Abstract
Variations in the host response during pneumonia caused by Streptococcus pneumoniae in susceptible (CBA/Ca) and resistant (BALB/c) inbred mouse strains were investigated. Significant differences were detected in survival time, core body temperature, lung-associated and systemic bacterial loads, mast cell numbers, magnitude and location of cytokine production, lung disruption, and ability of isolated lung cells to release the cytokine tumor necrosis factor (TNF) alpha in vitro. Overall, the results indicate that the reduced capacity of CBA/Ca mice to induce rapid TNF activity within the airways following infection with S. pneumoniae may be a factor in their elevated susceptibility to pneumococcal pneumonia.
Streptococcus pneumoniae, the pneumococcus, is an important human pathogen, causing both invasive diseases (including pneumonia, meningitis, and septicemia) as well as noninvasive infections, such as otitis media. The escalating problem of antibiotic-resistant pneumococci has led to enhanced interest in the vaccination of individuals at greatest risk for pneumococcal infection. However, the limited efficacy of the current vaccine has resulted in greater attention being paid to the discovery of novel methods of control.
Effective pulmonary host defense against respiratory pathogens is believed to be mainly mediated via phagocytosis by alveolar macrophages and recruited neutrophils (18). If pneumococci overcome these defenses and gain entry to the bloodstream, systemic protection is afforded by anticapsular antibodies (31). Such defenses are orchestrated by a rapid inflammatory response following infection. Cytokines have been shown to be important soluble mediators responsible for coordinating this response (reviewed in reference 38).
Many cytokines are known to be involved in antibacterial defenses within the lungs. Tumor necrosis factor (TNF) is capable of recruiting inflammatory cells to the site of infection both directly and via up regulation of adhesion molecules (34, 39). TNF is also capable of stimulating the release of chemokines, cytokines that are directly chemotactic for inflammatory cells. Macrophage inflammatory protein 1 α (MIP-1α) and MIP-2 are two chemokines known to be important in bacterial pneumonia (20, 49). Following recruitment of phagocytic cells, TNF can also promote antimicrobial activity by activating the respiratory burst (12) and by activating the capacity to degranulate (27). These effects of TNF have been shown to be required for effective in vivo host defense against a range of microorganisms, including Klebsiella pneumoniae and Pneumocystis carinii (10, 28).
Interleukin 1β (IL-1β) shares several of TNF's activities, including the promotion of cell recruitment and the activation of macrophages at the site of infection (45). In some situations the two cytokines act synergistically to exert their effects (41).
IL-6 has been ascribed both pro- and anti-inflammatory effects. IL-6 can activate monocytes (6), and it can synergize with TNF to increase the respiratory burst of neutrophils (37). However, in pneumococcal pneumonia IL-6−/− mice have been shown to mount significantly elevated inflammatory responses in comparison to wild-type animals (53).
Regulation of the inflammatory response by anti-inflammatory cytokines prevents damage to the host. IL-10 contributes by reducing the production of proinflammatory cytokines and chemokines (5, 24) and down-regulates the expression of adhesion molecules (55). These cytokines were chosen for investigation in this study to characterize the pro- and anti-inflammatory response during pneumococcal infection.
Several pneumococcal factors are known to induce the release of inflammatory mediators. Cell wall teichoic acids, capsular polysaccharides, and pneumolysin (the toxin released from pneumococci upon lysis) have been shown to induce cytokine production from cells in vitro (22, 43, 47). Pneumolysin has also been shown to be involved in cell recruitment during pneumococcal pneumonia as delayed and reduced neutrophil influx results following infection with pneumolysin-deficient bacteria (23).
We have recently investigated the possibility of a genetic component to host response to pneumococcal pneumonia (16). Currently, genetic factors responsible for resistance to infections due to certain intracellular bacteria (e.g., Listeria monocytogenes and Salmonella enterica serovar Typhimurium) and fungi are understood or at least recognized (3, 42, 48). Less is known about candidate genes for resistance to extracellular bacteria, although some mutations conveying susceptibility have been identified (Xid and complement deficiencies [8, 15]). For this reason, we have established models of pneumococcal pneumonia in inbred strains of mice that are genetically resistant or susceptible to the infection (16). Comparison of cell recruitment revealed significantly higher numbers of neutrophils during early infection in BALB/c lungs than in CBA/Ca lungs. We have now investigated the production of inflammatory mediators by resistant and susceptible mice. As the induction of the cytokine cascade and the recruitment of inflammatory cells are not restricted to pneumococcal pneumonia, this infection may also be utilized to better understand acute inflammation in general.
MATERIALS AND METHODS
Mice.
Female CBA/Ca (15 to 20 g), BALB/c (20 to 25 g), C57BL/6 (20 to 25 g), and MF1 (25 to 30 g) mice were purchased from Harlan Olac, Bicester, United Kingdom. TNF-α p55 receptor and p75 receptor knockout mice (25 to 30 g) were bred at the University of Glasgow. Mice were infected at ∼9 weeks old. CBA/Ca mice do not carry the Xid mutation present in other CBA strains and can therefore respond to capsular polysaccharide.
Bacteria.
S. pneumoniae D39, serotype 2, was obtained from the National Collection of Type Cultures (NCTC 7466; Central Public Health Laboratory, London, United Kingdom). Bacteria were grown on blood agar base number 2 (Oxoid, Basingstoke, United Kingdom) plus 5% (vol/vol) defibrinated horse blood (E&O Laboratories, Bonnybridge, United Kingdom) (BAB).
The challenge dose was prepared by passaging S. pneumoniae through mice as previously described (2), and aliquots were stored at −80°C. Pneumococci could be stored for at least 3 months at −80°C with no significant loss of viability. Strain validation was carried out by checking bacterial susceptibility to the antibiotic optochin (Difco, Detroit, Mich.) by serotyping via the Quellung reaction and by multilocus sequence typing by using the service provided by the Scottish Meningococcus and Pneumococcus Reference Laboratory (Stobhill Hospital, Glasgow, United Kingdom). When required, a sample was thawed rapidly and bacteria were harvested by centrifugation before resuspension in an appropriate volume of sterile phosphate-buffered saline (PBS) (Oxoid). Control mice were inoculated with PBS alone.
Telemetry chip implantation.
Telemetry chips (Minimitter, Sunriver, Oreg.) were presterilized by immersion in Cidex (Johnston & Johnston Medical Limited, Skipton, United Kingdom).
Two weeks prior to infection, mice to be implanted were deeply anesthetized with 3 to 5% (vol/vol) halothane (Zeneca Pharmaceuticals, Macclesfield, United Kingdom) over oxygen (1.5 liter/min), administered using a calibrated vaporizer. The telemetry chip was then placed inside the peritoneum, the inner wound was fastened with a 5-0 Dexon II suture (Cyanamid of Great Britain Ltd., Hampshire, United Kingdom), and the outer wound was fastened with surgical clips (Becton Dickinson, Oxford, United Kingdom).
Telemetry readings were gathered using the VitalView data acquisition system (Minimitter). The implanted chip captures energy from the field of radio waves generated by the coils of an energizer-receiver box placed underneath each individually caged animal. The powered chip emits a signal, and the frequency of this signal informs the receiver box of the core body temperature and activity of the animal. The VitalView software gathers the information and presents the data for individual mice.
Infection of mice.
For intranasal infection, mice were lightly anesthetized with 1.5% (vol/vol) halothane, and the infectious dose was administered in a 50-μl volume as previously described (23). For intravenous infection, the infectious dose was administered as a 50 μl volume injected into the lateral tail vein. A zero time point bleed was taken from a separate vein to ensure successful infection. Mice were monitored for signs of disease until they were deemed to have irreversibly succumbed to the infection (51), at which point they were humanely sacrificed. Moribund was used as the end point of certain death, which in these studies was taken as the unwillingness of the animal to move when encouraged. Mice displaying no signs of illness were monitored for 336 h, at which point they were considered to have survived the infection.
Bacteriological investigation.
At prechosen intervals after infection, groups of mice were sacrificed by cervical dislocation, ensuring an intact trachea, and a blood sample was removed via cardiac puncture.
For bronchoalveolar lavage, a 16-gauge nonpyrogenic angiocath (F. Baker Scientific, Runcorn, United Kingdom) was inserted into the trachea and the lungs were lavaged with a total volume of 2 ml (BALB/c) or 1.5 ml (CBA/Ca, due to smaller lung size) of PBS. Lavaged lungs were homogenized in 5 ml of sterile PBS with a glass handheld tissue homogenizer (Jencons, Leighton Buzzard, United Kingdom). Viable bacteria in lung samples were counted by plating out serial 10-fold dilutions on BAB. As some CBA/Ca mice died before the 36-h time point, time course infections were repeated in order to obtain the required group size at 36 h for statistical analysis.
Sampling for cytokines.
Blood samples were left to clot for 30 min at room temperature before being centrifuged at 3,800 × g. Serum was stored at −80°C until use.
Bronchoalveolar lavage was done as described above, but the fluid was snap-frozen by immersion in liquid nitrogen. The lungs were then removed, wrapped in aluminium foil, and snap-frozen (54). Both bronchoalveolar lavage fluid (BALF) and whole-lung samples were stored at −80°C until further processing.
Upon thawing, the whole lungs were homogenized as described previously. Homogenates were centrifuged at 1,600 × g for 30 min at 4°C. The supernatants were then centrifuged at 5,000 × g for 30 min at 4°C, filter sterilized (0.2 μM) (Gelman Sciences, Northampton, United Kingdom), and stored at −80°C (54). Upon thawing, lavage fluids were centrifuged at 17,900 × g for 3 min.
Alveolar cell recovery and in vitro stimulation.
For BALB/c mice, the lavage volume was increased to 5× 1-ml aliquots of nonpyrogenic saline (Baxter Healthcare Ltd., Norfolk, United Kingdom). For CBA/Ca cell sampling, 5× 0.75-ml volumes were instilled. Recovered cells were centrifuged at 1,000 × g and 4°C for 5 min. The cell concentration was adjusted to 2.0 × 105 cells/ml with RPMI 1640 medium (Gibco, Paisley, United Kingdom), and 100 μl was placed in each well of a flat-bottom 96-well plate. Cells were incubated overnight at 37°C in 5% CO2 before being washed twice with 100 μl of sterile PBS.
Lipopolysaccharide (LPS) (from E. coli 0127:B8) (Sigma, Dorset, United Kingdom) was prepared at concentrations ranging from 50 to 0.5 ng/ml in RPMI 1640. Pneumolysin was produced as previously described (35) and purified by perfusion chromatography using a Poros 20 PE hydrophobic interaction matrix on a BioCAD 700E (Perseptive Biosystems, Warrington, United Kingdom). Pneumolysin was diluted in RPMI 1640 to concentrations ranging from 100 ng to 3 pg per ml. Heat-killed pneumococci were prepared by incubation at 60°C for 10 min and dilution in RPMI 1640. Killing was measured by plating out the preparation on BAB. Stimulants were added at 200 μl/well.
Histological analysis.
Lungs were inflated with 1 ml of 10% (vol/vol) formal saline prior to their removal. Following fixation, the lungs were embedded in paraffin and blocked by utilizing standard histological protocols. Lung blocks were sectioned at 5 μm prior to staining with hematoxylin and eosin (BDH Laboratory Supplies, Poole, United Kingdom).
Mast cell staining.
Mast cells were stained by virtue of their acidic granules, and these granules were counted in order to quantify the mast cell population.
Lung sections (5 μm) were washed in Histoclear (National Diagnostics, Atlanta, Ga.), dehydrated, and stained overnight in 0.5% (vol/vol) toluidine blue (National Diagnostics) in 0.5 M HCl (BDH Laboratory Supplies). The sections were then counterstained in 0.5% (vol/vol) safranine O (Sigma) in 0.125 M HCl.
Measurement of immune modulators.
TNF activity was measured with the standard L929 cytotoxicity bioassay (1) with 1-(4,5-dimethylthiazol-2-yl)-3,5-diphylformazan (MTT; Sigma) used to assess cell viability. TNF-α and IL-1β proteins were measured using murine Quantikine enzyme-linked immunosorbent assay kits (R&D Systems, Abingdon, United Kingdom). IL-6 was measured by enzyme-linked immunosorbent assay by utilizing commercially available antibody pairs (clones MP5-20F3 and MP5-32C11, Pharmingen catalog no. 18071D and 19251V; Becton Dickinson). IL-10 was quantified by using a mouse IL-10 duoset (R&D Systems).
Modulation of TNF-α level.
Local TNF-α within lung airways was neutralized in MF1 mice by intranasal administration of 10 μg of affinity-purified neutralizing anti-TNF-α polyclonal antibody (R&D Systems) 2 h prior to intranasal infection with 106 CFU of D39. Ten micrograms of goat immunoglobulin G (IgG) (Sigma) was given as a negative control. The efficacy of this treatment was confirmed by TNF bioassay (anti-TNF-α treatment gave a significant reduction in TNF activity within BALF with no effect on systemic TNF levels). Mice were humanely sacrificed at 24 h postinfection, and bacterial loads were measured in BALF and lung tissue.
Statistical analysis.
Mast cell counts and levels of inflammatory mediators are expressed as the medians ± 1 median absolute deviation (MAD). The MAD is the median of the differences between each result and the median of the data (StatView, Abacus Concepts, Inc., Berkeley, Calif.). Survival times and mast cell granule counts were analyzed by using nonparametric Mann-Whitney U analysis with a P value of <0.05 considered statistically significant for all analyses. Levels of inflammatory mediators were compared by Mann-Whitney U analysis with Bonferroni's correction for multiple analyses.
Bacteriology results are expressed as geometric means ± 1 standard error of the mean (SEM). Comparisons of bacterial load data from time course experiments were compared using one-way analysis of variance with Scheffe's post hoc test. Where samples contained fewer CFU per milliliter than the lower detection limit for the viable counting assay (log 1.92 per ml of blood or whole-lung tissue and log 1.22 per BALB/c lung airway), they were ascribed a value just below the detection limit (log 1.91 or 1.21). Comparisons of bacterial loads between mouse strains were carried out using multiple unpaired Student t tests with Bonferroni's correction.
Statistical analyses were carried out using StatView 4.1 (Abacus Concepts).
RESULTS
CBA/Ca mice are more susceptible to pneumococcal pneumonia than are BALB/c mice and display sustained hypothermia during infection.
Following intranasal infection, all CBA/Ca mice developed piloerection and lethargy to reach the predetermined endpoint (51) around 36 h into the experiment (median survival time, 36 h). By comparison, in all of these studies only 24 of 166 BALB/c mice displayed any symptoms of infection (median survival time, 336 h, P < 0.01, longer than that for CBA/Ca mice).
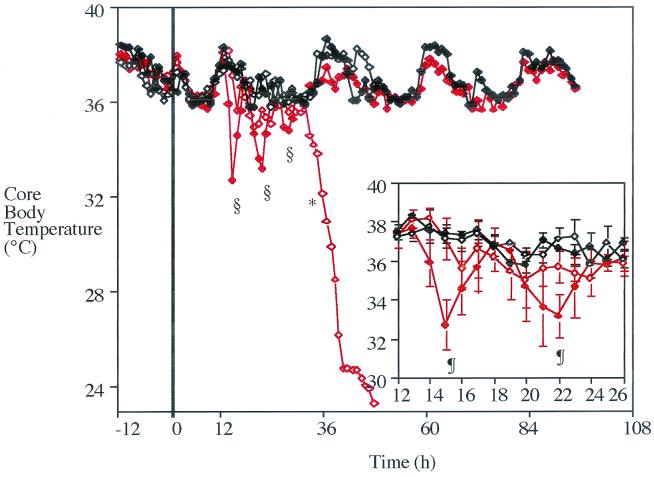
No significant differences were found in CBA/Ca core body temperatures until 36 h postchallenge (Fig. 1), when infected CBA/Ca mice displayed lower temperatures than sham-infected CBA/Ca mice and both groups of BALB/c animals (P < 0.01, lower for infected CBA/Ca mice). By the end of the experiment, the temperature of infected CBA/Ca mice had dropped to 24°C.
FIG. 1.
Median core body temperatures of BALB/c (⧫) and CBA/Ca (◊) mice from 12 h prior to infection to 96 h following intranasal administration of 106 CFU of S. pneumoniae (red diamonds [open and closed]) or PBS (⧫ and ◊). The thick vertical line represents the time of administration in each group. n, 4 to 8 mice; ∗, P < 0.01 (lower for infected CBA/Ca mice than for CBA/Ca mice administered PBS or infected BALB/c mice [from this time point until the end of infection]); §, P < 0.05 (lower for infected BALB/c mice than for BALB/c mice administered PBS). Insert represents median ± MAD for the period from 12 to 26 h in greater detail. ¶, P < 0.05 (lower for infected BALB/c mice than for infected CBA/Ca mice). No results are given for CBA/Ca mice after 48 h postchallenge as there were no survivors in this group past this time.
From 14 to 30 h postchallenge, resistant BALB/c mice displayed three transient periods of hypothermia, lasting approximately 4 h each. During these times, core body temperatures dropped between 4 and 5°C, significantly lower than that of BALB/c mice administered PBS (P < 0.05). During the first two periods of hypothermia, the core body temperatures of BALB/c mice also fell significantly below that of infected CBA/Ca mice at the same time postchallenge (P < 0.05).
Although CBA/Ca mice displayed symptoms of infection as early as 24 h postchallenge, their activity did not alter significantly until the end of the experiment (data not shown). The decline in movement corresponded to the drop in core body temperature. The activity of resistant BALB/c mice was not altered during the experiment (even during periods of hypothermia).
CBA/Ca mice are unable to control pneumococcal viability within the lungs and bloodstream.
Although there were significant differences between bacterial loads in BALB/c and CBA/Ca lungs during the initial 12 h of infection, these are difficult to interpret (Fig. 2A).
FIG. 2.
(A) Mean ± SEM bacterial loads within lavage fluid from BALB/c (⧫) and CBA/Ca (○) mice following intranasal infection with 106 CFU of S. pneumoniae. The SEM for CBA/Ca mice is within the area of symbol. n, 9 to 11 mice; ∗, P < 0.01; and §, P < 0.05 (different for BALB/c samples than for CBA/Ca samples). The thick horizontal line represents the detection limit of the viable count assay in BALB/c mice. No results are given for CBA/Ca mice after 36 h postchallenge as a full data set was not obtained past this time. (B) Mean ± SEM bacterial loads within lung tissue from BALB/c (⧫) and CBA/Ca (○) mice following intranasal infection with 106 CFU of S. pneumoniae. The SEM for CBA/Ca mice is within the area of symbol. n, 5 mice; ∗, P < 0.01; and §, P < 0.05 (different for BALB/c samples than for CBA/Ca samples). The thick horizontal line represents the detection limit of the viable count assay in BALB/c mice. No results are given for CBA/Ca mice after 36 h postchallenge as a full data set was not obtained past this time.
The overall trend was that pneumococcal viability within BALF increased between 0 and 6 h in both strains of mice. This was followed by a decline in bacterial loads. This decline was more rapid in BALB/c lungs than in CBA/Ca lungs. By 36 h postchallenge, bacterial loads were significantly lower than they were at 0 h in both strains of mice (P < 0.01). In BALB/c mice the decline in BALF-associated bacterial loads continued until no viable pneumococci were recovered in the airways.
The number of viable pneumococci recovered within CBA/Ca lung homogenates increased immediately following infection (Fig. 2B). This increase continued steadily throughout the experiment with levels significantly elevated above those at 0 h from 12 h postchallenge onwards (P < 0.01). During the infection the number of pneumococci in BALB/c lung homogenates did not alter significantly from the counts at 0 h.
Fifty percent of the CBA/Ca mice developed bacteremia by 6 h post-intranasal challenge. Once in the bloodstream, bacterial numbers increased until more than 107 CFU were recovered per milliliter of blood at 36 h postchallenge (results not shown). Bacteremia was only detected in 4 of 110 BALB/c mice during the experiment, with levels consistently below 104 CFU/ml. After 96 h postinfection, no BALB/c mice displayed bacteremia. These results are in keeping with previously published data (16).
CBA/Ca mice have a reduced inflammatory response within lung airways.
To determine whether a difference in the immune response could account for the inability of CBA/Ca mice to control the pneumococci, we measured the production of inflammatory mediators during the infection.
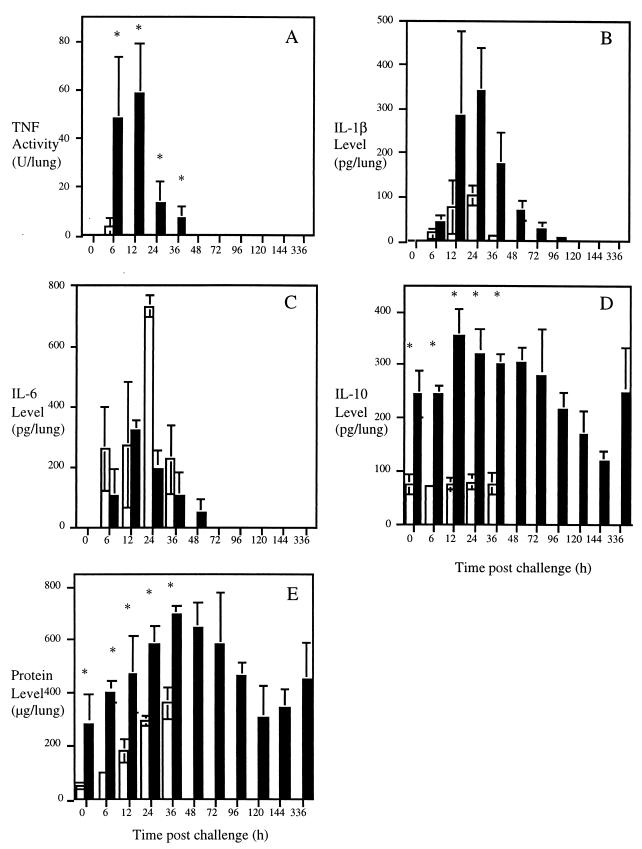
TNF activity increased in both BALB/c and CBA/Ca mice by 6 h postchallenge (Fig. 3A) (P < 0.05 for CBA/Ca and P < 0.01 for BALB/c when compared at 0 h). At this time significantly more TNF activity was associated with BALB/c samples than with CBA/Ca lavage fluid (BALB/c mice, 48 U of TNF/lung; CBA/Ca mice, 4 U of TNF/lung; P < 0.01). No TNF activity was detectable in all CBA/Ca BALF after 24 h and in BALB/c BALF from 72 h onwards.
FIG. 3.
Median ± MAD mediator levels in cell-free BALF from BALB/c (filled bars) and CBA/Ca (white bars) mice infected with 106 CFU of S. pneumoniae. (A) TNF activity; (B) IL-1β level; (C) IL-6 level; (D) IL-10 level; (E) total protein level. n, 4 to 10 mice; ∗, P < 0.01 (different for BALB/c mice than for CBA/Ca mice). No results are given for CBA/Ca mice after 36 h postchallenge as a full data set was not obtained past this time.
Transient production of IL-1β was detected in BALB/c and CBA/Ca lung airways (Fig. 3B). More IL-1β was recovered in BALB/c lavage fluid than in CBA/Ca lavage fluid throughout the experiment, although high levels of variation prevented statistical significance.
Similar concentrations of IL-6 were detected in BALF from both strains throughout the time course with no significant difference at any time point (Fig. 3C). Significant IL-6 concentrations remained associated with the pulmonary airways of CBA/Ca mice at their times of death (P < 0.01, compared to those at 0 h). In resistant BALB/c mice IL-6 production was undetectable by 96 h postchallenge.
High concentrations of IL-10 were detected in BALB/c samples throughout the infection (Fig. 3D). Concentrations were increased by 24 h following infection, remaining elevated until 96 h. This was followed by a reduction in IL-10 below the baseline from 120 to 144 h, with the level returning to the baseline by 336 h postchallenge. At all times investigated, the levels of IL-10 recovered in CBA/Ca BALF were significantly lower than those in BALB/c samples (P < 0.01). The level of IL-10 detected in CBA/Ca samples was not altered significantly during the experiment.
Total protein analysis of BALF monitored the integrity of the alveolar-capillary barrier during the infection (Fig. 3E). Higher protein levels were present within the airways of uninfected BALB/c mice than in those of CBA/Ca mice (P < 0.01). In CBA/Ca animals, the level increased more than sevenfold from ∼50 μg/lung at the time of infection to more than 360 μg/lung at 36 h (P < 0.01). Protein levels increased in BALB/c mice from 6 to 96 h postchallenge, following which they returned to the same levels as at 0 h.
CBA/Ca mice have an elevated inflammatory response within lung tissue.
Lung homogenate TNF activity profiles peaked at 6 h in BALB/c mice (Fig. 4A). Levels then decreased so that from 36 h postchallenge onwards TNF activity was undetectable in BALB/c lung tissues. In CBA/Ca lung homogenates there was a delayed TNF activity peak at 24 h (P < 0.01, compared to that at 0 h) with an activity level of greater than 160 U per lung.
FIG. 4.
Median ± MAD cytokine levels in lung homogenates from BALB/c (filled bars) and CBA/Ca (white bars) mice infected with 106 CFU of S. pneumoniae. (A) TNF activity; (B) IL-1β level; (C) IL-6 level; (D) IL-10 level. n, 4 to 10 mice; ∗, P < 0.01; §, P < 0.05 (different for BALB/c mice than for CBA/Ca mice). No results are given for CBA/Ca mice after 36 h postchallenge as a full data set was not obtained past this time.
The increased production of IL-1β within lung tissue during the infection was similar in both strains (Fig. 4B). Transient production peaked at around 12 h after infection and declined until the end of the experiment but with high concentrations still present in CBA/Ca lung homogenates immediately prior to death.
IL-6 levels increased transiently 24 h into the infection in BALB/c lung tissue (Fig. 4C) (P < 0.05). There was a significant reduction in concentrations from the baseline at 120 and 144 h postchallenge (P < 0.05, compared to those at 0 h) with these levels returning to the baseline by 336 h. From 12 h postchallenge onwards, CBA/Ca IL-6 lung homogenate levels were significantly higher than the baseline (P < 0.05) and higher than those in BALB/c samples (P < 0.05).
BALB/c mice produced higher amounts of IL-10 in their lung tissue than did CBA/Ca mice at all times investigated (Fig. 4D) (P < 0.01). By 12 h postchallenge, this level was significantly elevated when compared to that originally found (P < 0.05) and it remained higher until 144 h. By 336 h, the level was again higher than the baseline (P < 0.01). IL-10 concentrations within CBA/Ca lung homogenates were increased significantly at 12 and 24 h into the experiment (P < 0.05), but by 36 h, levels were no longer significantly different from the baseline.
Detection of systemic TNF activity.
TNF activity was not detected within the serum of BALB/c mice at any point during the experiment (the detection limit of the assay was around 38 U/ml for serum samples). At 24 and 36 h postchallenge, 50% of CBA/Ca mice displayed TNF activity, with positive samples containing between 38 and 440 U/ml (data not shown).
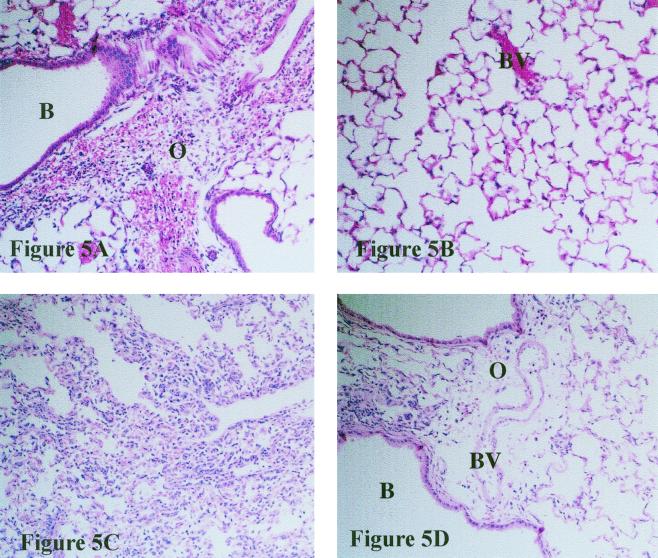
Pulmonary histopathology in CBA/Ca mice is located in perivascular areas.
Morphological examination of lungs from infected CBA/Ca mice revealed progressive edema of the organs accompanied by marked cell influx from 24 h postchallenge. This disruption was initially perivascular in regions neighboring infected bronchioles (Fig. 5A) (similar to previous results [16]), but in sections sampled later in the infection, large areas of the lung surface displayed signs of alteration. The airways of these mice did not display marked signs of inflammation 24 h postchallenge (Fig. 5B). By 6 h into the experiment, BALB/c lungs showed marked hemorrhage and edema with a slight cell influx in these same areas. Lungs sampled at 12 h contained more recruited cells within the airways. By 24 h postchallenge, large numbers of inflammatory cells were present within the airways, causing significant disruption to alveolar integrity (Fig. 5C), while perivascular areas remained less consolidated (Fig. 5D).
FIG. 5.
Histological sections from CBA/Ca (A and B) and BALB/c (C and D) mice 24 h postchallenge with 106 CFU of S. pneumoniae (magnification, ×200) stained with hematoxylin and eosin. B, bronchiole; BV, blood vessel; O, edema. Recruited inflammatory cells and edema are evident in a perivascular region of a CBA/Ca mouse in panel A while the airways of this mouse remain largely unaffected (panel B). High numbers of inflammatory cells can be seen within the alveoli of a BALB/c mouse in panel C. Less disruption is evident in perivascular areas (panel D).
Lungs of CBA/Ca mice contain fewer mast cells immediately following intranasal infection.
As mast cells store preformed TNF, we investigated whether BALB/c lungs contained more mast cells than did CBA/Ca lungs, helping to explain the rapidly elevated TNF levels.
Directly following infection with S. pneumoniae, resistant BALB/c mice had significantly more mast cell granules within their lung tissue than did CBA/Ca mice (Table 1) (P < 0.01). At this time the granules were generally found surrounding blood vessels. The number of mast cell granules decreased in BALB/c lung sections following infection (indicating release of granule contents from cells) and were significantly reduced by 12 h (P < 0.01). This reduction was maintained until after 36 h postchallenge as levels at 48, 96, and 120 h were not significantly different from the granule numbers detected at 0 h (data not shown).
TABLE 1.
Number of mast cells with positive staining granules per field of vision (magnification, ×200) in lung sections from BALB/c and CBA/Ca mice following intranasal infection with 106 CFU of S. pneumoniaea
| Mouse strain | No. of cells (median ± MAD) at time postchallenge (h):
|
||||
|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | 36 | |
| BALB/c | 13.0 ± 6.00b | 5.00 ± 1.00 | 2.00 ± 1.00 | 2.00 ± 1.00 | 4.00 ± 1.00 |
| CBA/Ca | 1.00 ± 1.00 | 6.00 ± 3.00 | 4.00 ± 1.00 | 2.00 ± 1.00 | 5.00 ± 1.00 |
n = 5 fields of vision per time point.
P < 0.01 for BALB/c lung sections compared to CBA/Ca lung sections at the same time (Mann-Whitney U test).
The number of mast cell granules detected within CBA/Ca lungs increased during early infection. By 6 h postchallenge, a significant increase above the numbers detected at 0 h had occurred (P < 0.01). Levels were also significantly elevated at 12 and 36 h postinfection (P < 0.05, higher than those at 0 h).
CBA/Ca airway cells release less TNF upon in vitro stimulation.
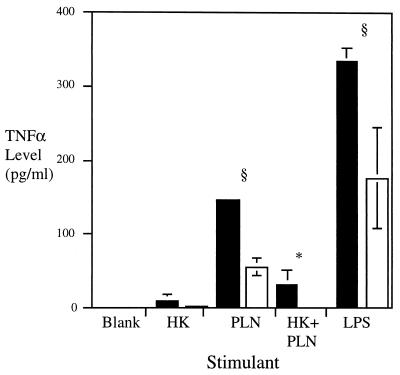
It was possible that CBA/Ca BALF contained less TNF as a result of a reduced capacity for airway cells to respond to stimuli. We investigated this hypothesis in vitro (Fig. 6). A stimulus of 106 CFU of heat-killed pneumococci induced TNF-α release by cells from both BALB/c and CBA/Ca mice (9 and 2 pg/ml, respectively). Stimulation with 104 and 105 CFU of heat-killed pneumococci did not result in detectable levels of TNF-α.
FIG. 6.
Median ± MAD TNF-α release from cells recovered from either BALB/c (filled bars) or CBA/Ca (white bars) airways and stimulated with various preparations. Blank, media alone; HK, 106 CFU of heat-killed pneumococci; PLN, 20 ng of pneumolysin; HK+PLN, 106 CFU of heat-killed pneumococci plus 0.2 ng of pneumolysin; LPS, 1 ng of LPS. n, 3 to 12 mice; ∗, P < 0.01; §, P < 0.05 (higher for BALB/c levels than for CBA/Ca levels).
Twenty nanograms of pneumolysin induced detectable TNF-α protein, with BALB/c cells releasing significantly more TNF-α (146 pg/ml) than CBA/Ca cells (55 pg/ml) (P < 0.05). Lower levels of pneumolysin (0.6 pg, 2 pg, and 0.2 ng) did not induce significant TNF-α levels.
The combination of 106 CFU of heat-killed bacteria and 0.2 ng of pneumolysin released significantly higher concentrations of TNF-α from BALB/c cells than from CBA/Ca cells (32 pg/ml compared to 0 pg/ml) (P < 0.01). These levels were higher than either stimulant used alone at these concentrations, suggesting that heat-killed bacteria and pneumolysin act in synergy to induce TNF-α production.
TNF-α was also produced in vitro when airway cells were stimulated with LPS (to investigate whether the above differences were also evident with gram-negative stimuli). One nanogram of LPS induced significant amounts of TNF-α in both cell populations. Again, airway cells from BALB/c mice released higher amounts of TNF-α than did CBA/Ca cells (P < 0.05).
TNF-α neutralization increases bacterial loads and decreases survival times.
TNF levels were modulated to confirm that TNF production is beneficial in our model of pneumococcal pneumonia. We used p55- and p75-deficient mice to evaluate the role of TNF signaling in inflammation. Mice deficient in p55 were significantly more susceptible to intranasal infection with S. pneumoniae (median time to reach experiment endpoint, 63 h; P < 0.01) than were C57BL/6 controls (all C57BL/6 mice survived infection). Mice deficient in p75 showed no significant differences from C57BL/6 mice (data not shown).
Twenty-four hours postchallenge, p55-deficient mice displayed significantly higher bacterial loads within the lung airways (P < 0.05) (Table 2), lung tissues (P < 0.05), and bloodstream (P < 0.01) than did wild-type C57BL/6 mice.
TABLE 2.
Bacterial loads in TNF p55−/− and C57BL/6 mice 24 h post-intranasal infection with 106 CFU of S. pneumoniae (n = 5 to 7 mice)
| Mouse strain | Bacterial load (log CFU/ml) (mean ± SEM) in:
|
||
|---|---|---|---|
| Lung airways | Lung tissue | Blood | |
| p55 knockout | 4.79 ± 0.20a | 4.76 ± 0.40a | 5.03 ± 0.94b |
| C57BL/6 | 3.72 ± 0.34 | 3.34 ± 0.32 | 0.65 ± 0.65 |
P < 0.05 (higher for p55−/− mice than for C57BL/6 mice).
P < 0.01.
The effects of pulmonary TNF activity were investigated via antibody neutralization. Local TNF-α neutralization with polyclonal anti-TNF antibody significantly increased bacterial loads within the BALF (P < 0.01) (Table 3) and lung tissue (P < 0.01) of MF1 mice 24 h following intranasal infection with 106 CFU of S. pneumoniae. This treatment had no effect on bacteremia.
TABLE 3.
Bacterial loads in MF1 mice 24 h post-intranasal infection with 106 CFU of S. pneumoniae following pulmonary TNF-α neutralization (n = 5 mice)
| Treatment | Bacterial load (log CFU/ml) (mean ± SEM) in:
|
||
|---|---|---|---|
| Lung airways | Lung tissue | Bloodstream | |
| Goat IgG | 4.42 ± 0.19 | 5.04 ± 0.27 | 6.02 ± 0.37 |
| anti-TNF-α | 5.74 ± 0.03a | 6.03 ± 0.23a | 6.62 ± 0.45 |
P < 0.01 (higher for anti-TNF treatment than for goat IgG treatment).
CBA/Ca mice are more susceptible to systemic infection with S. pneumoniae.
In order to determine whether the resistance of BALB/c mice in comparison to CBA/Ca mice was due to pulmonary defenses alone, intravenous infections were initiated with 2.4 × 103 or 5.0 × 104 CFU of S. pneumoniae.
At 24 h postchallenge, CBA/Ca mice infected with either dose displayed slight piloerection. They then passed through a similar set of symptoms as with the pulmonary challenge. All infected CBA/Ca mice were deemed to have irreversibly succumbed to the infection by 33 h postchallenge, at which point they were humanely sacrificed (median time to reach experiment endpoint, 32.5 h for 2.4 × 103 CFU of infectious dose and 27 h for 5.0 × 104 CFU of infectious dose). All BALB/c mice survived until the endpoint of infection and thus were significantly more resistant to systemic pneumococcal infection than were CBA/Ca mice (P < 0.01 for each dose). There was no difference between symptoms displayed after infection with 2.4 × 103 or 5.0 × 104 CFU of S. pneumoniae. BALB/c mice displayed mild clinical signs in comparison to CBA/Ca mice, with all mice displaying normal behavior and appearance by 49 h.
By 12 h following intravenous infection, the number of bacteria within CBA/Ca bloodstream had increased significantly (P < 0.01) while BALB/c mice displayed a slight reduction in bacterial numbers. This decrease was more marked by 24 h into the experiment when 4 of 5 BALB/c mice no longer had detectable bacteremia. In CBA/Ca mice, pneumococcal proliferation occurred unchecked until the end of the experiment when around 108 CFU were present per milliliter of blood.
DISCUSSION
Utilization of a telemetry system revealed the development of hypothermia upon onset of clinical signs of infection with S. pneumoniae. Hypothermia has been recognized previously in models of pneumococcal pneumonia (29) and may be beneficial to the host as pneumococci cannot multiply as quickly at hypothermic temperatures as they can at 37°C, both in vivo and in vitro (13, 14). Additionally, the immune response may be affected as low temperatures down-regulate cytokine levels (21) and reduce the phagocytic index (13, 14). Thus, transient hypothermia in BALB/c mice might regulate the inflammation before it becomes detrimental. The delayed onset of this response in CBA/Ca mice may allow overwhelming inflammation to occur before it can be controlled.
Although we could not detect bacteremia or systemic cytokines in BALB/c mice, the telemetry system revealed a systemic response to infection (hypothermia). The initiation of hypothermia in BALB/c and CBA/Ca mice occurred rapidly after peak pulmonary inflammation. One or more of the inflammatory mediators may thus play a role in the temperature alterations displayed by infected mice. Injection of high levels of TNF-α into mice induces hypothermia while lower levels of TNF-α induce febrile responses (26). IL-6 can also induce febrile responses (30). Cytokines expressed within the lungs might alter core body temperature indirectly by induction of phospholipase A2 (44). This in turn stimulates prostaglandin release and temperature change.
Counting pneumococcal levels within the lung airways and tissue following intranasal infection with S. pneumoniae (16) expanded previous investigations of bacterial loads within the whole lungs. From 12 h onwards, pneumococcal viability declined in the airways of both BALB/c and CBA/Ca mice (Fig. 2A). It is probable that the reduction in bacterial viability in BALB/c mice is a reflection of pneumococcal kill as homogenate-associated counts also dropped. The reduction in CBA/Ca BALF counts suggests greater association of the bacteria with lung tissues as homogenate counts increased during this time (Fig. 2B).
High numbers of pneumococci were recovered from the blood of infected CBA/Ca mice towards the end of the infection. By perfusing the blood from lungs prior to homogenization, it was previously confirmed that these high levels of bacteremia do not contribute to lung homogenate bacterial loads (25).
The reduction in bacterial loads within the lung airways and tissues of BALB/c mice correlated with elevated TNF activity levels (Fig. 2A and 3). At peak production, airways of BALB/c mice contained 12-fold-higher levels of this inflammatory cytokine than did CBA/Ca airways.
TNF may therefore contribute to protection from pneumococcal pneumonia. Significantly elevated production of TNF within the airways of BALB/c mice during Pseudomonas aeruginosa infection, when compared to susceptible C57BL/6 mice, has been suggested to play a role in resistance during that infection (19, 46). Elevated TNF levels in BALB/c airways may play a role in chemotaxis of phagocytic cells, which arrive earlier and in greater numbers than in CBA/Ca mice due to the ability of TNF to up-regulate VCAM-1 and E-selectin in lung tissue as soon as 4 h after stimulation (39). TNF will also activate these cells so that they are primed for antimicrobial activity upon arrival at the site of infection (12, 27).
TNF expression has also been implicated in susceptibility to human infections. A G-to-A polymorphism at position −308 in the promoter region of the TNF-α gene results in higher systemic TNF expression, a sevenfold increase in death or severe neurological sequelae from cerebral malaria (33), and a sevenfold increase in susceptibility to mucocutaneous leishmaniasis (9). Therefore, in contrast to elevated local TNF playing a protective role during infections, elevated systemic TNF is likely to be detrimental.
Our findings with TNF-α p55−/− mice reveal that a TNF response is necessary to control bacterial loads within the lungs and bloodstream during pneumococcal pneumonia. This is the first investigation of the role of the p55 receptor in the lungs during pneumococcal pneumonia. Our results in the pneumonia model are in agreement with the heightened susceptibility of p55−/− mice during systemic pneumococcal infection (40). Increased susceptibility and bacterial loads have also been found in mice following systemic neutralization of TNF during pneumococcal pneumonia (50, 52).
To confirm the importance of pulmonary TNF during pneumococcal pneumonia, we neutralized TNF activity within lung airways (using our well-characterized MF1 model [2, 23, 25]). We used the MF1 model because lower TNF levels than those in BALB/c mice allowed TNF neutralization. Neutralization of pulmonary TNF resulted in significantly higher lung-associated bacterial loads. Thus TNF is involved in the protective pulmonary response during pneumococcal pneumonia.
The differences in TNF production in BALB/c and CBA/Ca mice could be explained in several ways. TNF-α has two active forms, one is a surface-bound 26-kDa protein and the other is a 17-kDa secreted protein released from cell surfaces by cleavage of the 26-kDa form. This cleavage is regulated by TNF-α-converting enzyme (4, 36). A disruption to the activities of this enzyme in CBA/Ca mice would result in lower levels of secreted TNF-α, leading to impaired host defenses within the airways, while levels in lung tissue would be elevated (Fig. 3A and 4A).
Also, although low numbers of pneumococci left the airways in BALB/c mice, the organisms readily gained access to the lung tissues of CBA/Ca mice. Thus the inflammatory stimuli were in different locations in the mouse strains. Compartmentalized cytokine production occurs in human cases of pneumonia (11) and would explain why the highest levels of TNF were detected in BALB/c BALF and in CBA/Ca lung tissue.
Differences in the mast cell population in lung airways in BALB/c and CBA/Ca mice might also result in different TNF activities. Significantly higher numbers of mast cell granules were found in the lungs of BALB/c mice than in those of CBA/Ca mice immediately following infection (Table 1). Mast cells are primed for the release of granule contents within hours of infection (17), perhaps explaining the rapid nature of TNF response in BALB/c mice. A longer period of time was required for CBA/Ca mast cells to be recruited or to synthesize granule contents (including TNF). This recruitment or synthesis continued throughout the experiment (as granule numbers did not decrease significantly once elevated). Mast cell release of TNF has previously been implicated in host resistance to infectious disease (32). We are currently investigating the role of mast cells in initiating inflammation in pneumococcal pneumonia.
CBA/Ca airway cells may also have a reduced capacity to respond to stimuli by producing TNF than do BALB/c mice. With each stimulant tested in vitro, higher levels of TNF were released from BALB/c cells than from CBA/Ca cells (Fig. 6).
Lung tissues from CBA/Ca mice also contained significantly more IL-6 than did samples from BALB/c mice, in agreement with the spread of pneumococci into lung tissues and the more widespread inflammation in CBA/Ca mice (11). Previous studies have highlighted the involvement of high levels of IL-6 in the lethality of pneumococcal infection (56). Levels of IL-6 detected in CBA/Ca mice immediately prior to the death of the animals remained significantly higher than the baseline (Fig. 4C), consistent with the sustained expression of high levels of IL-6 in CBA/Ca mice being involved in the pathogenesis of the disease rather than protection.
Disruption to lung integrity (as revealed by histology) (Fig. 5) was quickly followed by the recruitment of phagocytic cells. The cell influx to infected BALB/c lungs was mainly contained in the lung airways while that in CBA/Ca lungs involved large areas of the lungs (correlating with sites of maximum TNF activity and bacterial loads) (Fig. 2, 3, and 4).
Our previous observations revealed that BALB/c mice had larger numbers of neutrophils within whole-lung homogenates than did CBA/Ca mice at both 12 and 24 h postchallenge with 106 CFU of D39 (16). Maximal neutrophil recruitment occurred between 6 and 12 h postinfection. Such timings indicate that neutrophils could be responsible for the initial, rapid pneumococcal kill evident within BALB/c airways and tissues.
As individual mice were sacrificed at each time point following intranasal infection, it was impossible to determine whether any BALB/c mice that displayed bacteremia would progress to develop the disease or survive. However, the ability of BALB/c mice to resist intravenous infection with 5.0 × 104 CFU (approximately the highest recorded level of bacteremia in a BALB/c mouse following intranasal infection with S. pneumoniae) indicates a lower level of bacteremia would not be fatal for BALB/c mice. Briles et al. have previously shown that the 50% lethal dose for intravenous infection of BALB/c mice with D39 was greater than 106 CFU (7). Therefore, with low numbers of type 2 pneumococci administered intravenously, BALB/c mice can control the infection and survive, but with larger inoculations or when bacteria are constantly shed from the lungs into the bloodstream, the defense mechanisms are overcome and the infection becomes fatal. Thus, the resistant status of BALB/c mice to intranasal infection with 106 CFU of D39 is likely to be due to both elevated pulmonary immunity and systemic defenses. TNF may be involved in both, with anti-TNF-α treatment resulting in higher pulmonary bacterial loads (Table 3) and p55-deficient mice developing greater bacteremia during pneumococcal infection (Table 2). As mentioned above, these experiments were done on different genetic backgrounds for logistical reasons.
Our results indicate that the susceptibility of CBA/Ca mice to pneumococcal pneumonia may be due to reduced expression of TNF in lung airways during early infection. This leads to reduced neutrophil influx, permitting pneumococcal numbers to increase within the lung tissue, and to the initiation of systemic disease.
Acknowledgments
A.R.K. was the recipient of an MRC industrial collaborative studentship with AstraZeneca (formerly Zeneca). Research in Glasgow, United Kingdom, is funded by the Wellcome Trust and the National Meningitis Trust. AstraZeneca and the Wellcome Trust support research in Leicester, United Kingdom.
Thanks to A. M. Mowat, Department of Immunology and Bacteriology, University of Glasgow, for supplying the TNF-α p55 receptor and p75 receptor knockout mice. Thanks to C. E. Lawrence, Department of Immunology, Strathclyde University, for the mast cell staining.
REFERENCES
- 1.Aggarwal, B. B., and W. J. Kohr. 1985. Human tumor necrosis factor. Methods Enzymol. 116:448-456. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, J. E., R. A. Lock, C. C. A. M. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansmann, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashman, R. B., and J. M. Papadimitriou. 1992. Genetic resistance to Candida albicans infection is conferred by cells derived from the bone marrow. J. Infect. Dis. 166:947-948. [DOI] [PubMed] [Google Scholar]
- 4.Black, R. A., C. T. Rauch, C. J. Kozlosky, J. J. Peschon, J. L. Slack, M. F. Wolfson, B. J. Castner, K. L. Stocking, P. Reddy, S. Srinivasan, N. Nelson, N. Boiani, K. A. Schooley, M. Gerhart, R. Davis, J. N. Fitzner, R. S. Johnson, R. J. Paxton, C. J. March, and D. P. Cerretti. 1997. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385:729-733. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan, C., Y. Vodovotz, and C. Nathan. 1991. Macrophage deactivation by interleukin 10. J. Exp. Med. 174:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borish, L., R. Rosenbaum, L. Albury, and S. Clark. 1989. Activation of neutrophils by recombinant interleukin 6. Cell. Immunol. 121:280-289. [DOI] [PubMed] [Google Scholar]
- 7.Briles, D. E., J. Horowitz, L. S. McDaniel, W. H. Benjamin, Jr., J. L. Claflin, C. L. Booker, G. Scott, and C. Forman. 1986. Genetic control of the susceptibility to pneumococcal infection. Curr. Top. Microbiol. Immunol. 124:103-120. [DOI] [PubMed] [Google Scholar]
- 8.Briles, D. E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J. Exp. Med. 153:694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabrera, M., M.-A. Shaw, C. Sharples, H. Williams, M. Castes, J. Convit, and J. M. Blackwell. 1995. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J. Exp. Med. 182:1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, W., E. A. Havell, and A. G. Harmsen. 1992. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect. Immun. 60:1279-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehoux, M. S., A. Boutten, J. Ostinelli, N. Seta, M. C. Dombret, B. Crestani, M. Deschenes, J. L. Trouillet, and M. Aubier. 1994. Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am. J. Respir. Crit. Care Med. 150:710-716. [DOI] [PubMed] [Google Scholar]
- 12.Dusi, S., V. D. Bianca, M. Donini, K. A. Nadalini, and F. Rossi. 1996. Mechanisms of stimulation of the respiratory burst by TNF in nonadherent neutrophils: its independance of lipidic transmembrane signalling and dependance on protein tyrosine phosphorylation and cytoskeleton. J. Immunol. 157:4615-4623. [PubMed] [Google Scholar]
- 13.Eiseman, B., W. G. Malette, R. S. Wotkyns, W. B. Summers, and J. L. Tong. 1956. Prolonged hypothermia in experimental pneumococcal peritonitis. J. Clin. Investig. 35:940-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eiseman, B., R. S. Wotkyns, and H. Hirose. 1964. Hypothermia and infection: three mechanisms of host protection in type III pneumococcal peritonitis. Ann. Surg. 160:994-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueroa, J. E., and P. Densen. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4:359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gingles, N. A., J. E. Alexander, A. Kadioglu, P. W. Andrew, A. Kerr, T. J. Mitchell, E. Hopes, P. Denny, S. Brown, H. B. Jones, S. Little, G. C. Booth, and W. L. McPheat. 2001. The role of genetic resistance in invasive pneumococcal infection: identification and study of susceptibility and resistance in inbred mouse strains. Infect. Immun. 69:426-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon, J. R., and S. J. Galli. 1990. Mast cells as a source of both preformed and immunologically inducible TNFα/cachectin. Nature 346:274-276. [DOI] [PubMed] [Google Scholar]
- 18.Gordon, S. B., G. R. B. Irving, R. A. Lawson, M. E. Lee, and R. C. Read. 2000. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect. Immun. 68:2286-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosselin, D., J. DeSanctis, M. Boule, E. Skamene, C. Matouk, and D. Radzioch. 1995. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect. Immun. 63:3272-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberger, M. J., R. M. Strieter, S. L. Kunkel, J. M. Danforth, L. L. Laichalk, D. C. McGillicuddy, and T. J. Standiford. 1996. Neutralisation of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J. Infect. Dis. 173:159-165. [DOI] [PubMed] [Google Scholar]
- 21.Hanson, D. F. 1997. Fever, temperature, and the immune response. Ann. N. Y. Acad. Sci. 813:453-464. [DOI] [PubMed] [Google Scholar]
- 22.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect. Immun. 62:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadioglu, A., N. A. Gingles, K. Grattan, A. Kerr, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasama, T., R. M. Strieter, N. W. Lukacs, M. D. Burdick, and S. L. Kunkel. 1994. Regulation of neutrophil-derived chemokine expression by IL-10. J. Immunol. 152:3559-3569. [PubMed] [Google Scholar]
- 25.Kerr, A. R. 2000. Ph.D. dissertation. The University of Glasgow, Glasgow, United Kingdom.
- 26.Kettlehut, A. C., W. Fiers, and A. L. Goldberg. 1987. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc. Natl. Acad. Sci. USA 84:4273-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klebanoff, S. J., M. A. Vadas, J. M. Harlan, L. H. Sparks, J. R. Gamble, J. M. Agosti, and A. M. Waltersdorph. 1986. Stimulation of neutrophils by tumour necrosis factor. J. Immunol. 136:4220-4225. [PubMed] [Google Scholar]
- 28.Laichalk, L. L., S. L. Kunkel, R. M. Strieter, J. M. Danforth, M. B. Bailie, and T. J. Standiford. 1996. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect. Immun. 64:5211-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson, W. P., R. N. Bieter, M. Levine, and W. F. McLimans. 1939. Temperature reactions in mice infected with pneumococci. Proc. Soc. Exp. Biol. Med. 42:649-651. [Google Scholar]
- 30.Leon, L. R., A. A. White, and M. J. Kluger. 1998. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am. J. Physiol. 275:R269-R277. [DOI] [PubMed] [Google Scholar]
- 31.MacLeod, C. M., R. G. Hodges, M. Heidelberger, and W. G. Bernhard. 1945. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J. Exp. Med. 82:445-465. [PMC free article] [PubMed] [Google Scholar]
- 32.Malaviya, R., T. Ikeda, E. Ross, and S. N. Abraham. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature 381:77-80. [DOI] [PubMed] [Google Scholar]
- 33.McGuire, W., A. V. S. Hill, C. E. M. Allsopp, B. M. Greenwood, and D. Kwiatkowski. 1994. Variation in the TNF-α promoter region associated with susceptibility to cerebral malaria. Nature 371:508-511. [DOI] [PubMed] [Google Scholar]
- 34.Ming, W. J., L. Bersani, and A. Mantovani. 1987. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J. Immunol. 138:1469-1474. [PubMed] [Google Scholar]
- 35.Mitchell, T. J., J. A. Walker, F. K. Saunders, P. W. Andrew, and G. J. Boulnois. 1988. Expression of the pneumolysin gene in Escherichia coli: rapid purification and biological properties. Biochim. Biophys. Acta 1007:67-72. [DOI] [PubMed] [Google Scholar]
- 36.Moss, M. L., S.-L. C. Jin, M. E. Milla, W. Burkhart, H. L. Carter, W.-J. Chen, W. C. Clay, J. R. Didsbury, D. Hassler, C. R. Hoffman, T. A. Kost, M. H. Lambert, M. A. Leesnitzer, P. McCauley, G. McGeehan, J. Mitchell, M. Moyer, G. Pahel, W. Rocque, L. K. Overton, F. Schoenen, T. Seaton, J.-L. Su, J. Warner, D. Willard, and J. D. Becherer. 1997. Cloning of a disintegrin metalloproteinase that processes precursor tumour necrosis factor-α. Nature 385:733-736. [DOI] [PubMed] [Google Scholar]
- 37.Mullen, P. G., A. C. J. Windsor, A. A. Fowler, and H. J. Sugerman. 1995. Tumor necrosis factor alpha and interleukin-6 selectively regulate neutrophil function in vitro. J. Surg. Res. 58:124-130. [DOI] [PubMed] [Google Scholar]
- 38.Nelson, S., and W. R. Summer. 1998. Innate immunity, cytokines and pulmonary host defense. Infect. Dis. Clin. N. Am. 12:555-567. [DOI] [PubMed] [Google Scholar]
- 39.Neumann, B., T. Machleidt, A. Lifka, K. Pfeffer, D. Vestweber, T. W. Mak, B. Holzmann, and M. Krönke. 1996. Crucial role of 55-kilodalton TNF receptor in TNF-induced adhesion molecule expression and leukocyte organ infiltration. J. Immunol. 156:1587-1593. [PubMed] [Google Scholar]
- 40.O'Brien, D. P., D. E. Briles, A. J. Szalai, A.-H. Tu, I. Sanz, and M. H. Nahm. 1999. Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infect. Immun. 67:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okusawa, S., J. A. Gelfand, T. Ikejima, R. J. Connolly, and C. A. Dinarello. 1988. Interleukin-1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J. Clin. Investig. 81:1162-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plant, J. E., J. M. Blackwell, A. D. O'Brien, D. J. Bradley, and A. A. Glynn. 1982. Are the Lsh and Ity disease resistance genes at one locus on mouse chromosome 1? Nature 297:510-511. [DOI] [PubMed] [Google Scholar]
- 43.Riesenfeld-Orn, I., S. Wolpe, J. F. Garcia-Bustos, M. K. Hoffmann, and E. Tuomanen. 1989. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect. Immun. 57:1890-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rintala, E. M., and T. J. Nevalainen. 1993. Group II phospholipase-A(2) in sera of febrile patients with microbiologically or clinically documented infections. Clin. Infect. Dis. 17:864-870. [DOI] [PubMed] [Google Scholar]
- 45.Rogers, H. W., C. S. Tripp, R. D. Schreiber, and E. R. Unanue. 1994. Endogenous IL-1 is required for neutrophil recruitment and macrophage activation during murine listeriosis. J. Immunol. 153:2093-2101. [PubMed] [Google Scholar]
- 46.Sapru, K., P. K. Stotland, and M. M. Stevenson. 1999. Quantitative and qualitative differences in bronchoalveolar inflammatory cells in Pseudomonas aeruginosa-resistant and -susceptible mice. Clin. Exp. Immunol. 115:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson, S. Q., R. Singh, and D. E. Bice. 1994. Heat-killed pneumococci and pneumococcal capsular polysaccharides stimulate tumor necrosis factor-alpha production by murine macrophages. Am. J. Respir. Cell Mol. Biol. 10:284-289. [DOI] [PubMed] [Google Scholar]
- 48.Skamene, E., P. A. L. Kongshavn, and D. H. Sachs. 1979. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J. Infect. Dis. 139:228-231. [DOI] [PubMed] [Google Scholar]
- 49.Standiford, T. J., S. L. Kunkel, M. J. Greenberger, L. L. Laichalk, and R. M. Strieter. 1996. Expression and regulation of chemokines in bacterial pneumonia. J. Leukoc. Biol. 59:24-28. [DOI] [PubMed] [Google Scholar]
- 50.Takashima, K., K. Tateda, T. Matsumoto, Y. Izzawa, M. Nakao, and K. Yamaguchi. 1997. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect. Immun. 65:257-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toth, L. A. 1997. The moribund state as an experimental endpoint. Contemp. Top. Lab. Anim. Sci. 36:44-48. [PubMed] [Google Scholar]
- 52.van der Poll, T., C. V. Keogh, W. A. Buurman, and S. F. Lowry. 1997. Passive immunization against tumor necrosis factor-α impairs host defense during pneumococcal pneumonia in mice. Am. J. Respir. Crit. Care Med. 155:603-608. [DOI] [PubMed] [Google Scholar]
- 53.van der Poll, T., C. V. Keogh, X. Guirao, W. A. Buurman, M. Kopf, and S. F. Lowry. 1997. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J. Infect. Dis. 176:439-444. [DOI] [PubMed] [Google Scholar]
- 54.van der Poll, T., A. Marchant, C. V. Keogh, M. Goldman, and S. F. Lowry. 1996. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J. Infect. Dis. 174:994-1000. [DOI] [PubMed] [Google Scholar]
- 55.Willems, F., A. Marchant, J.-P. Delville, C. Gérard, A. Delvaux, T. Velu, M. De Boer, and M. Goldman. 1994. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur. J. Immunol. 24:1007-1009. [DOI] [PubMed] [Google Scholar]
- 56.Ziegler-Heitbrock, H. W. L., B. Passlick, E. Käfferlein, P. G. Coulie, and J. R. Izbicki. 1992. Protection against lethal pneumococcal pneumonia septicemia in pigs is associated with decreased levels of interleukin-6 in blood. Infect. Immun. 60:1692-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]