Abstract
Mycobacterium microti is phylogenetically closely related to Mycobacterium tuberculosis and is a member of that complex of organisms. It is a curved, acid-fast bacillus that is naturally attenuated with a narrow host range for Microtus species only. In this study, we confirm the unique susceptibility of voles to infection with M. microti and the relative resistance of mice with a significantly lower organism burden after 8 weeks of infection. In addition, histopathologic examination of lungs reveals a lack of cellular, granulomatous aggregates characteristically seen in murine M. tuberculosis infection. In the past, M. microti has been used successfully in humans as a vaccine against tuberculosis but was associated with cutaneous reactions. In an attempt to circumvent this adverse effect, we report the efficacy of aerosol and oral vaccination with M. microti. High-dose orogastric vaccination with M. microti resulted in a statistically significant improvement in protection against aerosol challenge with virulent M. tuberculosis in the murine model compared with subcutaneous M. bovis BCG Pasteur vaccination.
Mycobacterium microti, a member of the Mycobacterium tuberculosis complex, was first discovered in wild voles by A. Q. Wells in 1937 in an investigation prompted by population cycling in Microtus species at the Scottish-British border (7, 26). The disease was described as a widespread caseous infection secondary to curved, acid-fast bacilli, especially in the lymphatic and subcutaneous tissues of infected voles. Despite its subsequent success as a vaccine candidate against tuberculosis, M. microti proved not to be the causative agent of the cyclical death of wild voles. Publications over the next decade elaborated the unique host susceptibility of M. microti, showing that voles succumb quickly to the organism but that guinea pigs, rabbits, mice, and rats were all relatively resistant to infection even at high doses (9, 11, 12, 25). Because of this narrow host range, M. microti was investigated in guinea pigs, calves, and finally humans as a vaccine against tuberculosis. Over 10,000 persons were vaccinated with the vole bacillus from 1946 to 1961, and it was shown to be immunogenic and safe (23, 27, 28). In the largest comparative trial of BCG and M. microti performed by the British Medical Research Council, both vaccines showed 77% protective efficacy (14).
The reasons for the abandonment of M. microti as a vaccine against tuberculosis remain unclear. One clear disadvantage of the vaccine was an infrequent, but severe, lupoid skin reaction that occurred in 3 to 17% of vaccinated patients (24, 28). An advantage of this vaccine, however, is its natural attenuation and the ability to passage vaccine strains in voles to maintain the same antigenic integrity.
In this study, we report the comparative susceptibilities of voles and mice to M. microti and a surprising absence of granuloma formation in the lungs of both animals. In an attempt to avoid the adverse effects noted with percutaneous vaccination, we also tested the protective efficacy of M. microti given by two novel routes of administration, orogastric and aerosol.
MATERIALS AND METHODS
Animals.
Eight- to nine-week-old, pathogen-free female BALB/c or C57BL/6 mice (Charles River Laboratories) were maintained in microisolator cages and fed commercial mouse chow and water ad libitum. Susan Carter and Nancy Cushing at the University of Maryland generously provided 6-month-old, pathogen-free female voles (Microtus ochrogaster). Voles were housed in microisolator cages and had free access to mouse chow and water. All animals were maintained in accordance with protocols approved by the Johns Hopkins University Institutional Animal Care and Use Committee.
Microorganisms.
M. microti ATCC 19422, a vole strain isolated by A. Q. Wells in 1950, was obtained from the American Type Tissue Collection (ATCC). M. microti was grown in Middlebrook 7H9 medium (Difco) without glycerol, 0.05% Tween 80, and 10% albumin dextrose complex (ADC; 25 g of bovine albumin, 10 g of dextrose, and 4.25 g of NaCl in 500 ml) as a stationary culture in the presence of 5% CO2. The challenge M. tuberculosis CDC1551 strain was passaged in mice and grown to an optical density of 1.0 in Middlebrook 7H9 supplemented with ADC and Tween 80. The mycobacteria were then bead vortexed for 1 min and frozen in aliquots at −70°C. Strains were frozen in titered aliquots, thawed at the time of use, diluted with phosphate-buffered saline (PBS), and bead vortexed. The titer of the inoculum was verified by plating on Middlebrook 7H10 agar supplemented with ADC.
Infection of voles and mice.
Voles were infected by intraperitoneal injection of 0.1 ml of 106 CFU of either vole-passaged M. microti ATCC 19422 or the original non-vole-passaged strain from the ATCC per ml. Voles were sacrificed at 4 and 8 weeks. Spleens and lungs were homogenized and plated on Middlebrook 7H10 supplemented with ADC to determine the number of organ CFU. Five uninfected voles were housed in the same room and kept in a microisolator cage and were held for the duration of the 8-week experiment to serve as negative controls.
BALB/c mice were infected by lateral tail vein injection with 105 CFU of M. microti, and mice were sacrificed at 4 and 8 weeks for organ homogenization and CFU determination. Other BALB/c mice were infected intraperitoneally with a 100-fold-higher dose and sacrificed at 4 and 8 weeks. Ten SCID mice were infected intraperitoneally, and ten SCID mice were infected by lateral tail vein injection with 103 organisms. In another SCID mouse experiment, 10 mice were infected intravenously with 105 CFU of M. microti, and another nine mice were infected intravenously with 105 CFU of M. tuberculosis.
Immunization.
In the first immunization experiment, BALB/c mice were vaccinated with 106organisms subcutaneously, 20 to 60 organisms by aerosol, or 107 organisms by orogastric gavage by using a PS20 gavage needle with either M. bovis BCG Pasteur (kindly provided by Frank Collins), M. microti (ATCC 19422), or sterile PBS. The mice were challenged by aerosol with M. tuberculosis CDC 1551 12 weeks after vaccination. Frozen aliquots were thawed and diluted with PBS to 106 CFU/ml to give 20 to 50 organisms in the lung at day 1 after a 30-min exposure in a Middlebrook chamber (Glas Col, Terre Haute, Ind.). Five mice were sacrificed on day 1 to verify the number of bacteria in the lung as a result of aerosolization. After 4, 8, or 12 weeks, the remaining mice were sacrificed, and lungs and spleens were homogenized in Ten Broeck homogenizers in 1 ml of 7H9 medium. The homogenates were serially diluted, and 100 μl was plated on Middlebrook 7H10 containing trimethoprim (20 μg/ml), carbenicillin (50 μg/ml), cycloheximide (50 μg/ml), and polymyxin (200 U/ml). Plates were then incubated at 37°C and 5% CO2 for 5 weeks, and the CFU were counted.
In the second immunization experiment, C57BL/6 mice were vaccinated with 106, 107, or 108 M. microti by orogastric gavage as described above. In addition, control animals were either sham vaccinated orogastrically with PBS or vaccinated subcutaneously with 106 CFU of M. bovis BCG Pasteur. After 12 weeks, animals were aerosol challenged with virulent M. tuberculosis CDC 1551 at a dose of 4 × 106 CFU/ml to give an average of 106 (±1) organisms in the lung at day 1 after a 30-min exposure as described above. Mice were then sacrificed 4 weeks later to determine the organism burden.
RESULTS
M. microti infection in voles versus mice.
M. microti-infected BALB/c mice appeared vigorous throughout the 8-week infection period, with no weight loss by the conclusion of the experiment. Among those animals infected both intravenously and intraperitoneally, no grossly visible lesions were seen in the lungs, although the spleens were mildly enlarged in most infected animals. After 8 weeks, the lungs revealed little histologic change and a distinct lack of cellular, granulomatous aggregates as seen in mice intravenously infected with M. tuberculosis (Fig. 1A). Spleens were uniformly enlarged but had few visible acid-fast bacilli. SCID mice infected with a low dose (103 organisms) of M. microti were still healthy and vigorous 6 months after intravenous and intraperitoneal infection. With high-dose intravenous infection (105 organisms), M. tuberculosis-infected SCID mice died at a mean of 32 days, whereas only 1 of 10 M. microti-infected mice had died through day 210 (experiment still on-going).
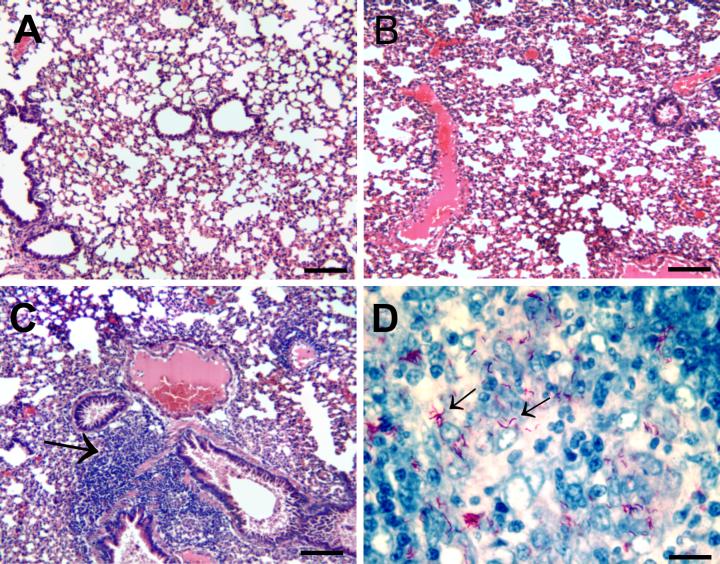
FIG. 1.
(A) 8-Week intraperitoneally M. microti-infected BALB/c mouse lung (hematoxylin and eosin staining; bar, 100 μm). (B) 8-Week intraperitoneally M. microti-infected vole lung (hematoxylin and eosin staining; bar, 100 μm). (C) Same as panel B, showing lymphocytic infiltrate around three bronchioles (bar, 50 μm). (D) 8-Week intraperitoneally M. microti-infected vole spleen acid-fast stain, showing hooked-rod appearance of M. microti in histology specimens (bar, 10 μm).
In contrast, the majority of the M. microti-infected voles were clearly ill by 8 weeks of infection. One vole died 2 days after the intraperitoneal infection for unclear reasons that were unrelated to infection. Among the remaining 14 voles, one vole from the unpassaged ATCC 19422 group died at 44 days with extensive caseous skin lesions. The other four voles in that group had multiple crusted caseous skin lesions and were moribund. The spleens were remarkably enlarged, and there was some evidence of caseous lymphadenopathy. Many curved, acid-fast bacilli were seen in splenic areas of macrophage infiltration (Fig. 1D, arrows). There were no grossly identifiable granulomata, and air spaces were patent with no evidence of consolidation or cellular, granulomatous aggregates (Fig. 1B). There were areas, especially in the hilar area, with intense lymphocytic infiltrate consistent with bronchial associated lymphoid tissue (Fig. 1C, arrow). The five control voles became secondarily infected, presumably due to handling with the same gloves as the infected voles. Voles were housed in microisolator cages but required leather gloves for handling. Three of these five voles had caseous skin lesions, and all had enlarged spleens. The organism burdens in the vole lungs and spleens overall were significantly higher (1.8 and 0.9 log, respectively) than in mice infected with the 100-fold-more organisms intraperitoneally (Table 1).
TABLE 1.
Comparative CFU in lung and spleens of voles and BALB/c mice
| Treatment (CFU [105]) | Mean log10 CFU ± SD (n) in:
|
||||
|---|---|---|---|---|---|
| Mousea
|
Vole
|
||||
| Lung | Spleen | Lung | Spleen | ||
| Intravenous M. microti (1.2) | 3.80 ± 0.23 (4) | 4.77 ± 0.53 (4) | |||
| Intraperitoneal M. microti (1.2) | 2.87 ± 0.99b (4) | 4.49 ± 0.62b (4) | 4.63 ± 0.48 (8) | 5.38 ± 0.51 (8) | |
| Intraperitoneal M. tuberculosis (1.0) | 3.13 ± 0.46 (5) | 3.67 ± 0.31 (5) | |||
Mice were sacrificed 8 weeks after infection.
The mice received 100 times the dose that the voles received intraperitoneally.
Subcutaneous, orogastric, and aerosol immunization with M. microti.
At 12 weeks after subcutaneous, orogastric, or aerosol immunization, vaccinated and naive mice were challenged by low-dose aerosol of M. tuberculosis CDC1551. At 4, 8, and 12 weeks after challenge, five mice per group were sacrificed. The number of bacteria in the spleens and lungs of BALB/c mice immunized by the orogastric and subcutaneous routes both with M. microti and M. bovis BCG was significantly lower than in the PBS-treated controls (P < 0.05). This protection against bacterial proliferation in these vaccination groups persisted through the 8- and 12-week time points except in the subcutaneous M. microti group (Table 2, Fig. 2). Low-dose aerosol immunization with M. bovis BCG Pasteur did not result in a significant decline in the number of bacteria in spleens and lungs from that of naive controls at any time point after aerosol challenge. M. microti aerosol-vaccinated mice showed statistically significant protection in the lungs only at the 8- and 12-week time points (Table 2, Fig. 2).
TABLE 2.
Mean log10 CFU of vaccination groups at 4, 8, and 12 weeks in lungs and spleens
| Treatmenta | Mean log10 CFU (SE)b in mouse:
|
||||||
|---|---|---|---|---|---|---|---|
| Lung at:
|
Spleen at:
|
||||||
| 4 wk | 8 wk | 12 wk | 4 wk | 8 wk | 12 wk | ||
| M. microti | |||||||
| SQ | 3.98 (0.45)∗ | 3.80 (0.33)∗ | 4.08 (0.39) | 2.41 (0.77)∗ | 2.70 (0.55)∗ | 3.06 (0.35)† | |
| OG | 3.88 (0.30)∗ | 3.54 (0.08)† | 3.76 (0.47)∗ | 2.45 (0.36)† | 2.78 (0.10)† | 2.66 (0.27)† | |
| Aerosol | 4.36 (0.51) | 3.62 (0.43)† | 3.80 (0.26)† | 3.55 (0.48) | 2.96 (0.50) | 3.54 (0.45) | |
| BCG | |||||||
| SQ | 3.73 (0.26)† | 3.28 (0.27)† | 3.37 (0.54)† | 2.15 (1.22)† | 2.19 (0.57)† | 2.82 (0.43)† | |
| OG | 3.62 (0.41)† | 3.09 (0.35)† | 3.53 (0.57)∗ | 2.10 (0.91)∗ | 2.18 (0.81)∗ | 2.96 (0.29)† | |
| Aerosol | 4.31 (0.43) | 3.98 (0.46) | 4.22 (0.08) | 3.09 (0.61) | 3.26 (0.43) | 3.62 (0.34) | |
| PBS | |||||||
| SQ | 4.78 (0.38) | 4.17 (0.12) | 4.27 (0.18) | 3.24 (0.32) | 3.49 (0.23) | 3.77 (0.50) | |
| OG | 4.50 (0.26) | 4.51 (0.42) | 4.70 (0.29) | 3.28 (0.46) | 3.49 (0.15) | 4.12 (0.48) | |
| Aerosol | 4.51 (0.22) | 4.14 (0.15) | 4.26 (0.42) | 3.62 (0.30) | 3.22 (0.28) | 3.99 (0.32) | |
SQ, subcutaneous; OG, orogastric.
∗, Statistically significant Student t test P value (≤0.05); vaccination group versus all PBS control groups; †, P ≤ 0.01 (Student t test).
FIG. 2.
(A) Lung CFU counts in BALB/c mice at 12 weeks post-aerosol challenge with M. tuberculosis after the 12-week vaccination period with either M. microti (shaded bars), M. bovis BCG Pasteur (white bars), or PBS control (black bars) given by the subcutaneous (SQ), orogastric (OG), or aerosol route of administration. (B) Spleen CFU counts of same samples at 12 weeks. Five mice were sacrificed per group per time point. Statistically significant differences are indicated by an asterisk.
Immunization with increasing doses of M. microti given orogastrically.
Because there was a trend toward better protection against aerosol M. tuberculosis challenge in the orally vaccinated mice, three different doses of M. microti were administered orally. The 108-CFU dose was statistically better than subcutaneous BCG, giving a 0.4-log additional reduction in the organism burden at 4 weeks postchallenge in both lungs and spleens (P = 0.05). The other two lower doses of M. microti showed protection equal to that of subcutaneous BCG at 4 weeks after virulent M. tuberculosis challenge (Fig. 3).
FIG. 3.
(A) Lung CFU counts at 4 weeks in C57BL/6 mice post-aerosol challenge with M. tuberculosis after a 12-week vaccination period with either subcutaneous doses of M. bovis BCG Pasteur (BCG) or an oral dose of 106 CFU of M. microti (MM6), 107 CFU of M. microti (MM7), or 108 CFU of M. microti (MM8). Five mice were sacrificed per group per time point. (B) Spleen CFU of the same mice at 4 weeks. A statistically significant difference compared to controls is denoted with one asterisk. A statistically significant difference compared to subcutaneous BCG is denoted with two asterisks.
DISCUSSION
M. microti or the vole bacillus, discovered in 1937 by A. Q. Wells as the causative agent of a caseous granulomatous disease of voles, was studied extensively in the 1940s (9-13, 25, 26). A large variety of animals, including hamsters, guinea pigs, and mice, were infected with M. microti. M. microti was virulent only for voles, however, and could infect other animals species only at high concentrations. In this study, we confirm the unique vole host predilection of the organism with extensive disease and death in voles at 8 weeks of infection, whereas a comparable dose in mice resulted in no significant illness clinically or microscopically. In addition, infection of SCID mice showed marked attenuation in the virulence of M. microti compared to that of M. tuberculosis. Voles appeared to die from widespread lymphatic and skin involvement with exuberant proliferation of the organism in the reticuloendothelial system as well. Interestingly, neither voles nor mice formed discrete granulomatous lung lesions or inflammatory progressive bronchopneumonia as is seen commonly in mice intravenously infected with M. tuberculosis within 4 to 8 weeks. Despite a high degree of genomic similarity to M. tuberculosis, it has been shown through bacterial artificial chromosome analysis and deletion analysis that M. microti is missing some genes present in H37Rv (8, 22). These absent genes may be important in the ability of M. tuberculosis to form granulomas in vivo.
Meta-analyses of BCG vaccine efficacy show remarkable heterogeneity in the quality and scope of BCG trials in the last century with variable efficacy of BCG (5). Taken as a whole, BCG appears to be efficacious against childhood forms of the disease but does little to prevent pulmonary tuberculosis in adults (4). Furthermore, BCG substrains show genomic variability with data to suggest that it continues to attenuate and lose protective efficacy with serial passage (2; M. A. Behr and P. M. Small, Letter, Nature 389:133-134, 1997). Without a natural host to exert selective pressure, it is likely that BCG will continue to attenuate and to lose protective efficacy with serial passage over time.
Because of its narrow host susceptibility spectrum, M. microti was tested in more than 10,000 humans for its efficacy as a tuberculosis vaccine, with results that showed it to be comparable to BCG in protective efficacy (24). It was subsequently abandoned for reasons that remain unclear. It has clear advantages over BCG since it is naturally attenuated for humans and, therefore, can be passaged in animals to maintain genomic integrity and its original virulence for voles. In addition, because M. microti has already been tested in so many humans, its safety profile has already been confirmed. Given the inherent complexity in testing new tuberculosis vaccines, reviving old vaccines that have previously been tested in humans has become more attractive. Because of reports in the literature of occasional severe cutaneous reactions to vaccine administered by multiple puncture method, alternative routes of administration may be preferable. For this reason, we investigated both the orogastric route and the aerosol route to administer M. microti vaccine.
Aerosol vaccination with BCG has been tested in the past and shown to be efficacious in mice, monkeys, and guinea pigs (1, 16, 18). Overall, the local activation of the bronchoalveolar macrophage was higher and, therefore, led to good protection against aerosol challenge with virulent M. tuberculosis (17). The systemic effects of aerosol vaccination as assessed by the adoptive transfer of immunity appeared to be slower in onset and ultimately no better than those in animals that had been intradermally vaccinated (19, 21). In our experiment, we vaccinated with lower aerosol doses than had been used in the past (107 CFU/10 ml in the Glas Col nebulizer), leaving ca. 25 organisms in the lungs of each animal at day 1. This dose of M. bovis BCG appears to be insufficient for any protection against low-dose aerosol challenge after 12 weeks. Interestingly, a similar dose of M. microti resulted in protection against proliferation in lung, again confirming previously published data that aerosol immunization shows more local protection.
Orally gavaged M. microti at a dose of 108 organisms was statistically more protective against M. tuberculosis aerosol challenge than subcutaneously administered M. bovis BCG. These data support previous observations that orally gavaged attenuated mycobacteria (BCG) can be protective against tuberculosis challenge (15). The oral route of administration was the first route tried in humans when the BCG vaccine was introduced in the 1920s (3). Complications of this route of administration have been cervical adenitis secondary to the high doses required to overcome gastric acidity, variable absorption, and variable purified protein derivative (PPD) response. Although it has subsequently been shown that the PPD response to vaccination does not correlate with protective immunity (6) and that cervical adenitis is not required for protection in a murine model (15), a synthetic vehicle or formulation that allows a smaller dose of mycobacteria to be given orally and dispersed beyond the acidic stomach environment may make neonatal oral vaccination even more attractive (20).
M. microti is a naturally attenuated mycobacterium with a curiously narrow host range. Histopathologically, the absence of granulomas in early infection in the mouse and vole infection models points to the possibility of important genetic differences between this and other members of the M. tuberculosis complex. Finally, when given at a high dose as a gastric gavage, M. microti is statistically better than subcutaneous M. bovis BCG Pasteur and warrants further investigation with improved delivery methods.
Acknowledgments
This work was supported by NIH grants K08 AI 01689-01, AI36973, AI37856, and AI43846 and grants from the National Vaccine Program Office and the Sequella Global Tuberculosis Foundation.
We thank Kathy Gabrielson for helping with the interpretation of the histopathologic specimens, Brian Schofield for photographic images of these specimens, and David Dowdy for technical assistance.
REFERENCES
- 1.Barclay, W. R., W. M. Busey, D. W. Dalgard, R. C. Good, B. W. Janicki, J. E. Kasik, E. Ribi, C. E. Ulrich, and E. Wolinsky. 1973. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am. Rev. Respir. Dis. 107:351-358. [DOI] [PubMed] [Google Scholar]
- 2.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 3.Calmette, A., B. Weill-Halle, A. Saenz, and L. Costil. 1933. Demonstration experimentale du passage des bacille-vaccins BCG a travers la muquese de l'intestin chez l'enfant et chez le singe. Bull. Acad. Med. 110:203-206. (In French.)
- 4.Colditz, G. A., C. S. Berkey, F. Mosteller, T. F. Brewer, M. E. Wilson, E. Burdick, and H. V. Fineberg. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29-35. [PubMed] [Google Scholar]
- 5.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 6.Comstock, G. W. 1994. Field trials of tuberculosis vaccines: how could we have done them better? Control Clin. Trials 15:247-276. [DOI] [PubMed] [Google Scholar]
- 7.Elton, C., and D. H. S. Davis. 1935. An epidemic among voles (Microtus agrestis) on the Scottish border in the spring of 1934. J. Anim. Ecol. 4:277-288. [Google Scholar]
- 8.Gordon, S. V., R. Brosch, A. Billault, T. Garnier, K. Eiglmeier, and S. T. Cole. 1999. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol. Microbiol. 32:643-655. [DOI] [PubMed] [Google Scholar]
- 9.Griffith, A. S. 1942. The cultural characters and pathogenicity for some laboratory animals of the vole strain of acid-fast bacillus. J. Hyg. 42:527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith, A. S. 1941. Further experiments of the field vole with tubercle bacilli. J. Hyg. 41:250-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffith, A. S. 1941. Further experiments on the golden hamster with tubercle bacilli and the vole strain of acid-fast bacillus. J. Hyg. 41:260-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith, A. S. 1939. The relative susceptibility of the field-vole to the bovine, human and avian types of tubercle bacilli and to the vole strain of acid-fast bacillus. J. Hyg. 39:244-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith, A. S. 1939. The susceptibility of the golden hamster to bovine, human and avian tubercle bacilli and to the vole stain of acid-fast bacillus. J. Hyg. 39:154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart, P. D. A., and I. Sutherland. 1977. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br. Med. J. 2:293-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagranderie, M., P. Chavarot, A. M. Balazuc, and G. Marchal. 2000. Immunogenicity and protective capacity of Mycobacteriumbovis BCG after oral or intragastric administration in mice .Vaccine 18:1186-1195. [DOI] [PubMed] [Google Scholar]
- 16.Lagranderie, M., C. Frehel, C. de Chastellier, and M. Gheorghiu. 1991. Cellular oxidative responses and mycobacterial growth inhibition in aerosol and intradermal BCG-immunized guinea-pigs. Biologicals 19:335-345. [DOI] [PubMed] [Google Scholar]
- 17.Lagranderie, M., P. Ravisse, G. Marchal, M. Gheorghiu, V. Balasubramanian, E. H. Weigeshaus, and D. W. Smith. 1993. BCG-induced protection in guinea pigs vaccinated and challenged via the respiratory route. Tuberc. Lung Dis. 74:38-46. [DOI] [PubMed] [Google Scholar]
- 18.Lefford, M. J. 1978. Immunization of mice after airborne infection with various strains of BCG. Am. Rev. Respir. Dis. 117:103-109. [DOI] [PubMed] [Google Scholar]
- 19.Lefford, M. J. 1977. Induction and expression of immunity after BCG immunization. Infect. Immun. 18:646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine, M. M., C. Ferreccio, P. Abrego, O. S. Martin, E. Ortiz, and S. Cryz. 1999. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine 2(Suppl. 17):S22-S27. [DOI] [PubMed] [Google Scholar]
- 21.Orme, I. M., and F. M. Collins. 1986. Aerogenic vaccination of mice with Mycobacterium bovis BCG. Tubercle 67:133-140. [DOI] [PubMed] [Google Scholar]
- 22.Parsons, L. M., R. Brosch, S. T. Cole, A. Somoskovi, J. E. Hotaling, T. Tratts, A. Loder, G. Bretzel, and M. Salfinger. 2000. Differentiation of members of the Mycobacterium tuberculosis complex using deletion analysis, p. 22. In Tuberculosis: past, present, and future. American Society for Microbiology, Washington, D.C.
- 23.Paul, R. 1961. The effects of vole bacillus vaccination of African mine workers in the Northern Rhodesian copper mines. Br. J. Ind. Med. 18:148-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuberculosis Vaccine Trials Committee. 1956. B.C.G. and vole bacillus vaccines in the prevention of tuberculosis in adolescents. Br. Med. J. 1:413-427. [PMC free article] [PubMed] [Google Scholar]
- 25.Wells, A. Q. 1938. The susceptibility of voles to human and bovine strains of tubercle bacilli. Br. J. Exp. Pathol. 19:324-328. [Google Scholar]
- 26.Wells, A. Q. 1937. Tuberculosis in wild voles. Lancet i:1221.
- 27.Wells, A. Q. 1949. Vaccination with the murine type of tubercle bacillus (vole bacillus). Lancet i:53-55. [DOI] [PubMed]
- 28.Wells, A. Q., and J. A. H. Wylie. 1954. Vaccination against tuberculosis with the vole bacillus. Br. Med. Bull. 10:96-100. [DOI] [PubMed] [Google Scholar]