Abstract
We have previously shown that Legionella pneumophila induces caspase 3-dependent apoptosis in mammalian cells during early stages of infection. In this report, we show that nine L. pneumophila strains with mutations in the dotA, dotDCB, icmT, icmGCD, and icmJB loci are completely defective in the induction of apoptosis, in addition to their severe defects in intracellular replication and pore formation-mediated cytotoxicity. Importantly, all nine dot/icm mutants were complemented for all their defective phenotypes with the respective wild-type loci. We show that the role of the Dot/Icm type IV secretion system in the induction of apoptosis is independent of the RtxA toxin, the dot/icm-regulated pore-forming toxin, and the type II secretion system. However, the pore-forming toxin, which is triggered upon entry into the postexponential growth phase, enhances the ability of L. pneumophila to induce apoptosis. Our data provide the first example of the role of a type IV secretion system of a bacterial pathogen in the induction of apoptosis in the host cell.
Legionella pneumophila is a gram-negative bacterium that is ubiquitous in the aquatic environment, where the bacterium invades and replicates within protozoa (3, 25). Upon transmission to the human host, L. pneumophila invades and replicates within alveolar macrophages and epithelial cells (1, 20). Within both host cells, the bacterial phagosome is blocked from maturation along the “default” endosomal-lysosomal degradation pathway (10, 30, 44) and is surrounded by the rough endoplasmic reticulum (2, 29, 52). Formation of this replicative niche is controlled by a type IV-like secretion machinery, designated Dot and Icm (48, 53).
The molecular mechanisms by which L. pneumophila kills mammalian cells have been recently characterized to occur by two mechanisms (13, 14). First, L. pneumophila induces apoptosis in macrophages and alveolar epithelial cells during early stages of the infection (14, 15, 17, 22), which is mediated by the activation of caspase 3 (13). The second mechanism is pore formation-mediated lysis of the host cell, which is triggered upon termination of intracellular replication (5, 7, 16).
A number of bacterial pathogens have been shown to induce apoptosis during infection, but the benefits of this modulation of apoptosis to the invading pathogen may vary (15, 17). In addition, pathogens have evolved distinct mechanisms to induce apoptosis within mammalian cells (11, 17, 21, 26-28, 31, 39, 40, 42, 47, 54).
To date, L. pneumophila has two major secretion systems known to be involved in pathogenesis. These are the Lsp type II (23, 43) and Dot/Icm type IV secretion systems (8). The induction of apoptosis could be a result of secreted molecules by either one or both of these systems or by another unknown mechanism. In this report, we show that the type II secretion system has no detectable role in the induction of apoptosis and the type IV secretion system is the sole machinery involved in the induction of mammalian cells to undergo programmed cell death. We also show that neither the RtxA toxin nor the pore-forming toxin is essential for the induction of programmed cell death. However, expression of the pore-forming toxin upon transition to the postexponential growth phase enhances the ability of L. pneumophila to induce apoptosis.
The virulent parental strain AA100 of L. pneumophila and construction of the mini-Tn10::kan bank of mutants have been described previously (kan is the kanamycin resistance cassette) (18, 19). The kan insertion mutant (AA100KmT), which has kan inserted within icmT, has been recently described (37). Bacterial growth on artificial media was performed as previously described (19, 51). The parental strain AA100 was grown on buffered charcoal-yeast extract (BCYE) agar plates or in buffered yeast extract (BYE) broth. For the mutants, the media were supplemented with 50 μg of kanamycin per ml (19). The PilD mutant with a kan insertion in the NarI site was recently constructed (35). The LspG mutant which contains a kanamycin resistance cassette was described previously (43). The complemented strains were created by electroporation (50) of the mutants with pMMB207-derived plasmids containing a chloramphenicol resistance marker and a region of the dot/icm loci. Complementing loci are dotA for GG105, GS95, and GL10 mutants; icmJB for GG46 and GM187; icmGCD for GN142 and GS111; and dotBCD for GQ262.
For infections, L. pneumophila were grown on BCYE plates and prepared for infections as described above. Infection of phorbol myristate acetate-differentiated U937 monolayers with a L. pneumophila strain was performed, in triplicate, in 96-well plates containing 105 cells/well at a multiplicity of infection (MOI) of 10. The plates were spun at 900 × g for 5 min and then incubated for 1 h at 37°C. At the end of this infection period, monolayers were washed three times with tissue culture medium to remove nonadherent bacteria. Subsequently, monolayers were incubated for 1 h at 37°C in the presence of 50 μg of gentamicin per ml to kill extracellular bacteria and further incubated for several time intervals in the absence of gentamicin. For measurements of the number of remaining viable cells in the monolayer, the cells were treated with 10% Alamar blue dye (Alamar Bioscience Inc., Sacramento, Calif.), which is reduced by viable cells. Measurements of optical density were performed at a wavelength of 570 nm and corrected for background at 600 nm, using a Molecular Devices Microplate Reader (Molecular Devices, Sunnyvale, Calif.). Cytopathogenicity of L. pneumophila to the monolayers was calculated as the ratio of the optical density (OD) of the reduced Alamar blue of infected cell monolayers to that of noninfected ones, and percent cytopathogenicity was calculated by the formula {[1 − (OD of infected cells/OD of noninfected cells)] × 100}. The viability of U937 cells was determined by trypan blue dye exclusion, and the percent cytopathogenicity was determined by comparing the infected cell monolayers to that of noninfected ones.
Transfections, restriction enzyme digestions, and DNA ligations were performed as described elsewhere, unless specified otherwise (4). Restriction enzymes and T4 DNA ligase were purchased from Promega (Madison, Wis.). Plasmid and cosmid DNA preparations were performed with the Bio-Rad Quantum miniprep kit (Bio-Rad Laboratories, Hercules, Calif.) and the polyethylene glycol DNA extraction protocol, as described elsewhere (45). Electroporations were performed with a Bio-Rad Gene Pulser, as recommended by the manufacturer. DNA probes for Southern hybridization were generated by PCR amplification with a Perkin-Elmer Gene Amp PCR system 2400 (Perkin-Elmer, Norwalk, Conn.). Purification of DNA fragments from agarose gels for subcloning or labeling was performed with a QIAquick gel purification kit (Qiagen Inc., Chatsworth, Calif.). Fluorescein labeling of DNA probes for Southern hybridization was performed with the ECL (enhanced chemiluminescence) Random Prime Labeling System, version II (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.). Transfer of DNA from agarose gels onto membranes, fluorescein labeling of DNA probes, hybridizations, and detection were performed as previously described (4). Oligonucleotide synthesis for PCR and sequencing were performed by Integrated DNA Technologies, Inc. (Coralville, Calif.).
Terminal deoxytransferase-mediated dUTP nick end labeling (TUNEL) assays were performed exactly as we described previously (14). Briefly, cells attached to 96-well plates were infected for 1 h with L. pneumophila at an MOI of 50, and then extracellular unattached bacteria were washed off. For labeling of apoptotic nuclei, the cells were subjected to fluorescein isothiocyanate-conjugated TUNEL using an apoptosis detection kit, according to the manufacturer's instructions (Boehringer Mannheim Corporation, Indianapolis, Ind.). Cells were examined using an Axiovert S100 Zeiss fluorescence microscope. A minimum of 100 cells per sample was counted, and apoptosis was quantitated as the percentage of apoptotic cells (TUNEL-positive nuclei). Multiple independent samples were examined.
U937 macrophage-like cells (105 CFU/well) in 96-well plates were infected with L. pneumophila in triplicate, at the MOI indicated for each experiment. The plates were centrifuged at 900 × g for 5 min and incubated for 1 h at 37°C. At the end of this infection period, monolayers were washed three times with tissue culture medium to remove nonadherent bacteria. Subsequently, monolayers were incubated for 1 h at 37°C in the presence of 50 mg of gentamicin per ml to kill extracellular bacteria and then incubated for several time intervals in the absence of gentamicin. The time point at the end of the gentamicin treatment was the initial time point (T0). Monolayers were lysed (at several time intervals) using sterile double-distilled water. The supernatants prior to lysis of the monolayers (which may contain released bacteria) were combined with the ones after lysis. Aliquots were diluted immediately in water and plated on BCYE agar plates for enumeration of intracellular bacteria.
Identification of L. pneumophila mutants defective in inducing apoptosis.
We have previously isolated and characterized two collections of mutants, one of which is defective in replication within both protozoa and macrophages (designated pmi for protozoa and macrophage infectivity loci) (19). The other collection contained mutants that are defective only within macrophages (designated mil for macrophage infectivity loci) (18). Southern hybridization of the 89 pmi mutants (19) and 32 mil mutants (18) was performed using PCR fragments that cover all the gene clusters encompassing the 23 dot/icm genes. The locations of the kan insertions were denoted by a 1.7-kb increase in the size of the DNA fragment that hybridized with the specific probe at high stringency. In addition to the three dotA mutants (GL10, GS95, and GG105), we identified five other pmi mutants that had insertions within the icmGCD (GN142 and GS111), icmJB (GG46 and GM187), and dotDCB (GQ262) loci (data not shown). All eight mutants had distinct kanamycin resistance cassette insertions, as determined by Southern analysis (data not shown). We have also recently constructed an insertion mutant with a mutation in icmT (37), which will be included in our analysis of the dot/icm mutants for their ability to induce apoptosis. None of the 32 mil mutants had kan insertion within the dot/icm loci (data not shown).
The Dot/Icm secretion system is essential for the induction of apoptosis.
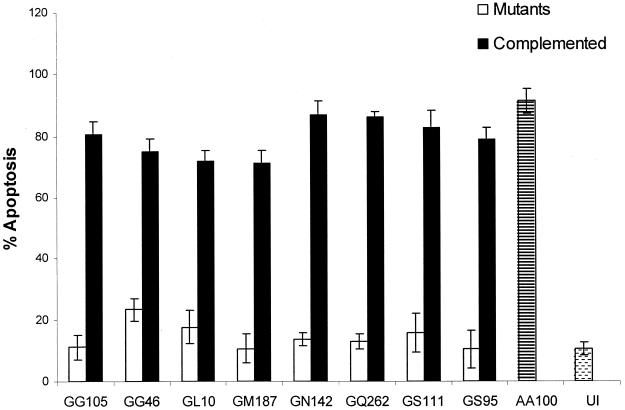
L. pneumophila causes a contact-dependent apoptosis within mammalian cells that does not require invasion or bacterial protein synthesis (14). To determine if the dot/icm loci were required to induce apoptosis, the nine mutants were examined along with the wild-type strain for their ability to induce apoptosis within macrophages by TUNEL assays. All apoptotic macrophages had a condensed nucleus that was labeled with a green fluorescent dUTP by TUNEL, and membrane blebbing was evident by phase-contrast microscopy, which further confirmed the presence of apoptotic cells. The wild-type strain AA100 induced more than 90% of the U937 macrophages to undergo apoptosis, while less than 10% of the uninfected cells underwent apoptosis (Fig. 1). The nine mutants with kan insertions within the dot/icm loci were severely defective in the induction of apoptosis, where less than 15% of the infected cells underwent programmed cell death (Fig. 1). Importantly, upon introduction of the respective wild-type dot/icm loci on a plasmid into the respective mutant, all of the mutants induced apoptosis in macrophages similar to the wild-type strain (Fig. 1) (data not shown). Thus, the Dot/Icm type IV secretion system is essential for the induction of apoptosis.
FIG. 1.
The dot/icm mutants are defective in the induction of U937 cells to undergo apoptosis. Apoptosis was measured by TUNEL assay at an MOI of 50 after infection for 1 h followed by further incubation for 3 h. Complementing loci are dotA for GG105, GS95, and GL10 mutants; icmJB for GG46 and GM187; icmGCD for GN142 and GS111; and dotBCD for GQ262. AA100 (the wild-type strain) and uninfected U937 cells (UI) were used as controls. The data are representative of three different experiments, and error bars represent the standard deviations of triplicate samples.
Characterization of the dot/icm mutants within U937 macrophages.
We examined whether the eight pmi mutants, which had kan insertions within the dot/icm loci, were also defective in intracellular growth. All the mutants showed a steady decline in viability to a value 100-fold less than the initial infecting dose during the 48-h infection period of U937 macrophages (data not shown). In contrast, infection by the wild-type strain showed a 100-fold increase in intracellular bacteria within 48 h of infection.
To confirm that the kan insertion within the dot/icm loci in the eight mutants from the pmi collection is responsible for the defect in intracellular replication, we examined whether the complemented strains were rescued for their defect in intracellular replication. The data indicate that the complemented strains are indeed rescued for this defect (data not shown). Similarly, the defect in intracellular replication of the icmT insertion mutant is also rescued by the wild-type icmT gene (37).
Cytopathogenicity and hemolytic properties of the dot/icm mutants.
The Dot/Icm secretion system of L. pneumophila is required for cytotoxicity to U937 macrophages, which is mediated by contact-dependent pore formation (5, 33, 48). It has been previously shown that mutations within the dotA, dotB, dotE/icmC, dotG/icmE, dotH/icmK, dotI/icmL, dotO/icmB (32), and icmT (37) loci are defective in their cytotoxicity to macrophages due to their defect in inserting pores in eukaryotic membranes. Therefore, we utilized two different assays to examine the mutants and their complemented counterparts for the pore-forming activity (5). First, we tested them for their ability to lyse sheep red blood cells upon contact. GL208, a mutant defective in the pore-forming toxin (5), was used as a negative control in these assays. The data showed that the mutant strains were defective in their pore-forming activity compared to the wild-type strain and were complemented by the wild-type loci for the defect in the pore-forming activity (data not shown). Similarly, the defect in contact-dependent hemolysis of the icmT insertion mutant is also rescued by the wild-type icmT gene (37).
In the second strategy, we examined cytopathogenicity to U937 macrophage-like cells at 24 and 48 h postinfection. The mutant strains were completely defective in their cytopathogenicity to macrophages, and introduction of the corresponding wild-type dot/icm region defective in each of the mutants restored the cytopathogenicity of the mutants to the wild-type level (data not shown). Similarly, the defect in cytopathogenicity of the icmT insertion mutant is also rescued by the wild-type icmT gene (37).
Role of the type II secretion system in apoptosis.
The type II secretion system Lsp (Legionella secretion pathway) of L. pneumophila is responsible for the secretion of many degradative enzymes including a metalloprotease, an acid phosphatase, a phospholipase A, and a lysophospholipase A (6, 23, 43). To examine whether the type II secretion system played a role in the induction of apoptosis by L. pneumophila, we utilized two mutants defective in this secretion system. NU259 is a mutant that has a kan insertion within lspG that encodes an inner membrane structural protein (46), and the NU243 mutant that has a kan insertion in pilD, which encodes the prepilin peptidase (40). Macrophages were infected with the two mutant strains along with AA100 and GL10 (dotA) and examined for their ability to induce apoptosis by the TUNEL assay. The strains were also assessed for their ability to activate caspase 3 by using a synthetic fluorometric substrate specific for caspase 3 (13). The data showed that the pilD mutants and lspG mutants were similar to the wild-type strain AA100 in their abilities to induce apoptosis and to activate caspase 3 (data not shown). In contrast, less than 15% of the cells infected by the dotA mutant control became apoptotic. These data showed that the type II secretion system is not required for the induction of apoptosis by L. pneumophila.
Role of pore formation in induction of apoptosis.
Pore-forming toxins of other gram-negative bacteria, such as the hemolysin A of Escherichia coli (12), alpha-toxin of Staphylococcus aureus (36), and PorB of Neisseria gonorrhoeae (41), have been shown to be involved in the induction of apoptosis. Therefore, we examined if the RtxA pore-forming toxin played a role in the induction of apoptosis by L. pneumophila. The rtxA in-frame deletion (ψLp24) (9) mutant was utilized to infect U937 cells, and apoptosis was analyzed by the TUNEL assay (data not shown). The data showed that there was no difference between the rtxA mutant and the wild-type strain in the induction of apoptosis, as both achieved greater than 85% induction of apoptosis within the cellular population. Thus, the RtxA pore-forming toxin plays no detectable role in the induction of apoptosis within macrophages by L. pneumophila.
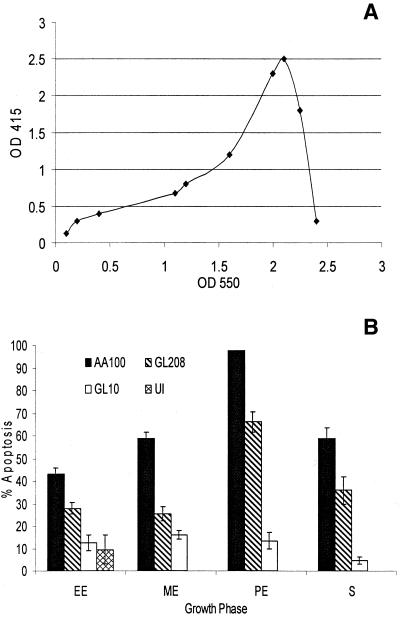
Many of L. pneumophila virulence properties are up-regulated upon entry into postexponential phase of growth, including motility, infectivity (8), and pore formation-mediated cytotoxicity (5). We examined whether the Dot/Icm-regulated pore-forming toxin, which is induced upon transition into the postexponential growth phase of L. pneumophila, has any effect on the ability to induce apoptosis phase (5, 24). The wild-type strain AA100 was grown to early exponential, mid-exponential, postexponential, and stationary growth phase. In addition, two other strains, GL208, a mutant defective in the pore-forming toxin (5), and GL10, a dotA mutant, were included as controls. AA100 showed a growth phase-dependent increase in its ability to induce apoptosis (Fig. 2). While 40% of the cells became apoptotic upon infection by early exponentially grown bacteria, 98% of the cells became apoptotic when infected by bacteria grown to the postexponential phase. The enhanced level of apoptosis at this phase correlated with maximal expression of the pore-forming activity (Fig. 2A). The amount of apoptosis was reduced to 55% when infection was carried out by AA100 in the stationary phase. The dotA mutant GL10 was completely defective in induction of apoptosis at all of the growth phases, inducing less than 15% of the cells to undergo programmed cell death.
FIG. 2.
(A) Kinetics of expression of the pore-forming toxin, measured by hemolysis of sheep red blood cells at an optical density at 415 nm (OD 415), at different growth phases, determined at an OD at 550 nm. Error bars were too small to be displayed by the software. (B) Apoptosis induced by L. pneumophila is enhanced upon entry into the postexponential phase of growth. The percentages of cells undergoing apoptosis were measured by TUNEL assay for AA100 (wild type), GL208 (rib mutant), GL10 (dotA mutant), and uninfected cells. Growth phase abbreviations: EE, early exponential; ME, midexponential; PE, postexponential; S, stationary. The data are representative of three different experiments, and error bars represent the standard deviations of triplicate samples.
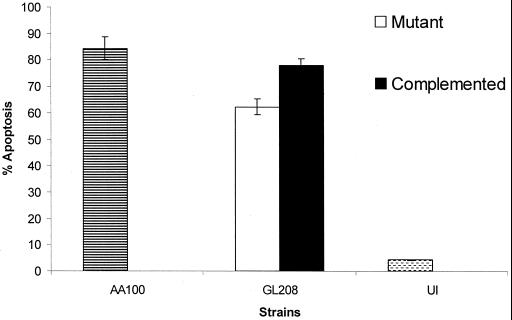
Infection by the mutant GL208, which is defective in the pore-forming toxin (5, 37), at the postexponential growth phase induced 60% of the cells to undergo apoptosis. This was significantly different from the wild-type strain AA100 that induced 95% of the cells to undergo apoptosis at the postexponential phase (P < 0.005 by the Student t test). To confirm this partial enhancement by the pore-forming toxin to induce apoptosis, the plasmid harboring the wild-type copy of the defective locus (icmT) in GL208 was examined for complementation of the partial reduction in apoptosis by the GL208 mutant. The GL208 mutant was complemented for the growth phase-dependent increase in induction of apoptosis upon entry into the postexponential phase, similar to the wild-type strain AA100 (Fig. 3). The data clearly showed that the pore-forming toxin is not the effector molecule responsible for the induction of apoptosis during early stages of the infection. However, the pore-forming toxin, which is triggered upon entry into the postexponential phase, is partially involved in the increased level of apoptosis seen at the postexponential phase.
FIG. 3.
The pore-forming toxin enhances the ability of L. pneumophila to induce apoptosis within U937 cells. The wild-type strain AA100, mutant GL208, and complemented GL208 were analyzed for their ability to induce apoptosis at the postexponential phase. The percentage of positive apoptotic cells was quantitated by TUNEL assay. Uninfected U937 cells (UI) were used as a control. The data are representative of three different experiments, and error bars represent the standard deviations of triplicate samples.
The type IV secretion system of a number of bacterial pathogens has been shown to export effector molecules that interfere with cellular signaling (8). The type IV Dot/Icm secretion system is critical to the ability of L. pneumophila to survive and replicate within mammalian cells. Consistent with previous observations (48, 53), we have shown that a defect in the Dot/Icm secretion system diminishes L. pneumophila's ability to replicate within macrophages and abolishes the pore-forming activity (33). Our data are also consistent with previous observations that the dot/icm loci are essential for intracellular replication within both mammalian and protozoan cells (19, 49). Our data clearly show that the type IV secretion system is essential for the induction of apoptosis and is the sole system responsible for this process. Since our dot/icm mutants are defective in several distinct phenotypes, it is most likely that the mutations are within genes encoding structural components of the secretion system and not individual effector molecules responsible for the individual phenotypes. Thus, in addition to the role of the Dot/Icm secretion system in endosomal trafficking, intracellular replication, pore-forming toxin-mediated egress from the host cell, it is also essential for the induction of apoptosis.
Our data show that the Dot/Icm type IV secretion system of L. pneumophila is not dependent on the type II secretion system for the induction of apoptosis. This is supported by our findings that a mutation within the type II secretion system apparatus or the peptidase-encoding gene does not affect the ability of L. pneumophila to induce apoptosis. The type II secretion system of L. pneumophila has been shown not to play a role in intracellular replication within HL-60 monocytes and only a minor role in U937 cells (23, 43). In contrast to the Bordetella pertussis Ptl system, the type IV secretion system of L. pneumophila seems to function independently of the general secretion machinery in the ability of the organism to replicate intracellularly (23), to manifest the pore-forming activity (37, 38), and to induce apoptosis within mammalian cells.
One mechanism by which several bacterial pathogens induce apoptosis within mammalian cells is through the formation of pores within the plasma membrane (34). This allows an influx of calcium ions into the cytoplasm that triggers a calcineurin-dependent response within the host cell and elicits apoptosis through a caspase-dependent mechanism (54). The alpha-hemolysin (HlyA) of E. coli is an RTX (repeats in toxin) pore-forming toxin that has been associated with the induction of apoptosis through the formation of pores within the plasma membrane of the host cell causing an influx of calcium into the cytosol, resulting in programmed cell death (12). The RTX leukotoxin (Ltx) of Actinobacillus actinomycetemcomitans also creates pores within the plasma membrane manifesting a similar effect to the E. coli toxin (34). In contrast, the RTX adenylate cyclase toxin of B. pertussis enters the cell via its hemolysin activity, which causes an increase in cellular cyclic AMP, resulting in programmed cell death (31). Our data show that the RtxA protein of L. pneumophila does not play a role in the induction of apoptosis. This may be due to the toxin not forming pores within the plasma membrane of the host cell or to the rapid repair of pores formed, not allowing a disruption in the calcium homeostasis. The induction of apoptosis that occurs during the early stages of L. pneumophila infection has been shown to be a distinct event from the cytotoxicity seen in the later stages of intracellular replication due to the pore-forming toxin (14). However, we have now shown that in the later stages of infection there is an increase in the amount of apoptotic induction due to the expression of the pore-forming toxin. This increase is possibly a result of insertion of pores within the plasma membrane, upon transition from the exponential to postexponential growth phase. However, the plasma membrane is likely to subsequently loose its integrity, and the cell is lysed, releasing the bacteria.
In summary, we have shown that in addition to the role of the Dot/Icm type IV secretion system of L. pneumophila in export of effector molecules required for evasion of phagosomal-lysosomal fusion and for export of the pore-forming toxin, it is also essential for the induction of apoptosis. The data also indicate that export of the apoptosis-inducing factor through the Dot/Icm type IV secretion system is independent of the Lsp type II secretion system. In addition, we show that RtxA is not involved in the induction of apoptosis, while the Dot/ Icm-regulated pore-forming toxin enhances the ability of L. pneumophila to induce apoptosis upon entry into the postexponential phase.
Acknowledgments
We thank Jeff Cirillo for the kind gift of the rtxA in-frame deletion mutant. We also thank O. A. Terry Alli for technical assistance and feedback.
N.P.C. is supported by Public Health Service Award RO1AI43987, and Y.A.K. is supported by Public Health Service Award RO1AI43965.
REFERENCES
- 1.Abu Kwaik, Y. 1998. Fatal attraction of mammalian cells to Legionella pneumophila. Mol. Microbiol. 30:689-696. [DOI] [PubMed] [Google Scholar]
- 2.Abu Kwaik, Y. 1996. The phagosome containing Legionella pneumophila within the protozoan Hartmanella vermiformis is surrounded by the rough endoplasmic reticulum. Appl. Environ. Microbiol. 62:2022-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu Kwaik, Y., L.-Y. Gao, B. J. Stone, C. Venkataraman, and O. S. Harb. 1998. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl. Environ. Microbiol. 64:3127-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Kwaik, Y., and L. L. Pederson. 1996. The use of differential display-PCR to isolate and characterize a Legionella pneumophila locus induced during the intracellular infection of macrophages. Mol. Microbiol. 21:543-556. [DOI] [PubMed] [Google Scholar]
- 5.Alli, O. A. T., L.-Y. Gao, L. L. Pedersen, S. Zink, M. Radulic, M. Doric, and Y. Abu Kwaik. 2000. Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila. Infect. Immun. 68:6431-6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aragon, V., S. Kurtz, A. Flieger, B. Neumeister, and N. P. Cianciotto. 2000. Secreted enzymatic activities of wild-type and pilD-deficient Legionella pneumophila. Infect. Immun. 68:1855-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christie, P. J., and J. P. Vogel. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cirillo, S. L. G., L. E. Bermudez, S. H. El-Etr, G. E. Duhamel, and J. D. Cirillo. 2001. Legionella pneumophila entry gene rtxA is involved in virulence. Infect. Immun. 69:508-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemens, D. L., and M. A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagolysosomal maturation is inhibited. J. Exp. Med. 181:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denecker, G., W. Declercq, C. A. W. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M.-P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of Bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Prada, C., B. D. Tall, S. E. Elliott, D. L. Hoover, J. P. Nataro, and M. M. Venkatesan. 1998. Hemolysin-positive enteroaggregative and cell-detaching Escherichia coli strains cause oncosis of human monocyte-derived macrophages and apoptosis of murine J774 cells. Infect. Immun. 66:3918-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, L.-Y., and Y. Abu Kwaik. 1999. Activation of caspase 3 in Legionella pneumophila-induced apoptosis in macrophages. Infect. Immun. 67:4886-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao, L.-Y., and Y. Abu Kwaik. 1999. Apoptosis in macrophages and alveolar epithelial cells during early stages of infection by Legionella pneumophila and its role in cytopathogenicity. Infect. Immun. 67:862-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao, L.-Y., and Y. Abu Kwaik. 2000. Hijacking the apoptotic pathways of the host cell by bacterial pathogens. Microb. Infect. 2:1705-1719. [DOI] [PubMed] [Google Scholar]
- 16.Gao, L.-Y., and Y. Abu Kwaik. 2000. The mechanism of killing and exiting the protozoan host Acanthamoeba polyphaga by Legionella pneumophila. Environ. Microbiol. 2:79-90. [DOI] [PubMed] [Google Scholar]
- 17.Gao, L.-Y., and Y. Abu Kwaik. 2000. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 8:306-313. [DOI] [PubMed] [Google Scholar]
- 18.Gao, L.-Y., O. S. Harb, and Y. Abu Kwaik. 1998. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect. Immun. 66:883-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao, L.-Y., O. S. Harb, and Y. Abu Kwaik. 1997. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant hosts, mammalian and protozoan cells. Infect. Immun. 65:4738-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao, L.-Y., B. J. Stone, J. K. Brieland, and Y. Abu Kwaik. 1998. Different fates of Legionella pneumophila pmi and mil mutants within human-derived macrophages and alveolar epithelial cells. Microb. Pathog. 25:291-306. [DOI] [PubMed] [Google Scholar]
- 21.Gueirard, P., A. Druilhe, M. Pretolani, and N. Guiso. 1998. Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo. Infect. Immun. 66:1718-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hägele, S., J. Hacker, and B. C. Brand. 1998. Legionella pneumophila kills human phagocytes but not protozoan host cells by inducing apoptotic cell death. FEMS Microbiol. Lett. 169:51-58. [DOI] [PubMed] [Google Scholar]
- 23.Hales, L. M., and H. A. Shuman. 1999. Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect. Immun. 67:3662-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammer, B. K., and M. S. Swanson. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33:721-731. [DOI] [PubMed] [Google Scholar]
- 25.Harb, O. S., L.-Y. Gao, and Y. Abu Kwaik. 2000. From protozoa to mammalian cells: a new paradigm in the life cycle of intracellular bacterial pathogens. Environ. Microbiol. 2:251-265. [DOI] [PubMed] [Google Scholar]
- 26.Hensel, M., J. E. Shea, S. R. Warterman, R. Mundy, T. Nikolaus, G. Banks, A. Vazuez-Torres, C. Gleeson, F. C. Fang, and D. W. Holden. 1998. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163-174. [DOI] [PubMed] [Google Scholar]
- 27.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilbi, H., J. E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R. A. Flavell, J. Yuan, P. J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895-32900. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz, M. A. 1983. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khelef, N., A. Zychlinsky, and N. Guiso. 1993. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect. Immun. 61:4064-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirby, J. E., and R. R. Isberg. 1998. Legionnaires' disease: the pore macrophage and the legion of terror within. Trends Microbiol. 6:256-258. [DOI] [PubMed] [Google Scholar]
- 33.Kirby, J. E., J. P. Vogel, H. L. Andrews, and R. R. Isberg. 1998. Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol. 27:323-336. [DOI] [PubMed] [Google Scholar]
- 34.Korostoff, J., J. F. Wang, I. Kieba, M. Miller, B. J. Shenker, and E. T. Lally. 1998. Actinobacillus actinomycetemcomitans leukotoxin induces apoptosis in HL-60 cells. Infect. Immun. 66:4474-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liles, M. R., P. H. Edelstein, and N. P. Cianciotto. 1999. The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol. Microbiol. 31:959-970. [DOI] [PubMed] [Google Scholar]
- 36.Menzies, B. E., and I. Kourteva. 2000. Staphylococcus aureus α-toxin induces apoptosis in endothelial cells. FEMS Immunol. Med. Microbiol. 29:39-45. [DOI] [PubMed] [Google Scholar]
- 37.Molmeret, M., O. A. T. Alli, S. Zink, A. Flieger, N. Cianciotto, and Y. Abu Kwaik. 2002. icmT is essential for pore formation-mediated egress of Legionella pneumophila from mammalian and protozoan cells. Infect. Immun. 70:69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molmeret, M., A. O. T. Alli, R. Marina, S. Milorad, D. Miljenko, and A. K. Yousef. The C-terminus of IcmT is essential for the pore formation and for intracellular trafficking of L. pneumophila within A. polyphaga. Mol. Microbiol., in press. [DOI] [PubMed]
- 39.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monack, D. M., B. Raupach, A. E. Hromockyj, and S. Falkow. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 93:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller, A., D. Gunther, F. Dux, M. Naumann, T. F. Meyer, and T. Rudel. 1999. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 18:339-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paddenberg, R., S. Wulf, A. Weber, P. Heimann, L. A. Beck, and H. G. Mannherz. 1996. Internucleosomal DNA fragmentation in cultured cells under conditions reported to induce apoptosis may be caused by mycoplasma endonucleases. Eur. J. Cell Biol. 71:105-119. [PubMed] [Google Scholar]
- 43.Rossier, O., and N. P. Cianciotto. 2001. Type II protein secretion is a subset of the PilD-dependent processes that facilitate intracellular infection by Legionella pneumophila. Infect. Immun. 69:2092-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy, C. R., K. H. Berger, and R. R. Isberg. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663-674. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 46.Schesser, K., J. M. Dukuzumuremyi, C. Cilio, S. Borg, T. S. Wallis, S. Pettersson, and E. E. Galyov. 2000. The Salmonella YopJ-homologue AvrA does not possess YopJ-like activity. Microb. Pathog. 28:59-70. [DOI] [PubMed] [Google Scholar]
- 47.Schesser, K., A. K. Spiik, J. M. Dukuzumuremyi, M. F. Neurath, S. Pettersson, and H. Wolf-Watz. 1998. The yopJ locus is required for Yersinia-mediated inhibition of NF-kappaB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 28:1067-1079. [DOI] [PubMed] [Google Scholar]
- 48.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila chromosome. Proc. Natl. Acad. Sci. USA 95:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segal, G., and H. A. Shuman. 1999. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 67:2117-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone, B. J., and Y. Abu Kwaik. 1999. Natural competency for DNA uptake by Legionella pneumophila and its association with expression of type IV pili. J. Bacteriol. 181:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stone, B. J., A. Brier, and Y. Abu Kwaik. 1999. The Legionella pneumophila prp locus; required during infection of macrophages and amoebae. Microb. Pathog. 27:369-376. [DOI] [PubMed] [Google Scholar]
- 52.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogel, J. P., H. L. Andrews, S. K. Wong, and R. R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873-876. [DOI] [PubMed] [Google Scholar]
- 54.Wang, H.-G., N. Pathan, I. M. Ethell, S. Krajewski, Y. Yamaguchi, F. Shibasaki, F. McKeon, T. Bobo, T. F. Franke, and J. C. Reed. 1999. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284:339-343. [DOI] [PubMed] [Google Scholar]