which S. flexneri can elicit mucosal inflammation.
Shigella spp. are a group of gram-negative enteric bacilli that cause acute bacillary dysentery in humans. The syndrome caused by Shigella consists of painful abdominal cramps, nausea, and fever, along with blood and mucus in the stool. In its most severe forms, shigellosis is associated with an intense inflammatory reaction that leads to the destruction of the colonic mucosa (4). The ability of this food-borne pathogen to invade and colonize the colonic epithelium is a key determinant in the establishment of the disease. Following entry into epithelial cells from the basolateral surface (35), Shigella flexneri lyses the primary vacuole and multiplies within the host cytoplasm. Dissemination occurs by both intracellular and intercellular spreading and results in the formation of “finger-like” protrusions that extend from infected cells and are endocytosed by adjacent cells (1, 9, 18, 47, 51). Thus, since Shigella can spread in cell monolayers without extracellular steps, these organisms are typically confined to the epithelial layer of the colonic mucosa.
The histopathology of shigellosis is defined by diffuse erythema, swelling of the mucosa, focal hemorrhages, and a purulent exudate. This response is largely characterized by the infiltration of polymorphonuclear leukocytes (PMN) into the intestinal epithelium, culminating in the formation of an intestinal crypt abscess (4, 29). The action of these PMN on the epithelium and the subsequent loss of barrier function are thought to be key events in mediating the clinical manifestations of shigellosis. Although relatively little is known pertaining to the nature of Shigella-induced signaling cascades that mediate PMN signaling, recent investigations are beginning to reveal some insight into this complex process. For example, previous findings have implicated interleukin-8 (IL-8) as an important chemokine in the control of Shigella-transepithelial translocation (47). IL-8 is a potent proinflammatory chemokine that is produced by epithelial cells in response to contact between S. flexneri and the epithelial basolateral membrane. This cytokine is secreted basolaterally by polarized model intestinal epithelia, such that it imprints a haptotactic gradient for PMN on the subepithelial matrix and likely guides the movement of extravasated PMN through the lamina propria (30). Insight into the mechanism of IL-8 secretion during Shigella infection of HeLa cells revealed that these interactions induced NF-κB binding activity. Since NF-κB is an important transcriptional regulator of IL-8, these findings emphasize the importance of NF-κB in regulating IL-8 induction during S. flexneri infection (41).
Furthermore, studies addressing the inflammatory event of PMN movement across model intestinal epithelia indicate that as a result of Shigella infection, PMN transmigration occurs and facilitates bacterial invasion at the basolateral membrane (40). This induction of PMN transepithelial migration appears to be dependent not only on the invasive ability and expression of a functional type III secretion system (33, 46), but also on the ability of the bacteria to spread to adjacent cells (11). Additionally, one key observation indicates that PMN transepithelial migration induced by S. flexneri-host cell interactions occurs following basolateral, but not apical, application of the pathogen (33), implying that host cell factors functioning at the basolateral domain of intestinal epithelial cells may modulate this event.
The intestinal epithelium is a highly polarized structure and plays an important role as a protective barrier to luminal threats. Under normal conditions, the apical surface not only encounters microbial threats, but also supports the presence of numerous commensals. The basolateral surface, on the other hand, interfaces the underlying immune system and provides mucosal protection by serving as a substrate for resident and emerging cells. Interestingly, although epithelial cells lining the colon are bathed in normal microbiota and their bacterial products, they remain generally refractory to lipopolysaccharide (LPS), a bacterial component associated with host inflammation (15, 17). Presumably, this is because detection of the resident microbiota by the colonic epithelium would have potential serious consequences, because the colon would be in a state of chronic inflammation (42). The basolateral membrane microenvironment, however, is most likely to be an LPS-free environment, considering that the intestinal epithelium is normally impenetrable to most bacteria. Since S. flexneri has evolved ways to overcome this epithelial barrier and gain access to the basolateral milieu, we hypothesize that interaction of S. flexneri with the basolateral surface of polarized epithelium may induce additional changes in signal transduction events that have not yet been characterized. Here we report that a mitogen-activated protein kinase (MAPK), extracellular regulated kinase (ERK), is used by S. flexneri to elicit neutrophil transmigration across a model intestinal epithelium. We also demonstrate that this inflammatory response is dependent on an intact bacterial LPS.
MATERIALS AND METHODS
Cell culture.
T84 intestinal epithelial cells (passages 46 to 66) were grown in a 1:1 mixture of Dulbecco-Vogt modified Eagle's medium and Ham's F-12 nutrient mixture supplemented with 15 mM HEPES (pH 7.5), 14 mM NaHCO3, 40 μg of penicillin per ml, 8 μg of streptomycin per ml, 8 μg of ampicillin per ml, and 5% newborn calf serum. Monolayers were grown on 0.33-cm2 (for invasion and transmigration assay) and on 4.7-cm2 (for cell harvest and immunoblotting) ring-supported collagen-coated permeable polycarbonated filters (Costar Corp., Cambridge, Mass.) and utilized 7 to 14 days after plating, as described previously (26, 27, 34, 37). Inverted monolayers used to study the invasion capacity of bacteria and PMN transmigration in the physiological basolateral-to-apical direction were constructed as previously described (26, 36-38). A steady-state transepithelial cell resistance of approximately 400 to 1,500 Ω · cm2 was reached in all monolayers used.
Bacterial strains and growth conditions.
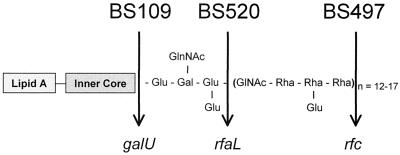
2457T, a wild-type strain of S. flexneri 2a, is invasive in HeLa cells and T84 cells (33) and Serény test positive (12). BS103 is a plasmid-cured derivative of 2457T and is noninvasive and completely avirulent. S. flexneri 2a BS109 (galU mutant) is invasive in HeLa cells (45). The galU gene codes for uridine diphosphoglucose (UDP-glucose) pyrophosphorylase and results in synthesis of an incomplete LPS core with loss of the attached O antigen. S. flexneri 2a BS497 and BS520 are both invasive in HeLa cells and are Sereny test negative (44, 45). Strain BS497 harbors a mutation in the rfc locus (encoding the O side chain polymerase) and is capable of attaching only one repeat of the O side chain. Strain BS520 contains a mutation in the rfaL locus (encoding the O side chain ligase), which results in a phenotype that contains a complete core but lacks the O side chains. The described mutations are depicted in Fig. 1.
FIG. 1.
LPS structure of S. flexneri 2a 2457T (wild type) and mutants BS109, BS520, and BS497. The arrow depicts the corresponding mutation in the LPS synthesis pathway.
Bacteria were routinely grown at 37°C in Trypticase soy broth (TSB) (Difco Laboratories, Detroit, Mich.). TSB was prepared according to the manufacturer's instructions. One hundred microliters of a stationary-phase culture was used to inoculate 10 ml of TSB, and the bacteria were grown in a shaking incubator for approximately 2 h at 37°C to the mid-exponential phase (optical density at 600 nm of 0.30). Trypticase soy agar is TSB containing 15 g of Bacto agar per liter (Difco Laboratories) (32, 33).
S. flexneri invasion of T84 intestinal epithelial monolayers.
Infection of T84 monolayers was performed as previously described (31) with slight modification (33). Inverted T84 cell monolayers were drained of medium and gently washed with Hanks balanced salt solution containing Ca2+ and Mg2+, supplemented with 10 mM HEPES (pH 7.4) (Sigma Chemical Co., St. Louis, Mo.), referred to as HBSS+. Bacterial samples representing a concentration of 5 × 108 CFU/ml were administered to the basolateral surface of T84 cell monolayers at a multiplicity of infection (MOI) of 25 bacteria per epithelial cell and incubated for 90 min at 37°C. After rigorous washing, the monolayers were either stored at 4°C (for associated bacteria) or treated with 480 μg of gentamicin per ml for 90 min at 37°C (for internalized bacteria). Next, the monolayers were extensively washed and lysed with Triton X-100 (Sigma), and the cell lysates were diluted and plated for CFU on MacConkey agar plates. Data are expressed as the percentage of CFU in the original inoculum.
PMN transepithelial migration assay.
The physiologically directed (basolateral to apical) PMN transepithelial migration in response to bacterial stimulus has been previously detailed (32, 33, 37). Briefly, human PMN were isolated from normal volunteers, as described elsewhere (21, 37). T84 cells were stimulated with S. flexneri from the basolateral surface at an MOI of 400 for 90 min. PMN (106 in 20 μl) were then added to the basolateral surface and allowed to transmigrate through the monolayer for 2 h. Transmigration was quantified by assaying for the PMN-specific azurophilic granule marker myeloperoxidase as described previously (37). PMN cell equivalents were estimated from daily PMN standards as the number of PMN that had completely traversed the monolayer into the apical chamber. In a subset of experiments, the ERK pathway was inhibited by incubation at 37°C for 45 min with the MEK 1 inhibitor PD98059 (Cell Signaling, Beverly, Mass.) at concentrations of 50, 10, and 1 μM, respectively (3).
Phosphorylation of ERK1/2.
The epithelial monolayers were infected with 1 ml of bacterial suspension at an MOI of 400 per epithelial cell (38) and incubated at 37°C. T84 cell monolayers were harvested at 30, 60, and 90 min on ice in 350 μl of lysis buffer (1% Triton X-100, 100 mM NaCl, 10 mM HEPES, 2 mM EDTA, 4 mM Na3VO4, 40 mM NaF, 200 mM PMSF, protease inhibitor cocktail [Complete Mini; Roche Molecular Biochemicals, Grenzach, Germany]). The lysates were centrifuged (14,000 × g for 30 min at 4°C), and the supernatant suspension, representing the cytosolic compartment, was collected and used immediately or stored at −80°C until further use. The protein concentration in each sample was determined by colorimetric Bradford protein assay (Bio-Rad, Hercules, Calif.). Samples (30 μg of protein per lane) were electrophoresed through gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8 to 16% polyacrylamide) and transferred to nitrocellulose. Immunoblots were performed with specific antibodies against phosphorylated ERK (p-ERK) (mouse immunoglobulin G2a [IgG2a]) and ERK1 (goat IgG) (Santa Cruz Biotechnology, St. Cruz, Calif.). Horseradish peroxidase-labeled secondary antibodies (Santa Cruz Biotechnology) in appropriate dilutions were used to detect the bands at 42 and 44 kDa and were visualized by enhanced chemiluminescence with the ECL system (Pierce, Rockford, Ill.).
Bacterium-induced IL-8 secretion assay.
IL-8 secretion by T84 intestinal epithelial cells in response to basolateral infection of S. flexneri was measured by enzyme-linked immunosorbent assay (ELISA) as previously described (31) with some minor modifications. Briefly, 96-well plates (Limbo/Titretek; ICN Biochemicals, Aurora, Ohio) were coated overnight with goat anti-human IL-8 (R&D Systems, Minneapolis, Minn.). Following the addition of samples and IL-8 standards, rabbit anti-human IL-8 (Endogen, Woburn, Mass.) was used as the detecting antibody. As a positive control, the monolayer was stimulated with tumor necrosis factor alpha (TNF-α) (final concentration, 30 ng/ml). To examine the effects of the ERK inhibitor on IL-8 secretion, the T84 cells were treated as described above for the PMN transmigration assays.
Statistical analysis.
Neutrophil isolation was limited to repetitive donations by 10 different donors over the course of these experiments. Due to variations in both neutrophils and transepithelial resistance between monolayers (baseline resistance of between 400 and 1,500 Ω · cm2), data were analyzed within an individual experiment and not between experiments. However, the overall trends associated with these data are reproducible between experiments. All results are expressed as the mean ± standard deviation of an individual experiment done in triplicate. P values were calculated according to Student's t test, and values <0.05 were considered statistically significant.
RESULTS
Role of LPS in S. flexneri invasion.
LPS is a principal component of gram-negative bacteria that activates the immune system (see Fig. 1 for LPS composition). While the effects of LPS on monocytes as one focal point of the host response to this key bacterial product have been well studied (55, 57), only recently have investigations begun to explore how LPS may induce specific responses in the intestinal epithelium (5), the frontline of the mucosal immune system. Therefore, given recent interest in LPS-epithelial interactions, we sought to determine whether LPS plays a role in S. flexneri infection of a model intestinal epithelium. Since most in vitro studies investigating the molecular requirements for S. flexneri-host interactions have utilized nonpolarizing tissue culture cells, we chose to characterize early events in S. flexneri-host cell interaction in a system that more accurately reflects the initial physiologic site of interaction with the pathogen (i.e., the basolateral surface).
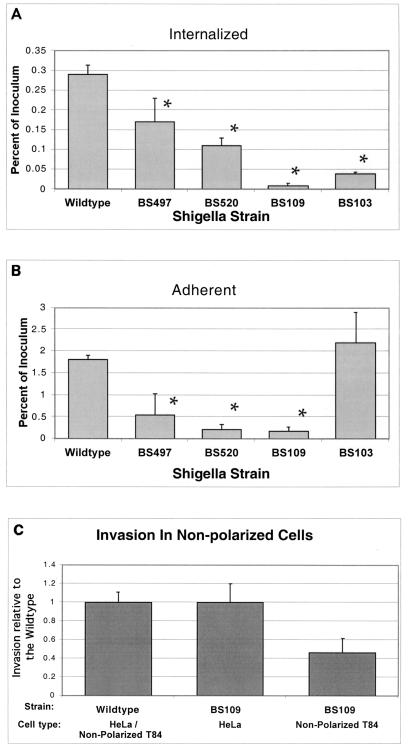
Polarized columnar T84 cell monolayers were infected with either wild-type S. flexneri or one of three isogenic LPS-defective derivatives (BS497, BS520, or BS109; see Fig. 1 for specific mutation) and assessed for their ability to attach to and subsequently enter into a model intestinal epithelium from the basolateral surface (see Materials and Methods for details). As shown in Fig. 2A and B, the S. flexneri LPS-defective strains exhibited a progressive decrease in their abilities to interact with the basolateral surface of polarized intestinal epithelium, depending on LPS chain length. In particular, we determined that while all Shigella mutant strains harboring a defect in the O side chain of the LPS showed an attenuated invasion profile, the most pronounced defect was observed for S. flexneri BS109, a strain that is missing both the outer core and the O antigen. When compared to the wild-type strain, BS109 exhibited a 30-fold reduction in its ability to invade the basolateral surface of T84 cell monolayers (Fig. 2A). As a negative control to establish background values, we included S. flexneri strain BS103, a plasmid-cured derivative of wild-type 2457T that is adherent but noninvasive and completely avirulent. We found that BS103 exhibited an almost 10-fold reduction in its ability to enter into the basolateral surface of polarized T84 cell monolayers. The other two LPS-defective strains, BS497 and BS520, also demonstrated an impaired ability to invade T84 cell monolayers (1.7- and 2.6-fold reductions, respectively, compared to the wild-type strain). These data contrast with other investigations (23, 44, 45), which examined the role of S. flexneri LPS in invasion by using several different LPS mutants and found no apparent invasion defect. Such studies, however, were performed with HeLa (cervical carcinoma) cells or other nonpolarizing and nonintestinal epithelial cells. Likewise, when experiments were performed with either nonpolarized T84 or HeLa cells, we found that the Shigella strain with the most severe LPS mutation (BS109) was rescued in its ability to invade the epithelial cells (Fig. 2C). Thus, by using a polarized model of the intestinal epithelium, our data are the first to demonstrate that defects in the S. flexneri LPS (primarily at the O side chain) can be directly linked to defects in the ability of this organism to interact with the basolateral membrane domain (i.e., the initial physiologic site of host interaction with the pathogen) (37).
FIG. 2.
S. flexneri 2a 2457T (wild-type) invades (A) and adheres to (B) polarized intestinal epithelial cells more efficiently than the LPS mutant strains. There is a strong correlation between the length of the LPS component and the mutant's ability to invade and adhere to intestinal epithelial cells. In panel C, we compare the relative invasiveness of the mutant with most severe LPS defect (BS109) with that of the Shigella wild type in nonpolarized T84 and HeLa cells. The data are expressed as the mean ± standard deviation of triplicate samples for all conditions tested and represent one of at least two experiments performed with similar results. *, P < 0.05 compared to the wild-type strain.
We next assessed the ability of the S. flexneri LPS-defective strains to adhere to the basolateral membrane domain of the polarized intestinal epithelium. Adherent bacteria are defined as the number of internalized bacteria subtracted from the number of cell-associated bacteria (the adherent plus the internalized populations). As shown in Fig. 2B, we observed a clear decline in the numbers of adherent bacteria to the basolateral epithelial surface for all LPS mutant strains to the cell monolayer. Specifically, for the wild-type strain, we typically found that about 2% of the original bacterial inoculum adhered to the cell monolayer. However, the LPS mutants failed to successfully adhere to the polarized epithelial monolayer when compared to wild-type S. flexneri (3-, 8-, and 11-fold reductions for S. flexneri strains BS497, BS52, and BS109, respectively). These findings suggest that the LPS-defective strains exhibit a reduced ability to adhere to the basolateral surface of a polarized intestinal epithelium. Indeed, this result is further substantiated by calculating the ratio of internalized to cell-adherent bacteria. We determined that 16% of the cell-associated S. flexneri wild-type strain had invaded the polarized monolayer. However, when we determined the ratio of internalized to adherent bacteria for the S. flexneri LPS-defective strains BS497 and BS520, we found that 32 and 50% of the adherent LPS-defective strains, respectively, had invaded the cells, in contrast to only 5% of the BS109 bacteria. These results suggest that of the LPS mutant strains examined, only S. flexneri strain BS109 exhibited an actual defect in invasiveness.
Dose-response studies showed that increasing the inoculum resulted in more equivalent numbers of epithelial-associated bacteria when the LPS-defective strains were compared to wild-type S. flexneri. In particular, increasing the inoculation input from 25 to 400 bacteria per epithelial cell yielded quantitatively similar levels of cell surface and internalized bacteria for wild-type S. flexneri when compared to BS497 and BS520 (2.31% ± 0.38%, 2.6% ± 0.39%, and 2.4% ± 0.03% and 0.61% ± 0.12%, 0.60% ± 0.11%, and 0.5% ± 0.17% of the original inoculum for attachment and internalization, respectively, for the wild type, BS497, and BS520). The only exception was for BS109, which displayed a comparable ability to attach to the basolateral surface of polarized intestinal epithelia (2.31% ± 0.38% and 3.1% ± 0.35% of the original inoculum, respectively, for the wild type and BS109), but failed to become internalized (0.61% ± 0.11% and 0.02% ± 0.007% of the original inoculum, respectively, for the wild type and BS109). These results confirm that of the S. flexneri LPS-defective strains examined, only strain BS109 exhibits an invasion defect.
Having demonstrated the importance of LPS on the interaction between S. flexneri and the basolateral membrane domain of polarized intestinal epithelium, we next sought to determine the influence of a well-known LPS antagonist on S. flexneri invasion. We found that when T84 cells were infected with S. flexneri at the basolateral surface in the presence of RsDPLA, a nontoxic anti-inflammatory LPS derived from Rhodobacter spheroides (43), bacterial invasion and adherence were reduced by nearly 50%. In addition, since CD14 is the surface receptor for LPS and its binding protein, we further examined whether this molecule influenced S. flexneri invasion profiles. Although antibodies to CD14 failed to inhibit bacterial invasion, this result was expected, since, at least to our knowledge, T84 cells do not possess CD14.
The role of LPS in S. flexneri-induced inflammatory responses.
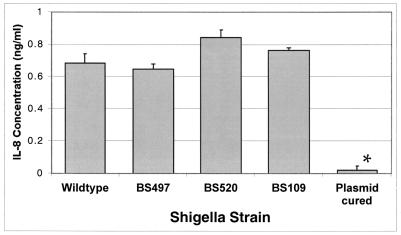
We next examined whether basolateral colonization of monolayers by the S. flexneri LPS-defective strains modulated the epithelial-induced inflammatory responses characterized by either elicitation of IL-8 secretion or induction of PMN transepithelial migration. The standard bacterial dose for assessing such proinflammatory responses has been previously determined to be 400 bacteria per epithelial cell (31, 33) and, unless stated otherwise, will be the infection dose used for the following studies. Initially, we examined whether polarized monolayers of T84 cells responded to the LPS-defective strains by eliciting the basolateral release of the proinflammatory cytokine IL-8. We found that all of the S. flexneri LPS-defective strains, regardless of the LPS deficiency, maintained the ability to elicit basolateral secretion of IL-8 from T84 cells when compared to the wild-type strain (Fig. 3). In contrast, the avirulent plasmid-cured strain, BS103, which is adherent but noninvasive, failed to induce IL-8 secretion. The failure of the BS103 strain to induce IL-8 and activate NF-κB has been previously reported (32, 33). Nevertheless, we determined that the polysaccharide core of LPS is dispensable for the polarized release of IL-8 secretion from the basolateral surface of T84 cell monolayers. It should also be noted that exogenous addition of S. flexneri LPS (10 μg/ml) to the basolateral surface failed to elicit IL-8 secretion from the T84 cells.
FIG. 3.
The S. flexneri LPS mutant strains induce IL-8 secretion from polarized T84 cell monolayers. The S. flexneri plasmid-cured strain BS103 induces significantly less IL-8 secretion from polarized T84 intestinal epithelial cells following a 1-h infection than the wild-type strain, 2457T, or any of the LPS mutants (BS109, BS497, and BS520). The data are expressed as the mean ± standard deviation of triplicate samples for all conditions tested and represent one of at least three experiments performed with similar results. ∗, P < 0.05 compared to the wild-type strain.
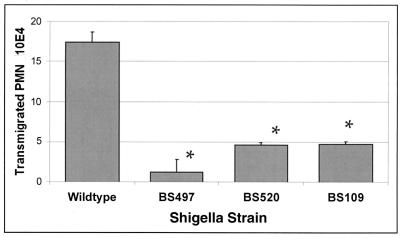
We next studied the role of LPS in transepithelial migration of PMN by using an in vitro assay that we have extensively characterized (4, 29, 33). As shown in Fig. 4, we determined that there was a marked defect (nearly 70%) in the ability of the LPS-defective strains to induce PMN transmigration when compared with wild-type S. flexneri, even with inocula in which the wild-type and LPS-defective strains achieved attachment and internalization in similar numbers. The only exception was for BS109, which adhered to the same extent as the wild type, but failed to enter. These results not only demonstrate that S. flexneri strain-specific attenuation of PMN transepithelial migration occurs even in the face of IL-8, but also suggest that the specific interaction between the basolateral surface of polarized monolayers, and S. flexneri LPS may mediate the epithelial direction of PMN transmigration.
FIG. 4.
The complete LPS of S. flexneri is essential for initiating PMN transepithelial migration. The mutant LPS strains elicited significantly less PMN transmigration than the wild-type strain. Although the BS497 strain appears to induce the lowest rate of PMN transmigration, this is not a consistent result. In every PMN transmigration assay that was performed, we found that all of the LPS-defective strains had virtually the same level of inhibition compared to the wild type, such that a definitive ranking could not be established. The data are expressed as the mean ± standard deviation of triplicate samples for all conditions tested and represent one of at least three experiments performed with similar results. ∗, P < 0.05 compared to the wild-type strain.
The role of S. flexneri LPS in mediating PMN signaling across intestinal monolayers was further addressed by examining the influence of RsDPLA (LPS antagonist) in this proinflammatory response. We found that addition of RsDPLA reduced, by over 50%, the ability of wild-type S. flexneri to induce PMN transepithelial migration (data not shown), whereas antibodies to CD14 failed to inhibit PMN signaling induced by this pathogen. This is in line with previous reports that RsDPLA functions independently of CD14 and soluble CD14 (sCD14) (24). Given that RsDPLA attenuated the ability of S. flexneri to invade T84 cells, it was possible that this reduction was responsible for the decrease in PMN transmigration. However, to reconcile this possibility, the level of the wild-type inoculum was decreased, so that the numbers of cell-associated bacteria matched that of wild-type S. flexneri plus RsDPLA. We found that reduction of the wild-type inoculum in this manner did not significantly reduce the epithelial promotion of PMN (data not shown). This result indicates that S. flexneri interaction with the epithelial basolateral surface, likely requiring LPS, rather than reduced cell-associated bacteria, results in activation of the epithelial signaling pathways that mediate PMN movement across the monolayer. It is interesting to note that this result is contrary to exogenous addition of purified S. flexneri LPS (10 μg/ml), which failed to induce PMN transepithelial migration when applied to either the apical or basolateral membrane domain (data not shown).
S. flexneri activates ERK pathways in intestinal epithelial cells.
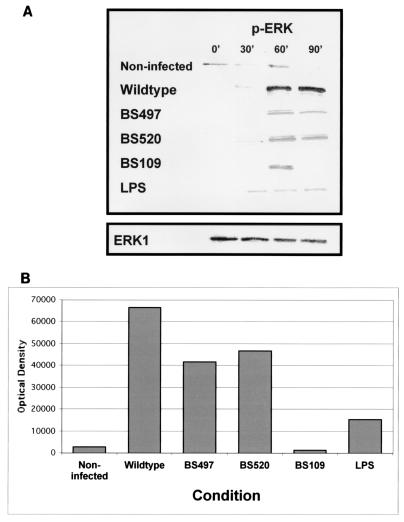
Our observations thus far suggest that S. flexneri LPS may play an important role in mediating events leading to PMN signaling across intestinal epithelium. Several bacterial pathogens have been demonstrated to induce inflammation via MAPK pathways (6, 8, 19, 28). There are three parallel MAPK cascades: ERK, JNK, and p38. Investigation of the impact of Shigella on the ERK pathway was of particular interest, since other studies of infectious processes caused by LPS have indicated a role for this kinase in the induction of proinflammatory signals by host cells (28). We therefore explored whether Shigella infection triggered activation of ERK1/2. It is important to point out that these experiments were normalized for the numbers of S. flexneri cells adherent to the basolateral surface of polarized monolayers. Figure 5A shows that phosphorylation of ERK1/2 increased as early as 30 min postinfection and at 90 min was 30-fold higher than in uninfected control monolayers. By comparison, the S. flexneri O side chain-defective strains exhibited a marked decrease in their ability to phosphorylate ERK1/2. Specifically, at 90 min postinfection, S. flexneri strains BS109, BS497, and BS520 were only either equal to the baseline or 15- and 17-fold higher than uninfected control monolayers, respectively (Fig. 5B). Again, the most profound reduction was observed for the BS109 strain. In keeping with our observations presented above, exogenous addition of purified S. flexneri LPS induced phosphorylation of ERK1/2 only fivefold compared to the baseline. Equal loading of protein was confirmed by probing the immunoblots for ERK1. These data indicate that the ERK1/2 pathway, likely stimulated by LPS, might be involved in Shigella-induced inflammatory events.
FIG. 5.
Time course of ERK phosphorylation by S. flexneri. T84 cells were exposed to wild-type S. flexneri or the LPS mutant strains at an MOI of 400 for various times prior to preparation of cellular lysates. (A) Western blot performed with specific antibodies for dually phosphorylated ERK1/2. Incubation with HBSS+ buffer served as the uninfected control. Equal loading of protein was confirmed by probing the immunoblots for ERK1. (B) Optical density tracing of phosphorylated ERK following a 90-min infection of T84 cells by S. flexneri. The data shown represent one of at least three experiments performed with similar results.
We next determined whether activation of ERK by S. flexneri occurred through the expected Raf/MEK pathway (52). Thus, we treated T84 cells with PD98059 (3), a specific MEK inhibitor, prior to infection with S. flexneri (see Materials and Methods for details). The S. flexneri inducible ERK phosphorylation was reduced by PD98059 treatment to levels not above background (data not shown). Such a reduction in the ERK1/2 signal was not attributed to a loss of S. flexneri host-cell interaction, since PD98059 at concentrations of 1 to 50 μM did not inhibit the adhesion or invasion of S. flexneri into the host cell (data not shown). It is important to note that for all the experiments carried out in the presence of PD98059, buffer containing 0.1% dimethyl sulfoxide was used as an appropriate vehicle control. Since no differences were observed between the buffer and vehicle controls, only the buffer controls have been reported.
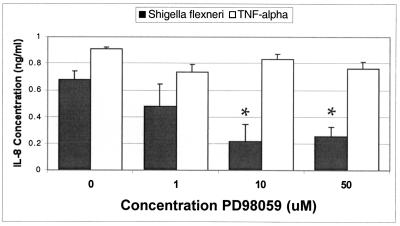
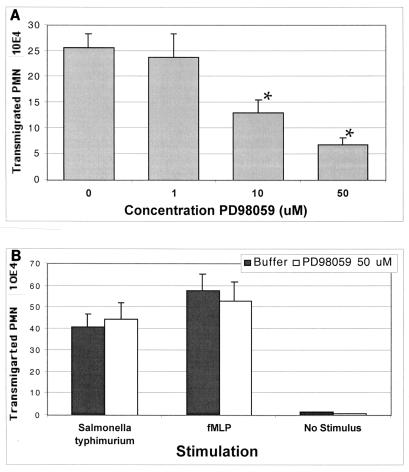
The next studies were aimed at determining whether the ERK1/2 pathway is directly involved in Shigella-induced inflammatory events. As shown in Fig. 6, we found that PD98059 at concentrations of 1 to 50 μM attenuated the ability of wild-type S. flexneri to induce IL-8 secretion from T84 cells in a dose-dependent manner. Interestingly, the TNF-α-induced IL-8 response was insensitive to PD98059 (Fig. 6). Likewise, PD98059 at concentrations of 10 and 50 μM also significantly reduced the ability of wild-type S. flexneri to induce PMN transepithelial migration (Fig. 5A). To ascertain whether PD98059 exhibited a general down-regulatory effect on PMN transepithelial migration or was specific to elicitation by Shigella infection, we measured whether PD98059 could inhibit PMN transepithelial migration induced by other physiological agonists. Salmonella enterica serovar Typhimurium and the potent PMN chemoattractant N-formylmethionyl-leucylphenylalanine (fMLP) were tested with PD98059 in our model system. We found that the pretreatment of T84 epithelial cell monolayers with PD98059 at concentrations that prevented S. flexneri-induced PMN transepithelial migration was ineffective at inhibiting PMN signaling induced by either S. enterica serovar Typhimurium or fMLP (Fig. 7B). Thus, these results illustrate that rather than exerting a global effect, the inhibitory action of PD98059 appears to be specific only for S. flexneri-induced proinflammatory responses defined by PMN transmigration. Further, these results also confirm our previous findings that S. flexneri and S. enterica serovar Typhimurium use different signaling pathways to induce proinflammatory responses that govern PMN trafficking across epithelia (32).
FIG. 6.
Effect of the MEK inhibitor PD98059 on S. flexneri-induced IL-8 secretion. IL-8 secretion by polarized intestinal epithelial cells infected with S. flexneri 2457T (wild type) is significantly decreased in the presence of 10 and 50 μM MEK inhibitor PD98059. However, IL-8 secretion by T84 intestinal epithelial cells maintains baseline levels when the cell monolayers are stimulated by 30 ng of TNF-α per ml. The data are expressed as the mean ± standard deviation of triplicate samples for all conditions tested and represent one of at least two experiments performed with similar results. ∗, P < 0.05 compared to the 0 μM PD98059 buffer control.
FIG. 7.
Effect of the MEK inhibitor PD98059 on S. flexneri-induced PMN transepithelial migration. (A) S. flexneri 2457T (wild type)-induced PMN transmigration is significantly decreased when the T84 cell monolayer is preincubated with 10 or 50 μM MEK inhibitor PD98059. (B) PMN transmigration induced by S. enterica serovar Typhimurium or fMLP is not affected by PD98059. The data are expressed as the mean and standard deviation of triplicate samples for all conditions tested and represent one of at least three experiments performed with similar results. *, P < 0.05 compared to the 0 μM PD98059 buffer or vehicle control.
DISCUSSION
In the present study, we investigated a potential role of LPS in the S. flexneri-induced inflammatory responses by considering the possibility that interaction of S. flexneri with the basolateral surface of polarized epithelium may induce signal transduction events that have not yet been characterized. We found that S. flexneri LPS plays an important role in mediating events leading to PMN signaling across a model intestinal epithelium most likely involving the activation of the ERK1/2 pathway. Since ERK activation is involved in modulating proinflammatory events, our findings have profound implications on the understanding of the mechanisms by which S. flexneri can elicit mucosal inflammation.
The involvement of LPS in S. flexneri invasion was previously examined in nonpolarized cell lines of both epithelial (HeLa) and fibroblastic (L2) lineages. In these cell lines, Hong and Payne (26) determined that the LPS rfaL, rfaX, and rfb loci are required for serum resistance and intercellular spread, but not for bacterial invasion. In addition, Sandlin et al. (44, 45) found that the same S. flexneri LPS mutant strains used here in our report (i.e., BS497, BS520, and BS109) displayed wild-type invasion capabilities upon infection of HeLa cells. However, our results obtained with polarized intestinal epithelial cells revealed that the S. flexneri LPS mutants exhibited a progressive decrease in the ability to interact with the basolateral surface of the intestinal epithelium, depending on chain length. These results suggest that S. flexneri invasion into polarized epithelium may have different molecular requirements from entry into nonpolarized epithelia (7, 13).
The mechanism of Shigella adherence to the host basolateral cell surface prior to entry is incompletely understood. Although the Ipa complex has been shown to bind to the fibronectin receptor α5β1, integrins do not appear to be exclusive receptors for the IpaB-C complex (54). Moreover, IpaB may bind directly to the extracellular domain of CD44 (49). Nonetheless, since S. flexneri cells require contact with the host cell before Ipa proteins are secreted by the Mxi/Spa secretion apparatus (39), this opens up the possibility that S. flexneri LPS might be involved in the initial contact between the pathogen and basolateral membrane domain of the intestinal epithelium. In this regard, additional experiments will need to be performed before we understand why the invasive ability (at a higher MOI) of S. flexneri strains BS497 and BS520 was rescued, yet that of strain BS109 was not. One explanation might be a surface charge difference, since BS109 is a deep rough mutant.
In the human system, the TLR4-MD2-CD14 complex has been demonstrated to serve as a surface receptor for LPS (2). In addition to the cell surface TLR4 complex, there is mounting evidence to suggest that mammalian cells also have an intracellular receptor that detects LPS in the cytoplasm of bacterium-infected cells (41) and activates NF-κB. Indeed, recent studies suggest that this response is mediated by a cytosolic plant disease resistance-like protein termed CARD4/NOD1 (17). In particular, CARD4/NOD1 has been found to mediate NF-κB and JNK activation by invasive S. flexneri. The presence of an intracellular detection system for bacterial LPS would be expected at epithelial surfaces, such as those of the gut lumen that are highly exposed to bacteria and bacterial products. Indeed, triggering of an inflammatory response to bacterial products through surface receptors, such as TLR4, would most likely be detrimental to the host (i.e., elicit chronic states of inflammation).
However, since the epithelium is normally impenetrable to most bacteria, the basolateral membrane milieu (i.e., the lamina propria) is an LPS-free environment. Considering that S. flexneri enters epithelial cells by means of basolateral ligand(s), this organism, by virtue of its invasion strategy, is able to deliver LPS specifically to the epithelial cell basolateral membrane domain. For instance, evidence indicates that epithelial cells may have evolved proinflammatory “sensors” at the basolateral membrane as a means of the host realizing that its primary barrier has been breached and that a mucosal response is warranted (15, 16, 20). For example, expression of TLR5 at the basolateral surface of the intestinal epithelia not only recognizes bacterial flagellin, but also mobilizes the activation of NF-κB (15, 16, 20). The indication that LPS may also target the basolateral surface is consistent with finding that the cellular distribution of LPS in the intestine demonstrated a basal interaction for the uptake of LPS into enterocytes (56), arguing that LPS most likely enters the basolateral surface of enterocytes from the lamina propria. Furthermore, our results herein provide evidence to suggest that S. flexneri LPS-basolateral membrane interactions play a critical role in mediating the proinflammatory events that govern PMN transepithelial migration. Mechanistic evaluation of this response implicates the activation of the ERK1/2 pathway. Based on our evidence that exogenous soluble LPS failed to stimulate PMN transmigration on its own, we favor the possibility that an intracellular LPS receptor may modulate these responses. Alternative possibilities are that the presentation of purified LPS to target cells differs from the way native LPS (in particular the oligosaccharide chain) is presented or that bacterial proteins may be expressed differently in the membrane of the LPS-defective strains.
Several gastrointestinal pathogens have been shown to utilize MAPK to promote inflammatory responses (14, 22, 25, 53). However, we provide the first demonstration that S. flexneri LPS plays an important role in mediating events leading to PMN signaling across a model intestinal epithelium, most likely involving the activation of the ERK1/2 pathway. Our evidence clearly shows that the S. flexneri LPS mutant strains exhibited an attenuated ability to activate the ERK pathway, and in addition, failed to elicit PMN transepithelial migration even in the face of the ability to induce IL-8 secretion. We have previously reported that induction of PMN transepithelial migration by S. flexneri appears to be dependent not only on invasive ability and expression of a functional type III secretion system, but also on the ability to spread to adjacent cells (11, 32). Based on our observations herein, we conclude that the activation of the ERK pathway might be a common pathway for S. flexneri-mediated PMN transepithelial migration.
Finally, since Shigella-epithelial cell interactions are clearly required to evoke the transcellular signaling cascade that coordinates the initiation of the mucosal inflammatory process (10, 32, 33, 47, 48), the involvement of ERK activation in this proinflammatory response is not surprising. However, while we determined that blocking the ERK1/2 pathway with the inhibitor PD98059 diminished the neutrophil transmigration response elicited by wild-type Shigella in a dose-dependent manner, activation of ERK did not seem to be necessary for the entry of Shigella into the host cell. This is in contrast to what was found by Tang et al. (50), who described that Listeria monocytogenes requires an activation of the ERK pathway to enter the host cell.
In summary, our findings reported herein are the first to indicate that the ERK1/2 signal transduction pathway is stimulated by S. flexneri to elicit neutrophil transmigration across a model intestinal epithelium. Interestingly, this inflammatory response appears to be dependent on an intact bacterial LPS polysaccharide chain. Thus, we envisage that epithelial cells have evolved a response to the polysaccharide portion of LPS at its basolateral surface as a means of the host realizing its primary barrier has been breached and that a mucosal immune inflammatory response is necessary. S. flexneri may have evolved a such a mechanism utilizing its LPS, since these organisms lack flagella.
Acknowledgments
The S. flexneri strains 2457T, BS109, BS497, and BS520 were generously provided by A. T. Maurelli, Uniformed Services of Health Science, Bethesda, Md. We are grateful to Andrew Gewirtz, Emory University, Atlanta, Ga., for his help with the IL-8 ELISA and to Nilofer Qureshi, University of Wisconsin—Madison, for providing the RsDPLA. We also thank Milton Silva for technical assistance and William J. Nadeau for critically reading the manuscript.
The study was supported by National Institutes of Health grants DK56754 and DK33506 Project 3 (B. A. McCormick). Henrik Köhler was supported by the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Adam, T., M. Arpin, M. C. Prevost, P. Guonon, and P. J. Sansonetti. 1995. Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J. Cell Biol. 129:367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 3.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 4.Bennish, M. L. 1991. Potentially lethal complications of shigellosis. Rev. Infect. Dis. 13:319-324. [DOI] [PubMed] [Google Scholar]
- 5.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Bech, H.C. Reinecker, and D. K. Podolsky. 2000. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966-972. [DOI] [PubMed] [Google Scholar]
- 6.Chen, W., M. M. Monick, A. B. Carter, and G. W. Hunninghake. 1999. Activation of ERK1/2 by respiratory syncytial virus in A549 cells is linked to the production of interleukin-8. Exp. Lung Res. 26:13-26. [DOI] [PubMed] [Google Scholar]
- 7.Criss, A. K., D. M. Ahlgren, T. Z. Jou, B.A. McCormick, and J. E. Casanova. 2001. The GTPase Rac1 selectively regulates Salmonella invasion at the apical plasma membrane of polarized epithelial cells. J. Cell Sci. 114:1331-1341. [DOI] [PubMed] [Google Scholar]
- 8.Denning, G. M., L. A. Wollenweber, M. A. Railsback, C. D. Cox, L.L. Stoll, and B. E. Britigan. 1998. Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infect. Immun. 66:5777-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.d'Hauteville, H., R. D. Lagelouse, F. Nato, and P. J. Sansonetti. 1996. Lack of cleavage of IcsA in Shigella flexneri causes aberrant movement and allows demonstration of a cross-reactive eukaryotic protein. Infect. Immun. 64:511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumenil, G., J. C. Olivo, S. Pelligrini, M. Fellous, P. J. Sansonetti, and G. T. Nhieu. 1998. Interferon alpha inhibits a Src-mediated pathway necessary for Shigella-induced cytoskeletal rearrangements in epithelial cells. J. Cell Biol. 143:1003-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez, I. M., M. Silva, R. Schuch, W. A. Walker, A. M. Siber, A. T. Maurelli, and B.A. McCormick. 2001. Cadaverine prevents the escape of Shigella flexneri from the phagolysosome: a connection between bacterial dissemination and neutrophil transepithelial signaling. J. Infect. Dis. 184:743-753. [DOI] [PubMed] [Google Scholar]
- 12.Formal, S. B., G. J. Dammin, E. H. LaBrec, and H. Schneider. 1958. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J. Bacteriol. 75:604-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gewirtz, A. T., A. M. Siber, J. L. Madara, and B.A. McCormick. 1999. Orchestration of neutrophil movement by intestinal epithelial cells in response to Salmonella typhimurium can be uncoupled from bacterial internalization. Infect. Immun. 67:608-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gewirtz, A. T., A. S. Rao, P.O. Simon, D. Merlin, D. Carnes, J. L. Madara, and A. S. Neish. 2000. Salmonella typhimurium induces epithelial IL-8 expression via Ca (2+)- mediated activation of the NF-kappaB pathway. J. Clin. Investig. 105:79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gewirtz, A. T., P. O. Simon, C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A.D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory genes expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 17.Girardin, S. E., R. Tournebize, M. Mavris, A. L. Page, X. Li, G. R. Stark, J. Bertin, P. S. DiStefano, M. Yaniv, P. J. Sansonetti, and D. J. Philpott. 2001. CARD4/NOD1 mediates NF-κB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2:736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg, M. B., and P.J. Sansonetti. 1993. Shigella subversion of the cellular cytoskeleton: a strategy for epithelial colonization. Infect. Immun. 61:4941-4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 21.Henson, P., and Z. G. Oades. 1975. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. J. Clin. Investig. 56:1053-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobbie, S. I., M. Chen, R. J. Davis, and J. E. Galan. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550.. [PubMed] [Google Scholar]
- 23.Hong, M., and S.M. Payne. 1997. Effect of mutations in Shigella flexneri chromosomal and plasmid-encoded lipolysaccharide genes on invasion and resistance. Mol. Microbiol. 24:779-791. [DOI] [PubMed] [Google Scholar]
- 24.Kirikae, T., F. Kirikae, and K. Tominaga. 1997. Rhodobacter sphaeroides diphosphotyl lipid A inhibits interleukin-6 production in CD14-negative murine marrow stromal cells stimulated with lipopolysaccharide or Paclitaxel. J. Endotoxin Res. 4:115-122.
- 25.Li, J. D., W. Feng, M. Gallup, J. H. Kim, J. Gum, Y. Kim, and C. Basbaum. 1998. Activation of NF-kappaB via a src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc. Natl. Acad. Sci. USA 95:5718-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madara, J. L., C. A. Parkos, S. P. Colgan, R. J. MacLeod, S. Nash, J. Matthews, C. Delp, and W. S. Lencer. 1992. Cl- secretion in a model intestinal epithelium induced by a neutrophil-derived secretagogue. J. Clin. Investig. 89:1938-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madara, J. L., and K. Dharmsathaphorn. 1985. Occluding junction structure-function relationships in a cultured monolayer. J. Cell Biol. 101:2124-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marie, C., S. Roman-Roman, and G. Rawadi. 1999. Involvement of mitogen-activated protein kinase pathways in interleukin-8 production by human monocytes and polymorphonuclear cells stimulated with lipopolysaccharide or Mycoplasma fermentans membrane lipoproteins. Infect. Immun. 67:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathan, M. M., and V. I. Mathan. 1986. Ultrastructural pathology of the rectal mucosa in Shigella dysentery. Am. J. Pathol. 123:25-38. [PMC free article] [PubMed] [Google Scholar]
- 30.McCormick, B. A., P. M. Hofman, J. Kim, D. K. Carnes, S. I. Miller, and J. L. Madara. 1995. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J. Cell Biol. 131:1599-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormick, B. A., S. P. Colgan, C. D. Archer, S. I. Miller, and J. L. Madara. 1993. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J. Cell Biol. 123:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormick, B. A., M. I. Fernandez, A. M. Siber, and A. T. Maurelli. 1999. Inhibition of Shigella flexneri-induced transepithelial migration of polymorphonuclear leukocytes by cadaverine. Cell Microbiol. 1:143-156. [DOI] [PubMed] [Google Scholar]
- 33.McCormick, B. A., A. M. Siber, and A. T. Maurelli. 1998. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect. Immun. 66:4237-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormick, B. A., C. A. Parkos, S. P. Colgan, D. K. Carnes, and J. L. Madara. 1998. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J. Immunol. 160:455-466. [PubMed] [Google Scholar]
- 35.Mounier, J., T. Vasselon, R. Hellio, M. Lesourd, and P. J. Sansonetti. 1992. Shigella enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect. Immun. 60:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nash, S., J. Stafford, and J. L. Madara. 1987. Effects of polymorphonuclear leukocyte transmigration on barrier function of cultured intestinal epithelial monolayers. J. Clin. Investig. 80:1104-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parkos, C. A., S. P. Colgan, C. Delp, M. A. Arnaout, and J. L. Madara. 1992. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J. Cell Biol. 117:757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkos, C. A., C. Delp, M. A. Arnaout, and J. L. Madara. 1991. Neutrophil migration across a cultured intestinal epithelium: dependence on a CD11b/CD18-mediated event and enhanced efficiency in the physiologic direction. J. Clin. Investig. 88:1605-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsot, C., and P. J. Sansonetti. 1996. Invasion and pathogenesis of Shigella infections. Curr. Top. Microbiol. Immunol. 209:25-42. [DOI] [PubMed] [Google Scholar]
- 40.Perdomo, J. J., P. Gounon, and P. J. Sansonetti. 1994. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J. Clin. Investig. 93:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philpott, D. J., S. Yamaokao, A. Israël, and P. J. Sansonetti. 2000. Invasive Shigella flexneri activates NF-κB through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J. Immunol. 165:903-914. [DOI] [PubMed] [Google Scholar]
- 42.Pugin, J., C. C. Schurer-Maly, D. Leturcq, A. Moriarty, R. J. Ulevitch, and P. S. Tobias. 1993. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc. Natl. Acad. Sci. USA 90:2744.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qureshi, N., B. W. Jarvis, and K. Takayama. 1999. Nontoxic RsDPLA as a potent antagonist of toxic lipopolysaccharide, p. 689-698. In H. Brade, S. M. Opal, S. N. Vogel, and D. C. Morrison (ed.), Endotoxin in health and disease. Marcel Dekker, Inc., New York, N.Y.
- 44.Sandlin, R. C., M. B. Goldberg, and A. T. Maurelli. 1996. Effect of O-side chain length and composition on the virulence of Shigella flexneri 2a. Mol. Microbiol. 22:63-73. [DOI] [PubMed] [Google Scholar]
- 45.Sandlin, R. C., K. A. Lampel, S. P. Keasler, M. B. Goldberg, A. L. Stolzer, and A. T. Maurelli. 1995. Avirulence of rough mutants of Shigella flexneri: requirement of O antigen for correct unipolar localization of IcsA in the bacterial outer membrane. Infect. Immun. 63:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sansonetti, P. J. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. III. Shigellosis: from symptoms to molecular basis. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G319-G323. [DOI] [PubMed] [Google Scholar]
- 47.Sansonetti, P. J., J. Arondel, M. Huerre, A. Harada, and K. Matsushima. 1999. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect. Immun. 67:1471-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skoudy, A., G. T. Nhieu, N. Mantis, M. Arpin, J. Mounier, P. Gounon, and P. J. Sansonetti. 1999. A functional role for ezrin during Shigella flexneri entry into epithelial cells. J. Cell Sci. 112:2059-2068. [DOI] [PubMed] [Google Scholar]
- 49.Skoudy, A., J. Mounier, A. Aruffo, H. Ohayon, P. Gounon, P. Sansonetti, and G. Tran Van Nhieu. 2000. CD44 binds to the Shigella IpaB protein and participates in bacterial invasion of epithelial cells. Cell. Microbiol. 2:19-33. [DOI] [PubMed] [Google Scholar]
- 50.Tang, P., C. L. Sutherland, M. R. Gold, and B. B. Finlay. 1998. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect. Immun. 66:1106-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasselon, T., J. Mounier, M. C. Prevost, R. Hellio, and P. J. Sansonetti. 1991. Stress fiber-based movement of Shigella flexneri within cells. Infect. Immun. 59:1723-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vojtek, A. B., and C. J. Der. 1998. Increasing complexity of the ras signaling pathway. J. Biol. Chem. 273:19925-19928. [DOI] [PubMed] [Google Scholar]
- 53.Warny, M., A. C. Keates, L. Castagliulo, J. L. Zacks, S. Aboudola, A. Qamar, C. Pothoulakis, J. T. Lamont, and C. P. Kelly. 2000. P38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Investig. 105:1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watari, M., S. Funato, and C. Sasakawa. 1996. Interaction of Ipa proteins of Shigella flexneri with alpha5beta1 integrin promotes entry of the bacteria into mammalian cells. J. Exp. Med. 183:991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 56.Yimin, G., R. M. Ezzell, and H. S. Warren. 2000. Localization of endotoxin in the rat intestinal epithelium. J. Infect. Dis. 182:873-881. [DOI] [PubMed] [Google Scholar]
- 57.Ziegler-Heitbrock, H. W., and R. J. Ulevitch. 1993. CD14: cell surface receptor and differentiation marker. Immunol. Today 14:121-125. [DOI] [PubMed] [Google Scholar]