Abstract
Stachybotrys chartarum is a toxigenic fungus that has been associated with human health concerns such as nasal bleeding in adults and pulmonary hemosiderosis (PH) in infants. Seven of eight strains of S. chartarum isolated from homes of infants with PH in Cleveland, Ohio, and the strain from the lung of an infant with PH in Texas produced stachylysin in tryptic soy broth (TSB), whereas only one out of eight strains isolated from control homes produced stachylysin. However, all strains produced stachylysin when grown on TSB with 0.7% sheep's blood. When stachylysin was injected into Lumbricus terrestis, the erythrocruorin hemoglobin (absorbance peaks at 280 and 415 nm) was released, resulting in a lethal effect. These results support the hypothesis that stachylysin may be one agent responsible for hemorrhaging in humans.
Pulmonary hemosiderosis (PH) in infants has been associated with exposure to Stachybotrys chartarum (Ehrenb. ex Link) Hughes(= S. atra Corda) in some studies (9, 12, 13). PH is characterized by a chronic bleeding which leads to the observation of hemosiderin-laden macrophages in the infant lungs and can be fatal (4, 5). Agricultural workers exposed to S. chartarum report nasal and tracheal bleeding (17, 31).
Consumption of S. chartarum by domestic or experimental animals can be lethal (15, 28). Generally, low-molecular-weight toxins, e.g., macrocyclic trichothecenes, are considered to be responsible for the lethality (10). Hemorrhaging is not a typical response to purified trichothecenes in experimental animals (32), but it is known that consumption of S. chartarum-contaminated fodder by horses leads to hemorrhaging (14). Perhaps factors other than low-molecular-weight toxins are responsible for the hemorrhaging.
High-molecular-weight cytotoxins, called hemolysins, are produced by both bacteria and fungi and can cause hemorrhaging. For example, β-hemolysin produced by the group B streptococci produce hemorrhages in infant lungs (16, 22, 23). Exposure of experimental animals to the purified hemolysin from Aspergillus fumigatus caused hemorrhagic lesions in experimental animals (27).
Recently, the hemolysin from S. chartarum, designated stachylysin, was initially characterized (35, 36, 37). Strains of S. chartarum isolated from PH case houses in Cleveland, Ohio (18), and the isolate from the lung of a PH patient in Texas (9) generally produced significantly more hemolysin under the conditions tested than most strains isolated from control houses in Cleveland (36). In this report, an explanation for the hemorrhaging after S. chartarum exposure is suggested, based on the role of stachylysin in vascular leakage in a model organism, Lumbricus terrestis.
MATERIALS AND METHODS
Strains of Stachybotrys.
Eight strains of S. chartarum isolated from PH control houses in Cleveland and eight strains from case houses (18) and the Houston strain (9) were used in this study. Stachylysin was purified from strain 58-06.
Purification of stachylysin.
Stachylysin was purified as previously described (37) with the following modifications. The culture supernatant was centrifuged in a Millipore Centricon Plus 80 filter apparatus with a molecular mass cutoff of 30 kDa (Millipore, Bedford, Mass.) at 4,000 × g for 15 min in an RC5 centrifuge. The concentrate was recovered according to the manufacturer's instructions. The concentrate was then subjected to ion-exchange chromatography. DEAE-cellulose (Sigma, St. Louis, Mo.) was hydrated in 20 mM Tris-HCl (pH 5.5) for 1 h (with multiple changes of buffer during the hydration) and then poured into a column, giving a final bed of 3 by 0.5 cm, and then 0.5 ml of the concentrate was introduced on the top of the column and allowed to bind. The bed was sequentially eluted with 10 ml of 20 mM Tris-HCl buffer containing 0, 0.1, 0.2, and 0.5 M sodium azide. Five-drop fractions were collected throughout the elution, and then 10 μl of each fraction was plated on sheep's blood agar (SBA) (Becton Dickinson, Sparks, Md.) and hemolysis was noted.
The five hemolytically active fractions from the ion-exchange chromatography were then subjected to gel filtration using Sephadex G 100-50 (Sigma) hydrated for 5 days in running buffer containing 0.4 M sodium azide, 5 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride and poured into a chromatography column to give a final bed of 0.5 by 14 cm. Five-drop fractions were collected at 1.5 ml per h using a fraction collector (ISCO, Lincoln, Nebr.). Ten microliters of each fraction was then plated on SBA and incubated at 37°C, and hemolysis was noted at 48 h.
After the first gel filtration, the four hemolytically active fractions were again subjected to gel filtration as described above but with a column with the dimensions 0.25 by 24 cm. The three most hemolytically active fractions from this second gel filtration were combined and then desalted twice using a D-Salt Polyacrylamide 6000 desalting column (Pierce, Rockford, Ill.). The final desalted solution was frozen at −80°C and lyophilized using a Spin Vac (Savant Instruments, Farmingdale, N.Y.), resulting in a lyophilized pellet.
Electrophoresis analysis.
Native protein electrophoresis and sodium dodecyl sulfate-polyacrylamide gel electrophoresis were performed using a Mini-Protean 3 Cell and precast 4 to 15% Tris-HCl gels (Bio-Rad Laboratories, Hercules, Calif.), as per the manufacturer's instructions. Gels were stained with silver using the Bio-Rad Silver Stain Plus kit by following the manufacturer's instructions. The native stachylysin band did not stain initially with this silver reagent, indicating that it was likely glycosylated. Non-silver-staining protein gels were treated by oxidizing with 1% hydrogen peroxide for 15 min, cleared with 3 mM potassium dichromate in 3 mM nitric acid, and then restained with Silver Stain Plus as described above (21).
To determine if the non-silver-staining band was hemolytically active stachylysin, a purified preparation was divided in half and run in two separate wells in a precast 4 to 15% Tris-HCl gel. After native protein electrophoresis, half of the gel was stained as described above; the other half was placed on an SBA plate and incubated at 37°C for 48 h, and the hemolysis process was photographed.
Strain differences in production of stachylysin.
Each of the 17 strains of S. chartarum was grown on wet wall board for 5 weeks, and the spores were harvested as described earlier (35). Approximately 4 × 104 conidia of each of the 17 strains was added to 100 ml of tryptic soy broth (TSB) (Becton Dickinson) in 250-ml flasks with and without 0.7 ml of sheep's blood (Becton Dickinson). The flasks were placed on an incubator-shaker (LabLine Inc., Melrose Park, Ill.) set at 36 ± 1°C and mixed at 200 rpm/min. After 7 days of incubation, the concentrate was prepared as described above. The concentrates were then filtered through a 0.2-μm-pore-size Acrodisc syringe filter (Pall Gelman Laboratory, Ann Arbor, Mich.), and 100 μl was plated on SBA and incubated at 37°C for 48 h, at which time hemolysis was noted.
Stachylysin effect on L. terrestis.
L. terrestis earthworms were obtained from a local bait shop. The earthworms were washed in cold water, dried, placed in standard 100- by 15-mm sterile polystyrene petri dishes, and weighed.
Concentrates were prepared from 500-ml TSB cultures (without blood) of strains 58-06 and 58-17, as described above. The concentrates were then filtered through a 0.2-μm-pore-size Acrodisc filter, and 100 μl was plated on SBA and incubated at 37°C for 48 h, at which time hemolysis was noted. Half of the concentrate from the 58-06 culture was boiled for 15 min to inactivate the hemolysin. Aliquots (100 μl) of each concentrate (the 58-06 concentrate, boiled and not boiled, and the 58-17 concentrate) were injected into each of five earthworms (each 4.5 ± 0.5 g) within the so-called heart region, segments 7 to 11. The earthworms were maintained at 23°C. The health of the worms was noted at 6, 24, and 48 h. Hemolytic activity or inactivity for each of the injection solutions was confirmed by plating on SBA (35).
The purified stachylysin was prepared as described above and then resuspended in sterile, deionized water. Protein quantification was performed using the Bio-Rad DC protein assay. The 50% lethal dose (LD50) was determined by injecting, into each of six worms (each 3.5 ± 0.5 g), 100 μl of purified stachylysin diluted in sterile water to 24, 6, 1.5, and 0 μg/100 μl. The earthworms were maintained at 23°C, and their health was noted at 72 h. The estimate of the LD50 was made by a logistic regression of log10 dose of stachylysin versus survival.
Excretions from the dead worms that accumulated in the petri dish were collected in microcentrifuge tubes, and the debris was removed by centrifugation. The supernatant was analyzed spectrophotometrically by scanning from 200 to 600 nm using a DU-600 spectrophotometer (Beckman Coulter, Fullerton, Calif.).
RESULTS
Gel electrophoresis.
Purified stachylysin showed a single non-silver-staining band when run in loading buffer (without β-mercaptoethanol, sodium dodecyl sulfate, or boiling) (Fig. 1A). When this protein was oxidized with hydrogen peroxide and then restained, a single silver-stained band was produced (Fig. 1B), indicating that stachylysin is glycosylated.
FIG. 1.
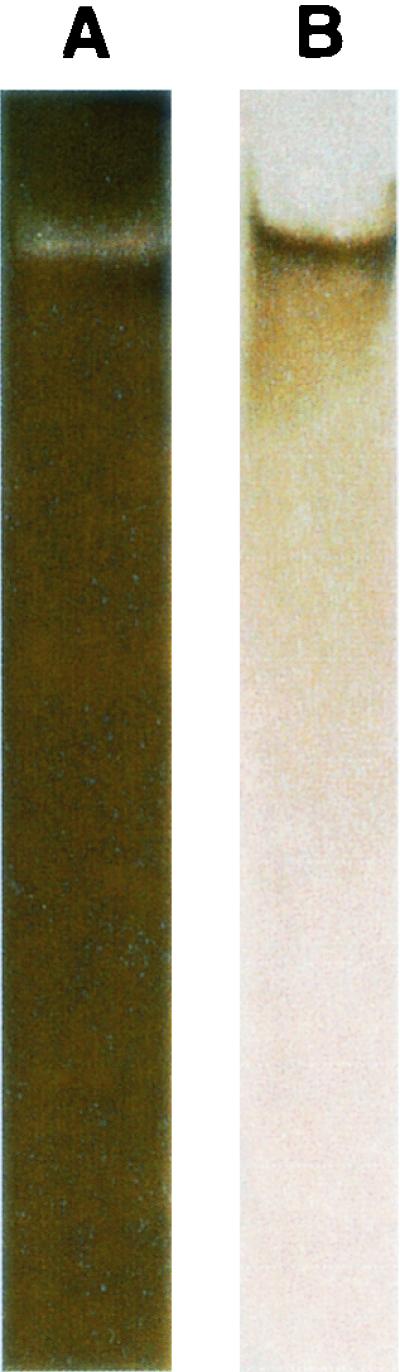
(A) Purified stachylysin was subjected to native protein electrophoresis with a 4 to 15% Tris-HCl gel stained with silver (using the Bio-Rad Silver Stain Plus kit). (B) The native stachylysin band did not stain initially with this silver reagent used for panel A and was treated by oxidizing with 1% hydrogen peroxide for 15 min. The gel cleared with 3 mM potassium dichromate in 3 mM nitric acid and then restained.
The band in the native gel (Fig. 2A) was found to be hemolytically active when a comparable unstained part of the gel was placed on the surface of an SBA plate and then incubated at 37°C. A dark area, corresponding to the band, was apparent at 24 h (Fig. 2B), and then at 48 h the area was clear, demonstrating complete hemolysis (Fig. 2C).
FIG. 2.
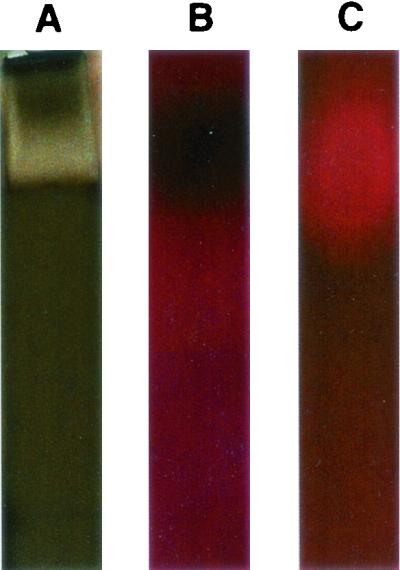
Purified stachylysin was subjected to native protein electrophoresis using a 4 to 15% Tris-HCl gel. Half of the gel was then silver stained (using the Bio-Rad Silver Stain Plus kit) (A), and the other half was placed on SBA and then photographed at 24 h (B) and 48 h (C).
Strain differences in production of stachylysin.
Table 1 shows hemolysis of the concentrates from different strains of S. chartarum after growth in TSB with or without sheep's blood. In TSB alone, seven of the eight strains isolated from PH case houses in Cleveland and the Houston strain, but only one of eight of the control house strains, were positive for hemolysis. The concentrates from all strains were positive when sheep's blood was added to the TSB (Table 1).
TABLE 1.
Observations of hemolytic activity of concentrates from S. chartarum strains grown in TSB with or without 0.7% sheep's blooda
| Strainb | Hemolysis
|
|
|---|---|---|
| Without blood | With blood | |
| 51-05 (Ca) | + | + |
| 51-06 (Ca) | + | + |
| 51-08 (Ca) | + | + |
| 51-11 (Ca) | − | + |
| 51-19 (Ca) | + | + |
| 58-02 (Ca) | + | + |
| 58-06 (Ca) | + | + |
| 63-07 (Ca) | + | + |
| Houston | + | + |
| 58-07 (Ct) | − | + |
| 58-08 (Ct) | − | + |
| 58-15 (Ct) | − | + |
| 58-16 (Ct) | − | + |
| 58-17 (Ct) | − | + |
| 58-18 (Ct) | + | + |
| 58-19 (Ct) | − | + |
| 63-01 (Ct) | − | + |
In each case, 100 μl of each concentrate was plated on SBA and incubated at 37°C.
Ca, case house strain; Ct, control house strain
Stachylysin effect on L. terrestis.
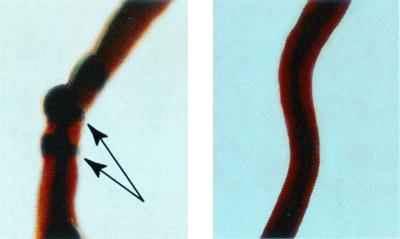
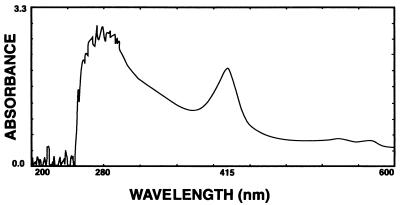
All of the earthworms that received the injection of concentrate from the TSB (without blood) culture of 58-06 died by 6 h after injection, and death was marked by the excretion of a reddish fluid (Fig. 3). In some cases, what appear to be aneurisms on the dorsal vessel were observed (Fig. 4). Those earthworms receiving the injections of the boiled 58-06 culture or the 58-17 culture concentrate survived through the 48-h experiment. When the reddish fluid was examined spectrophotometrically, it was found to have absorbance peaks at 280 and 415 nm (Fig. 5).
FIG. 3.
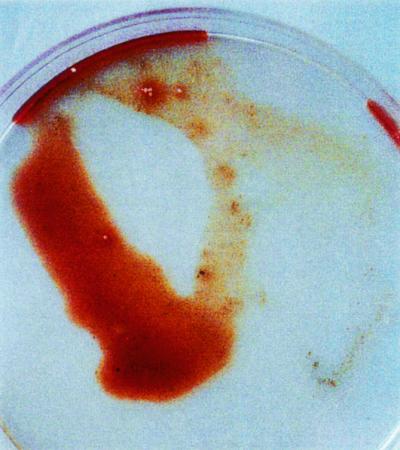
Appearance of reddish excretions from L. terrestis 6 h after injection with 100 μl of concentrate from strain 58-06 grown for 7 days in TSB.
FIG. 4.
Appearance of a dorsal vessel of L. terrestis 6 h after injection with 100 μl of concentrate from S. chartarum strain 58-06 grown for 7 days in TSB (left) and of an untreated earthworm (right). The arrows indicate possible aneurisms. The worms are back-lighted to reveal internal structures.
FIG. 5.
Spectrum of excretions from L. terrestis treated with stachylysin after removal of the debris by centrifugation. The supernatant was analyzed spectrophotometrically by scanning from 200 to 600 nm with a Beckman DU-600 spectrophotometer. Peaks at 280 and 415 are characteristic of the earthworm hemoglobin.
The results of the LD50 test are shown in Table 2. The estimated LD50 for L. terrestis is 1.1 μg/g of worm based on a logistic regression of log10 dose of stachylysin versus survival.
TABLE 2.
LD50 determinationa
| Stachylysin injected (μg/100 μl) | No. of worms injected | No. of worms surviving |
|---|---|---|
| 24 | 6 | 0 |
| 6 | 6 | 2 |
| 1.5 | 6 | 5 |
| 0 | 6 | 6 |
The LD50 was determined by injecting into each worm (3.5 ± 0.5 g) 100μl of purified stachylysin diluted in sterile water to 24, 6, 1.5, and 0 μg. The earthworms were maintained at 23°C, and their health was noted at 72 h. The estimated LD50 for L. terrestis is 1.1 μg/g of worm based on a logistic regression of log10 dose of stachylysin versus survival.
DISCUSSION
Hemolysins have been shown to have great significance in the virulence of many bacteria (1, 2, 3, 11, 19, 24, 25, 29, 33) and of the few fungal pathogens for which they have been examined (6, 7, 8, 20, 26, 27). Many of these hemolysins damage vascular tissue, resulting in hemorrhaging.
Asp-hemolysin is the only well-characterized fungal hemolysin. Asp-hemolysin binds to arterial walls (7) and injections (intraperitoneal) of the purified Asp-hemolysin produced hemorrhagic lesions in experimental animals (26). Inoculation of viable spores of A. fumigatus, together with Asp-hemolysin, helped to establish and develop A. fumigatus infections in mice (6). The Asp-hemolysin LD50 is 750 μg/kg of body weight in mice (27), which is comparable to that of stachylysin (1,100 μg/kg) in L. terrestis. Like stachylysin, Asp-hemolysin is a glycosylated protein (38), and, since they are both released into the growth medium, they are both secretory proteins. The fact that stachylysin is glycosylated may hold great significance for its activity (34).
The protein band seen in Fig. 1A and 2A is the hemolytically active form of stachylysin, since the changes in SBA upon exposure to the purified band are identical to those observed with culture concentrates (37), i.e., a darkening of the SBA initially, followed by a clearing by 48 h (Fig. 2). However, it cannot be ruled out that there are other hemolytic agents produced by S. chartarum.
To test the effects of stachylysin, we chose a simple experimental model, the earthworm L. terrestis. Earthworms have a closed vascular system consisting of the dorsal and ventral vessels connected by commissural vessels in each segment. L. terrestis has no oxygen-carrying cell but instead has a respiratory hemoglobin, erythrocruorin, dissolved in the plasma (30).
The injection of stachylysin concentrate into L. terrestis resulted in the leakage of the hemoglobin from the vascular system (Fig. 3), with no such exudate produced by the worms injected with heat-inactivated concentrate or the concentrate from the culture of 58-17 (with no blood added). The absorbance peaks at 280 and 415 nm (Fig. 5) along with the reddish color are consistent with the erythrocruorin hemoglobin of earthworms (30). This indicates that stachylysin can cause hemorrhaging in earthworms.
If stachylysin is involved in hemorrhaging, it is important to know which strains of S. chartarum produce it and under what conditions it is produced and released. Previously it was shown that all strains used in this study, when grown on SBA or TSB with blood, produced a hemolytic agent (35, 36). If blood is not added to the TSB, most strains isolated from the homes of infants with PH and the Houston strain isolated from the lung of a PH victim still produced stachylysin. However, most strains isolated from control homes, when grown in TSB without blood, did not produce detectable hemolysis. Since TSB is a complex medium, it is not clear what the controlling factor is or what factor in blood is important in the induction for some strains. These strain differences may help explain why PH occurs in some homes infested with S. chartarum and not in others.
Unfortunately, the production characteristics of other fungal hemolysins have not been well studied. Asp-hemolysin was shown to be produced only during late logarithmic phases of growth and apparently by only some strains of A. fumigatus (8). Candida albicans hemolysin production is apparently under the control of glucose, at least in the medium tested (20). The important question remains as to whether stachylysin is released when S. chartarum spores enter the nasopharynx and/or human lung or if ingested.
We believe that stachylysin exposure may be proposed here as a reasonable mechanism to explain the bloody noses in adults and hemorrhaging in infants exposed to S. chartarum. This exposure model is now being tested by measuring the antibody response to stachylysin in exposed individuals. It is also possible that other indoor fungi also produce hemolysins with potentially similar effects.
Acknowledgments
We thank Teresa Ruby for preparation of the figures and Armah de la Cruz for much helpful advice.
This work was supported by funding from the Children at Risk Program of the U.S. Environmental Protection Agency's National Center for Environmental Assessment, which is gratefully acknowledged.
REFERENCES
- 1.Bhakdi, S., H. Bayley, A. Valeva, I. Walev, B. Walker, U. Weller, M. Kehoe, and M. Palmer. 1996. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin prototypes of pore-forming bacterial cytolysins. Arch. Microbiol. 165:73-79. [DOI] [PubMed] [Google Scholar]
- 2.Bhakdi, S., and J. Tranum-Jensen. 1991. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 55:733-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavalieri, S. J., G. A. Bohach, and I. S. Snyder. 1984. Escherichia coli α-hemolysin: characteristics and probable role in pathogenicity. Microbiol. Rev. 48:326-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dearborn, D. 1997. Pulmonary hemorrhage in infants and children. Curr. Opin. Pediatr. 9:219-224. [DOI] [PubMed] [Google Scholar]
- 5.Dearborn, D. G., I. Yike, W. G. Sorenson, M. J. Miller, and R. A. Etzel. 1999. An overview of the investigations into pulmonary hemorrhage among infants in Cleveland, Ohio. Environ. Health Perspect. Suppl. 107:495-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebina, K., K. Yokota, and O. Sakaguchi. 1982. Studies on the toxin of Aspergillus fumigatus. XIV. Relationship between Asp-hemolysin and experimental infection of mice. Jpn. J. Med. Mycol. 23:246-252. [Google Scholar]
- 7.Ebina, K., K. Yokota, and O. Sakaguchi. 1983. Studies on the toxin of Aspergillus fumigatus. XVI. Biological properties of Asp-hemolysin as a parasitic factor. Jpn. J. Med. Mycol. 24:245-252. [Google Scholar]
- 8.Ebina, K., H. Sakagami, K. Yokota, and H. Kondo. 1994. Cloning and nucleotide sequence of cDNA encoding Asp-hemolysin from Aspergillus fumigatus. Biochim. Biophys. Acta 1219:148-150. [DOI] [PubMed] [Google Scholar]
- 9.Elidemir, O., G. N. Colasurdo, S. N. Rossmann, and L. L. Fan. 1999. Isolation of Stachybotrys from the lung of a child with pulmonary hemosiderosis. Pediatrics 102:964-966. [DOI] [PubMed] [Google Scholar]
- 10.Eppley, R. M., and W. J. Bailey. 1973. 12,13-Epoxy-9-trichothecenes as the probable mycotoxins responsible for stachybotryotoxicosis. Science 181:758-760. [DOI] [PubMed] [Google Scholar]
- 11.Ermert. L., S. Rousseau, H. Schutte, R. G. Birkenmeyer, F. Grimminger, S. Bhakdi, H. R. Duncker, and W. Seeger. 1992. Induction of severe vascular leakage by low doses of Escherichia coli hemolysin in profused rabbit lungs. Lab. Investig. 66:362-369. [PubMed] [Google Scholar]
- 12.Etzel, R. A., E. Montana, W. G. Sorenson, G. J. Kullman, T. M. Allan, and D. G. Dearborn. 1998. Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Arch. Pediatr. Adolesc. Med. 152:757-762. [DOI] [PubMed] [Google Scholar]
- 13.Flappan, S. M., J. Portnoy, P. Jones, and C. Barnes. 1999. Infant pulmonary hemorrhage in a suburban home with water damage and mold (Stachybotrys atra). Environ. Health Perspect. 107:927-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forgacs, J. 1965. Stachybotrytoxicosis and moldy corn toxicosis, p. 87-104. In G. N. Wogan (ed.), Mycotoxins in foodstuffs. MIT Press, Cambridge, Mass.
- 15.Forgacs, J. 1972. Stachybotryotoxicosis, p. 95-128. In S. Kadis, A. Ciegler, and S. J. Ajl (ed.), Microbial toxins, vol. VIII. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 16.Gibson, R. L., V. Nizet, and C. E. Rubens. 1999. Group B streptococcal β-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr. Res. 45:626-634. [DOI] [PubMed] [Google Scholar]
- 17.Hintikka, E.-L. 1978. Human stachybotrytoxicosis, p 87-89. In T. D. Willie and L. G. Morehouse (ed.), Mycotoxic fungi, mycotoxins, mycotoxicoses, an encyclopedic handbook, vol. 3. Marcel Dekker, Inc., New York, N.Y.
- 18.Jarvis, B. B., W. G. Sorenson, E.-L. Hintikka, M. Nikulin, Y. Zhou, J. Jiang, S. Wang, S. Hinkley, R. A. Etzel, and D. G. Dearborn. 1998. Study of toxin production by isolates of Stachybotrys atra and Memnoniella echinata isolated during a study of pulmonary hemosiderosis in infants. Appl. Environ. Microbiol. 64:3620-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, P., M. Lindberg, I. Haraldsson, and T. Waldstrom. 1985. Virulence of Staphylococcus aureus in a mouse mastitis model: studies of alpha hemolysin, coagulase and protein A as possible virulence determinants with protoplast fusion and gene cloning. Infect. Immun. 49:765-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manns, J. M., D. M. Mosser, and H. R. Buckley. 1994. Production of a hemolytic factor by Candida albicans. Infect. Immun. 62:5154-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merril, C. R., D. Goldman, S. A. Sedman, and M. H. Ebert. 1981. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variations in cerebrospinal fluid proteins. Science 211:1437-1438. [DOI] [PubMed] [Google Scholar]
- 22.Nizet, V., R. L. Gibson, E. Y. Chi, P. E. Framson, M. Hulse, and C. E. Rubens. 1996. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect. Immun. 64:3818-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nizet, V., R. L. Gibson, and C. E. Rubens. 1997. The role of group B streptococci β-hemolysin expression in newborn lung injury, p. 627-630. In: T. Horaud et al. (ed.), Streptococci and the host. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 24.Nomura, T., Y. Fujii, and K. Okamota. 1999. Secretion of hemolysin of Aeromonas sobria as protoxin and contribution of the propeptide region removed from the protoxin to the proteolytic stability of the toxin. Microbiol. Immunol. 43:29-38. [DOI] [PubMed] [Google Scholar]
- 25.Ou Said, A. M., M. G. Contrepois, M. D. Vartanian, and J. Girardeau. 1988. Virulence factors and markers in Escherichia coli from calves with bacteremia. Am. J. Vet. Res. 49:1657-1660. [PubMed] [Google Scholar]
- 26.Sakaguchi, O., and K. Yokota. 1972. Studies on the toxin of Aspergillus fumigatus. III. Fraction causing dermonecrosis and hemorrhage-like changes. Jpn. J. Bacteriol. 27:649-655. [PubMed] [Google Scholar]
- 27.Sakaguchi, O., H. Shimada, and K. Yokota. 1975. Purification and characteristics of hemolytic toxin from Aspergillus fumigatus. Jpn. J. Med. Sci. Biol. 28:328-331. [PubMed] [Google Scholar]
- 28.Sarkisov, A. K., and V. N. Orshanskaiya. 1944. Laboratory diagnosis of toxic strains of Stachybotrys alternans. Veterinariya 21:38-40. [Google Scholar]
- 29.Seeger, W., R. Obernitz, M. Thomas, D. Walmrath, N. Suttorn, I. B. Holland, F. Grimminger, B. Eberspacher, F. Hugo, and S. Bhakdi. 1991. Lung vascular injury after administration of viable hemolysin-forming Escherichia coli in isolated rabbit lungs. Am. Rev. Respir. Dis. 143:797-805. [DOI] [PubMed] [Google Scholar]
- 30.Shlom, J. M., and S. N. Vinogradov. 1973. A study of the subunit structure of the extracellular hemoglobin of Lumbricus terrestis. J. Biol. Chem. 248:7904-7912. [PubMed] [Google Scholar]
- 31.Sorenson, W. G., and D. M. Lewis. 1996. Organic dust toxic syndrome, p. 159-172 In K. Esser and P. A. Lempke (ed.), The mycota, vol. VII. Animal and human relationships. Springer Verlag, Berlin, Germany. [Google Scholar]
- 32.Ueno, Y. 1983. Toxicology, p. 135-177. In Y. Ueno (ed.), Developments in food science, vol. 4. Trichothecenes: chemical, biological and toxicological aspects. Elsevier Scientific Publishing, Amsterdam, The Netherlands. [Google Scholar]
- 33.Van Der Vijer, J. C. M., M. M. Van Es-Boom, and M. F. Michel. 1975. A study of the virulence factors with induced mutants of Staphylococcus aureus. J. Med. Microbiol. 8:279-287. [DOI] [PubMed] [Google Scholar]
- 34.Varki, A. 1993. Biological roles of oligosaccharides: all the theories are correct. Glycobiology 3:97-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vesper, S. J., D. G. Dearborn, I. Yike, W. G. Sorenson, and R. A. Haugland. 1999. Hemolysis, toxicity, and randomly amplified polymorphic DNA analysis of Stachybotrys chartarum strains. Appl. Environ. Microbiol. 65:3175-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vesper, S. J., D. G. Dearborn, O. Elidemir, and R. A. Haugland. 2000. Quantification of siderophore and hemolysin from Stachybotrys chartarum strains, including a strain isolated from the lung of a child with pulmonary hemorrhage and hemosiderosis. Appl. Environ. Microbiol. 66:2678-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vesper, S. J., M. L. Magnuson, D. G. Dearborn, I. Yike, and R. A. Haugland. 2001. Initial characterization of the hemolysin stachylysin from Stachybotrys chartarum. Infect. Immun. 69:912-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokota, K., H. Shimada, A. Kamaguchi, and O. Sakaguchi. 1977. Studies on the toxin of Aspergillus fumigatus. VII. Purification and some properties of hemolytic toxin (Asp-hemolysin) from culture filtrates and mycelia. Microbiol. Immunol. 21:11-22. [DOI] [PubMed] [Google Scholar]