Abstract
Vβ10+ and Vβ14+ T cells were selectively increased 7 to 14 days following infection in the lungs of naive mice infected with Histoplasma capsulatum. Following secondary challenge of immune mice, Vβ1+ and Vβ8.1+ cells were sporadically increased. Elimination of Vβ10+ and Vβ14+ cells from naive mice did not alter the course of infection over a period of 21 days. Thus, overexpression of Vβ families does not necessarily signify a key role in host defense.
Among the many elements of the immune system that contribute to host resistance to Histoplasma capsulatum, T cells are one of the most critically important. In primary infection, the absence of T-cell receptor (TCR) α/β+ or CD4+ cells converts a sublethal pulmonary challenge into a fatal infection. Elimination of CD8+ cells modestly impairs the course of infection (1, 2). In secondary pulmonary histoplasmosis, progressive infection develops after challenge with a sublethal inoculum only upon depletion of both CD4+ and CD8+ cells (1).
T cells exhibit marked heterogeneity; the variable region of the TCR imparts clonal diversity that is necessary to combat the untoward effects of a multitude of foreign antigens. Biases in the TCR repertoire have been detected in a number of infectious diseases, including histoplasmosis (2, 3, 6, 7, 9, 10). Previous reports from our laboratory have documented the selective expansion of Vβ4+ and Vβ6+ cells in the lungs of C57BL/6 mice with primary and secondary histoplasmosis, respectively. Elimination of these cells impaired the efficiency of clearance of the fungus from the lungs and spleens of mice (6, 7). Here, we analyzed TCR usage in pulmonary histoplasmosis in BALB/c mice to determine if a different strain of mouse would manifest a similar profile of TCR expression in the lungs.
Male BALB/c and athymic nude mice, 5 weeks of age, were purchased from the National Cancer Institute (Frederick, Md.). H. capsulatum yeasts (strain G217B) were prepared as described previously (1). In primary infection, animals were inoculated intranasally (i.n.) with 2.0 × 106 yeasts in 30 μl of balanced salt solution. Secondary infection was generated by infecting mice with 104 yeasts i.n. and 8 weeks later rechallenging them with 2 × 106 yeasts i.n. Monoclonal antibody (MAb) to Vβ10 (clone B21.5; rat immunoglobulin G2a [IgG2a]) was purchased from BD Pharmingen (San Diego, Calif.). MAb to Vβ14 (rat IgM) was generated in athymic nude mice from clone 14.2 (from David Raulet, University of California at Berkeley). IgM was purified using the Immunopure IgM purification kit (Pierce Endogen, Rockford, Ill.). MAb to TCR α/β+ cells (H57-597; hamster IgG) was prepared in serum-free medium using Nutridoma (Roche Biochemicals, Indianapolis, Ind.).
For TCR quantitation, the lungs of mice (n = 6) were flushed of circulating cells by injecting 2 ml of phosphate-buffered saline free of Ca2+ and Mg2+ into the right ventricle. Lungs were homogenized in 4 ml of RNAzol (BIOTECKX, Friendwood, Tex.), and RNA was extracted with chloroform and precipitated. RNA was resuspended in nuclease-free water, and the nucleic acid yield and purity were determined by the optical density at 260 nm (OD260) and the OD260/OD280 ratio. The relative abundance of each Vβ transcript in organs has been described previously (6). cDNA was synthesized from total RNA (6 μg) that was annealed with 50 ng of an antisense primer complementary to the constant region of the β chain of TCR. One microliter of cDNA was used as a template for PCR. Each tube contained a common nested antisense primer specific to the constant region of the β chain and Vβ-specific sense primers (4), except Vβ3, -5, -11, and -12, which are absent in BALB/c mice (8). The abundance of each Vβ-specific PCR product was determined by Southern blotting using a digoxigenin-labeled DNA probe specific to the Cβ region of the TCR. Signal was revealed with alkaline phosphatase-conjugated anti-digoxigenin Fab signal and the chemiluminescent substrate CDP-Star (Roche). Light production was measured directly with a ChemiImager 4000 instrument (Alpha Innotech Corp., San Leandro, Calif.). To determine the complementarity-determining region 3 (CDR3) sequence, PCR products amplified with Vβ10- and Vβ14-specific primers were reamplified using a nested common antisense primer complementary to the constant region of the β chain. The DNA was ligated in Topo PCR2.1 (Invitrogen, La Jolla, Calif.). At least five random colonies were picked for each of three mice and sequenced.
Lung leukocytes were prepared as described previously (6). Cells were incubated with 0.25 μg of phycoerythrin-conjugated MAb to either Vβ10, Vβ14, or Vβ6 (BD Pharmingen) and with fluorescein-conjugated MAb to CD3 for 30 min at 4°C. The cells were washed and fixed in 2% paraformaldehyde in phosphate-buffered saline.
Groups of mice were depleted of Vβ10+ or Vβ14+ T cells or both by intraperitoneal injection of 100 μg of MAb, produced from each of the respective hybridomas, on days −7 and −3 and on the day of infection. Mice depleted of TCR α/β+ cells were given 300 μg of MAb or control Ab on the same schedule described above. Subsequently, the mice were injected each week until the end of each experiment. Control mice were given an equal amount of rat IgG, rat IgM, or hamster IgG (Pierce Endogen) intraperitoneally. The depleting activity of each MAb was ≥95% in both lungs and spleens as determined by flow cytometry.
Student's t test was used to compare the means of two groups. One-way analysis of variance was used to compare more than two groups. Dunnet's correction was used for multiple comparisons against a control group. Chi-square analysis was used for comparison of CDR3 usage.
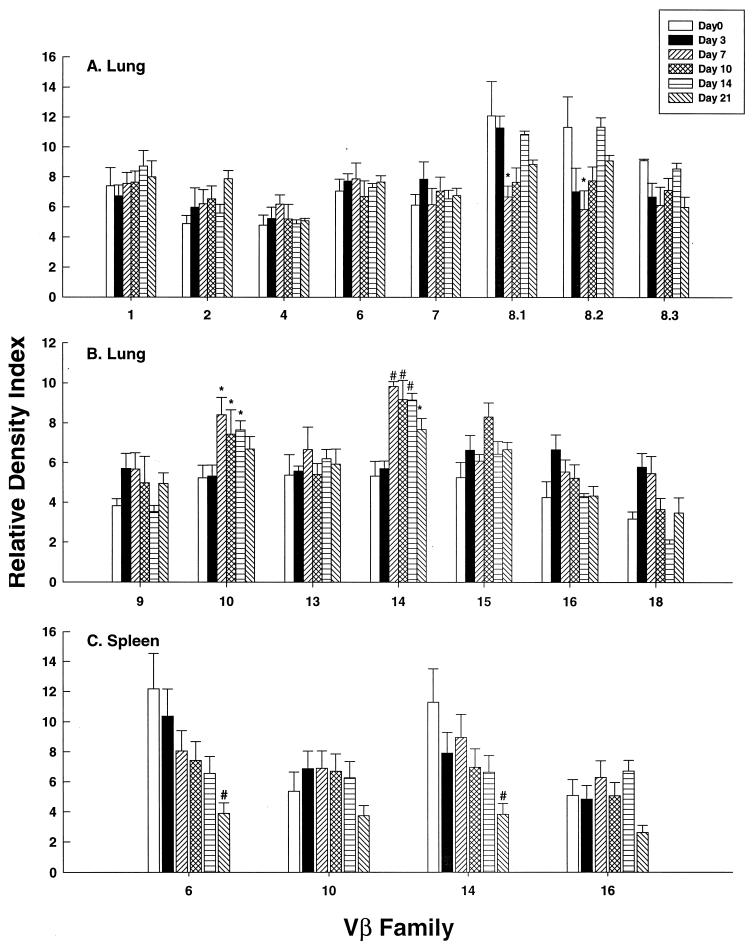
In primary infection, the proportion of Vβ10+ and Vβ14+ cells significantly exceeded (P < 0.01) uninfected controls (day zero) on days 7, 10, and 14 postinfection (Fig. 1A and B). On day 21, Vβ14+ cells were increased (P < 0.05). To determine if increased expression of these families was exclusive to the lungs and did not occur in lymphoid tissue, we analyzed Vβ10, -14, and -16 expression in spleens. None of the surveyed families was modulated compared to controls (Fig. 1C). We confirmed PCR quantitation by using flow cytometry on lung leukocytes from mice infected for 7 days. The mean percentages (± standard error of the mean [SEM]) of CD3+ Vβ10+ (8.1 ± 0.8) and CD3+ Vβ14+ (4.3 ± 0.6) cells in infected lungs exceeded (P < 0.01) these populations in normal lungs (2.7 ± 0.3 and 1.8 ± 0.1, respectively). In contrast, the mean percentages of Vβ6+ cells were similar (P > 0.05) in lungs from infected (3.7 ± 0.4) and uninfected (3.1 ± 0.9) mice.
FIG. 1.
TCR usage in the lungs of BALB/c mice with primary histoplasmosis. The data are expressed as the relative density index at serial intervals after infection. Uninfected controls are denoted by day zero. The data represent the mean ± SEM of six animals at each time point. *, P < 0.05; #, P < 0.01.
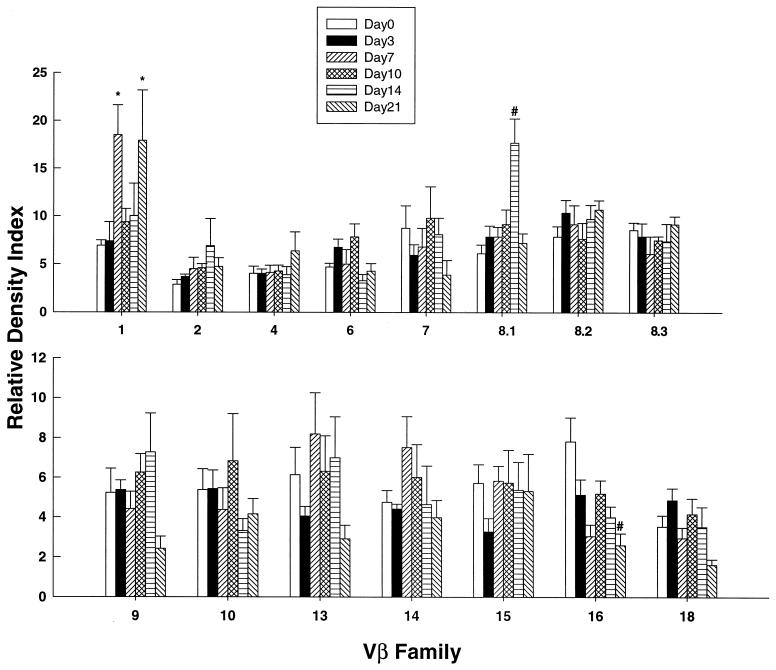
Analysis of the response in secondary infection revealed that Vβ1+ cells were significantly elevated (P < 0.05) on days 7 and 21, Vβ8.1+ cells were significantly elevated on day 14 (P < 0.01), and Vβ16+ cells were depressed (P < 0.01) on day 21 (Fig. 2).
FIG. 2.
TCR usage in lungs of BALB/c mice with secondary infection. The data are expressed as the relative density index at serial intervals of infection. Immunized animals that were not rechallenged are denoted by day zero. The data represent the mean ± SEM of six mice at each time point. *, P < 0.05; #, P < 0.01.
Analysis of Jβ usage did not reveal preferential expression of a particular Jβ family in either the Vβ10+ or Vβ14+ lung cells during the course of infection (data not shown). We searched for dominant amino acid motifs in the CDR3s of both Vβ10+ and Vβ14+ cells. Among sequences from Vβ10+ cells, GTGGX was seen in 1 of 15 sequences from uninfected mice (day zero). In infected mice, 1 of 15 sequences from three individual mice expressed this motif on day 3 postinfection, 3 of 15 sequences expressed it on day 7, 5 of 15 sequences expressed it on day 10 (P < 0.05), 3 of 15 sequences expressed it on day 14, and 2 of 15 sequences expressed it on day 21. The motif found during the course of infection was found in at least two mice for each of the days analyzed. Among Vβ14+ cells, the GTGGX motif was detected in 1 of 15 sequences from uninfected mice. On day 3 postinfection, 1 of 15 sequences contained this motif; 6 of 15 sequences contained the motif on day 7 (P < 0.01), 1 of 15 sequences contained the motif on day 10, 1 of 15 sequences contained the motif on day 14, and 3 of 15 sequences contained the motif on day 21. No other common motifs were found among the amino acid sequences of the respective CDR3s.
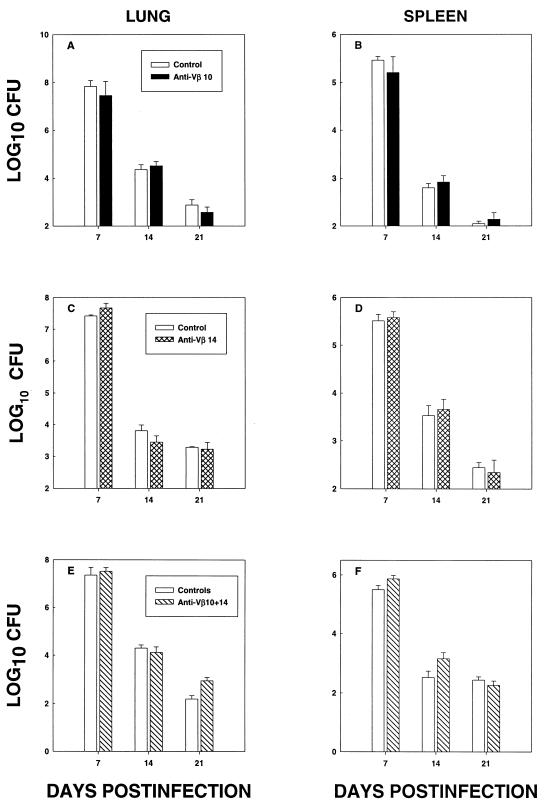
The elimination of Vβ10+ (Fig. 3A and B) or Vβ14+ (Fig. 3C and D) cells did not alter the number of CFU (P > 0.05) in either spleens or lungs. Concomitant elimination of both subsets did not significantly modify (P > 0.05) the fungal burden (Fig. 3E and F). To ensure that T cells were important to host resistance, BALB/c mice (n = 8) were depleted of TCR α/β+ cells and followed for survival. All BALB/c mice that lacked TCR α/β+ died by day 20 with a mean (±SEM) time to death of 12 ± 4 days. All mice given control antibody survived for 45 days.
FIG. 3.
Fungal burden in lungs and spleens of H. capsulatum-infected mice given MAb to Vβ10 (A and B), Vβ14 (C and D), or both (E and F). The mice were given MAb or an equal amount of rat IgG and/or rat IgM and infected with H. capsulatum. At days 7, 14, and 21, mice (n = 6/group) were sacrificed and lungs and spleens were assessed for the presence of H. capsulatum CFU.
In the lungs of BALB/c mice, the TCR repertoire to H. capsulatum manifests a pronounced skewing during the phase of infection (days 7 to 21) when cell-mediated immunity is activated and the organism is being cleared (5). Levels of both Vβ10+ and Vβ14+ cells were markedly higher than in uninfected controls. Since this elevation was observed in lungs and not spleens, the results indicate that these cells undergo a compartmentalization within the lungs.
Analyses of the CDR3s from these cells demonstrated limited heterogeneity rather than polyclonal expansion. Preferential accumulation of a limited population of Vβ10+ and Vβ14+ cells suggests that expansion is driven by a single antigenic epitope or a small number of epitopes.
Elimination of Vβ10+ or Vβ14+ cells or both did not modulate the course of infection. This finding was unanticipated, since previous work in our model of pulmonary histoplasmosis unequivocally demonstrated that the overexpressed TCR families played a functional role in host resistance. In primary infection of C57BL/6 mice, depletion of Vβ4+ cells produced a pronounced impairment in clearance of yeasts (6). Likewise, in secondary infection, C57BL/6 mice that lacked Vβ6+ cells or Vβ6+ and Vβ4+ cells manifest delayed clearance of H. capsulatum (7). The reason(s) for the differences between the two strains of mice is not known, but the differences strongly suggest that the TCR repertoire to infection with this fungus is dependent on the genetic background of a mouse strain. The results do indicate that BALB/c mice depleted of the two expanded families can mount an effective compensatory response. Thus, the detection of expanded clonotypes does not necessarily signify that these cells are critically important in host defenses. These findings do not exclude the possibility that Vβ10+ or Vβ14+ cells contribute to other parameters of infection, such as the inflammatory response.
In murine listeriosis, the TCR repertoire is conserved in primary and secondary infection (2). In pulmonary histoplasmosis, the biased TCR repertoire in the lungs of mice demonstrates a shift. Vβ4+ cells are increased in primary infection, whereas Vβ6+ cells are elevated in secondary histoplasmosis (6, 7). BALB/c mice manifested a completely different pattern. Hence, skewing of the TCR repertoire in primary infection is not predictive of a similar event in secondary disease.
Acknowledgments
This work was supported by grant AI-42747 from the National Institute of Allergy and Infectious Diseases.
Editor:J. D. Clements
REFERENCES
- 1.Allendörfer, R., G. D. Brunner, and G. S. Deepe, Jr. 1999. Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J. Immunol. 162:7389-7396. [PubMed] [Google Scholar]
- 2.Busch, D. H., I. Pilip, and E. G. Pamer. 1998. Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection. J. Exp. Med. 188:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callan, M. F. C., N. Steven, P. Krausa, J. D. Wilson, P. A. Moss, G. M. Gillespie, J. I. Bell, A. B. Rickinson, and A. J. McMichael. 1996. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat. Med. 2:906-911. [DOI] [PubMed] [Google Scholar]
- 4.Casanova, J. L., P. Romero, C. Widman, P. Kourilsky, and J. L. Maryanski. 1991. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J. Exp. Med. 174:1371-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deepe, G. S., Jr., R. Gibbons, G. D. Brunner, and F. J. Gomez. 1996. A protective domain of heat-shock protein 60 from Histoplasma capsulatum. J. Infect. Dis. 174:828-834. [DOI] [PubMed] [Google Scholar]
- 6.Gomez, F. J., J. A. Cain, R. Gibbons, R. Allendoerfer, and G. S. Deepe, Jr. 1998. Vβ4+ T cells promote clearance of infection in murine pulmonary histoplasmosis. J. Clin. Investig. 102:984-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez, F. J., E. O. Woodward, R. Pilcher-Roberts, R. S. Gibbons, and G. S. Deepe, Jr. 2001. Vβ6+ and Vβ4+ T cells exert cooperative activity in clearance of secondary infection with Histoplasma capsulatum. J. Immunol. 166:2855-2862. [DOI] [PubMed] [Google Scholar]
- 8.Okada, C. Y., and I. L. Weissman. 1989. Relative Vβ transcript levels in thymus and peripheral lymphoid tissues from various mouse strains. Inverse correlation of I-E and Mls expression with relative abundance of several Vβ transcripts in peripheral lymphoid tissues. J. Exp. Med. 169:1703-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiner, S. L., Z.-E. Wang, F. Hatam, P. Scott, and R. M. Locksley. 1993. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science 259:1457-1460. [DOI] [PubMed] [Google Scholar]
- 10.Zhou, P., and R. A. Seder. 1999. CD40 ligand is not essential for induction of type 1 cytokine responses or protective immunity after primary or secondary infection with Histoplasma capsulatum. J. Exp. Med. 187:1315-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]