Abstract
Neisseria meningitidis (meningococcus [MC]) is able to enter and replicate within epithelial cells. Iron, an essential nutrient for nearly all organisms, is an important determinant in the ability of MC to cause disease; however, its role in MC intracellular replication has not been investigated. We analyzed the growth of MC within the A431 human epithelial cell line and the dependence of this growth on iron uptake. We present evidence here that chelation of iron from infected tissue culture cells with Desferal strongly inhibited intracellular replication of wild-type (wt) MC. We also provide genetic evidence that iron must be acquired by MC from the host cell in order for it to replicate. An hmbR mutant that is unable to use hemoglobin iron and could not grow in tissue culture media without iron supplementation replicated more rapidly within epithelial cells than its wt parent strain. An fbpA mutant that is unable to utilize human transferrin iron or lactoferrin iron replicated normally within cells. In contrast, a tonB mutant could not replicate intracellularly unless infected cultures were supplemented with ferric nitrate. Taken together, these findings strongly suggest that MC intracellular replication requires TonB-dependent uptake of a novel host cell iron source.
The gram-negative diplococcus Neisseria meningitidis remains a significant cause of morbidity and mortality in developed and developing nations. The meningococcus (MC) is a common inhabitant of the human nasopharynx that can cross the epithelial barrier to enter the bloodstream. Within the bloodstream, MC can replicate, causing septicemia. From this site, it can also cross the blood-brain barrier to enter the cerebrospinal fluid, where it causes meningitis. The mechanisms by which MC crosses the epithelial and blood-brain barriers are poorly understood.
Humans are the only reservoir for MC, and this strict host tropism has prevented the development of a suitable animal model for studying the mechanisms by which MC colonizes and crosses the epithelial barrier. Most of the knowledge of MC infection has been derived from studies of its interactions with organ cultures, primary cell cultures, and immortalized cell lines. These studies reveal that MC is able to adhere to, enter, and traffic through human epithelial cells (15). MC can also replicate within cultured epithelial cell lines (14). Transmission electron micrographs of infected nasopharyngeal organ cultures reveal MC in large numbers, apparently within phagosomes, suggesting that intracellular replication also occurs in this ex vivo setting (26). The significance of intracellular MC replication is unclear, although it is likely to play a role in promoting disease. A mutant was recently identified in a closely related species, Neisseria gonorhoeae, which trafficked through polarized epithelial cells at an enhanced rate (9). This fast intracellular trafficking mutant, fitA, also had an accelerated intracellular replication phenotype, suggesting that replication may be part of the transcytotic process for pathogenic Neisseria spp.
The requirements for MC replication within cultured epithelial cells have not been addressed, but iron acquisition is likely to be a key attribute. Iron is an essential element for nearly all organisms, facilitating fundamental processes such as nucleotide biosynthesis and electron transport (5). The importance of iron for MC pathogenesis has been well documented, beginning over two decades ago when inorganic iron was found to greatly enhance the lethality of MC injected into mice (3). Mammals withhold iron from invading microbes as a nonspecific means of defense. Thus, unbound or free iron is in low concentration in the body. The glycoproteins transferrin and lactoferrin effectively chelate iron on mucosal surfaces and in the bloodstream. Iron availability in the blood is further decreased during hypoferremia, a mammalian response to infection in which the reticuloendothelial system removes transferrin from the circulation (31).
MC is able to circumvent the iron-withholding tactics of its human host (25). It can assimilate iron via outer membrane receptors for human transferrin and lactoferrin. The MC transferrin receptor, TbpA/B, and the lactoferrin receptor, LbpA/B, bind their iron-loaded ligands at the bacterial outer membrane. They next facilitate the removal of iron from these carriers and its subsequent internalization into the bacterial periplasm (8). Utilization of transferrin and lactoferrin iron by MC also requires FbpA, a 37-kDa ferric binding protein that is thought to deliver iron from these receptors to the inner membrane transporter FbpB/C (11). Heme, either free or as a component of hemoglobin, is also an iron source for MC. Two outer membrane receptors, HmbR and/or HpuA/B, allow MC to bind hemoglobin and remove and internalize heme (12, 13, 28). The uptake of free heme by MC is poorly understood. Numerous attempts to identify MC mutants that cannot utilize free heme-iron have been unsuccessful, suggesting that redundant heme-iron uptake systems may exist.
MC differs from most pathogenic bacteria in that it does not produce siderophores, small secreted ferric iron chelators that extract iron from host molecules and deliver it to the pathogen via membrane-spanning siderophore receptors. MC does, however, produce FetA, an outer membrane receptor that is capable of binding enterobactin, a siderophore secreted by E. coli. FetA has been postulated to allow MC to scavenge iron by binding siderophores secreted by other bacteria colonizing the same mucosal surfaces (4).
The uptake of iron by all of the aforementioned outer membrane receptors requires the TonB complex, a highly conserved macromolecule located in the inner membrane and periplasm of gram-negative bacteria. Proteins in this complex, TonB, ExbB, and ExbD, cooperate in translating the proton motive force across the inner membrane into conformational changes in TonB-dependent outer membrane receptors (16). MC lacking TonB are unable to grow in media with transferrin, lactoferrin, or hemoglobin as a sole iron source but retain their ability to grow in media supplemented with free heme or ferric nitrate.
The diverse array of systems utilized by MC to take up iron is evidence of the important role this element plays in promoting its survival within the host. The importance of iron in MC pathogenicity was demonstrated in the infant rat model for MC septicemia. An hmbR mutant that could not assimilate hemoglobin iron was injected intraperitoneally into infant rats. This mutant was cleared from the bloodstream of the animal much more efficiently that its wild-type (wt) parent strain, indicating that iron acquisition strongly influences the in vivo survival of MC in the bloodstream (27).
The extracellular environments exploited by MC, such as the nasopharyngeal mucosa, bloodstream, and cerebrospinal fluid are supplied with well-characterized iron-containing compounds that are likely to meet the growth requirements of the bacteria (25). In contrast, the iron requirements of intracellular MC and the iron sources available to them in this environment are unknown. Many intracellular pathogens, such as Chlamydia spp., Legionella pneumophila, and Mycobacterium tuberculosis must acquire iron from their host cell in order to replicate (1, 18, 20, 22). We hypothesized that MC replication in the intracellular niche would also require acquisition of iron from the host cell. We present evidence here that the replication of MC within cultured A431 human endocervical epithelial cells requires iron uptake. We demonstrate that iron is derived from the host cell and not the media. We show that mutants unable to utilize hemoglobin, transferrin, or lactoferrin replicate normally within cells. In contrast, a tonB mutant is deficient in intracellular growth. These data strongly suggest that MC replication within epithelial cells requires a novel iron source and that uptake of this iron source is TonB dependent.
MATERIALS AND METHODS
Cell culture.
The A431 human endocervical epithelial cell line, obtained from S. Schmid, was maintained in Dulbecco modified Eagle's medium (DMEM; Gibco-BRL) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco-BRL). According to the manufacturer's specifications, DMEM contains only picomolar quantities of iron, whereas FCS contains approximately 15 μM concentrations of both bovine transferrin and bovine hemoglobin. Cells were used for bacterial infection experiments between passages 8 and 15.
Bacterial strains.
Information for all strains used in this study is shown in Table 1. MC strain 8013.6 is a serogroup C isolate from a patient at the Pasteur Hospital (17). 8013.6 is a capsulated, piliated strain producing high-adhesive pilin. All mutants constructed for this study were obtained by transformation of 8013.6 with chromosomal DNA from published mutants (Table 1). All mutants were piliated, as judged by colony morphology and immunoblotting with antipilin antisera. All strains were Opa negative as judged by Western immunoblotting, and their iron-uptake phenotypes were verified by growth on Desferal plates spotted with the appropriate iron source. All strains were phase off for HpuA/B, the neisserial hemoglobin-haptoglobin receptor. Inactivation of the relevant genes was verified by PCR. All MC strains were maintained on GCB agar plates with Kellog's Supplements I and II. Strains were passed no more than twice before assays, and bacterial inocula were harvested from plates at 12 to 16 h after passage.
TABLE 1.
MC Strains used in this study and relevant phenotypes
| Strain | Replication in DMEM with iron sourcea
|
Intracellular replication | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hb | hTf | bTf | hLf | Fe | Heme | FCS | |||
| MC 8013.6 | + | + | − | + | + | + | + | + | 17 |
| MC 8013.6 hmbR::Kan | − | + | − | + | + | + | − | + | 27 |
| MC 8013.6 tonB::Kan | − | − | − | − | + | + | − | − | 29 |
| MC 8013.6 fbpA::Ω | + | − | − | − | + | + | + | + | 11 |
Hb, hemoglobin; hTf, human transferrin; bTf, bovine transferrin; hLf, human lactoferrin; Fe, ferric nitrate.
Intracellular growth rate assay.
A431 cells were seeded into 12-well plates and grown to 80% confluency before infection. wt 8013.6 or its mutant derivatives were swabbed from GCB plates, directly suspended in DMEM-10% FCS, and diluted in the same medium to allow for infection at a multiplicity of infection of two (i.e., two bacteria per cell). Infected cultures were incubated for 12 h and then rinsed three times in phosphate-buffered saline (PBS; Gibco-BRL) to remove nonadherent bacteria. Cultures were then incubated for 1 h in fresh prewarmed medium containing 50 μg of gentamicin (Gibco-BRL)/ml to kill extracellular bacteria. Gentamicin was then removed, and the cells were gently rinsed three times in PBS. Epithelial cells were lifted and disrupted by incubating them for 3 min in PBS containing 2 mM EDTA and 0.5% saponin (Sigma), followed by transfer to Eppendorf tubes and high-speed vortexing for 30 s. The lysed host cells were plated at appropriate dilutions on gonococcal base medium (GCB) agar to determine MC CFU. Ferric nitrate (10 μM; Sigma) or Desferal (100 μM; Ciba-Geigy) were added to selected assays as described in the text and in Fig. 1. Values for each time point were derived from three to five infected cultures. Error bars represent the standard deviation of the mean. All assays were repeated at least three times with similar results.
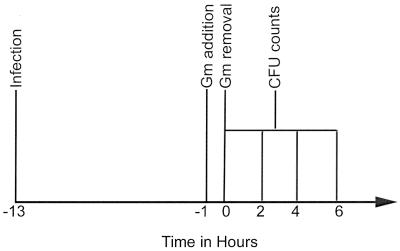
FIG. 1.
Gentamicin protection assays were initiated by infecting A431 cells with bacteria as noted (T = −13). After 12 h of infection cultures were incubated with gentamicin for 1 h. They were then rinsed thoroughly and incubated further without gentamicin (T = 0). At various times, cells were disrupted, and intracellular bacteria were quantified as described in Materials and Methods. Desferal or ferric nitrate was added to some cultures after gentamicin removal (T = 0). Some mutants required ferric nitrate to promote growth during the invasion phase of the assay (T = −13 to −1).
MC growth rate in liquid media.
To monitor the growth of MC 8013.6 and MC 8013.6 hmbR::Kan in tissue culture media (in the absence of epithelial cells), bacteria were swabbed from GCB plates and suspended in DMEM-10% FCS (without phenol red) to an initial optical density at 600 nm (OD600) of 0.05 as measured with a Beckman 600 series spectrophotometer, and the OD600 of the cultures was monitored at various time points over 8 h. The numbers of CFU were also determined at the beginning and ending of each assay to verify that the OD600 readings corresponded to viable bacteria. In order to examine the effect of Desferal treatment on the replication of MC 8013.6 in the absence of cells, the bacteria were preincubated in DMEM-10% FCS for 12 h. Bacteria were then isolated by centrifugation and resuspended in fresh DMEM-10% FCS lacking phenol red. The OD600 was monitored with or without 100 μM Desferal.
RESULTS
Iron chelation inhibits MC replication within epithelial cells.
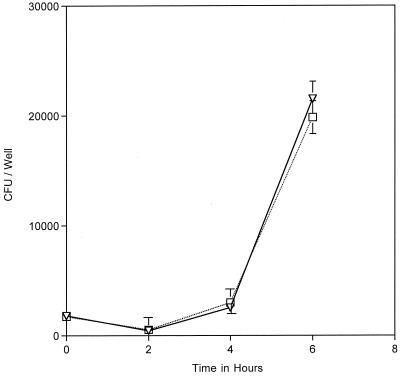
In order to study the role of iron in MC intracellular replication, we first examined its replication within cells. A431 epithelial cells, which have been shown previously to support MC cell entry and subsequent intracellular replication (14), were infected with MC strain 8013.6, a serogroup C isolate (17). Intracellular growth was quantified by means of the gentamicin protection assay (10) (Fig. 1). Briefly, A431cells were infected with MC at a multiplicity of infection of two. After 12 h, extracellular bacteria were killed by incubating the cultures with gentamicin for 1 h. The termination point of the gentamicin treatment was designated “0 h.” At various times after removal of gentamicin, intracellular CFU were quantified. Under such conditions, MC 8013.6 replicated within A431 cells, their intracellular numbers increasing fivefold over the 6 h after gentamicin treatment (Fig. 2). These results are similar to those of earlier reports (14). Examination of MC-infected cells by immunofluorescence microscopy verified the intracellular location of MC, as well as the increase in intracellular cell counts (data not shown).
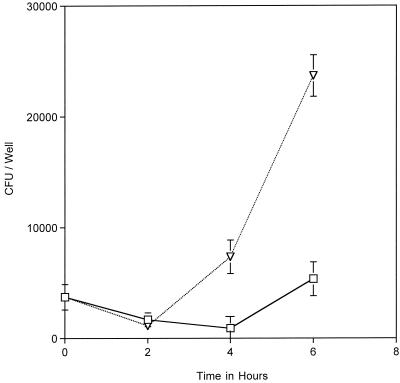
FIG. 2.
Intraepithelial replication of MC 8013.6 was monitored by using a gentamicin protection assay (described in Fig. 1). The addition of 100 μM Desferal to the infected cultures after gentamicin removal (T = 0 in Fig. 1) resulted in an inhibition of intracellular replication (□) compared to the no-Desferal control (▿). The datum points represent the averages of three or more cultures, with error bars representing the standard deviation.
We next determined whether MC intracellular growth required iron acquisition. Iron uptake of infected cultures was blocked pharmacologically by incubating the cultures with the iron-specific chelator Desferal (i.e., deferoxamine mesylate). Desferal forms stable chelates with ferric iron, and the chelated iron cannot be assimilated by MC (32). Desferal is a hydrophobic molecule, which enters cells through fluid-phase endocytosis, where it can chelate iron in the endosomal and lysosomal compartments. Desferal diminishes cytosolic iron levels via an indirect mechanism (6). This treatment has been shown to inhibit intracellular replication of several bacterial pathogens, including Chlamydia spp. and L. pneumophila (1, 20, 22). Desferal is routinely used to treat human iron overload disorders and is not toxic to tissue culture cells at the concentrations used in our assays (6). Desferal was added to infected cultures after gentamicin removal and at various times the numbers of intracellular CFU were determined. The results show that growth of 8013.6 within Desferal-treated epithelial cells was significantly decreased compared to the growth in the control untreated cells (Fig. 2). This effect was reversed when Desferal-treated cells were incubated with an equimolar amount of ferric nitrate (data not shown).
One possible explanation for the negative effect of Desferal on MC intracellular growth was the toxicity of the chelator to the infecting bacteria. However, Desferal did not affect bacterial growth in tissue culture media alone (data not shown). Taken together, these results suggest that iron is necessary for MC replication within epithelial cells and that Desferal chelates iron from an intracellular source.
Our results additionally suggest that iron stored within the bacteria during the extracellular growth period cannot fulfill the iron requirements of intracellular growth. MC can take up iron from bovine hemoglobin via its hemoglobin receptor, HmbR (28). The infection medium, DMEM-10% FCS, contains ∼15 μM bovine hemoglobin, a concentration that can fulfill the iron requirements of MC 8013.6 via HmbR (28). The bacteria in these experiments were, therefore, in iron-replete media prior to entering the epithelial cells.
Iron for intracellular replication of MC is not derived from tissue culture media.
DMEM contains only picomolar amounts of iron, whereas FCS contains significant amounts of iron, mostly in the form of bovine transferrin and bovine hemoglobin, each at ∼15 μM. Bovine transferrin is not an iron source for MC due to the specificity of the Neisseria transferrin receptor, TbpA/B, for human transferrin (24). On the other hand, bovine hemoglobin may provide the necessary iron, since the neisserial hemoglobin receptor, HmbR, permits the utilization of iron from bovine and other animal hemoglobin (28). Desferal does not chelate hemoglobin iron, which led us to hypothesize that the inability of this chemical to affect MC replication in tissue culture media was due the fact that hemoglobin is the only significant MC iron source in DMEM-FCS.
To test our hypothesis that bovine hemoglobin provides the only significant iron source for MC in tissue culture media, we examined the replication rate of a hemoglobin uptake mutant in DMEM-10% FCS. MC 8013.6 hmbR::Kan lacks a functional hemoglobin receptor and is unable to grow in media in which hemoglobin is the sole iron source (27). The 8013.6 hmbR::Kan mutant replicated very slowly in DMEM-FCS compared to the growth of MC 8013.6, its parent strain with a functional HmbR (Fig. 3). Addition of ferric nitrate to the medium rescued the growth defect of 8013.6 hmbR::Kan, allowing the mutant to grow at nearly the wt rate. These results strongly suggest that bovine hemoglobin is the only significant iron source for MC in the infection assay provided by the media. Since hemoglobin iron is not chelatable by Desferal (23), the effect of this compound on intracellular replication strongly suggests that the iron required for intracellular replication by MC is derived from the host cell and not from the cell culture medium.
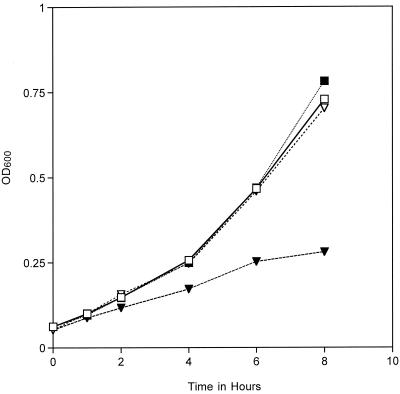
FIG. 3.
Replication rates of MC 8013.6 (▪), 8013.6 hmbR::Kan (▾) in DMEM-10% FCS (without phenol red) were monitored by measuring the OD600 for 8 h. Iron supplementation of the media with ferric nitrate rescued the replication defect of the hmbR mutant (▿). Growth of the wt strain was not enhanced by ferric nitrate supplementation (□). The viability of the bacteria was determined by plating cultures for the enumeration of CFU. An increase in the OD600 corresponded with a comparable increase in the numbers of CFU (data not shown).
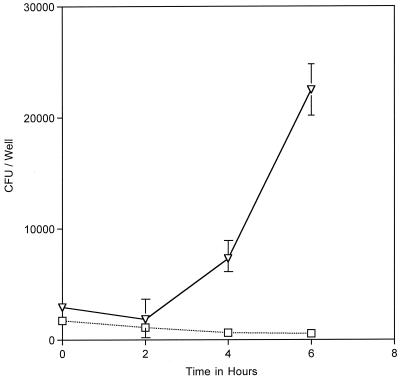
An MC mutant unable to acquire iron from tissue culture medium replicates faster than the wt parent strain within epithelial cells.
To provide further evidence that iron acquired from the host cell is required for intracellular replication of MC, we quantified the intracellular replication rate of MC 8013.6 hmbR::Kan by using the gentamicin protection assay. This mutant replicated poorly in media without iron supplementation; therefore, it should not replicate well within cultured A431 cells unless an additional iron source is derived from the host cell. As shown in the previous experiment, this mutant cannot replicate effectively in the DMEM-FCS. It therefore does not grow well during the infection stage, prior to entering the epithelial cells, compared to the wt strain. Our intracellular growth protocol was therefore modified to increase the invasion frequency of the mutant by the addition of ferric nitrate to the infection medium. Ferric nitrate was removed by extensive rinsing of the infected cultures prior to gentamicin treatment and withheld during the subsequent 6 h of the intracellular growth phase of the assay (Fig. 1). Under these conditions, 8013.6 hmbR::Kan replicated faster than its wt parent strain within cells (Fig. 4). The significant increase in the rate of replication of this mutant within cells compared to its growth rate in tissue culture medium (compare Fig. 3 and 4) lends further support to our hypothesis that a host cell iron source is required for intracellular replication of MC. Since the mutant cannot utilize hemoglobin iron, this experiment also rules out the possibility that bovine hemoglobin can reach the gentamicin-protected bacteria to serve as an intracellular iron source. The enhanced replication displayed by this mutant may be due to the fact that, unlike wt MC, it is not conditioned to utilize heme prior to entering cells. The transition to an intracellular iron source may be more efficient for this mutant.
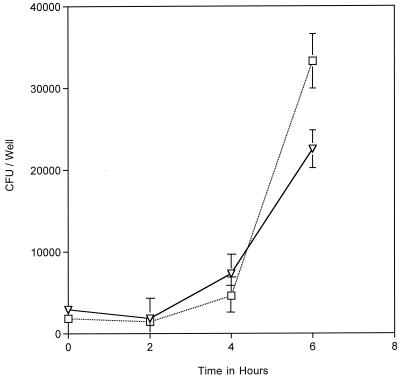
FIG. 4.
Intracellular replication rates of MC 8013.6 (▿) and MC 8013.6 hmbR::Kan (□) as determined by gentamicin protection assay. Ferric nitrate was included in the invasion media (T = −13 to −1 in Fig. 1) to allow efficient growth and entry of the mutant. MC 8013.6 hmbR::Kan replicated faster than wt MC 8013.6 within cells. The datum points represent the averages of three or more cultures, with error bars representing the standard deviation.
Transferrin is not the intracellular MC iron source.
Transferrin has been shown to serve as an intracellular iron source for several pathogenic bacteria including Ehrlichia chaffeensis, M. tuberculosis, M. avium complex, and C. pneumoniae (1, 2, 18). As stated above, the transferrin in our assays is bovine in origin and, therefore, cannot support MC growth in liquid media (24). The formal possibility, however, remained that bovine transferrin may serve as an intracellular iron source for MC. Transferrin enters cells via endocytosis. During endosomal acidification, transferrin undergoes conformational changes and releases its cargo of iron (7, 19). It was possible that bovine transferrin in a lower pH compartment could undergo a conformational change that would enable its binding to the MC transferrin receptor, TbpA/B. To explore this possibility, we studied the intracellular replication of the MC 8013.6 mutant fbpA::Ω. This mutant is unable to utilize transferrin or lactoferrin as an iron source because it lacks the periplasmic iron-binding protein FbpA, which is thought to transfer iron to the inner membrane transporter, FbpB/C (11). In the gentamicin protection assay, fbpA::Ω replicated as well as its wt parent strain within A431 cells (Fig. 5). These results lend further support to the argument that transferrin is not the intracellular iron source for MC.
FIG. 5.
Intracellular replication rates of MC 8013.6 (▿) and MC 8013.6 fbpA::Ω (□) as determined by gentamicin protection assay. MC 8013.6 fbpA::Ω replicated similarly to wt MC 8013.6 inside of cells. The datum points represent the averages of three or more cultures, with error bars representing the standard deviation.
The MC tonB mutant is defective for intracellular replication, but the defect can be rescued by iron supplementation.
Having ruled out both hemoglobin and transferrin as intracellular iron sources for MC, we next examined the replication of an MC mutant that is more broadly deficient in iron acquisition. MC 8013.6 tonB::Kan is unable to utilize hemoglobin, transferrin, or lactoferrin iron (29). This mutant is, however, able to grow in media supplemented with free heme or inorganic iron such as ferric nitrate. The intracellular growth of 8013.6 tonB::Kan was monitored by using the modified gentamicin protection assay as described above. Like the hmbR mutant, the tonB mutant is unable to use hemoglobin iron. To maintain bacterial viability, it was necessary to supplement the medium with ferric nitrate in the first 12 h of infection, prior to addition of gentamicin. Under these conditions, the intracellular counts reached wt levels at 0 h, indicating that iron supplementation during the infection process permitted vigorous extracellular growth and cell invasion. However, the ability of the mutant to replicate within cells after gentamicin protection was impaired, and its intracellular CFU declined significantly over time (Fig. 6). This result suggests that at least one TonB-dependent receptor is necessary for MC to replicate within A431 cells.
FIG. 6.
Intracellular replication rates of MC 8013.6 (▿) and MC 8013.6 tonB::Kan (□) determined by the gentamicin protection assay. Ferric nitrate was included in the invasion media (T = −13 to −1 in Fig. 1) to allow efficient growth and entry of the mutant. MC 8013.6 tonB::Kan failed to replicate within A431 cells. The datum points represent the averages of three or more cultures, with error bars representing the standard deviation.
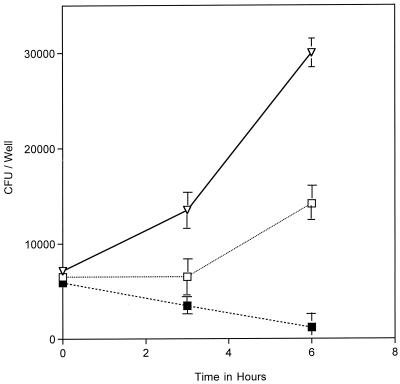
In other gram-negative bacteria, TonB energizes outer membrane receptors that are not involved in iron uptake (16). Although the neisserial TonB has not been demonstrated to function in uptake of non-iron compounds, the possibility remained that the intracellular growth defect of the tonB mutant reflected a defect in the uptake of other compounds besides iron. To demonstrate that the inability of the tonB mutant to replicate intracellularly was indeed due to an iron uptake defect, we attempted to rescue replication of the tonB mutant by supplementing the infected cultures with ferric nitrate after gentamicin removal. Iron supplementation had a marked effect, partially restoring the intracellular growth lesion of the mutant. (Fig. 7). The inability of the tonB mutant to replicate intracellularly is therefore at least partially due to an iron uptake defect within cells. The inability of ferric nitrate to fully rescue the intracellular replication defect may indicate that TonB serves an additional function aside from iron uptake. Alternately, it may mean that ferric nitrate is not delivered efficiently to intracellular MC.
FIG. 7.
Intracellular replication of MC 8013.6 tonB::Kan was rescued by ferric nitrate supplementation after gentamicin killing (T = 0 to 6 in Fig. 1). Supplementation of the media with ferric nitrate after gentamicin killing (T = 0 in Fig. 1) rescued intracellular replication (□) compared to non-iron-supplemented cultures (▪). Replication was not rescued to wt (▿) level. The datum points represent the average of three or more cultures, with error bars representing the standard deviation.
DISCUSSION
N. meningitidis is a successful pathogen in part because it can exploit multiple environments within the human host, acquiring nutrients at each site for its persistence, propagation, and dissemination. Iron is a key nutrient in determining bacterial pathogenicity (21). Multiple iron uptake systems-hemoglobin, transferrin, and lactoferrin-have been shown to perform essential functions for MC replication at extracellular sites within the human host (25). We provided in the present study both pharmacological and genetic evidence for a novel iron uptake system that plays a crucial role in MC replication within cultured epithelial cells. The iron chelator Desferal inhibited intracellular replication of MC while leaving its extracellular growth unaffected. An hmbR mutant, which was defective in growth in liquid media, replicated more rapidly than its wt parent strain within epithelial cells. These two results suggest that iron uptake is essential for intracellular growth of MC and that the element is derived from a host cell source. Most importantly, a tonB mutant was unable to replicate within epithelial cells without ferric nitrate supplementation. This result implicates a TonB-dependent receptor in the acquisition of this intracellular iron.
The intracellular iron source remains unidentified; however, transferrin, lactoferrin, hemoglobin, free heme, and free iron are unlikely candidates (Table 1). An fbpA mutant that cannot utilize transferrin or lactoferrin iron replicated normally within cells, thus ruling out these iron-containing compounds as intracellular iron sources. An hmbR mutant replicated faster than its wt parent within cells, thus ruling out hemoglobin. A tonB mutant that grows well on free heme or free iron sources was unable to grow within epithelial cells without iron supplementation, indicating that these iron sources may not be available to intracellular MC. Taken together, our results predict that MC obtains its intracellular iron from a novel source.
A heme-based iron source appears to be an unlikely candidate for two reasons. First, it has been demonstrated that a Neisseria mutant that requires exogenous heme for growth is unable to replicate within epithelial cells (30). Second, Desferal cannot chelate heme-iron and therefore should not interfere with heme-iron uptake. However, it strongly inhibits MC intracellular growth. While the identity of the intracellular iron source is unknown, clues to its identify may lie in the recently published MC genome sequences. An examination of these databases revealed several candidates for TonB-dependent receptors whose functions are unknown. Their role in MC intracellular growth is being examined.
In summary, we have provided evidence of a novel iron uptake system in N. meningitidis that utilizes a host cell iron source for its growth within human epithelial cells in culture. Intracellular replication may promote its crossing of the epithelial barrier and the development of septicemia and/or meningitis. The identification of this iron uptake system in MC may therefore provide an additional target for antimeningococcal therapy. In addition, identification of the host cell iron source may shed new light on mammalian iron metabolism.
Acknowledgments
We thank Heng Khun for providing the insertionally inactivated fpbA DNA. We also thank Eldine Argyle for technical support. We are also grateful to Laura Potter and Robert Bonnah for review of the manuscript.
This work was supported in part by NIH grant AI32493 to M.S.
REFERENCES
- 1.Al-Younes, H. M., T. Rudel, V. Brinkmann, A. J. Szczepek, and T. F. Meyer. 2001. Low iron availability modulates the course of Chlamydia pneumoniae infection. Cell Microbiol. 3:427-437. [DOI] [PubMed] [Google Scholar]
- 2.Barnewall, R. E., and Y. Rikihisa. 1994. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron-transferrin. Infect. Immun. 62:4804-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calver, G. A., C. P. Kenny, and G. Lavergne. 1976. Iron as a replacement for mucin in the establishment of meningococcal infection in mice. Can J. Microbiol. 22:832-838. [DOI] [PubMed] [Google Scholar]
- 4.Carson, S. D., P. E. Klebba, S. M. Newton, and P. F. Sparling. 1999. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J. Bacteriol. 181:2895-2901. [DOI] [PMC free article] [PubMed]
- 5.Conrad, M. E., and J. N. Umbreit. 2000. Iron absorption and transport: an update. Am. J. Hematol. 64:287-298. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, C. E., G. R. Lynagh, K. P. Hoyes, R. C. Hider, R. Cammack, and J. B. Porter. 1996. The relationship of intracellular iron chelation to the inhibition and regeneration of human ribonucleotide reductase. J. Biol. Chem. 271:20291-20299. [DOI] [PubMed] [Google Scholar]
- 7.Dautry-Varsat, A., A. Ciechanover, and H. F. Lodish. 1983. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 80:2258-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray-Owen, S. D., and A. B. Schryvers. 1996. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 4:185-191. [DOI] [PubMed] [Google Scholar]
- 9.Hopper, S., J. S. Wilbur, B. L. Vasquez, J. Larson, S. Clary, I. J. Mehr, H. S. Seifert, and M. So. 2000. Isolation of Neisseria gonorrhoeae mutants that show enhanced trafficking across polarized T84 epithelial monolayers. Infect. Immun. 68:896-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isberg, R. R., and S. Falkow. 1985. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured cells by Escherichia coli K-12. Nature 317:262-264. [DOI] [PubMed] [Google Scholar]
- 11.Khun, H. H., S. D. Kirby, and B. C. Lee. 1998. A Neisseria meningitidis fbpABC mutant is incapable of using nonheme iron for growth. Infect. Immun. 66:2330-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis, L. A., E. Gray, Y. P. Wang, B. A. Roe, and D. W. Dyer. 1997. Molecular characterization of hpuAB, the haemoglobin-haptoglobin- utilization operon of Neisseria meningitidis. Mol. Microbiol. 23:737-749. [DOI] [PubMed] [Google Scholar]
- 13.Lewis, L. A., M. H. Sung, M. Gipson, K. Hartman, and D. W. Dyer. 1998. Transport of intact porphyrin by HpuAB, the hemoglobin-haptoglobin utilization system of Neisseria meningitidis. J. Bacteriol. 180:6043-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin, L., P. Ayala, J. Larson, M. Mulks, M. Fukuda, S. R. Carlsson, C. Enns, and M. So. 1997. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol. Microbiol. 24:1083-1094. [DOI] [PubMed] [Google Scholar]
- 15.Merz, A. J., and M. So. 2000. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423-457. [DOI] [PubMed] [Google Scholar]
- 16.Moeck, G. S., and J. W. Coulton. 1998. TonB-dependent iron acquisition: mechanisms of siderophore-mediated active transport. Mol. Microbiol. 28:675-681. [DOI] [PubMed] [Google Scholar]
- 17.Nassif, X., J. Lowy, P. Stenberg, P. O'Gaora, A. Ganji, and M. So. 1993. Antigenic variation of pilin regulates adhesion of Neisseria meningitidis to human epithelial cells. Mol. Microbiol. 8:719-725. [DOI] [PubMed] [Google Scholar]
- 18.Olakanmi, O., B. E. Britigan, and L. S. Schlesinger. 2000. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect. Immun. 68:5619-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponka, P., and C. N. Lok. 1999. The transferrin receptor: role in health and disease. Int. J. Biochem. Cell Biol. 31:1111-1137. [DOI] [PubMed] [Google Scholar]
- 20.Pope, C. D., W. O'Connell, and N. P. Cianciotto. 1996. Legionella pneumophila mutants that are defective for iron acquisition and assimilation and intracellular infection. Infect. Immun. 64:629-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 22.Raulston, J. E. 1997. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect. Immun. 65:4539-4547. [DOI] [PMC free article] [PubMed]
- 23.Rouault, T., K. Rao, J. Harford, E. Mattia, and R. D. Klausner. 1985. Hemin, chelatable iron, and the regulation of transferrin receptor biosynthesis. J. Biol. Chem. 260:14862-14866. [PubMed] [Google Scholar]
- 24.Schryvers, A. B., and G. C. Gonzalez. 1990. Receptors for transferrin in pathogenic bacteria are specific for the host's protein. Can. J. Microbiol. 36:145-147. [DOI] [PubMed] [Google Scholar]
- 25.Schryvers, A. B., and I. Stojiljkovic. 1999. Iron acquisition systems in the pathogenic Neisseria. Mol. Microbiol. 32:1117-1123. [DOI] [PubMed] [Google Scholar]
- 26.Stephens, D. S., L. H. Hoffman, and Z. A. McGee. 1983. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells. J. Infect. Dis. 148:369-376. [DOI] [PubMed] [Google Scholar]
- 27.Stojiljkovic, I., V. Hwa, L. de Saint Martin, P. O'Gaora, X. Nassif, F. Heffron, and M. So. 1995. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol. Microbiol. 15:531-541. [DOI] [PubMed] [Google Scholar]
- 28.Stojiljkovic, I., J. Larson, V. Hwa, S. Anic, and M. So. 1996. HmbR outer membrane receptors of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J. Bacteriol. 178:4670-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stojiljkovic, I., and N. Srinivasan. 1997. Neisseria meningitidis tonB, exbB, and exbD genes: Ton-dependent utilization of protein-bound iron in neisseriae. J. Bacteriol. 179:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner, P. C., C. E. Thomas, C. Elkins, S. Clary, and P. F. Sparling. 1998. Neisseria gonorrhoeae heme biosynthetic mutants utilize heme and hemoglobin as a heme source but fail to grow within epithelial cells. Infect. Immun. 66:5215-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberg, E. D. 1985. Roles of iron in infection and neoplasia. J. Pharmacol. 16:358-364. [PubMed] [Google Scholar]
- 32.Yancey, R. J., and R. A. Finkelstein. 1981. Assmilation of iron by pathogenic Neisseria spp. Infect. Immun. 32:592-599. [DOI] [PMC free article] [PubMed] [Google Scholar]