Abstract
The type III secretion system encoded by Salmonella pathogenicity island 2 (SPI2) translocates Salmonella translocated effectors (STE) into host cells. STE are encoded by genes outside of SPI2. The distribution of STE loci within the salmonellae was investigated. In contrast to the SPI2 locus that is conserved within Salmonella enterica, STE loci show a variable distribution. In addition to other virulence determinants, the possession of various sets of STE loci may contribute to the different host ranges and pathogenic potentials of S. enterica serovars.
Salmonella enterica is a highly successful enteric pathogen of humans and animals that has evolved fine-tuned mechanisms by which to interact with eukaryotic cells. Infections with S. enterica display a remarkable range of different clinical outcomes. Furthermore, the host ranges of different Salmonella serotypes vary between highly host-adapted forms and serotypes that can cause infections in a wide variety of hosts. The molecular basis of the different disease outcomes and host specificity is not understood. It is currently discussed whether the different distribution of virulence determinants within the salmonellae may be important for these phenomena (4).
S. enterica uses two type III secretion systems (TTSS) for different modes of interaction with the infected host during pathogenesis. Both TTSS are encoded by pathogenicity islands. The TTSS encoded by Salmonella pathogenicity island 1 (SPI1) mediates the invasion by Salmonella of nonphagocytic cells such as epithelial cells of the intestinal mucosa and is involved in enteropathogenesis (reviewed in references 7 and 22). The second TTSS encoded by SPI2 is required for intracellular survival and replication of S. enterica (for a review, see reference 9).
A cluster of effector proteins of SPI2 termed Salmonella translocated effectors (STE) has been identified by virtue of the N-terminal conserved domain (13). Studies using fusions to the reporter CyaA indicated that intracellular Salmonella bacteria translocate STE into host cells via the TTSS of SPI2. STE genes are all outside of the SPI2 locus, and several of these loci are associated with prophage genes, indicating that the STE genes are part of the variable assortment of virulence factors of S. enterica. We questioned whether these loci represent separate acquisitions by horizontal gene transfer events that show a more diverse distribution than the SPI2 locus, which is conserved within S. enterica (10, 11).
Genetic organization of STE loci
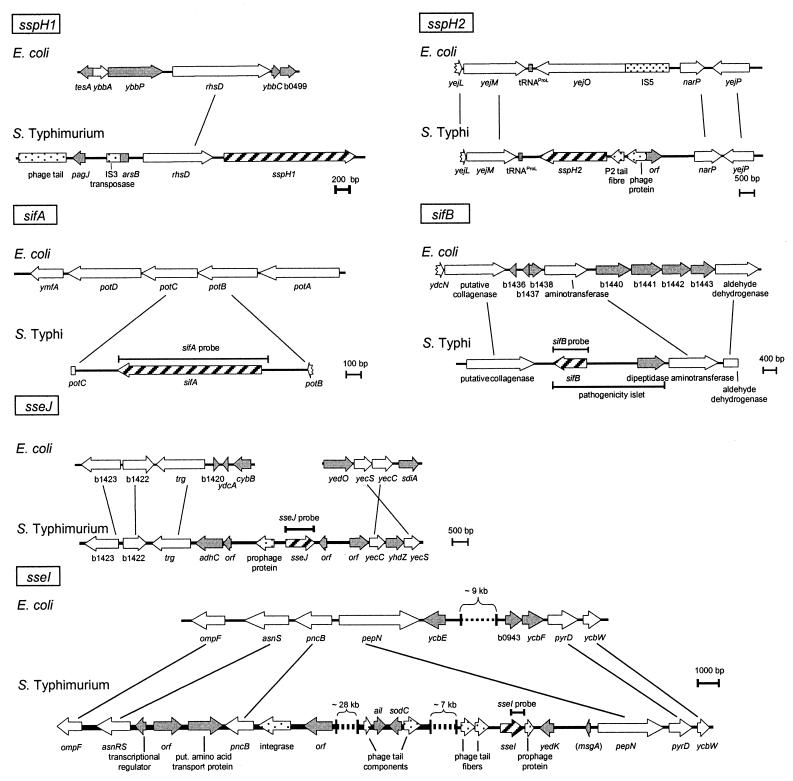
The genomic organization of STE loci was analyzed based on genome data available for serovars Typhi and Typhimurium. DNA sequence data for serovar Typhi CT18 were generated by the Salmonella Typhi Sequencing Group at the Sanger Centre and can be obtained from ftp://ftp.sanger.ac.uk/pub/pathogens/st/St.dna. DNA sequence data for serovar Typhimurium LT2 were obtained from the Genome Sequencing Center, Washington University, St. Louis, Mo., and can be retrieved from http://genome.wustl.edu/gsc/Projects/bacteria.shtml. The organization of the various loci was also compared to that of the corresponding region of the Escherichia coli K-12 chromosome (Fig. 1). The STE loci are insertions specific to Salmonella spp. sifA forms an integration of a single gene interrupting the potABCD operon of serovars Typhi and Typhimurium (18). The sifB locus represents a small insertion in the serovar Typhi genome that contains two open reading frames. This locus is also present in S. enterica serotype Enteritidis and has been referred to as a pathogenicity islet (GenBank accession no. AF128835). Comparison to the corresponding regions of the E. coli K-12 chromosome revealed a more complex organization of the other STE loci. sspH1, sspH2, sseI, and sseJ (6, 13) are located in the immediate vicinity of genes encoding phage-related proteins. In addition, the tRNA gene proL is located adjacent to sspH2 in the serovar Typhi genome but not in the serovar Typhimurium genome. The presence of phage genes, as well as tRNA genes, may indicate that the STE loci were located on mobile genetic elements and have been acquired by horizontal gene transfer.
FIG. 1.
Genomic organization of STE genes in S. enterica and positions of hybridization probes. The organization of the chromosomal regions of various STE genes was compared to that of the corresponding regions of the E. coli K-12 chromosome. Sequence data available for S. enterica serotypes Typhimurium and Typhi were obtained from the Genome Sequencing Center of Washington University and the Sanger Centre, respectively. Genes shared between E. coli and serovar Typhimurium or specific for one species are depicted by open and filled symbols, respectively. STE genes and genes with similarity to phage genes are shown by hatched and dotted symbols, respectively. The positions of the PCR fragments used as specific hybridization probes in this study are indicated. Probes were constructed by PCR with primers SifA-For-EcoRI and SifA-Rev-EcoRV (sifA), SifB-For-EcoRI and SifB-Rev-EcoRV (sifB), SseI2-For and SseI-Rev-EcoRV (sseI), and SseJ-For-EcoRI and SseJ-Rev-EcoRV (sseJ).
The contribution of these genetic elements to horizontal transfer of virulence genes has been frequently observed (reviewed in reference 1) and may also be relevant for the variable distribution of sspH1, sspH2, sseI, and sseJ. sseI is located within the Gifsy-2 prophage. Gifsy-2 is inducible and fully functional as a vehicle for the transfer of genetic elements (5). Thus, this prophage may still be active and contribute to the distribution of genes for effector proteins between various serovars of S. enterica. The distribution of sspH1 within the salmonellae has been described before (20), and recent work showed that sspH1 is located on the Gifsy-3 prophage (6). In contrast, the absence of prophage genes or insertion sequence elements in the sifA and sifB loci may explain the stability of these loci.
The role of a bacteriophage as a means of horizontal gene transfer has recently been described for SPI1. While genes for the TTSS of SPI1 are conserved among the salmonellae, genes for effector proteins of this TTSS have a variable distribution within Salmonella subspecies (14). The association of prophage SopEϕ with a virulence gene (sopE) for an effector protein of the TTSS of SPI1 was observed (15).
Phylogenetic distribution of STE genes
The SPI1 locus is present in both S. enterica and S. bongori, whereas SPI2 was observed in all of the subspecies of S. enterica but not in S. bongori (11, 16). In all of the S. enterica serotypes analyzed so far, the TTSS of SPI2 is functional in secreting substrate proteins in vitro (I.H.-W. and M.H., unpublished observations). Since STE genes are located outside of SPI2, we questioned whether these genes are also present in all of the subspecies of S. enterica or if a distinct distribution of STE genes is characteristic of certain subspecies.
The distribution of STE genes within the salmonellae was analyzed by using strains of the SARB (2) and SARC (3) reference collections that were obtained from the Salmonella Genetic Stock Centre (Calgary, Alberta, Canada). Gene-specific hybridization probes for STE genes were obtained by PCR using the primers indicated in Table 1 and the legend to Fig. 1 with the genomic DNA of S. enterica subspecies I serotype Typhimurium (serovar Typhimurium) strain ATCC 14028 as the template. PCR fragments were labeled by using the DIG DNA Labeling Kit (Roche). Hybridization was carried out at 42°C overnight with 20% (vol/vol) formamide in 1× hybridization buffer (250 mM NaHPO4, 1 mM EDTA, 7% sodium dodecyl sulfate). Two washing steps were performed under nonstringent conditions (washing buffer: 40 mM NaHPO4, 1 mM EDTA, 1% sodium dodecyl sulfate) for 20 min at room temperature.
TABLE 1.
Oligonucleotides used in this study
| Designation | Sequence |
|---|---|
| SifA-Rev-EcoRV | 5′-ACGGATATCAAAACAACATAAACAGCCGC-3′ |
| SifA-For-EcoRI | 5′-CCGGAATTCATTTTTACTCCAGTATAAG-3′ |
| SifB-For-EcoRI | 5′-CCGGAATTCATATAATCACTTGTGGTC-3′ |
| SifB-Rev-EcoRV | 5′-ACGGATATCACTCTGGTGATGAGCCTC-3′ |
| SseI2-For | 5′-TAACCATGGCAACAGAACCG-3′ |
| SseI-Rev-EcoRV | 5′-ACGGATATCCATTTTACCTATTAAGGA-3′ |
| SseJ-For-EcoRI | 5′-CCGGAATTCGTAAGGAGGACACTATGCC-3′ |
| SseJ-Rev-EcoRV | 5′-ACGGATATCTTCAGTGGAATAATGATGAGC-3′ |
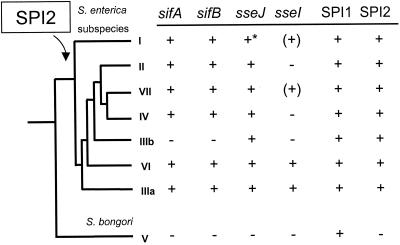
We analyzed the distribution of sifA, sifB, sseI, and sseJ in the SARC collection that represents S. bongori and various subspecies of S. enterica (Fig. 2). None of these genes was detected in S. bongori. sifA and sifB were detected in all subspecies apart from subspecies IIIb. Hybridization signals for sseI were detected in subspecies IIIa and only in one of the two strains each of subspecies I and VII but were absent in the other subspecies. sseJ is present in all S. enterica subspecies, except serovar Typhi, belonging to subspecies I (this study and reference 13).
FIG. 2.
Distribution of STE genes within the salmonellae. The SARC collection, representing S. enterica subspecies I, II, IIIa, IIIb, IV, VI, and VII and S. bongori, was analyzed for the presence of sifA, sifB, sseJ, and sseI. A phylogenetic tree of the salmonella subspecies based on multilocus enzyme electrophoresis was adapted from reference 17. The presence or absence of the genes is indicated by a plus or minus sign, respectively. Brackets indicate that sseI is only present in a low number of serotypes of subspecies I and in only one of two representatives of subspecies VII. The asterisk indicates that sseJ is absent from subspecies I serotypes Typhi and Paratyphi (for details, see Fig. 3). The distribution of SPI1 and SPI2 has been reported previously (11, 16).
slrP, a member of the STE family of proteins, contributes to the host range of serovar Typhimurium (21), and further STE genes may contribute to the host adaptation of S. enterica serotypes. The absence of genes for SPI2-specific substrate proteins parallels the absence of the SPI2-encoded TTSS in S. bongori. sseI, sseJ, sifA, and sifB (this study), as well as slrP and sspH2 (21), are absent in S. bongori. In contrast, sspH1, a substrate for the TTSS of both SPI1 and SPI2, was detected in S. bongori (21). The sspH1, sspH2, sseJ, and sseI loci have a more variable distribution within S. enterica subspecies (this study and references 6 and 21). These differences may contribute to host specificity and the pathogenic profiles of Salmonella spp.
Distribution of STE genes within S. enterica subspecies I
A subset of strains of the SARB collection representing 36 serovars of S. enterica subspecies I, as well as a collection of clinical isolates of the Max von Pettenkofer Institute, was analyzed by Southern blot hybridization (Fig. 3). sifA and sifB were detected in all serovars of S. enterica subspecies I. sseJ was absent from serovars Typhi and Paratyphi A and B but present in serovar Paratyphi C and all of the other serovars analyzed. In contrast, sseI was detected in only 9 of the 36 serovars investigated and showed a rather heterogeneous distribution among the isolates from patients, although it was not detectable in serovar Paratyphi A, B, or C.
FIG. 3.
Distribution of STE genes within S. enterica subspecies I. Strains of the SARB collection that are representative of various serotypes of S. enterica subspecies I were analyzed by Southern hybridization for the presence of sifA, sifB, sseI, and sseJ. Positive and negative hybridization signals are indicated by plus and minus signs, respectively. The SARB collection strain numbers of the various serotypes are in parentheses.
A large number of virulence determinants with variable distribution has been identified in S. enterica, such as fimbrial genes (19), SPI1 effector proteins (14), and the virulence plasmid (8, 12). We showed here that genes for SPI2 effectors also belong to this group of variable virulence determinants. It can be speculated that the specific combination of these known and putative unknown virulence factors may determine the virulence characteristics and host specificity of various S. enterica serotypes. However, the role of most SPI2 effector proteins is not understood. Continuing detailed analyses of the role of STE proteins in Salmonella virulence by different models of infection, as well as further characterization of the cellular phenotypes of various STE mutant strains, will contribute to the understanding of these phenomena.
Acknowledgments
Imke Hansen-Wester and Bärbel Stecher contributed equally to this work.
The project was supported by the Deutsche Forschungsgemeinschaft (grants HE1964/2-3 and HE1964/4-2).
We are grateful to Jürgen Heesemann for generous support of this work at the Max von Pettenkofer Institute in Munich and to Cosima Pelludat and Brad Taylor for critical review of the manuscript. We thank Wolf-Dietrich Hardt for stimulating discussions. We thank the Genome Sequencing Center, Washington University, St. Louis, Mo., and the Salmonella Typhi Sequencing Group at the Sanger Centre for communication of DNA sequence data prior to publication.
REFERENCES
- 1.Boyd, E. F., B. M. Davis, and B. Hochhut. 2001. Bacteriophage-bacteriophage interactions in the evolution of pathogenic bacteria. Trends Microbiol. 9:137-144. [DOI] [PubMed]
- 2.Boyd, E. F., F. S. Wang, P. Beltran, S. A. Plock, K. Nelson, and R. K. Selander. 1993. Salmonella reference collection B (SARB): strains of 37 serovars of subspecies I. J. Gen. Microbiol. 139:1125-1132. [DOI] [PubMed] [Google Scholar]
- 3.Boyd, E. F., F. S. Wang, T. S. Whittam, and R. K. Selander. 1996. Molecular genetic relationships of the salmonellae. Appl. Environ. Microbiol. 62:804-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fierer, J., and D. G. Guiney. 2001. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J. Clin. Investig. 107:775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figueroa-Bossi, N., and L. Bossi. 1999. Inducible prophages contribute to Salmonella virulence in mice. Mol. Microbiol. 33:167-176. [DOI] [PubMed] [Google Scholar]
- 6.Figueroa-Bossi, N., S. Uzzau, D. Maloriol, and L. Bossi. 2001. Variable assortment of prophages provides a transferable repertoire of pathogenic determinants in Salmonella. Mol. Microbiol. 39:260-271. [DOI] [PubMed] [Google Scholar]
- 7.Galan, J. E. 1999. Interaction of Salmonella with host cells through the centisome 63 type III secretion system. Curr. Opin. Microbiol. 2:46-50. [DOI] [PubMed] [Google Scholar]
- 8.Gulig, P. A., H. Danbara, D. G. Guiney, A. J. Lax, F. Norel, and M. Rhen. 1993. Molecular analysis of spv virulence genes of the Salmonella virulence plasmids. Mol. Microbiol. 7:825-830. [DOI] [PubMed] [Google Scholar]
- 9.Hensel, M. 2000. Salmonella pathogenicity island 2. Mol. Microbiol 36:1015-1023. [DOI] [PubMed] [Google Scholar]
- 10.Hensel, M., T. Nikolaus, and C. Egelseer. 1999. Molecular and functional analysis indicates a mosaic structure of Salmonella pathogenicity island 2. Mol. Microbiol. 31:489-498. [DOI] [PubMed] [Google Scholar]
- 11.Hensel, M., J. E. Shea, A. J. Bäumler, C. Gleeson, F. R. Blattner, and D. W. Holden. 1997. Analysis of the boundaries of Salmonella pathogenicity island 2 and the corresponding chromosomal region of Escherichia coli K-12. J. Bacteriol. 179:1105-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby, S. J., L. G. Adams, T. A. Ficht, C. Allen, H. A. Whitford, N. A. Buchmeier, S. Bossie, and D. G. Guiney. 1997. The spv genes on the Salmonella dublin virulence plasmid are required for severe enteritis and systemic infection in the natural host. Infect. Immun. 65:1786-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao, E. A., and S. I. Miller. 2000. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 97:7539-7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirold, S., K. Ehrbar, A. Weissmüller, R. Prager, H. Tschäpe, H. Rüssmann, and W. D. Hardt. 2001. Salmonella host cell invasion emerged by acquisition of a mosaic of separate genetic elements, including Salmonella pathogenicity island 1 (SPI1), SPI5, and sopE2. J. Bacteriol. 183:2348-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirold, S., W. Rabsch, M. Rohde, S. Stender, H. Tschäpe, H. Rüssmann, E. Igwe, and W. D. Hardt. 1999. Isolation of a temperate bacteriophage encoding the type III effector protein SopE from an epidemic Salmonella typhimurium strain. Proc. Natl. Acad. Sci. USA 96:9845-9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochman, H., and E. A. Groisman. 1996. Distribution of pathogenicity islands in Salmonella spp. Infect. Immun. 64:5410-5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves, M. W., G. M. Evins, A. A. Heiba, B. D. Plikaytis, and J. J. Farmer III. 1989. Clonal nature of Salmonella typhi and its genetic relatedness to other salmonellae as shown by multilocus enzyme electrophoresis, and proposal of Salmonella bongori comb. nov. J. Clin. Microbiol. 27:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein, M. A., K. Y. Leung, M. Zwick, F. Garcia del Portillo, and B. B. Finlay. 1996. Identification of a Salmonella virulence gene required for formation of filamentous structures containing lysosomal membrane glycoproteins within epithelial cells. Mol. Microbiol. 20:151-164. [DOI] [PubMed] [Google Scholar]
- 19.Townsend, S. M., N. E. Kramer, R. Edwards, S. Baker, N. Hamlin, M. Simmonds, K. Stevens, S. Maloy, J. Parkhill, G. Dougan, and A. J. Bäumler. 2001. Salmonella enterica serovar Typhi possesses a unique repertoire of fimbrial gene sequences. Infect. Immun. 69:2894-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsolis, R. M., L. G. Adams, T. A. Ficht, and A. J. Bäumler. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67:4879-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsolis, R. M., S. M. Townsend, E. A. Miao, S. I. Miller, T. A. Ficht, L. G. Adams, and A. J. Bäumler. 1999. Identification of a putative Salmonella enterica serotype Typhimurium host range factor with homology to IpaH and YopM by signature-tagged mutagenesis. Infect. Immun. 67:6385-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallis, T. S., and E. E. Galyov. 2000. Molecular basis of Salmonella-induced enteritis. Mol. Microbiol. 36:997-1005. [DOI] [PubMed] [Google Scholar]