Abstract
Several genetic loci in the mouse have been identified that regulate the severity of Lyme arthritis. The region of chromosome 5 including the osteopontin (OPN) gene (Opn) has been identified in intercross populations of C3H/HeN × C57BL/6 and C3H/HeJ × BALB/cAnN mice. OPN is of particular interest as it is involved in the maintenance and remodeling of tissue during inflammation, it regulates production of interleukin-10 (IL-10) and IL-12 (cytokines implicated in Lyme arthritis), it is necessary for host control of certain bacterial infections, and mice displaying different severities of Lyme arthritis possess different alleles of the OPN gene. Macrophages and splenocytes from OPN-deficient mice on mixed C57BL/6J-129S or inbred 129S backgrounds were stimulated with the Pam3Cys modified lipoprotein from Borrelia burgdorferi, OspA. OPN was not required for OspA-induced cytokine production; however, macrophages from 129S-Opn−/− mice displayed a reduced level of IL-10 production. OPN was also not required for resistance to severe arthritis, as B. burgdorferi-infected 129S-Opn−/− mice developed mild arthritis, as did their wild-type littermates. Arthritis was more severe in OPN-deficient mice on the mixed C57BL/6J-129S backgrounds than in inbred mice of either strain. This increase was most likely due to a gene(s) closely linked to Opn on chromosome 5 in conjunction with other randomly assorting genes. Deficiency in OPN did not influence the numbers of spirochetes in tissues from B. burgdorferi-infected mice, indicating OPN is not part of the host defense to this pathogen. Interestingly, there was no alteration in the B. burgdorferi-specific antibody isotypes in OPN-deficient mice, indicating that its effect on helper T-cell responses is not relevant to the host response to B. burgdorferi.
Lyme disease is a tick-transmitted infection caused by the spirochete Borrelia burgdorferi (11, 21, 34). Upon transmission, the bacteria disseminate from the site of inoculation and are responsible for multiple symptoms in the human host. In the absence of antibiotic treatment, arthritis, carditis, neurological complications, and skin abnormalities may develop, with great variability in the severity of disease in different individuals (34). Arthritis is a late-stage symptom seen in approximately 60% of individuals who are not treated with antibiotics at the time of infection. This subacute arthritis is associated with the presence of spirochetes in the joint tissue and is characterized by edema, tendonitis, synovitis, and inflammatory cell infiltrate of predominantly neutrophils and mononuclear cells (33, 34, 42).
Mice infected with B. burgdorferi develop arthritis similar to that found in humans, including tendonitis, synovitis, infiltration of inflammatory cells, and, in the most severe cases, new bone formation (5). Further, there is variability in the severity of disease among mouse strains, similar to that of the spectrum of disease seen in the human population. The mouse model of Lyme disease has allowed exploration of the role that the host inflammatory response plays in the development of Lyme arthritis and established that this is an inflammatory process not requiring the presence of T and B lymphocytes (52). Infected C3H mice develop severe arthritis, which occurs in the presence of large numbers of infiltrating spirochetes (6, 8, 54). C57BL/6 mice also harbor large numbers of spirochetes in their joints; they characteristically develop mild to moderate arthritis (6, 7, 26). The difference in arthritis severity between these strains of mice does not appear to be regulated by the mice's ability to control spirochete numbers but by their ability to control the inflammatory process in the joint.
A mapping project has been carried out in this laboratory, using the F2 intercross generation of C3H/HeNCr and C57BL/6NCr mice, with the goal of identifying genes regulating the severity of Lyme arthritis (51). Four chromosomal regions were linked to arthritis development by quantitative trait locus (QTL) analysis, identifying arthritis development as a complex, multigenic trait. Two of the QTL identified, Bb2 and Bb3, are located on chromosome 5. Composite interval mapping analysis of four reciprocal backcross populations, B6C3F1 × C57BL/6N, B6C3F1 × C3H/HeN, and (C3H/HeJ × BALB/cAnN) × C3H/HeN as well as the original (C3H/HeN × C57BL/6N) F2 intercross population, suggests the presence of four QTL on chromosome 5 regulating arthritis severity (46). These QTL span the previously mapped Bb2 and Bb3 loci and are found at 45, 53 to 60, 66, and 72 to73 centimorgans. Osteopontin (Opn), or spp1, has been identified as a candidate gene located at 56 centimorgans.
The pleiotropic effects on biological, immune, and inflammatory processes of Opn are attested to by the diverse names assigned to this locus: osteopontin (OPN), secreted phosphoprotein 1 (spp1), early T-cell activation factor-1 (eta-1), 2ar, and transformation-associated phosphoprotein (14, 36, 43, 50). The Rickettsia resistance locus (ric) and OPN (spp1) map to the same region of chromosome 5 (16, 38). In fact, the polymorphic alleles of spp1 are proposed to be responsible for different T-cell responses to and host defense against Rickettsia tsutsugamushi and for resistance in C57BL/6 mice and susceptibility in C3H/HeN mice (35, 38, 39). Importantly, this involvement of OPN alleles in host defense against Rickettsia has not been confirmed with purified proteins or with OPN-deficient mice.
OPN is a secreted phosphoprotein which contains an RGD domain and interacts with a number of integrin receptors and CD44. It appears to be involved in the maintenance and remodeling of tissue during inflammation, including wound healing, bone remodeling, and cell-mediated immunity (14, 36, 50). OPN is involved in the control of a variety of pathogens which cause chronic disease, as mice lacking the OPN gene show increased susceptibility to Mycobacterium bovis and Listeria monocytogenes (4, 32). Intradermal injection of OPN leads to the infiltration of inflammatory cells, largely macrophages, as well as a small increase in polymorphonuclear cells (17, 48). This suggests that early in infection or wounding, the production of OPN may recruit inflammatory cells to the site of infection.
In vitro, OPN has both pro- and anti-inflammatory properties. Nitric oxide production by macrophages is suppressed in the presence of OPN, suggesting an anti-inflammatory role (19, 45). Recently, Ashkar and colleagues demonstrated a proinflammatory action of OPN (4). OPN independently stimulates the production of interleukin-12 (IL-12) by macrophages while downregulating the production of IL-10 by macrophages stimulated with lipopolysaccharide (LPS). Both IL-10 and IL-12 have been implicated in the regulation of murine Lyme arthritis (2, 3, 10).
Many of the properties of Opn suggest it as a candidate gene for regulation of the host response to B. burgdorferi infection and development of Lyme arthritis: (i) the involvement of OPN in inflammation and regulation of IL-12 and IL-10, bone remodeling, wound repair, and the immune response to pathogens; (ii) the physical position of OPN within a linkage group on chromosome 5; and (iii) the fact that severely and mildly arthritic strains of mice possess distinct alleles for OPN implicated in resistance to other bacteria. The development of mice with a targeted disruption in the OPN gene allowed assessment of its involvement in host response to B. burgdorferi.
MATERIALS AND METHODS
Mice.
Female C3H/HeN mice were obtained from the National Cancer Institute. Female C57BL/6J-129S and B6129SF2/J mice were obtained from the Jackson Laboratories (Bar Harbor, Maine). B6129SF2/J mice are an F2 intercross of the C57BL/6J and 129S mouse strains. OPN-deficient mice were generated as previously described (44). Briefly, gene-targeting techniques were used to interrupt the OPN gene in 129S-derived AB2.1 embryonic stem cells. Chimeric males were mated to either C57BL/6J or 129S females, and the subsequent heterozygous F1 animals were crossed to generate Opn+/+ or Opn−/− lines. Hereafter, mice from the C57BL/6J line are designated as (C57BL/6J × 129S)-Opn and mice from the 129S line as 129S-Opn. The (C57BL/6J × 129S)-Opn−/− line was crossed with C57BL/6J mice to produce heterozygous offspring, which were mated to generate litters with Opn−/− and Opn+/+ littermates. This line was designated C57BL/6J × (C57BL/6J × 129S)-Opn. Mice were housed in the Animal Resource Center at the University of Utah Medical Center according to the National Institutes of Health guidelines for care and use of laboratory animals.
Reagents.
Lipidated recombinant OspA (OspA) from the B31 strain of B. burgdorferi was a gift from Robert Huebner (Connaught Laboratories, Swiftwater, Pa.). OspA has been shown to possess stimulatory properties similar to those of native OspA purified from B. burgdorferi. OspA contained less than 0.3 endotoxin unit/500 ng of protein as determined by the Limulus amoebocyte lysate assay (Associates of Cape Cod, Woods Hole, Mass.). Glutathione transferase (GST)-OPN was produced as described by Xuan et al. (53) and its bioactivity established in our laboratories with in vitro cellular adhesion, apoptosis, and proliferative assays (data not shown). Commercial anti-mouse OPN antibody (Ab) was from R&D Systems (Minneapolis, Minn.) and has been shown to neutralize the bioactivity of mouse OPN with in vitro cellular adhesion assays. Recombinant standards and paired monoclonal Abs for murine IL-6, IL-10, and IL-12p40 were purchased from Pharmingen (San Diego, Calif.). The OptEIA mouse IL-12p70 set from Pharmingen was used to assess IL-12p70. Avidin-horseradish peroxidase was purchased from Vector Laboratories (Burlingame, Calif.).
Cytokine production by splenocyte cultures.
Splenocytes were cultured in 24-well plates at a concentration of 107 cells per ml in serum-free medium containing the indicated stimuli. Serum-free medium is composed of RPMI (Gibco-BRL, Gaithersburg, Md.) containing 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, 1% (vol) of the serum replacement Nutridoma SP (Boehringer Mannheim, Indianapolis, Ind.), and 0.01 mg of gentamicin sulfate per ml. Recombinant OPN was added 1 h after stimulation as described by Ashkar et al. (4). Supernatants were harvested at 24 to 36 h after stimulation and assayed. Polymyxin B (Sigma, St. Louis, Mo.) was used at a concentration of 5 μg/ml.
Cytokine production by macrophage cultures.
Murine macrophages were obtained from femur and tibia bone marrow as previously described (27). Briefly, bone marrow cells were cultured in macrophage medium composed of RPMI containing 20% horse serum, 30% L929-conditioned medium, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, and 0.01 mg of gentamicin sulfate per ml for 5 days at 37°C. Macrophages were recovered using ice-cold PBS and replated in 24-well culture dishes at 6 × 105 cells/ml in serum-free medium containing Nutridoma SP as described above. Following overnight incubation at 37°C, nonadherent cells were removed and various stimuli were added. Again, GST-OPN and anti-OPN Ab were added 1 h after stimulation with OspA. Gamma interferon (IFN-γ) is required for optimal macrophage production of nitric oxide; therefore, samples were run in duplicate, with one set containing 10 U of recombinant IFN-γ/ml (Pharmingen).
Cytokine and nitric oxide assays.
All cytokines were assayed immediately upon harvesting supernatants or were frozen at −20°C. Supernatants were assayed for IL-6, IL-10, IL-12p40, and IL-12p70 by enzyme-linked immunosorbent assay (ELISA) using paired monoclonal Abs. Values were obtained by comparison to a recombinant protein standard within the linear range. Although IL-12p70 was not detected in the supernatants described in this paper, the standard was detectable.
Nitrite levels in supernatants were assayed as previously described (15). Briefly, 100 μl of Griess reagent [equal volumes of 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride in 60% acetic acid and 1% sulfanilamide in 30% acetic acid] was added to 50 μl of supernatant. Samples were read at 570 nm on a 96-well microtest plate spectrophotometer (Molecular Devices, Sunnyvale, Calif.). Values were obtained by comparison to a sodium nitrite standard.
Bacteria and infection.
Age-matched mice between 5 and 8 weeks of age were infected by intradermal injection in the shaven back with 2 × 103 bacteria of the N40 isolate of B. burgdorferi (provided by S. Barthold, University of California at Davis, at passage 3 from an infected mouse). This mode of infection is reported to require the fewest spirochetes and to most closely mimic tick transmission. Passage 4 spirochetes were grown in Barbour-Stoenner-Kelly H (BSK-H) medium containing 6% rabbit serum (Sigma, St. Louis, Mo.) for 3 to 5 days prior to injection, enumerated using a Petroff-Hausser chamber, and diluted with sterile medium. Mock-infected animals received intradermal injections of sterile BSK-H medium in the shaven back and tested negative for B. burgdorferi infection by both PCR and serology.
Measurement of ankle joints.
Rear ankle joints of mice anesthetized with methoxyflurane (Schering-Plough, Union, N.J.) were measured with a metric caliper (Mitutoyo, Tokyo, Japan) during each week of infection or at the times of infection and sacrifice. Measurements were taken in the anterior-to-posterior position, with the ankle extended, through the thickest portion of the ankle. Numerous factors can influence normal joint measurements, including age, sex, and strain of mice; therefore, findings are reported as the change in joint measurement relative to the same animal's joint measurement prior to infection. This measurement of arthritis severity primarily reflects edema and has been used to identify several QTL in intercross populations of C3H/HeN × C57BL/6 mice and C3H/HeJ × BALB/cAnN mice. Two QTL for ankle swelling were identified on chromosome 5 (46, 51).
Histopathology of ankle joints.
The rear ankle joint displaying the greatest swelling at the time of sacrifice was taken from each mouse for histological analysis. Samples were fixed in 10% formalin, decalcified, and embedded in paraffin, and sections were stained with hematoxylin and eosin. Sections were viewed in a blind fashion and given a score for arthritis severity on a scale ranging from 0 to 4+. A score of 4+ was characterized by a large region of edema, with the presence of many neutrophils, thickening of the tendon sheath, and evidence of bone and cartilage abnormalities within the tendon sheath. A joint with a score of 1+ displayed slight thickening of the tendon sheath with little edema and neutrophil infiltration, whereas a score of 0 was given to samples indistinguishable from mock-infected controls. Scores of 2 and 3 were assigned to samples with intermediate pathologies (26). Several QTL regulating histopathologically determined arthritis severity have been identified in mouse intercross populations, including at least two distinct QTL on chromosome 5 (46, 51).
Preparation of DNA from infected tissues.
Mice were sacrificed at 4 weeks postinfection, and rear ankle joint, heart, and ear tissues were prepared as previously described (26). Briefly, tissue specimens were incubated in 0.1% collagenase A (Boehringer Mannheim) solution at 37°C overnight and were then mixed with an equal volume of proteinase K solution (Gibco-BRL) and incubated at 55°C overnight. DNA was then recovered by extraction with an equal volume of phenol-chloroform and precipitated with ethanol. After digestion with 1.0 mg of DNase-free RNase (Gibco-BRL) per ml, the samples were extracted again and DNA was recovered by precipitation. The precipitated DNA was resuspended in 0.5 to 1 ml of Tris-EDTA and diluted to 50 μg/ml.
Quantification of DNA from infected tissues.
The levels of B. burgdorferi DNA in joints, hearts, and ears were determined by continuous-monitoring PCR using the LightCycler (Idaho Technologies, Idaho Falls, Idaho) as previously described (31). Briefly, 200 ng of sample DNA was amplified in a final volume of 10 μl containing 50 mM Tris (pH 8.3), 3 mM MgCl2, 4.5 μg of bovine serum albumin, 200 μM deoxynucleoside triphosphates, an approximately 1:30,000 dilution of SYBR Green I (Molecular Probes, Eugene, Oreg.), 5 μM concentrations of each primer, 0.5 U of Taq polymerase (Gibco-BRL), and 110 ng of TaqStart Ab (ClonTech, Palo Alto, Calif.). Forty cycles of amplification were performed, with each cycle consisting of three steps: heating at 20°C/s to 95°C with a 1-s hold, cooling at 20°C/s to 60°C with a 1-s hold, and heating at 1°C/s to 84°C. This technique monitors the cycle-by-cycle accumulation of fluorescently labeled product. The cycle at which the product is first detected is an indicator of relative starting copy number and was calculated using the LightCycler analysis software. B. burgdorferi was quantified using the chromosomally encoded recA gene, and values were normalized to 103 copies of a single-copy mouse gene, nidogen. The oligonucleotide primers used to detect mouse nidogen were nido.F (5′-CCA GCC ACA GAA TAC CAT CC-3′) and nido.R (5′-GGA CAT ACT CTG CTG CCA TC-3′). The oligonucleotide primers used to detect B. burgdorferi recA were nTM17.F (5′-GTG GAT CTA TTG TAT TAG ATG AGG CTC TCG-3′) and nTM17.R (5′-GCC AAA GTT CTG CAA CAT TAA CAC CTA AAG-3′). DNA prepared from mock-infected mice sacrificed at the same time as infected mice served as a negative control for contamination during tissue preparations and was free of Borrelia-specific amplification products.
Detection of B. burgdorferi-specific immunoglobulin (Ig) levels.
Serum obtained by retro-orbital bleeding of experimental animals at sacrifice was analyzed for B. burgdorferi-specific Ab using an Ab capture ELISA. Eleven columns per 96-well plate were coated with B. burgdorferi sonicate at a concentration of 5 μg of sonicate/ml. The 12th column was coated with a polyclonal rabbit anti-mouse immunoglobulin, IgG plus IgA plus IgM (Zymed, South San Francisco, Calif.), at a concentration of 5.0 μg/ml. Serum samples were added to B. burgdorferi sonicate-coated wells and analyzed for total IgG at 1:320,000 and 1:640,000 dilutions and for isotype-specific Ab content at the following dilutions: 1:10, 1:50, 1:100, and 1:500. In all cases the dilutions chosen for estimation were within the linear range of a standard curve of total IgG, IgG1, IgG2a, IgG2b, or IgG3 run in parallel. Serum samples from uninfected mice did not possess Borrelia-specific Ig.
Statistical analysis.
The degrees of statistical significance of the quantitative differences between sample groups were determined by application of Student's t test.
RESULTS
Cytokine regulation in bone marrow-derived macrophages from OPN-deficient mice.
The stimulatory properties of the B. burgdorferi outer-surface protein OspA have been well documented, and the various lipoproteins associated with the bacteria are responsible for the majority of the inflammatory activity of the organism, as demonstrated with both living bacteria and sonicated preparations of bacteria (28, 47). Although the OspA lipoprotein is expressed abundantly on cultured organisms, more than 10% of the genome of B. burgdorferi encodes proteins with the Pam3Cys modification that are thought to be sequentially expressed during infection. Bone marrow-derived macrophages from OPN-deficient mice on the C57BL/6J × (C57BL/6J × 129S) background were cultured and stimulated with OspA in the presence or absence of recombinant OPN. ELISA assays were used to determine the quantity of IL-6, IL-10, and IL-12p40 present in 24-hour supernatants. The amount of nitric oxide produced, in the form of nitrite, was determined using the Griess assay. In multiple experiments, no significant differences in the production of cytokines or nitric oxide were seen between the OPN-deficient and wild-type macrophages on the C57BL/6J × (C57BL/6J × 129S) background (Table 1). Further, the addition of recombinant OPN had no effect on cytokine production. IL-12p70 production was below detectable levels.
TABLE 1.
OspA-induced cytokine and nitrite production by bone marrow macrophages from Opn+/+ and Opn−/− mice on the C57BL/6J × (C57BL/6J × 129S) backgrounda
| Cytokine or nitrite | Level produced in:b
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Opn+/+ mice
|
Opn−/− mice
|
||||||||
| Medium | OPN | OspA | OspA + OPN | Medium | OPN | OspA | OspA + OPN | ||
| Nitritec | 2.7 ± 0.3 | 3.3 ± 0.0 | 62.1 ± 0.9 | 68.5 ± 1.2 | 1.4 ± 0.6 | 3.8 ± 2.8 | 94.4 ± 3.8 | 88.1 ± 0.6 | |
| IL-6 | 0 ± 0 | 0 ± 0 | 6.5 ± 0.5 | 7.0 ± 0.8 | 0 ± 0 | 0 ± 0 | 5.0 ± 0.2 | 6.1 ± 0.3 | |
| IL-10 | 11 ± 5 | 11 ± 26 | 614 ± 42 | 584 ± 0.0 | 0 ± 10 | 0 ± 0 | 464 ± 64 | 562 ± 53 | |
| IL-12p40 | 0 ± .02 | 0 ± .01 | 1.5 ± 0.004 | 1.8 ± 0.04 | 0 ± .01 | 0 ± .02 | 1.3 ± 0.03 | 1.4 ± 0.07 | |
Macrophages were cultured at 6 × 105 cells/ml in 24-well culture plates and treated with medium containing 500 ng of OspA/ml or 500 ng of OspA/ml plus 1 μg of GST-OPN/ml. Supernatants were harvested at 24 h and analyzed by ELISA or Griess assay as described in Materials and Methods. Results are presented as the arithmetic means ± standard deviations of duplicate samples.
Values for nitrites, IL-6, IL-10, and IL-12p40 in μM, ng/ml, pg/ml, and ng/ml, respectively.
Macrophages stimulated in the presence of 10 U of IFN-γ/ml.
Stimulation of bone marrow macrophages on the 129S background with OspA revealed a two- to fourfold increase in IL-10 production in the OPN-deficient mice when compared to that of wild-type controls (Table 2). However, IL-10 production was not influenced by the presence of exogenous OPN or neutralizing Abs to OPN. No significant differences were seen in nitric oxide, IL-6, or IL-12p40 production between the OPN-deficient and wild-type macrophages stimulated with OspA on the 129S background (Table 2). IL-12p70 production was again below the threshold of detection. These data indicate that IL-10 is upregulated in 129S-Opn−/− macrophages stimulated with OspA.
TABLE 2.
OspA-induced cytokine and nitrite production by bone marrow macrophages from Opn+/+ and Opn−/− mice on the inbred 129S backgrounda
| Cytokine or nitrite | Level produced in:b
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Opn+/+ mice
|
Opn−/− mice
|
||||||||||||
| Medium | OPN | Anti-OPN | OspA | OspA + OPN | OspA + anti-OPN | Medium | OPN | Anti-OPN | OspA | OspA + OPN | OspA + anti-OPN | ||
| Nitritec | 2.2 ± 0.3 | 1.8 ± 1.0 | 2.4 ± 1.3 | 58 ± 3.0 | 60 ± 0.6 | 52 ± 2.0 | 2.9 ± 0.3 | 3.1 ± 1.2 | 2.9 ± 1.5 | 64 ± 2.0 | 63 ± 1.0 | 59 ± 0.9 | |
| IL-6 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 11 ± 0.4 | 15 ± 0.9 | 12 ± 1.4 | 0 ± 1.5 | 0 ± 0 | 0.1 ± 0.4 | 15 ± 2.2 | 18 ± 5.4 | 18 ± 1.9 | |
| IL-10 | 0 ± 0 | 0 ± 5 | 28 ± 5 | 214 ± 5.0d | 226 ± 0.0 | 288 ± 11 | 0 ± 0 | 0 ± 44 | 43 ± 5 | 743 ± 5.0 | 626 ± 5.0 | 630 ± 0.0 | |
| IL-12p40 | 0 ± 19 | 0 ± 9 | 9 ± 5 | 470 ± 14 | 640 ± 5.0 | 536 ± 23 | 0 ± 10 | 0 ± 14 | 22 ± 14 | 608 ± 23 | 776 ± 19 | 645 ± 23 | |
Macrophages were cultured at 6 × 105 cells/ml in 24-well culture plates and treated with medium containing 500 ng of OspA/ml, 500 ng of OspA/ml plus 1 μg of GST-OPN/ml or 500 ng of OspA/ml plus 10 μg of anti-OPN antibody/ml. Supernatants were harvested at 24 h and analyzed by ELISA or Griess assay as described in Materials and Methods. Results are presented as the arithmetic means ± standard deviations of duplicate samples.
Values for nitrites, IL-6, IL-10, and IL-12p40 in μM, ng/ml, pg/ml, and pg/ml, respectively.
Macrophages were stimulated in the presence of 10 U of IFN-γ/ml.
Boldface type indicates a significant difference between wild-type and OPN−/− macrophages.
Cytokine regulation in splenocytes from OPN-deficient mice.
Bone marrow macrophages derived from OPN-deficient mice and stimulated with OspA did not reveal any alteration in IL-12 production. Interestingly, Ashkar and colleagues reported regulation of IL-12p40 and p70 in peritoneal macrophages, draining lymph node cells, and splenic macrophages (4). Others have found that OPN regulation of IL-12 production by human monocytes is T-cell dependent (37). Therefore, we examined cytokine production by splenocytes, a mixed population of cells including macrophages, B cells, and T cells.
Splenocytes were isolated from OPN-deficient and wild-type mice of both the C57BL/6J × (C57BL/6J × 129S) and inbred 129S backgrounds. OspA was added to the splenocytes and OPN or anti-OPN Ab was added one hour later, as indicated. Supernatants were removed 24 to 36 h after stimulation and assessed for the production of IL-6, IL-10, and IL-12p40. No differences in cytokine production were seen on the C57BL/6J × (C57BL/6J × 129S) background (data not shown) or the 129S background (Table 3). The addition of recombinant OPN or neutralizing Ab had no effect on cytokine production. Therefore, the absence of OPN has no effect on cytokine production by splenocytes stimulated with OspA.
TABLE 3.
OspA-induced cytokine production by splenocytes from Opn+/+ and Opn−/− mice on the inbred 129S backgrounda
| Cytokine | Level produced in:b
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Opn+/+ mice
|
Opn−/− mice
|
||||||||
| Medium | OspA | OspA + OPN | OspA + anti-OPN | Medium | OspA | OspA + OPN | OspA + anti-OPN | ||
| IL-6 | 0.01 ± 0.27 | 1.7 ± 0.12 | 1.5 ± 0.13 | 1.7 ± 0.15 | 0.2 ± 0.05 | 2.1 ± 0.02 | 2.1 ± 0.09 | 2.4 ± 0.11 | |
| IL-10 | 105 ± 39 | 536 ± 11 | 545 ± 53 | 495 ± 29 | 61 ± 6 | 418 ± 15 | 442 ± 36 | 409 ± 17 | |
| IL-12p40 | 0.9 ± 0.08 | 1.6 ± 0.007 | 1.6 ± 0.053 | 1.5 ± 0.107 | 0.5 ± 0.011 | 1.3 ± 0.019 | 1.2 ± 0.027 | 1.3 ± 0.096 | |
Splenocytes were cultured at 107 cells/ml in 24-well culture plates and treated with medium containing 500 ng of OspA/ml, 500 ng of OspA/ml plus 1 μg of GST-OPN/ml, or 500 ng of OspA/ml plus 10 μg of anti-OPN antibody/ml. Supernatants were harvested at 36 h and analyzed by ELISA as described in Materials and Methods. Results are presented as the arithmetic means ± standard deviations of duplicate samples.
Values for IL-6, IL-10, and IL-12p40 in ng/ml, pg/ml, and ng/ml, respectively.
Effects of genetic deletion of Opn on B. burgdorferi-induced arthritis on the C57BL/6J × 129S, C57BL/6J × (C57BL/6J × 129S) backgrounds.
OPN-deficient mice were developed using gene-targeting techniques in the 129S-derived embryonic stem cell line AB2.1. Chimeric males were mated to C57BL/6J females to create an OPN-deficient line (44). The initial infection experiments were performed using these C57BL/6J × 129S-Opn−/− mice (Fig. 1). Because wild-type littermates were unavailable for use as a control in these experiments, a variety of mouse strains were utilized to control for the background of the mice, including C3H/HeN, C57BL/6J, 129S, and B6129SF2/J mice. C3H/HeN mice develop severe arthritis upon infection with B. burgdorferi, while C57BL/6 mice are resistant and develop mild arthritis (6-8, 26, 54). It has been reported that 129 strains also develop mild arthritis (18). B6129SF2/J mice were used as an additional control for the mixed background of the C57BL/6J × 129S-Opn−/− mice. These mice would be expected to develop mild arthritis, as both parental backgrounds are resistant to severe arthritis.
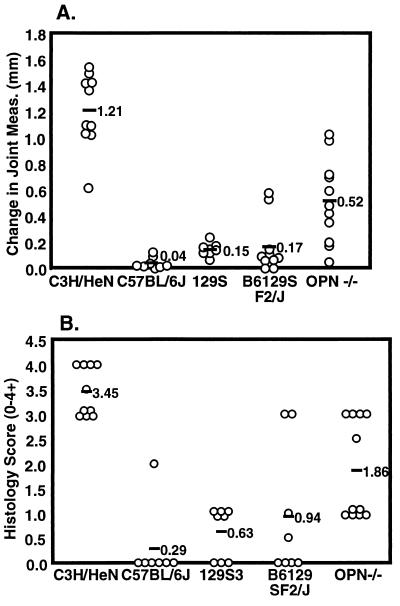
FIG. 1.
Arthritis severity in B. burgdorferi-infected C57BL/6J × 129S-Opn−/− mice. Mice were infected with 2 × 103 B. burgdorferi bacteria, and joint swelling (A) and histopathology (B) were assessed as described in Materials and Methods.
Mice were each infected with 2 × 103 B. burgdorferi intradermally in the shaven back. Infection was allowed to proceed for 4 weeks, at which time the mice were sacrificed. Arthritis severity was assessed blindly using two methods, joint measurement and histopathology. Joints were measured prior to infection and either weekly or at the time of sacrifice. Joint swelling is reported as the change in joint measurement relative to the same animal's joint measurement prior to infection. Swelling was most severe at week 4 of infection (Fig. 1a). C3H/HeN mice developed severe joint swelling while C57BL/6J and 129S mice developed mild joint swelling, as expected. The B6129SF2/J mice showed a range of joint swelling, with most animals having mild swelling while two mice developed moderate to severe joint swelling. The C57BL/6J × 129S-Opn−/− mice show a range of joint swelling from mild to severe.
As a second assessment of arthritis, one joint from each animal was taken at sacrifice for histological analysis and examined for lesion severity. C3H/HeN mice had severe arthritis lesion scores (3+ to 4+), indicating hyperproliferation of the tendon sheath surrounding the cranial tibial tendon and inflammatory cell infiltrate predominated by neutrophils. The C57BL/6J mice displayed no arthritis (0), with the exception of one mouse that had a moderate arthritis lesion score (2+). 129S mice had mild arthritis scores (0 to 1+) (Fig. 1b). The B6129SF2/J mice had mild arthritis lesions (0 to 1+), with the exception of the two mice that had severe joint swelling and also showed severe arthritis lesions (3+). The C57BL/6J × 129S-Opn−/− mice developed a range of arthritis lesion severity from mild to severe (1+ to 3+).
It was surprising to find that two mice within the wild-type B6129SF2/J group developed severe arthritis. Although both the parental strains are resistant to severe arthritis, it appears that there must be combinations of C57BL/6J and 129S genes which promote severe arthritis upon infection with B. burgdorferi. Each mouse of the F2 generation possesses a unique random mixture of C57BL/6J and 129S alleles, which could allow exposure of hidden genetic susceptibilities. In fact, in the previously published C3H/HeNCr × C57BL/6NCr mapping study, susceptibility genes were identified on the C57BL/6 background and were only unmasked when those mice were intercrossed with C3H/HeNCr mice (46).
A tremendous range of arthritis severity, from mild to severe, was seen in the infected C57BL/6J × 129S-Opn−/− mice (Fig. 1). The finding that resistance to severe arthritis was maintained in over half of the infected C57BL/6J × 129S-Opn−/− mice argued that OPN itself was not responsible for arthritis resistance and that genes at other loci could be influencing the OPN effect. In an effort to better control for background effects, the C57BL/6J × 129S-Opn−/− mice were backcrossed with C57BL/6J mice and the heterozygous pups mated to produce an F2 generation, C57BL/6J × (C57BL/6J × 129S)-Opn (Fig. 2), with all genes except Opn and closely linked genes randomly assorting. OPN-deficient and wild-type littermates from the F2 generation were infected and arthritis severity examined as described above.
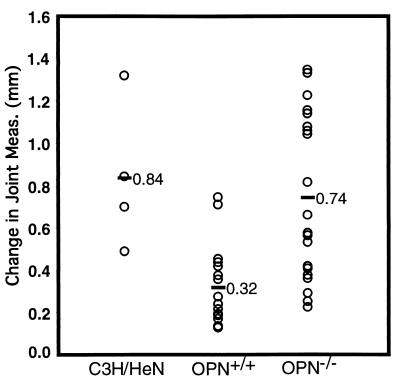
FIG. 2.
Joint swelling in B. burgdorferi-infected C57BL/6 × (C57BL/6J × 129S)-Opn−/− and -Opn+/+ mice. Mice were infected with 2 × 103 B. burgdorferi bacteria, and joint swelling was assessed as described in Materials and Methods.
C3H/HeN mice infected with B. burgdorferi were included as a control for joint swelling and severe arthritis in this experiment (Fig. 2; Table 4). The C57BL/6J × (C57BL/6J × 129S)-Opn+/+ mice displayed mild to moderate joint swelling and arthritis, while the C57BL/6J × (C57BL/6J × 129S)-Opn−/− mice showed a greater range of arthritis, from mild to severe. Although both wild-type and OPN-deficient mice showed a range of arthritis severity, there were more mice with moderate to severe arthritis in the OPN-deficient group than in their wild-type littermates. This difference was statistically significant, with a P value of ≤0.0002 for joint swelling and ≤0.02 for histopathology. The arthritis results from the wild-type littermates suggest that the mixed C57BL/6J-129S background is moderately resistant to B. burgdorferi-induced arthritis. OPN-deficient mice on this background fell into two groups, one developing mild to moderate arthritis, similar to wild-type littermates, and the other with severe arthritis not seen in mice containing the wild-type OPN gene. It is important to note that the wild-type and OPN-deficient mice were randomly assorted throughout cages and both measurements of arthritis severity were made in a blind manner.
TABLE 4.
Histopathological assessment of arthritis severity at 4 weeks postinfection in OPN-deficient mice on the C57BL/6J × (C57BL/6J × 129S) backgrounda
| Mouse strain | No.b | Avg histopathology scorec |
|---|---|---|
| C3H/HeN | 4 | 3.75 ± 0.50 |
| Opn+/+ | 17 | 1.32 ± 1.37 |
| Opn−/− | 18 | 2.34 ± 1.17 |
Mice were infected with 2 × 103 B. burgdorferi bacteria. Arthritis severity was assessed as described in Materials and Methods.
No., number of mice per group.
Results are presented as the arithmetic means ± standard deviations.
The results in Fig. 2 and Table 4 were quite perplexing, as some OPN-deficient mice developed severe arthritis, while others were resistant, making it difficult to assess whether the increase in arthritis severity in the OPN null mice was due to the deficiency. Instead, the increase in severity could have been due to the random mix of C57BL/6J and 129S genes within the OPN-deficient mice, as suggested by the results for wild-type B6129SF2/J mice presented in Fig. 1. Examining infection in wild-type littermates controlled for the random assortment of C57BL/6J and 129S genes in the background of the mice. However, in the OPN-deficient mice, chromosomal DNA linked to the deletion would be from the 129S background, while those 129S alleles would not be found in wild-type littermates. Previous mapping studies have shown that as many as four QTL for arthritis susceptibility are located on chromosome 5, suggesting that it is quite possible that a closely linked gene may have been responsible for the change in phenotype, in conjunction with other randomly assorting genes. Experiments in which the wild-type and OPN-deficient mice are more nearly genetically identical should allow assessment of the role of OPN in Lyme disease.
Effects of genetic deletion of Opn on B. burgdorferi-induced arthritis on the inbred 129S background.
In order to determine whether it was the absence of OPN or the presence of closely linked genes that was responsible for the increase in arthritis severity, OPN-deficient mice on an inbred 129S background were infected with B. burgdorferi.
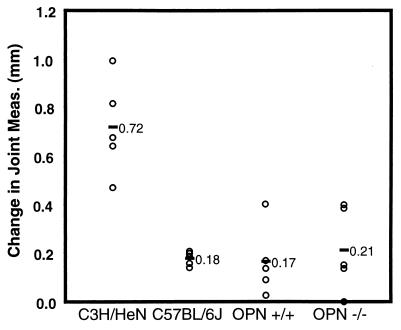
In this experiment, C3H/HeN mice developed severe arthritis, while C57BL/6J mice developed mild arthritis as assessed by both joint swelling and lesion severity (Fig. 3; Table 5). Both wild-type and OPN-deficient mice on the 129S background were resistant to severe arthritis, showing mild joint swelling and mild to moderate lesion severity (0 to 2+). These data suggest that OPN is not required for resistance to severe arthritis on the inbred 129S background. Closely linked genes on chromosome 5, in conjunction with other randomly assorting genes, are most likely responsible for the increase in arthritis severity seen in OPN-deficient mice on the C57BL/6J-129S mixed backgrounds in Fig. 2, not the absence of OPN.
FIG. 3.
Joint swelling in B. burgdorferi-infected 129S-Opn−/− and -Opn+/+ mice. Mice were infected with 2 × 103 B. burgdorferi bacteria, and joint swelling was assessed as described in Materials and Methods.
TABLE 5.
Histopathological assessment of arthritis severity at 4 weeks postinfection in OPN-deficient mice on the inbred 129S backgrounda
| Mouse strain | Avg histopathology score |
|---|---|
| C3H/HeNb | 2.40 ± 0.55 |
| C57BL/6J | 0.90 ± 0.22 |
| Opn+/+ | 1.00 ± 1.00 |
| Opn−/− | 1.11 ± 0.55 |
Mice were infected with 2 × 103 B. burgdorferi. Arthritis severity was assessed as described in Materials and Methods. Results are presented as the arithmetic means ± standard deviations.
Five mice per group.
Spirochete numbers in the joint, heart, and ear tissues of OPN-deficient C57BL/6J × (C57BL/6J × 129S) and 129S mice.
OPN-deficient mice show an increase in bacterial numbers when infected with the intracellular pathogens Listeria monocytogenes and Mycobacterium bovis BCG, which cause chronic infection (4, 32). B. burgdorferi is an extracellular pathogen that causes chronic infection; thus, OPN could be required for the control of their numbers as well (5). To determine the numbers of B. burgdorferi in tissues, we performed continuous monitoring PCR on DNA collected from tissues 4 weeks postinfection. Borrelia-specific products were not detected in tissues from mock-infected mice (not shown). Spirochete numbers were similar in the joint tissues of C3H/HeN, C57BL/6J × (C57BL/6J × 129S)-Opn+/+, and C57BL/6J × (C57BL/6J × 129S)-Opn−/− mice (Table 6). C3H/HeN mice harbored fivefold greater numbers of spirochetes in heart tissues than either the OPN-deficient or wild-type mice, indicating that differences between mouse strains can be detected by this assay. The ability to detect effects of genetic mutation on host defense against B. burgdorferi was also shown in a previous study using IL-10-deficient mice (10). Similar numbers of B. burgdorferi were seen in the ear tissues of C3H/HeN, C57BL/6J × (C57BL/6J × 129S)-Opn+/+, and C57BL/6J × (C57BL/6J × 129S)-Opn−/− mice. Therefore, OPN did not influence spirochete numbers on the C57BL/6J × (C57BL/6J × 129S) background.
TABLE 6.
B. burgdorferi numbers in joint, heart, and ear tissues of OPN-deficient micea
| Mouse strain | No. of copies from:
|
||
|---|---|---|---|
| Joint | Heart | Ear | |
| C3H/HeN | 30.75 ± 24.36 | 1.72 ± 0.45 | 1.80 ± 0.71 |
| C57 × 129S-Opn+/+b | 18.73 ± 16.60 | 0.29 ± 0.15 | 1.76 ± 2.07 |
| C57 × 129S-Opn−/−b | 20.69 ± 22.18 | 0.33 ± 0.20 | 1.74 ± 1.81 |
| C3H/HeN | 11.40 ± 11.58 | 1.27 ± 0.54 | 0.64 ± 0.35 |
| 129S-Opn+/+ | 32.16 ± 27.09 | 1.13 ± 1.36 | 1.41 ± 2.20 |
| 129S-Opn−/− | 32.69 ± 10.22 | 1.47 ± 1.17 | 3.17 ± 2.46 |
Mice were infected with 2 × 103 B. burgdorferi bacteria, and tissues were harvested at 4 weeks postinfection. DNA was isolated from tissues and assessed using continuous monitoring PCR. Uninfected controls were negative for B. burgdorferi. Values are the number of copies of B. burgdorferi per 103 single copy mouse gene. Results are presented as arithmetic means ± standard deviations. Nidogen was the single copy mouse gene used for normalization.
C57BL/6J × (C57BL/6J × 129S)-Opn background.
Spirochete numbers were also assessed for OPN-deficient mice on the 129S background. Joint tissues from both OPN-deficient and wild-type mice contained approximately threefold more bacteria than joint tissue from C3H/HeN mice infected simultaneously (Table 6). This is the first time continuous monitoring PCR has been used to determine the number of spirochetes in joint tissue from mice on a 129S background. It is interesting that these arthritis-resistant mice harbor threefold more bacteria in the joints than either C3H/HeN or C57BL/6J mice, further suggesting that control of the inflammatory response may be key to the control of arthritis severity rather than control of B. burgdorferi numbers. Equivalent numbers of bacteria were present in the heart tissues of C3H/HeN and both OPN-deficient and wild-type mice. There was not a statistically significant difference in spirochete numbers in the ear tissues of 129S-Opn+/+ or -Opn−/− mice. The lack of difference in spirochete numbers between any of the OPN-deficient mice and their wild-type controls demonstrates that OPN is not required for control of B. burgdorferi numbers in joint, heart, or ear tissues of mice.
Immunoglobulin isotype profiles in mice lacking OPN.
OPN has been described as a molecule that initiates signals for the production of IL-12 while downregulating IL-10 production in order to allow for the development of type 1, or Th1, immunity (4). The induction of a Th1 response enhances the production of IgG2a and inhibits the production of IgG1, a Th2-associated Ab. Therefore, OPN-deficient mice might undergo a shift in Ab production away from IgG2a and toward IgG1.
Ab isotype profiles were examined in the serum of OPN-deficient mice, obtained at 4 weeks postinfection, to determine whether there was a change in the isotypes of IgG being produced in response to B. burgdorferi infection. No difference was seen in the total amounts of B. burgdorferi-specific IgG produced by the OPN-deficient and wild-type mouse strains (data not shown). Mice on the 129S background produced approximately fourfold more IgG1 than mice on the C57BL/6J × (C57BL/6J × 129S) background, yet there was no difference in production between OPN-deficient and wild-type mice of either background (Table 7). No differences were seen in the amounts of IgG2a, IgG2b, or IgG3 produced by OPN-deficient or wild-type mice. The amounts of IgG2a in serum were not determined in mice with a C57BL/6 background, because C57BL/6 mice do not possess the gene for IgG2a(γ) but instead possess a novel IgG2c(γ) gene which is not recognized by commercial Abs to IgG2a (22, 29, 30). Overall, the absence of OPN does not have an effect on the isotypes of Ab produced during infection with B. burgdorferi.
TABLE 7.
Effect of OPN genotype on B. burgdorferi-specific IgG isotypes in sera from OPN-deficient micea
| Mouse strain | IgG1 | IgG2a | IgG2b | IgG3 |
|---|---|---|---|---|
| C57 × 129S-Opn+/+b | 12.8 ± 3.4 | NDc | 2,249 ± 672 | 1,294 ± 453 |
| C57 × 129S-Opn−/−b | 13.3 ± 9.0 | ND | 2,272 ± 392 | 1,098 ± 496 |
| 129S-Opn+/+ | 41.2 ± 11.1 | 482.7 ± 197.8 | 3,065 ± 827 | 1,499 ± 1,337 |
| 129S-Opn−/− | 53.3 ± 43.7 | 585.7 ± 157.4 | 2,744 ± 534 | 1,311 ± 954 |
Mice were infected with 2,000 B. burgdorferi bacteria. Serum collected at 4 weeks postinfection was assessed by ELISA for IgG content as described in Materials and Methods. Values represent levels of Ig in nanograms per milliliter. Results are presented as arithmetic means ± standard deviations.
C57BL/6J × (C57BL/6J × 129S)-Opn background.
ND, not determined.
All samples had detectable IgG of each isotype.
DISCUSSION
Severity of Lyme arthritis is genetically regulated in mice, with C3H mice developing severe arthritis and C57BL/6 mice displaying mild to moderate arthritis. Intercross populations have implicated chromosome 5 in the regulation of disease, with up to four separate QTL on chromosome 5 identified in 4 intercross populations (46, 51). The gene encoding OPN is within the boundaries of one of these QTL and was considered a candidate for Lyme arthritis severity, as it is polymorphic between C3H and C57BL/6 mice and the resistance alleles for another mouse pathogen, Rickettsia tsutsugamushi, map to the same locus on chromosome 5 as the OPN gene (16, 38). The biological properties of the OPN gene include wound repair and bone remodeling (13, 24), both intriguing due to the development of sites of ossification in murine and human Lyme arthritis. Furthermore, both deletion of the IL-10 gene and blocking of IL-12 with Abs influence the severity of murine Lyme arthritis (2, 3, 10), and the in vitro production of these cytokines by LPS- and IL-4-stimulated murine macrophages is regulated by OPN (4, 19, 45). These findings suggested that Opn could be a candidate gene regulating the murine response to infection with B. burgdorferi. This hypothesis was tested in murine macrophages with in vitro assays that have previously been demonstrated to be relevant to the inflammatory response to B. burgdorferi and with in vivo infection of OPN-deficient mice to assess the involvement of OPN in disease development (10).
In vitro production of inflammatory mediators by macrophages and other cell types in response to B. burgdorferi lipoproteins has provided insight into the inflammatory potential of the organism (52). Previous experiments have shown that bone marrow-derived macrophages from the arthritis-resistant C57BL/6 mouse produce significantly more IL-10 than macrophages from arthritis-susceptible C3H/HeN mice, which produce large amounts of proinflammatory IL-6 and nitric oxide (10). Although IL-10 does not fall within any of the QTL regulating Lyme arthritis, the genes regulating its production could (46). The importance of IL-10 production was confirmed in vivo when it was shown that IL-10-deficient mice on the C57BL/6 background were more susceptible to severe B. burgdorferi-induced arthritis than wild-type C57BL/6 mice (10). Ab depletion of IL-12 presents a more complicated picture, as IL-12-depleted, B. burgdorferi-infected C3H/He mice develop less severe arthritis than untreated controls while IL-12-depleted SCID mice develop more severe arthritis than untreated controls (2, 3). Both studies implicate IL-12 as a regulator of Lyme arthritis and reinforce the importance of considering the context of the whole animal in interpreting the effects of individual gene products.
In vitro studies have identified OPN as a regulator of nitric oxide, IL-10, and IL-12 production by macrophages (4, 19, 45). Therefore, we began by examining cytokine production by OspA-stimulated bone marrow-derived macrophages from OPN-deficient mice. Interestingly, we saw a two- to fourfold increase in IL-10 production by bone marrow macrophages from 129S-Opn−/− mice compared to that of 129S-Opn+/+ mice. Yet we did not see any alterations in the production of IL-10 from C57BL/6J × (C57BL/6J × 129S)-Opn−/− macrophages. The increase in IL-10 production on the OPN-deficient-129S background is consistent with previously published results and might be expected to increase the resistance of the mice to severe arthritis. No alteration was seen in other cytokines produced by OPN-deficient macrophages.
In order to test for the possible requirement of T cells in OPN regulation of IL-12, we examined cytokine production by splenocyte cultures containing macrophages, B cells, and T cells. When splenocytes from wild-type and OPN-deficient mice of both the C57BL/6J × (C57BL/6J × 129S) and inbred 129S backgrounds were stimulated with OspA, no differences were seen in IL-6, IL-10, and IL-12p40 production.
In these studies we did not observe OPN regulation of nitric oxide or IL-12 production by macrophages and splenocytes stimulated by OspA from either the C57BL/6J × (C57BL/6J × 129S) or inbred 129S background, although others have reported OPN regulation of LPS-stimulated macrophages (4, 19, 45). The maturation stage of macrophages, as well as the potential need for mixed cultures, can influence cytokine production in response to OPN (12, 37). Previous studies have been performed stimulating macrophages with LPS. LPS signals through Toll-like receptor 4 (40, 41), while OspA signals through Toll-like receptor 2 (1, 9, 20, 25), potentially altering the effect OPN has on the regulation of cytokines. Nitric oxide regulation appears to be extremely dose dependent, while the regulation of IL-12 is phosphorylation dependent (45, 49). Because OPN is regulated at multiple levels, it is possible that within these experiments we were unable to create the correct conditions to examine nitric oxide and IL-12 regulation. Further, this may explain why we did not see OPN regulation of cytokine production upon the addition of exogenous OPN and anti-OPN Ab. However, the greater question to be answered was whether B. burgdorferi-induced arthritis severity would be altered in the absence of OPN in vivo.
OPN-deficient mice on the inbred 129S background infected with 2 × 103 B. burgdorferi developed mild to moderate arthritis, similar to that seen in genetically similar wild-type mice. Therefore, OPN is not required for resistance to B. burgdorferi-induced arthritis, as defined by joint swelling and histopathology. Yet why do OPN-deficient mice on the mixed C57BL/6J-129S backgrounds develop more severe arthritis than either of the parental strains?
Initial experiments with B. burgdorferi-infected B6129SF2/J mice suggest that a combination of C57BL/6J and 129S genes exists which can lead to increased arthritis severity. The previous finding that arthritis susceptibility alleles exist in the resistant C57BL/6 background and are only seen in conjunction with C3H/He susceptibility alleles strengthens this hypothesis (46, 51). Concerned that the trend toward the development of severe arthritis was due to the random assortment of C57BL/6J and 129S genes in the OPN-null mice rather than the absence of OPN, we backcrossed the C57BL/6J × 129S-Opn−/− mice with C57BL/6J mice. The heterozygous pups were mated to obtain C57BL/6J × (C57BL/6J × 129S)-Opn−/− and -Opn+/+ littermates. Within the wild-type and OPN-deficient littermates, all genes are randomly assorted except for OPN and closely linked genes. Both wild-type and OPN-deficient littermates showed a range of joint swelling upon infection with B. burgdorferi, with the OPN-deficient mice having more severe arthritis than wild-type littermates, although while some OPN-deficient mice developed severe arthritis, others maintained the arthritis-resistant phenotype. These results confirm that OPN is not required for resistance to severe arthritis. Four loci on chromosome 5 regulate Lyme arthritis severity as well as loci on several other chromosomes, 1, 4, 12, 15, and 17 (46). It is likely that a gene on chromosome 5 closely linked to OPN, in association with other randomly associating genes, is responsible for the increase in arthritis severity in a portion of the OPN-deficient mice on the C57BL/6J-129S mixed backgrounds. Testing the effect of OPN deficiency in genetically similar mice on the inbred 129S background was the only way to clearly define the role of OPN without the confounding effect of other randomly assorting genes. Another explanation for these results is that, while OPN alone is not required for resistance to disease, it is part of a haplotype effect, or overall combined effect, of the QTL on chromosome 5.
These experiments are a reminder that the background of the mouse strain being studied can lead to spurious conclusions when studying disease. Knockout mice may show a phenotype that is due to their mixed background rather than their genetic deficiency. For example, after 13 backcross generations, a locus regulating protection from the development of diabetes was separated from that of a deficiency in the IFN-γ receptor (23).
OPN has been shown to control the growth of intracellular bacteria, including Listeria monocytogenes and Mycobacterium tuberculosis BCG (4, 32). We examined B. burgdorferi numbers in joint, heart, and ear tissues of infected mice and saw no differences in bacterial numbers between wild-type and OPN-deficient mice on any background. Overall, the absence of OPN had no effect on the bacterial load in mouse tissues; therefore, OPN does not appear to be necessary for host control of the extracellular pathogen B. burgdorferi during the first 4 weeks of infection.
A recent study suggested that OPN is involved at an early time point in the host response to pathogens and the establishment of Th1 immunity. By upregulating production of IL-12 and downregulating IL-10, OPN provides an environment where T cells take on a Th1 phenotype, enhancing the production of IgG2a while inhibiting the production of IgG1, a Th2-associated Ab (4). Therefore, B. burgdorferi-specific Ab isotypes were examined. Within the B. burgdorferi-infected OPN-deficient mice we did not see any alteration in the amounts of B. burgdorferi-specific IgG1, IgG2a, IgG2b, or IgG3. Therefore, the absence of OPN has no effect on class switching during infection with B. burgdorferi.
Opn can now be eliminated as a candidate gene required for resistance to severe Lyme arthritis. However, it is still possible that the presence of the C3H/HeN OPN allele leads to severe arthritis during infection with B. burgdorferi. This will be tested in the congenic mice presently being developed by the laboratory. The experiments with OPN-deficient mice on the mixed C57BL/6J-129S background have confirmed the importance of arthritis susceptibility genes on chromosome 5 and suggest that arthritis-resistant mice carry susceptibility alleles. Future studies, most importantly the analysis of congenic lines, will allow for the eventual identification of arthritis severity genes within the QTL identified on chromosome 5.
Acknowledgments
This work was supported by National Institutes of Health grants AR43521 (J.J.W and C.T.), CA 72740 (S.R.R.), AR44434, and ES06897 (D.T.D). The project described was also supported in part by an award from the American Lung Association (J.H.W.). M.R.P. was supported as a predoctoral trainee by National Institutes of Health Genetics Training Grant 5T32 GM07464. R.J.R. was partially supported by NIH Cell and Molecular Biology Training Grant T32-GM-07283.
We thank Keith Hruska and Jena Strauss-Schoenberger from George Washington University, St. Louis, Mo., for providing the C57BL/6J × 129S-Opn−/− mice. We also thank Associated Regional and University Pathologists (ARUP).
Editor: J. D. Clements
REFERENCES
- 1.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 2.Anguita, J., D. H. Persing, M. Rincon, S. W. Barthold, and E. Fikrig. 1996. Effect of anti-interleukin 12 treatment on murine lyme borreliosis. J. Clin. Investig. 97:1028-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anguita, J., S. Samanta, S. W. Barthold, and E. Fikrig. 1997. Ablation of interleukin-12 exacerbates Lyme arthritis in SCID mice. Infect. Immun. 65:4334-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashkar, S., G. F. Weber, V. Panoutsakopoulou, M. E. Sanchirico, M. Jansson, S. Zawaideh, S. R. Rittling, D. T. Denhardt, M. J. Glimcher, and H. Cantor. 2000. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287:860-864. [DOI] [PubMed] [Google Scholar]
- 5.Barthold, S. W. 1996. Lyme borreliosis in the laboratory mouse. J. Spirochetes Tick-Borne Dis. 3:22-44.
- 6.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162: 133-138. [DOI] [PubMed] [Google Scholar]
- 7.Barthold, S. W., and M. de Souza. 1995. Exacerbation of Lyme arthritis in beige mice. J. Infect. Dis. 172: 778-784. [DOI] [PubMed] [Google Scholar]
- 8.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 9.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 10.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 67:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease: a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 12.Denhardt, D. T., C. M. Giachelli, and S. R. Rittling. 2001. Role of osteopontin in cellular signaling and toxicant injury. Annu. Rev. Pharmacol. Toxicol. 41:723-749. [DOI] [PubMed] [Google Scholar]
- 13.Denhardt, D. T., and M. Noda. 1998. Osteopontin expression and function: role in bone remodeling. J. Cell. Biochem. Suppl. 31:92-102. [PubMed] [Google Scholar]
- 14.Denhardt, D. T., M. Noda, A. W. O'Regan, D. Pavlin, and J. S. Berman. 2001. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 107:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 16.Fet, V., M. E. Dickinson, and B. L. Hogan. 1989. Localization of the mouse gene for secreted phosphoprotein 1 (Spp-1) (2ar, osteopontin, bone sialoprotein 1, 44-kDa bone phosphoprotein, tumor-secreted phosphoprotein) to chromosome 5, closely linked to Ric (Rickettsia resistance). Genomics 5:375-377. [DOI] [PubMed] [Google Scholar]
- 17.Giachelli, C. M., D. Lombardi, R. J. Johnson, C. E. Murry, and M. Almeida. 1998. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am. J. Pathol. 152:353-358. [PMC free article] [PubMed] [Google Scholar]
- 18.Glickstein, L., M. Edelstein, and J. Z. Dong. 2001. Gamma interferon is not required for arthritis resistance in the murine Lyme disease model. Infect. Immun. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo, H., C. Q. Cai, R. A. Schroeder, and P. C. Kuo. 2001. Osteopontin is a negative feedback regulator of nitric oxide synthesis in murine macrophages. J. Immunol. 166:1079-1086. [DOI] [PubMed] [Google Scholar]
- 20.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 21.Johnson, R. C., G. P. Schmid, F. W. Hyde, A. G. Steigwalt, and D. J. Brenner. 1984. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int. J. Syst. Bacteriol. 34:496-497. [Google Scholar]
- 22.Jouvin-Marche, E., M. G. Morgado, C. Leguern, D. Voegtle, F. Bonhomme, and P. A. Cazenave. 1989. The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics 29:92-97. [DOI] [PubMed] [Google Scholar]
- 23.Kanagawa, O., G. Xu, A. Tevaarwerk, and B. A. Vaupel. 2000. Protection of nonobese diabetic mice from diabetes by gene(s) closely linked to IFN-gamma receptor loci. J. Immunol. 164:3919-3923. [DOI] [PubMed] [Google Scholar]
- 24.Liaw, L., D. E. Birk, C. B. Ballas, J. S. Whitsitt, J. M. Davidson, and B. L. Hogan. 1998. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J. Clin. Investig. 101:1468-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 26.Ma, Y., K. P. Seiler, E. J. Eichwald, J. H. Weis, C. Teuscher, and J. J. Weis. 1998. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 66:161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma, Y., K. P. Seiler, K. F. Tai, L. Yang, M. Woods, and J. J. Weis. 1994. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect. Immun. 62:3663-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma, Y., and J. J. Weis. 1993. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect. Immun. 61:3843-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin, R. M., and A. M. Lew. 1998. Is IgG2a a good Th1 marker in mice? Immunol. Today 19:49.. [DOI] [PubMed] [Google Scholar]
- 30.Martin, R. M., A. Silva, and A. M. Lew. 1997. The Igh-1 sequence of the non-obese diabetic (NOD) mouse assigns it to the IgG2c isotype. Immunogenetics 46:167-168. [DOI] [PubMed] [Google Scholar]
- 31.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nau, G. J., L. Liaw, G. L. Chupp, J. S. Berman, B. L. Hogan, and R. A. Young. 1999. Attenuated host resistance against Mycobacterium bovis BCG infection in mice lacking osteopontin. Infect. Immun. 67:4223-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nocton, J. J., F. Dressler, B. J. Rutledge, P. N. Rys, D. H. Persing, and A. C. Steere. 1994. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 330:229-234. [DOI] [PubMed] [Google Scholar]
- 34.Nocton, J. J., and A. C. Steere. 1995. Lyme disease. Adv. Intern. Med. 40:69-117. [PubMed] [Google Scholar]
- 35.Ono, M., T. Yamamoto, and M. Nose. 1995. Allelic difference in the nucleotide sequence of the Eta-1/Op gene transcript. Mol. Immunol. 32:447-448. [DOI] [PubMed] [Google Scholar]
- 36.O'Regan, A., and J. S. Berman. 2000. Osteopontin: a key cytokine in cell-mediated and granulomatous inflammation. Int. J. Exp. Pathol. 81:373-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Regan, A. W., J. M. Hayden, and J. S. Berman. 2000. Osteopontin augments CD3-mediated interferon-gamma and CD40 ligand expression by T cells, which results in IL-12 production from peripheral blood mononuclear cells. J. Leukoc. Biol. 68:495-502. [PubMed] [Google Scholar]
- 38.Patarca, R., G. J. Freeman, R. P. Singh, F. Y. Wei, T. Durfee, F. Blattner, D. C. Regnier, C. A. Kozak, B. A. Mock, H. C. Morse III, et al. 1989. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J. Exp. Med. 170:145-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patarca, R., R. A. Saavedra, and H. Cantor. 1993. Molecular and cellular basis of genetic resistance to bacterial infection: the role of the early T-lymphocyte activation-1/osteopontin gene. Crit. Rev. Immunol. 13:225-246. [PubMed] [Google Scholar]
- 40.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahn, D. W., and S. E. Malawista. 1991. Lyme disease. West. J. Med. 154:706-714. [PMC free article] [PubMed] [Google Scholar]
- 43.Rittling, S. R., and D. T. Denhardt. 1999. Osteopontin function in pathology: lessons from osteopontin-deficient mice. Exp. Nephrol. 7:103-113. [DOI] [PubMed] [Google Scholar]
- 44.Rittling, S. R., H. N. Matsumoto, M. D. McKee, A. Nanci, X. R. An, K. E. Novick, A. J. Kowalski, M. Noda, and D. T. Denhardt. 1998. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J. Bone Miner. Res. 13:1101-1111. [DOI] [PubMed] [Google Scholar]
- 45.Rollo, E. E., D. L. Laskin, and D. T. Denhardt. 1996. Osteopontin inhibits nitric oxide production and cytotoxicity by activated RAW264.7 macrophages. J. Leukoc. Biol. 60:397-404. [DOI] [PubMed] [Google Scholar]
- 46.Roper, R. J., J. J. Weis, B. A. McCracken, C. B. Green, Y. Ma, S. Weber, D. Fairbairn, R. J. Butterfield, M. R. Potter, J. F. Zachary, R. W. Doerge, and C. Teuscher. 2001. Genetic control of susceptibility to experimental Lyme arthritis is polygenic and exhibits consistent linkage to multiple loci on chromosome 5 in four independent mouse crosses. Genes Immun. 2:388-397. [DOI] [PubMed] [Google Scholar]
- 47.Sellati, T. J., M. J. Burns, M. A. Ficazzola, and M. B. Furie. 1995. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect. Immun. 63:4439-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh, R. P., R. Patarca, J. Schwartz, P. Singh, and H. Cantor. 1990. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J. Exp. Med. 171:1931-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian, J. Y., E. S. Sorensen, W. T. Butler, C. A. Lopez, M. S. Sy, N. K. Desai, and D. T. Denhardt. 2000. Regulation of NO synthesis induced by inflammatory mediators in RAW264.7 cells: collagen prevents inhibition by osteopontin. Cytokine 12:450-457. [DOI] [PubMed] [Google Scholar]
- 50.Weber, G. F., and H. Cantor. 1996. The immunology of Eta-1/osteopontin. Cytokine Growth Factor Rev. 7:241-248. [DOI] [PubMed] [Google Scholar]
- 51.Weis, J. J., B. A. McCracken, Y. Ma, D. Fairbairn, R. J. Roper, T. B. Morrison, J. H. Weis, J. F. Zachary, R. W. Doerge, and C. Teuscher. 1999. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J. Immunol. 162:948-956. [PubMed] [Google Scholar]
- 52.Wooten, R. M., and J. J. Weis. 2001. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr. Opin. Microbiol. 4:274-279. [DOI] [PubMed] [Google Scholar]
- 53.Xuan, J. W., C. Hota, and A. F. Chambers. 1994. Recombinant GST-human osteopontin fusion protein is functional in RGD-dependent cell adhesion. J. Cell. Biochem. 54:247-255. [DOI] [PubMed] [Google Scholar]
- 54.Yang, L., J. H. Weis, E. Eichwald, C. P. Kolbert, D. H. Persing, and J. J. Weis. 1994. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 62:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]