Abstract
Brucella species are gram-negative, facultative intracellular bacteria that infect humans and animals. These organisms can survive and replicate within a membrane-bound compartment inside professional and nonprofessional phagocytic cells. Inhibition of phagosome-lysosome fusion has been proposed as a mechanism for intracellular survival in both types of cells. We have previously shown that the maturation inhibition of the Brucella-containing phagosome appears to be restricted at the phagosomal membrane, but the precise molecular mechanisms and factors involved in this inhibition have yet to be identified. Interestingly, recent studies have revealed that caveolae or lipid rafts are implicated in the entry of some microorganisms into host cells and mediate an endocytic pathway avoiding fusion with lysosomes. In this study, we investigated the role of cholesterol and the ganglioside GM1, two components of lipid rafts, in entry and short-term survival of Brucella suis in murine macrophages, by using cholesterol-sequestering (filipin and β-methyl cyclodextrin) and GM1-binding (cholera toxin B) molecules. Our results suggest that lipid rafts may provide a portal for entry of Brucella into murine macrophages under nonopsonic conditions, thus allowing phagosome-lysosome fusion inhibition, and provide further evidence to support the idea that the phagosome maturation inhibition is restricted at the phagosomal membrane.
Brucella species are gram-negative, facultative intracellular bacteria that infect humans and animals. These organisms can survive and replicate within a membrane-bound compartment inside professional (4, 14, 16, 26) and nonprofessional (8, 23, 24) phagocytic cells. In the classic endocytic pathway, phagosomes interact with lysosomes, allowing degradation by lysosomal enzymes. In the case of Brucella, inhibition of phagosome-lysosome fusion has been proposed as a mechanism for intracellular survival of the bacteria in both types of cells (1, 10, 14, 18, 23, 24).
The ability to inhibit phagosome maturation into a phagolysosome is shared by several bacteria and parasites, but the molecular mechanisms and the responsible microbial factors are poorly understood. This maturation inhibition has been associated with proteins secreted in the macrophage cytosol; for example, Salmonella SpiC protein is exported into the host cell cytosol and inhibits cellular trafficking (33). For Legionella pneumophila, the dot/icm gene products are required to avoid normal trafficking of the L. pneumophila phagosome (28, 35). For some other parasites, inhibition is associated with the presence of particular surface molecules on the organism's membrane or on the phagosomal membrane. Hence, for Leishmania, maturation inhibition requires lipophosphoglycan expression at the parasite surface (7, 29), and in mycobacteria, the TACO host protein present on the phagosomal membrane in a cholesterol-dependent manner prevents lysosomal delivery (9). It has previously been shown that the maturation inhibition of the Brucella-containing phagosome appears to be restricted at the phagosomal membrane (17), but the molecular mechanisms and factors implicated in this phenomenon have yet to be clearly defined.
Interestingly, recent studies have revealed that caveolae or lipid rafts may be implicated in the entry of some microorganisms into host cells (30, 31). Lipid rafts are lateral assemblies (rafts) of cholesterol, glycolipids such as the glycosphingolipid GM1, and glycosylphosphatidylinositol-anchored molecules (30, 31). For example, cholesterol is essential for entry of Mycobacterium bovis into macrophages (12). Moreover, cholesterol mediates the phagosomal association of TACO, a host protein that prevents phagosome-lysosome fusion (9, 12, 22). Thus, by entering host cells at cholesterol-rich domains, mycobacteria may ensure their intracellular survival within macrophages. The maturation inhibition of the Brucella-containing phagosome, which is restricted at the phagosomal membrane (17), may therefore be due to bacterial entry by lipid rafts. In this study, we investigated the roles of cholesterol and the ganglioside GM1, two components of lipid rafts, in entry and short-term survival of Brucella suis in murine macrophages.
J774.A1 cells (from a murine macrophage-like cell line) were grown in RPMI 1640 medium with Glutamax I (Gibco/BRL) containing 10% heat-inactivated fetal calf serum (complete medium) at 37°C and 5% CO2. Cells were resuspended at 105 cells/ml and cultured for 2 days in 24-well plates.
Bacterial strains used throughout the experiments were either B. suis 1330 (ATCC 23444) or, in microscopy experiments, B. suis 1330 p/sog, which constitutively expresses a green fluorescent protein, prepared as described elsewhere (15, 20). Bacteria were grown in tryptic soy broth (Difco Laboratories) at 37°C overnight to stationary phase. Bacteria were opsonized with polyclonal murine anti-Brucella antibodies for 30 min at 37°C.
We investigated the roles of cholesterol- and glycosphingolipid-enriched microdomains by treating cells with cholesterol-binding (filipin) or -depleting (β-methyl cyclodextrin) molecules or the ganglioside GM1-binding molecule (cholera toxin B subunit). Filipin, β-methyl cyclodextrin, and cholera toxin B subunit were obtained from Sigma-Aldrich (St Quentin Fallavier, France). After treatment at 37°C for 30 min in complete medium, cells were washed once with phosphate-buffered saline (PBS) before infection. As a control, the viability of the cells following treatment was assessed with dextran blue staining. We also ensured that the viability of bacteria was not affected by the treatments with drugs.
Cells were then infected with B. suis at a multiplicity of infection of 100 bacteria per cell for 45 min at 37°C. After three washes with PBS, cells were reincubated in culture medium containing gentamicin at 30 μg/ml for 1 h (1 h postinfection).
Phagocytosis measurement by fluorescence microscopy.
Entry of B. suis in murine macrophages was measured by using fluorescence microscopy. Cells were grown on glass coverslips, and following treatment with the relevant drug and infection with B. suis, which expresses green fluorescent protein, the cells were fixed for 20 min with 3% paraformaldehyde and washed twice in PBS. Extracellular bacteria were distinguished from intracellular bacteria by labeling extracellular particles with polyclonal murine anti-Brucella antibodies (1/1,000), revealed by rhodamine-conjugated second antibodies (1/100) [goat F(ab′)2 fragment mouse immunoglobulin (heavy plus light chains)-rhodamine was obtained from Immunotech (Marseille, France)]. Then, coverslips were mounted in Mowiol medium and examined by classical fluorescence microscopy with an inverted Leica DM IRB microscope. Phagocytosis was calculated by counting cells that had ingested at least one bacterium.
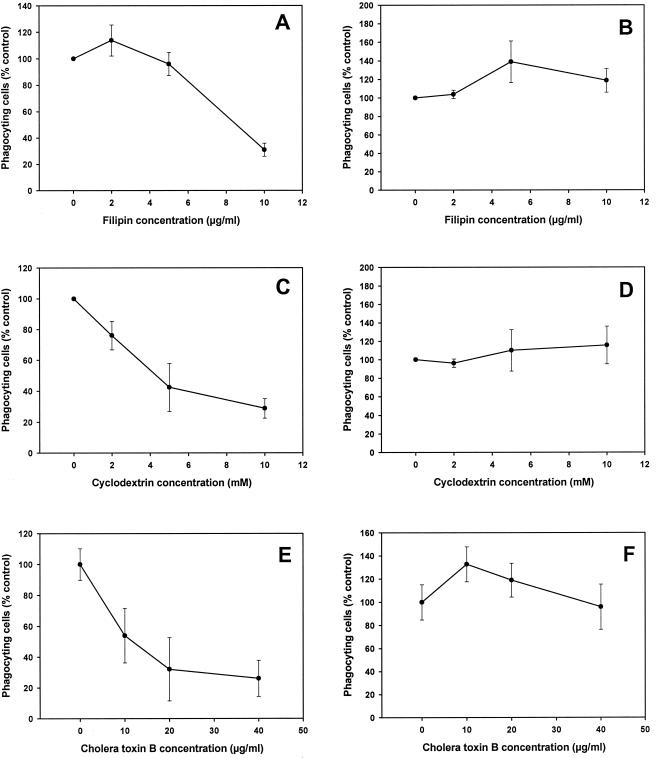
To test the involvement of cholesterol in the phagocytosis of B. suis, we treated cells with either cholesterol-binding (filipin) or -depleting (β-methyl cyclodextrin) molecules, which affect the function of lipid rafts. Following pretreatment with increasing concentrations of filipin or β-methyl cyclodextrin, phagocytosis of nonopsonized bacteria, as visualized by fluorescence microscopy, was strongly inhibited (Fig. 1A and C). In comparison, phagocytosis of opsonized bacteria was not affected by these treatments (Fig. 1B and D). Rather, we observed a slight increase, and the number of internalized bacteria per cell was always enhanced (data not shown). Cholesterol depletion of membranes decreases their rigidity, which may explain the increase in phagocytosis. Consistent with a role for cholesterol in providing rigidity to the cell surface, Gatfield and Pieters (12) observed a moderate enhancement of cellular motility after cholesterol depletion. Similar results were obtained with filipin and β-methyl cyclodextrin, despite their different modes of action. Thus, the entry of nonopsonized B. suis into J774 macrophages required cholesterol, while internalization via opsonic receptors did not.
FIG. 1.
Effects of filipin, β-methyl cyclodextrin, and cholera toxin B subunit on phagocytosis of nonopsonized and opsonized B. suis by murine macrophages. Cells were pretreated with cholesterol-binding (filipin) or -depleting (β-methyl cyclodextrin) molecules or the ganglioside GM1-binding molecule (cholera toxin B subunit) at the indicated concentrations. (A, C, and E) Phagocytosis under nonopsonic conditions. (B, D, and F) Phagocytosis under opsonic conditions. Phagocytosis was measured by microscopy experiments as described in the text. Values represent means ± standard deviations, obtained by counting three series of 100 (infection with opsonized bacteria) or 300 (infection with nonopsonized bacteria) cells. The percentages of cells having ingested nonopsonized and opsonized B. suis were 22 ± 5 and 49 ± 7, respectively. These values referred to untreated phagocytes. There was generally one bacterium per cell under nonopsonic conditions. The number of bacteria per cell was 3.4 ± 0.7 under opsonic conditions. Experiments were performed in duplicate and repeated twice.
We also investigated the role of lipid rafts in bacterial entry by treating cells with the ganglioside GM1-binding molecule (cholera toxin B subunit) (32). After pretreatment with increasing concentrations of drug, phagocytosis of nonopsonized bacteria was strongly inhibited (Fig. 1E). In comparison, phagocytosis of opsonized bacteria was not affected by cholera toxin B treatment (Fig. 1F). Thus, entry of nonopsonized B. suis into J774 macrophages required the ganglioside GM1, while internalization via opsonic receptors did not.
Bacteria can enter macrophages via a variety of surface receptors. The host cell receptor involved in the binding and internalization of B. suis into macrophages under nonopsonic conditions is not well characterized. Nonopsonic entry of Brucella abortus into bovine macrophages is found to be inhibited by fibronectin, mannan, and lipopolysaccharide (3), but the receptor has not yet been identified. Under opsonic conditions, phagocytosis is mediated by the Fc receptor. Moreover, while nonopsonized Brucella is poorly ingested by macrophages, opsonization with antibodies greatly increases the uptake of bacteria. In this work, we have shown that the uptake of nonopsonized B. suis by murine macrophages is mediated by lipid rafts and that at least two components of these membrane microdomains, cholesterol and the glycosphingolipid GM1, are involved. Lipid rafts could serve as “docking” sites for Brucella to stabilize the bacterium-macrophage interaction, after which internalization occurs. Bacterial uptake by lipid rafts is of interest in that the endocytic pathway used in this case is distinct from the classical endosome-lysosome pathway because the compartment formed by lipid-rich microdomains does not fuse with lysosomes (30, 31). Thus, Brucella uptake at lipid rafts could be related to the phagosome-lysosome fusion inhibition observed with these bacteria (1, 10, 14, 18, 23, 24). In comparison, by entering host cells at cholesterol-rich domains, mycobacteria may ensure their intracellular survival within macrophages (9, 12). Recently, another bacterium, L. pneumophila, which inhibits phagosome-lysosome fusion, was found to be internalized via a pathway involving the ganglioside GM1 (34). Moreover, a study using a proteomic approach reported the presence of some lipid raft proteins, flotillin-1 and stomatin, in latex bead-containing phagosomes (11), suggesting that lipid rafts might be present on the phagosomal membrane.
When we compared the uptakes of nonopsonized and opsonized B. suis into murine macrophages, phagocytosis of opsonized bacteria was not affected by treatment of cells with cholesterol-sequestering (filipin and β-methyl cyclodextrin) and GM1-binding (cholera toxin B) molecules, in contrast to the results obtained with nonopsonized bacteria. Cholesterol and GM1 are not required for bacterial uptake under opsonic conditions. Brucella entry into murine macrophages via the Fc receptor is considerably enhanced and can mask entry by lipid rafts. If we consider that inhibition of phagosome-lysosome fusion is reported for both nonopsonized and opsonized bacteria (1) and that lipid rafts might be involved in the phagosome maturation pathway, we cannot exclude that lipid rafts and Fc receptors were used simultaneously as a portal of entry for opsonized B. suis. Such differences in the effects of drugs on internalization between nonopsonized and opsonized bacteria have been reported in the literature. Phagocytosis of Mycobacterium kansasii by human neutrophils is strongly inhibited when cells are treated with cyclodextrin and filipin, but when M. kansasii is serum opsonized, the inhibitory effects of the drugs are not observed (21). Moreover, the inhibitor concentrations found to be required in our experiments were generally comparable to previous observations by others (12, 13, 21, 32).
Short-term survival of B. suis in macrophages.
In the above experiments, phagocytosis was measured by counting intracellular bacteria by fluorescence microscopy. In some studies, bacterial uptake was quantified by intracellular survival in host cells (13). For comparison, we studied the effects of drugs on the short-term survival of B. suis inside macrophages. Following treatment with the relevant drug, infection with B. suis, and postinfection cells were washed once with PBS and lysed in 0.2% Triton X-100. Bacterial counts (in CFU) were determined by plating appropriate dilutions on tryptic soy agar and incubation at 37°C for 3 days.
After pretreatment with increasing concentrations of filipin (Fig. 2A), β-methyl cyclodextrin (Fig. 2C), and cholera toxin B subunit (Fig. 2E), intracellular survival of nonopsonized bacteria was strongly inhibited. These results directly correlated to the decreased phagocytosis observed in the microscopy experiments (Fig. 1A, C, and E). When bacteria were internalized via opsonic receptors, we also observed a strong inhibition of bacterial survival with increasing concentrations of filipin (Fig. 2B), β-methyl cyclodextrin (Fig. 2D), and cholera toxin B subunit (Fig. 2F), in contrast to the phagocytosis results observed in the microscopy experiments (Fig. 1B, D, and F).
FIG. 2.
Effects of filipin, β-methyl cyclodextrin, and cholera toxin B subunit on short-term survival of nonopsonized and opsonized B. suis inside murine macrophages. Cells were pretreated with cholesterol-binding (filipin) or -depleting (β-methyl cyclodextrin) molecules, or the ganglioside GM1-binding molecule (cholera toxin B subunit) at the indicated concentrations. (A, C, and E) Survival of nonopsonized bacteria. (B, D, and F) Survival of opsonized bacteria. The number of surviving bacteria was determined 3 h postinfection (A and B) and 1 h postinfection (C, D, E, and F). Experiments were performed in triplicate, and the values represent means ± standard deviations. The experiments were repeated at least twice.
In these experiments, we investigated the role of lipid rafts in short-term survival of nonopsonized and opsonized B. suis inside macrophages. A strong inhibition of bacterial survival was always observed with increasing concentrations of cholesterol-sequestering (filipin and β-methyl cyclodextrin) and GM1-binding (cholera toxin B) molecules. In the case of nonopsonized bacteria, the decreased survival might be directly related to uptake inhibition observed in microscopy experiments, but we cannot exclude any adverse effect of these drugs on short-term survival. Opsonized bacteria could enter into the cells via the Fc receptors despite the presence of blocking molecules. However, in the presence of these molecules, we observed a strong inhibition of bacterial survival. We could therefore speculate that the brucellae must associate with cholesterol and GM1 directly to escape phagosome-lysosome fusion and survive inside the cell. These results provide support for the hypothesis that lipid rafts may be implicated in the first steps of Brucella-containing phagosome maturation. Moreover, it should be noted that bacterial uptake measured by microscopy experiments and short-term intracellular survival are two distinct phenomena and that our assay of short-term survival did not allow us to measure bacterial internalization, as proposed in some studies (13).
The ability to inhibit the maturation of a phagosome into a phagolysosome is a feature shared by several bacteria and parasites, but the molecular mechanisms and the factors involved have been identified in only a few cases (7, 9, 28, 29, 33, 35). We have shown that the maturation inhibition of Brucella-containing phagosomes appears to be restricted at the phagosomal membrane (17), but the mechanisms involved in this phenomenon have yet to be elucidated. Our results have suggested that lipid rafts may act as a portal for Brucella entry into macrophages, thus allowing phagosome-lysosome fusion inhibition and providing support for the idea that this inhibition is restricted at the phagosomal membrane (17).
Bacterial entry is probably essential in the maturation of the nascent vacuole. Entry of Brucella into tight compartments (1, 27) may reduce fusogenicity of phagosomes and delay their maturation, as observed for mycobacteria (5). Following uptake, Brucella-containing phagosomes rapidly acidify, and this early acidification is essential for survival and replication of the bacteria within the macrophage (25) and particularly for the induction of virB (2). Other results indicate that the first events in the biogenesis of Brucella-containing vacuoles, penetration and inhibition of phagolysosomal fusion, are not affected by mutations in three virB genes (6). Thus, the type IV secretion system, identified as occurring in Brucella suis (19), might be implicated in later steps of maturation.
In this study, we clearly show that two components of lipid rafts, cholesterol and the ganglioside GM1, are involved in the entry of B. suis into murine macrophages under nonopsonic conditions. As the endocytic pathway mediated by lipid rafts appears to avoid fusion with lysosomes, this portal of entry used by Brucella may explain the observed phagosome-lysosome fusion inhibition.
Acknowledgments
We thank J. P. Liautard for helpful discussions and J. Oliaro for critical reading of the manuscript.
A. Naroeni was supported by a fellowship from the French government and by a grant from the Fondation pour La Recherche Médicale. This work was partly supported by grant QLK2-CT-1999-00014 from the European Union.
REFERENCES
- 1.Arenas, G. N., A. S. Staskevich, A. Aballay, and L. S. Mayorga. 2000. Intracellular trafficking of Brucella abortus in J774 macrophages. Infect. Immun. 68:4255-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, A. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevielle, J. P. Liautard, M. Ramuz, and D. O'Callaghan. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 3.Campbell, G. A., L. G. Adams, and B. A. Sowa. 1994. Mechanisms of binding of Brucella abortus to mononuclear phagocytes from cows naturally resistant or susceptible to brucellosis. Vet. Immunol. Immunopathol. 41:295-306. [DOI] [PubMed] [Google Scholar]
- 4.Caron, E., J. P. Liautard, and S. Kohler. 1994. Differentiated U937 cells exhibit increased bactericidal activity upon LPS activation and discriminate between virulent and avirulent Listeria and Brucella species. J. Leukoc. Biol. 56:174-181. [DOI] [PubMed] [Google Scholar]
- 5.de Chastellier, C., and L. Thilo. 1997. Phagosome maturation and fusion with lysosomes in relation to surface property and size of the phagocytic particle. Eur. J. Cell Biol. 74:49-62. [PubMed] [Google Scholar]
- 6.Delrue, R. M., M. Martinez-Lorenzo, P. Lestrate, I. Danese, V. Bielarz, P. Mertens, X. De Bolle, A. Tibor, J. P. Gorvel, and J. J. Letesson. 2001. Identification of Brucella spp. genes involved in intracellular trafficking. Cell. Microbiol. 3:487-497. [DOI] [PubMed] [Google Scholar]
- 7.Desjardins, M., and A. Descoteaux. 1997. Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J. Exp. Med. 185:2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Detilleux, P. G., B. L. Deyoe, and N. F. Cheville. 1990. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect. Immun. 58:2320-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari, G., H. Langen, M. Naito, and J. Pieters. 1999. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97:435-447. [DOI] [PubMed] [Google Scholar]
- 10.Frenchick, P. J., R. J. Markham, and A. H. Cochrane. 1985. Inhibition of phagosome-lysosome fusion in macrophages by soluble extracts of virulent Brucella abortus. Am. J. Vet. Res. 46:332-335. [PubMed] [Google Scholar]
- 11.Garin, J., R. Diez, S. Kieffer, J. F. Dermine, S. Duclos, E. Gagnon, R. Sadoul, C. Rondeau, and M. Desjardins. 2001. The phagosome proteome: insight into phagosome functions. J. Cell Biol. 152:165-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatfield, J., and J. Pieters. 2000. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288:1647-1650. [DOI] [PubMed] [Google Scholar]
- 13.Guignot, J., M. F. Bernet-Camard, C. Pous, L. Plancon, C. Le Bouguenec, and A. L. Servin. 2001. Polarized entry of uropathogenic Afa/Dr diffusely adhering Escherichia coli strain IH11128 into human epithelial cells: evidence for α5β1 integrin recognition and subsequent internalization through a pathway involving caveolae and dynamic unstable microtubules. Infect. Immun. 69:1856-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harmon, B. G., L. G. Adams, and M. Frey. 1988. Survival of rough and smooth strains of Brucella abortus in bovine mammary gland macrophages. Am. J. Vet. Res. 49:1092-1097. [PubMed] [Google Scholar]
- 15.Kohler, S., S. Ouahrani-Bettache, M. Layssac, J. Teyssier, and J. P. Liautard. 1999. Constitutive and inducible expression of green fluorescent protein in Brucella suis. Infect. Immun. 67:6695-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liautard, J. P., A. Gross, J. Dornand, and S. Kohler. 1996. Interactions between professional phagocytes and Brucella spp. Microbiologia 12:197-206. [PubMed] [Google Scholar]
- 17.Naroeni, A., N. Jouy, S. Ouahrani-Bettache, J. P. Liautard, and F. Porte. 2001. Brucella suis-impaired specific recognition of phagosomes by lysosomes due to phagosomal membrane modifications. Infect. Immun. 69:486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberti, J., R. Caravano, and J. Roux. 1981. Attempts of quantitative determination of phagosome-lysosome fusion during infection of mouse macrophages by Brucella suis. Ann. Inst. Pasteur Immunol. 132D:201-206.
- 19.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 20.Ouahrani-Bettache, S., F. Porte, J. Teyssier, J. P. Liautard, and S. Kohler. 1999. pBBR1-GFP: a broad-host-range vector for prokaryotic promoter studies. BioTechniques 26:620-622. [DOI] [PubMed] [Google Scholar]
- 21.Peyron, P., C. Bordier, E. N. N'Diaye, and I. Maridonneau-Parini. 2000. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J. Immunol. 165:5186-5191. [DOI] [PubMed] [Google Scholar]
- 22.Pieters, J. 2001. Evasion of host cell defense mechanisms by pathogenic bacteria. Curr. Opin. Immunol. 13:37-44. [DOI] [PubMed] [Google Scholar]
- 23.Pizarro-Cerda, J., S. Meresse, R. G. Parton, G. van der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J. P. Gorvel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizarro-Cerda, J., E. Moreno, V. Sanguedolce, J. L. Mege, and J. P. Gorvel. 1998. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect. Immun. 66:2387-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porte, F., J. P. Liautard, and S. Kohler. 1999. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 67:4041-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price, R. E., J. W. Templeton, R. Smith III, and L. G. Adams. 1990. Ability of mononuclear phagocytes from cattle naturally resistant or susceptible to brucellosis to control in vitro intracellular survival of Brucella abortus. Infect. Immun. 58:879-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rittig, M. G., M. T. Alvarez-Martinez, F. Porte, J. P. Liautard, and B. Rouot. 2001. Intracellular survival of Brucella spp. in human monocytes involves conventional uptake but special phagosomes. Infect. Immun. 69:3995-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy, C. R., K. H. Berger, and R. R. Isberg. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663-674. [DOI] [PubMed] [Google Scholar]
- 29.Scianimanico, S., M. Desrosiers, J. F. Dermine, S. Meresse, A. Descoteaux, and M. Desjardins. 1999. Impaired recruitment of the small GTPase rab7 correlates with the inhibition of phagosome maturation by Leishmania donovani promastigotes. Cell. Microbiol. 1:19-32. [DOI] [PubMed] [Google Scholar]
- 30.Shin, J., and S. N. Abraham. 2001. Caveolae as portals of entry for microbes. Microbes Infect. 3:755-761. [DOI] [PubMed] [Google Scholar]
- 31.Shin, J. S., and S. N. Abraham. 2001. Co-option of endocytic functions of cellular caveolae by pathogens. Immunology 102:2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin, J. S., Z. Gao, and S. N. Abraham. 2000. Involvement of cellular caveolae in bacterial entry into mast cells. Science 289:785-788. [DOI] [PubMed] [Google Scholar]
- 33.Uchiya, K., M. A. Barbieri, K. Funato, A. H. Shah, P. D. Stahl, and E. A. Groisman. 1999. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 18:3924-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watarai, M., I. Derre, J. Kirby, J. D. Growney, W. F. Dietrich, and R. R. Isberg. 2001. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus(*). J. Exp. Med. 194:1081-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuckman, D. M., J. B. Hung, and C. R. Roy. 1999. Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Mol. Microbiol. 32:990-1001. [DOI] [PubMed] [Google Scholar]