Abstract
Leptospira interrogans glycolipoprotein (GLP) has been implicated in pathological and functional derangement seen in leptospirosis. The goal of this study was to evaluate GLP's ability to induce cellular activation, as assessed by cytokine production and expression of surface activation markers. GLP extracted from either pathogenic L. interrogans serovar Copenhageni or nonpathogenic Leptospira biflexa serovar Patoc (GLPp) was used to stimulate peripheral blood mononuclear cell cultures from healthy donors. Supernatant cytokine levels were measured by enzyme-linked immunosorbent assay. Expression of CD69 and HLA-DR on lymphocytes and monocytes, as well as lipopolysaccharide (LPS) binding, were measured by flow cytometry. At 6 h of incubation, GLP induced a significant rise in tumor necrosis factor alpha levels, which dropped progressively until 72 h of incubation. Interleukin-10 peak levels were obtained at between 24 and 48 h, with sustained levels until 72 h of incubation. The response magnitude was proportional to the GLP dose. CD69 expression on T lymphocytes and monocytes increased significantly, as did HLA-DR expression on monocytes. GLPp induced no CD69 or HLA-DR expression. GLP did not block biotinylated LPS binding to monocytes, suggesting that different pathways are used to induce cell activation. In conclusion, GLP induces cellular activation and may play a major role in the pathogenesis of leptospirosis.
Leptospirosis is a zooanthroponosis caused by pathogenic spirochetes of the genus Leptospira. Infection due to those microorganisms may either be nonsymptomatic or result in different clinical pictures. The anicteric form is a flu-like disease with low morbidity but sometimes evolves to respiratory failure or meningitis, resulting in death. The severe or icteric form, also known as Weil's syndrome, clinically resembles sepsis, with multiple organ dysfunction, mainly renal failure, acute respiratory distress syndrome, coagulopathy, and shock with hemodynamic alterations similar to hyperdynamic septic shock (15, 24).
Like in classical bacterial sepsis of a different etiology, plasma cytokine levels are elevated in severe leptospirosis (13). In the acute phase, tumor necrosis factor alpha (TNF-α) levels rise and are associated with the severity of the disease (14, 39). Interleukin-10 (IL-10) levels are also elevated but are not associated with the severity of the disease. However, a high IL-10/TNF-α ratio is associated with a better prognosis, suggesting that an anti-inflammatory response may be protective (40).
Small-vessel vasculitis is the characteristic lesion found in leptospirosis, leading to fluid and cell leakage and eventually to severe hemorrhage. Tissue lesions are characterized by the occurrence of major cellular damage in the presence of few microorganisms, suggesting the involvement of toxic factors from either the spirochete or the host, associated with bacterial-cellular adherence phenomena (5, 15, 29). Cytotoxic activity of Leptospira interrogans cellular components was demonstrated in the past (10, 32). The glycolipoprotein (GLP) complex, extracted from L. interrogans by Tris-lysozyme treatment followed by acetic acid precipitation, exerts toxic effects through its lipid portion, leading to cell membrane perforation, leakage of cell contents, and cell death (43). In rabbit renal tubule epithelial cells, GLP inhibits sodium-potassium ATPase (Na,K-ATPase) pump activity in a dose-dependent manner (47). This may explain some electrolytic alterations observed in leptospirosis patients with acute renal failure (1, 26, 36). In experimental models with guinea pigs injected with pathogenic L. interrogans, GLP can be detected in the damaged tissues adhering to endothelial cells and to epithelial membranes, accompanying other antigen deposits resulting from bacterial destruction by the immune system (3, 25). Therefore, the GLP complex may contain toxins potentially involved in the pathogenesis of leptospirosis.
The objective of this work was to verify whether GLP extracted from either pathogenic or nonpathogenic Leptospira was able to induce cellular activation, as assessed by cytokine secretion and cell surface antigen expression.
MATERIALS AND METHODS
Toxins.
The GLP complex was extracted from pathogenic L. interrogans serovar Copenhageni obtained from a patient with Weil's syndrome and from nonpathogenic Leptospira biflexa serovar Patoc (GLPp), using the method described by Vihn et al. (43). Lipopolysaccharide (LPS) from Salmonella enterica serovar Abortus Equi (kindly supplied by C. Galanos, Max Planck Institute für Immunbiologie, Freiburg, Germany) was separated by the phenol-water method and purified by the phenol-chloroform-petroleum ether method (17). Biotinylated S. enterica serovar Abortus Equi LPS (LPSb) (kindly provided by M. Freunderberg, Max Planck Institute für Immunbiologie) was prepared by using the biotin reagent biotinamidocaproate N-hydroxysuccinimide ester (Sigma, St. Louis, Mo.) according to the instructions of the manufacturer.
Blood collection and cell separation.
For all experiments, 15 ml of peripheral venous blood was collected from healthy volunteers in heparin-treated tubes and 5 ml was collected in dry tubes (Becton Dickinson, Franklin Lakes, N.J.). Peripheral blood mononuclear cells (PBMC) were obtained by the Ficoll gradient method (Ficollpaque; Amersham Pharmacia Biotech, Uppsala, Sweden) and suspended in RPMI 1640 medium (Sigma). The standard cell concentration was 2 × 106 cells/ml.
Cytokine kinetics and dose response.
PBMC were cultivated in 300 μl of RPMI 1640 supplemented with 30 μl of autologous serum in 24-well plates (Nunclon; Nalge Nunc International, Roskilde, Denmark). Cells were stimulated with 1 μg of LPS per ml and 0.3, 0.03, and 0.003 μg of GLP per ml. Supernatants were collected after 6, 24, 48, and 72 h of incubation, and cells were discarded after centrifugation at 6,500 × g for 5 min. Cell-free supernatants were stored at −80°C until used for cytokine determination.
Measurement of cytokines.
TNF-α and IL-10 were measured by capture enzyme-linked immunosorbent assay (ELISA). Antibody pairs and reagents were obtained from Genzyme (Cambridge, Mass.) for TNF-α assays and from PharMingen (San Diego, Calif.) for IL-10 assays. Samples were tested in duplicate, and a standard curve with human recombinant cytokine was made in each plate. Tests were performed according to the manufacturer's instructions. The sensitivity was 5 pg/ml for TNF-α and IL-10.
Induction of cell surface activation marker expression on lymphocytes and monocytes.
Cellular activation was performed by incubating 500 μl of blood in 12- by 75-mm sterile tubes (Becton Dickinson Immunocytometry Systems [BDIS]) for 6 h at 37°C and 5% CO2 with LPS at 1 μg/ml, with GLP at 0.3 μg/ml, and without toxins as control. Monoclonal antibodies conjugated to fluorochromes were used for surface staining of lymphocytes and monocytes. Monoclonal antibodies were the following: CD14-fluorescein isothiocyanate (FITC) (BDIS), CD14-phycoerythrin (PE) (BDIS), CD69-FITC (PharMingen), HLA-DR-PE (BDIS or PharMingen), CD8-perinidin cholorophyll protein (PerCP) (BDIS), CD3-allophycocyanin (APC) (BDIS), murine immunoglobulin G1 (mIgG1)-FITC (PharMingen) and mIgG2a-PE (PharMingen). After incubation, 100 μl of stimulated blood was distributed into four tubes containing the following monoclonal antibodies: (i) mIgG1-FITC isotype for CD69, (ii) CD69-FITC, (iii) mIgG2a-PE isotype for HLA-DR, and (iv) HLA-DR-PE. CD14-PE, CD8-PerCP, and CD3-APC were added to tubes i and ii, and CD14-FITC was added to tubes iii and iv, and the tubes were incubated at room temperature (RT) in the dark for 15 min. After this procedure, 2 ml of phosphate-buffered saline (PBS) was added to each tube, followed by centrifugation at 2,500 × g for 5 min at 4°C. Supernatants were discarded, and cells were washed again with 2 ml of PBS and resuspended in 300 μl of PBS-1% NaN3 (Sigma). Samples were run in a FACSCalibur flow cytometer (BDIS). For each condition, 20,000 events were acquired and analyzed using CellQuest software (BDIS). Lymphocyte acquisition was accomplished using a forward- and side-scatter plot to establish a gate over the low side scatter and low to moderate forward scatter. T-lymphocyte subpopulations were identified using CD3 and CD8, where appropriate. CD3+/CD8+ lymphocytes were considered T-cytotoxic and CD3+/CD8− lymphocytes were considered T-helper subpopulations. Monocytes were acquired using a combination of side scatter and CD14 plotting. Expression of surface markers was evaluated using dot plots or histograms.
Blockade of LPSb binding to its receptor by LPS or GLP.
One hundred microliters of blood was distributed in 12- by 75-mm tubes (BDIS) and incubated at 37°C with 5% CO2 for 5 min in the presence of LPS or GLP. Both toxins were used at increasing concentrations of 500, 5,000, and 10,000 ng/ml. After incubation, 5,000 ng of LPSb per ml was added and incubated for a further 5 min. As a positive control for LPS binding, one tube was treated only with LPSb, and as a negative control, one tube received neither toxins nor LPSb. Samples were then washed with 2 ml of PBS, and tubes were centrifuged at 2,500 × g for 5 min. Supernatants were discarded, and cells were resuspended in 2 ml of lysis solution (BDIS) and incubated for 15 min at RT in the dark. Next, tubes were centrifuged at 2,500 × g for 5 min and supernatants were discarded. Cells were washed with 2 ml of PBS and stained with CD14-FITC (BDIS) and streptavidin-APC (BDIS). After incubation for 15 min at RT in the dark, cells were washed with 2 ml of PBS and resuspended in 300 μl of PBS-1% NaN3 (Sigma) for flow cytometry. The test was performed as described above. Previous studies in our lab showed that anti-CD14 monoclonal antibody (clone MφP9) binding to CD14 molecules on the monocyte surface was not influenced by the presence of LPS, even at concentrations of as high as 50,000 ng/ml (8).
Statistical analysis.
One-way analysis of variance was used to compare GLP-induced TNF-α and IL-10 with those of controls, followed by post hoc analysis by the Student-Neuman-Keuls method for all pairwise multiple comparisons. Adherence to normality was tested with the Levene variance homogeneity test with the Brown-Forsythe modification. Wilcoxon matched-pair tests were used to compare GLP stimuli and controls in the flow cytometry experiments (CD69 and HLA-DR expression on cell surfaces). The significance level was established as 5% in all analyses.
RESULTS
GLP-induced TNF-α kinetics and dose response.
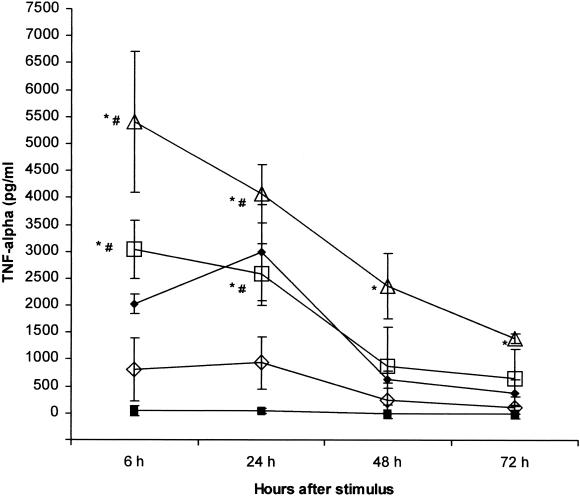
Increasing doses of GLP, from 0.003 to 0.3 μg/ml, induced an evident dose-response effect, which was detected at any incubation time. GLP-induced TNF-α secretion was already detected at high levels as early as 6 h of incubation. At between 6 and 24 h of incubation, TNF-α remained stable, with a mild decrease with 0.3 and 0.03 μg/ml and a slight increase with 0.003 μg/ml. Progressively lower levels were observed at 48 and 72 h. LPS produced similar kinetics, but TNF-α peaked at 24 h (Fig. 1).
FIG. 1.
TNF-α kinetics and dose response after stimulation with GLP. LPS was used as positive control. PBMC were stimulated with either S. enterica serovar Abortus Equi LPS or L. interrogans serovar Copenhageni GLP at the indicated doses and incubated for 72 h. Supernatants were collected at the specified time-points and TNF-α was measured by ELISA. Results are means ± SDs from three experiments. Closed squares, control; closed diamonds, LPS at 1 μg/ml; open triangles, GLP at 0.3 μg/ml; open squares, GLP at 0.03 μg/ml; open diamonds, TLP at 0.003 μg/ml. For all GLP stimuli compared with controls, P = 0.02; ∗, P < 0.05 compared to control; #, P < 0.05 compared to 0.003 μg/ml.
GLP-induced IL-10 kinetics and dose response.
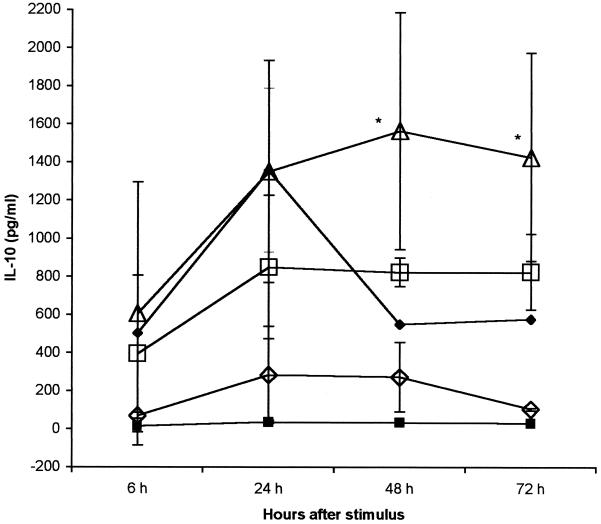
IL-10 secretion was dose dependent but at lower levels than TNF-α secretion. IL-10 reached peak levels at between 24 and 48 h of incubation with all doses and sustained a plateau until 72 h. LPS induced a similar kinetics, but IL-10 peaked at 24 h, decreasing thereafter (Fig. 2).
FIG. 2.
IL-10 kinetics and dose response after stimulation with GLP. LPS was used as positive control. PBMC were stimulated with either S. enterica serovar Abortus Equi LPS or L. interrogans serovar Copenhageni GLP in the indicated doses and incubated for 72 h. Supernatants were collected at the specified time points, and TNF-α was measured by ELISA. Results are means ± SDs from two experiments. Closed squares. control; closed diamonds, LPS at 1 μg/ml; open triangles, GLP at 0.3 μg/ml; open squares, GLP at 0.03 μg/ml; open diamonds, GLP at 0.003 μg/ml. For all GLP stimuli compared with controls, P = 0.05; ∗, P < 0.05 compared to control.
CD69 expression on lymphocytes and monocytes.
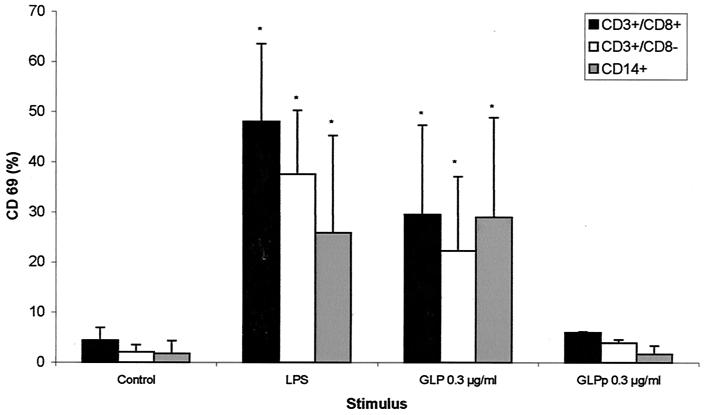
After 6 h of stimulation with 0.3 μg of GLP per ml or 1 μg of LPS per ml, there was an increase in the surface expression of CD69, a marker of cellular activation, in the CD3+/CD8−, CD3+/CD8+, and CD14+ subpopulations (Fig. 3). GLPp at the same doses induced no lymphocyte or monocyte CD69 expression (Fig. 3).
FIG. 3.
CD69 expression on LPS- or GLP-stimulated lymphocytes and monocytes. Whole blood was incubated in the presence of LPS, GLP, or medium alone for 6 h as described in Materials and Methods. CD69 is depicted as the percentage of positive cells acquired on the gate. Results are means ± SDs from five experiments with GLP and two experiments with GLPp. ∗, P < 0.05 compared to controls.
HLA-DR expression on monocytes.
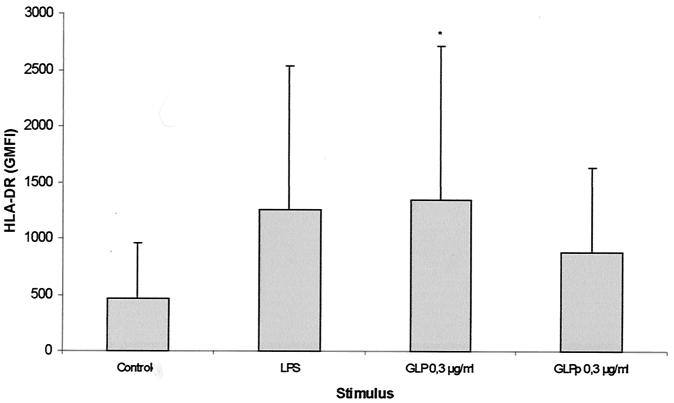
When stimulated with 0.3 μg of GLP per ml, monocytes showed an increase in HLA-DR surface expression, from a geometric mean fluorescence intensity (± standard deviation [SD]) of 224.19 ± 104.55 to 767.73 ± 126.40. GLPp at 0.3, 0.03, or 0.003 μg/ml produced no HLA-DR up-regulatory response. As expected, LPS induced an increase in HLA-DR expression on monocytes (Fig. 4).
FIG. 4.
HLA-DR expression on LPS- or GLP-stimulated monocytes. Whole blood was incubated in the presence of LPS, GLP, or medium alone for 6 h as described in Materials and Methods. HLA-DR is depicted as geometric mean fluorescence intensity (GMFI). Results are means ± SDs from five experiments for GLP and two experiments for GLPp. ∗, P < 0.05 compared to controls.
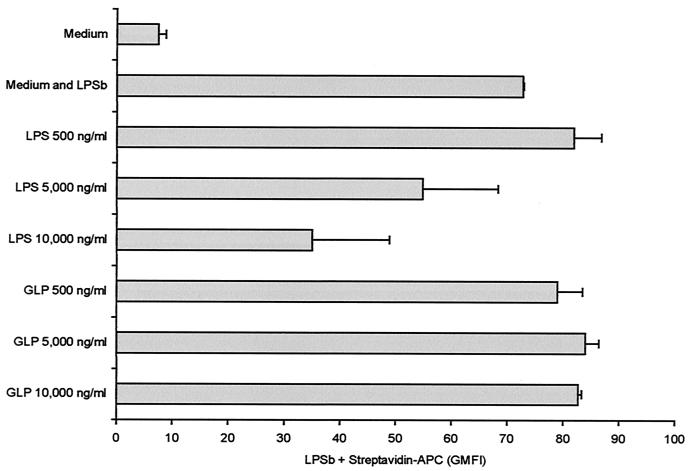
Lack of blockade of LPSb binding to its receptor by GLP.
LPS blocked binding of biotinylated LPS to monocytes at concentrations of 5,000 and 10,000 ng/ml. However, preincubation with GLP led to no blocking effect at the same concentrations (Fig. 5).
FIG. 5.
LPSb binding blockade by LPS or GLP. PBMC were preincubated with increasing concentrations of LPS or GLP and then incubated with 5,000 ng of LPSb per ml. There was a decrease in the LPSb binding to PBMC preincubated with 5,000 and 10,000 ng of LPS per ml. GLP produced no blocking effect on LPSb binding to monocytes. Results are means ± SDs from two experiments. Streptavidin-APC is depicted as the geometric mean fluorescence intensity (GMFI).
DISCUSSION
The results presented here demonstrated that GLP extracted from pathogenic L. interrogans serovar Copenhageni induces cellular activation of PBMC. This was demonstrated through the secretion of TNF-α and IL-10 and the increase in the expression of CD69 and HLA-DR markers for cellular activation. It was also demonstrated that GLP extracted from nonpathogenic L. biflexa serovar Patoc failed to induce cellular activation under the same experimental conditions. These results also suggested that cellular activation induced by GLP seems not to occur through the same LPS pathway, since the former does not block LPSb binding to monocytes.
Leptospirosis is a toxin-mediated disease, in which toxins are released by the spirochetes into the affected tissues (3, 4, 6). From the pathology point of view, in addition to specific tissue lesions, like interstitial nephritis and hepatic central-lobular necrosis, a generalized vasculitis accompanied by hemorrhagic phenomena is seen in all tissues (24, 29). Few spirochetes can be observed on the damaged tissue, strongly suggesting a toxic nature of the disease process (3). Toxic vasculitis (5) could be triggered by one or more factors that generate acute systemic inflammation, which is clinically found in the most severe cases such as in sepsis or systemic inflammatory response syndrome of infectious origin. Among the many antigens isolated from pathogenic Leptospira, GLP is likely to be involved in the pathogenesis of the disease, as demonstrated by the blocking of the tubular epithelial cell sodium pump in the kidneys (47). This may be one of the mechanisms of renal dysfunction that is characteristic of the disease (36). Other toxic effects exerted by GLP may be observed in cultures of Vero and L929 cells, leading to cell membrane perforations followed by cell death (43). The presence of GLP in damaged tissues of patients and experimental animals further supports the role of this molecule in leptospirosis (25, 33).
In this study it was demonstrated that GLP could induce in vitro and ex vivo lymphocyte and monocyte activation, leading to cytokine secretion and membrane antigen expression of activation markers. GLP induced TNF-α secretion by PBMC cultures, detectable at 6 h of incubation, suggesting that GLP may be the antigen that plays a pivotal role leading to TNF-α production by patients with leptospirosis. Monocyte-secreted TNF-α induces local inflammation, and, if present in sufficient amounts in the circulation, it could unleash systemic inflammation (38, 42). TNF-α induces monocytes to secrete other cytokines, such as IL-1, IL-6, and IL-8, all of which are essential in the control of infection but are also involved in tissue lesions (35). Leptospirosis patients with higher TNF-α have been shown to have a poor prognosis (39).
In the present experiments, GLP also induced IL-10 production in PBMC cultures, which showed a typically delayed kinetics compared to TNF-α production. The latter can be detected at as early as 6 hours of incubation, and its levels decrease in the following 24 to 72 h. IL-10 was detectable at low levels at 6 hours, increasing thereafter and reaching a plateau at 48 to 72 h of incubation. This sequence of events observed in vitro resembles cytokine patterns observed in patients with sepsis (9, 41). The induction of anti-inflammatory cytokines, such as IL-10, may be essential to modulate the inflammatory process, reducing tissue damage and stimulating regeneration and healing. We have previously found the IL-10/TNF-α ratio to be higher among patients with less severe leptospirosis (40).
Further evidence that GLP can induce cellular activation is the increased ex vivo expression of CD69 on lymphocytes and monocytes and of HLA-DR on monocytes. In the present experiments, toxins were added to whole blood, preserving the cell-toxin interaction microenvironment, like the case in vivo. Resting lymphocytes present a low level of CD69 expression. Nonspecific stimuli, such as phytohemagglutinin or phorbol ester, cytokines, and anti-CD69 antibodies, can induce an increase in its expression, detected as early as 30 to 60 min after incubation and sustained for more than 72 h. Activated cells proliferate and secrete cytokines, producing a positive feedback effect on the lymphocyte response to the stimulus (28). In our experiments, we were able to demonstrate increased CD69 expression on T lymphocytes within 6 h of incubation. Increased CD69 expression was also demonstrated on monocytes. In these cells, CD69 acts as a signal transducer molecule. Activation of monocytes through CD69 induces the secretion of inflammatory mediators, such as prostaglandins E2 and F1 and leukotriene B4, and increased production of nitric oxide (NO) (12). Increased intracellular NO production is essential to control intracellular infection. However, excess NO secretion in inflamed tissues results in cellular damage and vasodilatation. GLP-induced monocyte activation was further demonstrated by the up-regulation of HLA-DR expression on cell surface.
Vihn and coworkers (43) demonstrated that GLP extracted from L. biflexa serovar Patoc had the same biological effects as GLP extracted from pathogenic Leptospira, such as fibroblast cytotoxicity, hemagglutination, and erythrocyte crenation. In our hands, however, GLPp failed to induce the cellular activation observed with GLP under the same experimental conditions. This discrepancy could be a result of different approaches used to detect cellular activation or differences in the GLP extraction process. However, structural differences between GLP and GLPp may exist and could be related to the pathophysiology of leptospirosis.
Other toxic factors, such as LPS, peptidoglycan, and phospholipase, may also play a role in the pathogenesis of leptospirosis, but their roles are not well established (11, 15, 22, 37, 44, 46). Lipoproteins are also potential toxic factors in leptospirosis as well as in other spirochetal diseases. Lipoproteins are recognized to be the most abundant proteins in spirochetes and are involved in disease pathogenesis through their ability to trigger the host inflammatory response (19). Expression of leptospiral lipoproteins Lip L32 and Lip L41 was demonstrated by immune histochemistry of kidney tissue following infection with virulent Leptospira kirschneri (4, 19, 20). Treponemal and borrelial lipoproteins are capable of activating monocytes in vitro, and cellular activation has been shown to involve CD14 and Toll-like receptor 2 molecules (2, 18, 21, 34, 45).
In the present experiments GLP did not block LPSb binding to monocytes, suggesting that cellular activation may follow a distinct pathway. GLP inhibits renal tubular cell Na,K-ATPase and could have the same effect on monocytes. If this is true, it could be a mechanism involved in GLP-induced TNF-α secretion. In the PBMC, Na,K-ATPase inhibition induces proinflammatory cytokine secretion by monocytes and abolishes lymphocyte proliferation, with no influence on its early activation (7, 27, 30, 31). Foey and coworkers (16) showed that blocking mononuclear cell Na,K-ATPase with ouabain induced TNF-α and IL-1 secretion. Blocking of Na,K-ATPase, by its specific inhibitor bufalin, on TPH-1 monocytic leukemic cell lines induces the secretion of TNF-α and IL-1 secondary to increased intracellular calcium influx and activation of kinases and cytokine gene expression (23). Taken together, the data suggest that inhibition of Na,K-ATPase could be a possible mechanism of action for GLP.
In conclusion, GLP extracted from pathogenic L. interrogans activates PBMC, as demonstrated by the secretion of TNF-α and IL-10 and expression of CD69 and HLA-DR. GLP extracted from nonpathogenic L. biflexa has no activation effect under the same experimental conditions. GLP does not block LPS binding to monocytes, suggesting that activation is induced by a different pathway.
Acknowledgments
We are in debt to Marta Anesia Viana and Helena T. I. Tomiyama for excellent laboratory technical support, to Carla Cristiane Da Mota Bernardo for help with GLP extraction, and to Marcelo N. Burattini for statistical support.
This work was sponsored by the Brazilian Ministry of Science and Technology, PRONEX 41.96.0943.00, and Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (National Council for the Scientific and Technological Development), grant no. 524088/96-9.
Editor: R. N. Moore
REFERENCES
- 1.Abdulkader, R. C. R., A. C. Seguro, P. S. Malheiro, E. A. Burdmann, and M. Marcondes. 1996. Peculiar electrolytic and hormonal abnormalities in acute renal failure due to leptospirosis. Am. J. Trop. Med. Hyg. 54:1-6. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Alves, V. A. F., L. C. C. Gayotto, P. H. Yasuda, A. Wakamatsu, C. T. Kanamura, and T. Brito. 1991. Leptospiral antigens (L. interrogans serogroup ictero-haemorrhagiae) in the kidney of experimentally infected guinea pigs and their relation to the pathogenesis of the renal injury. Exp. Pathol. 42:81-93. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, J. K., D. Barnett, C. A. Bolin, T. A. Summers, E. A. Wagar, N. F. Cheville, R. A. Hartskeerl, and D. A. Haake. 1999. Expression and distribution of leptospiral outer membrane components during renal infection of hamsters. Infect. Immun. 67:853-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brito, T., G. M. Bohm, and P. H. Yasuda. 1979. Vascular damage in acute experimental leptospirosis of the guinea-pig. J. Pathol. 128:177-181. [DOI] [PubMed] [Google Scholar]
- 6.Brito, T., C. F. Morais, P. H. Yasuda, C. P. Lancellotti, S. Hoshino-Shimizu, E. Yamashiro, and V. A. F. Alves. 1987. Cardiovascular involvement in human and experimental leptospirosis: pathologic findings and immunohistochemical detection of leptospiral antigen. Ann. Trop. Med. Parasitol. 81:207-214. [DOI] [PubMed] [Google Scholar]
- 7.Brodie, C., A. Tordai, J. Saloga, J. Domenico, and E. W. Gelfand. 1995. Ouabain induces inhibition of the progression phase in human T-cell proliferation. J. Cell Physiol. 165:246-253. [DOI] [PubMed] [Google Scholar]
- 8.Brunialti, M. K. C., E. G. Kallás, M. Freudenberg, C. Galanos, and R. Salomao. Influence of EDTA and heparin on lipopolysaccharide binding and cell activation, evaluated at single cell-level in whole blood. Cytometry, in press. [PubMed]
- 9.Casey, L. C. 2000. Immunologic response to infection and its role in septic shock. Crit. Care Clin. 16:193-214. [DOI] [PubMed] [Google Scholar]
- 10.Cinco, M., E. Banfi, A. Furlani, and V. Scarcia. 1980. Cytotoxic activity of supernatant extracts of virulent and saprophytic leptospires. Zbl. Bakteriol. Hyg. I A 248:260-267. [PubMed] [Google Scholar]
- 11.Cinco, M., E. Vecile, R. Murgia, P. Dobrina, and A. Dobrina. 1996. Leptospira interrogans and Leptospira peptidoglycans induce the release of tumor necrosis factor α from human monocytes. FEMS Microbiol. Lett. 138:211-214. [DOI] [PubMed] [Google Scholar]
- 12.De Maria, R., M. G. Cifone, R. Trotta, M. R. Rippo, C. Festuccia, A. Santoni, and R. Testi. 1994. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. J. Exp. Med. 180:1999-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diament, D. 1995. Plasma levels of tumor necrosis factor α in leptospirosis (Weil's disease). Rev. Soc. Bras. Med. Trop. 28:157-158. [Google Scholar]
- 14.Estavoyer, J. M., E. Racadot, G. Couetdic, J. Leroy, and L. Grosperrin. 1991. Tumor Necrosis Factor in patients with leptospirosis. Rev. Infect. Dis. 13:1245.. [DOI] [PubMed] [Google Scholar]
- 15.Faine, S. 1994. Leptospira and leptospirosis. CRC Press, Boca Raton, Fla.
- 16.Foey, A., A. Crawford, and N. D. Hall. 1997. Modulation of cytokine production by human mononuclear cells following impairment of Na,K-ATPase activity. Biochim. Biophys. Acta 1355:43-49. [DOI] [PubMed] [Google Scholar]
- 17.Galanos, C., O. Lüderitz, E. T. Rietschel, and O. Westphal. 1977. Newer aspects of the chemistry and biology of bacterial lipopolysaccharides, with special reference to their lipid A component. Int. Rev. Biochem. 14:239-335. [Google Scholar]
- 18.Giambartolomei, G. H., V. A. Dennis, B. L. Lasater, and M. T. Philipp. 1999. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect. Immun. 67:140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haake, D. A. 2000. Spirochaetal lipoproteins and pathogenesis. Microbiology 146:1491-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haake, D. A., G. Chao, R. L. Zuerner, J. K. Barnett, D. Barnett, M. Mazel, J. Matsunaga, P. N. Levett, and C. A. Bolin. 2000. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 68:2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 22.Isogai, E., H. Isogai, Y. Kurebayashi, and N. Ito. 1986. Biological activities of leptospiral lipopolysaccharide. Zbl. Bakteriol. Hyg. A 261:53-64. [DOI] [PubMed] [Google Scholar]
- 23.Kurosawa, M., S. Numazawa, Y. Tani, and T. Yoshida. 2000. ERK signaling mediates the induction of inflammatory cytokines by bufalin in human monocytic cells. Am. J. Physiol. Cell. Physiol. 278:C500-C508. [DOI] [PubMed] [Google Scholar]
- 24.Lomar, A. V., D. Diament, and J. R. Torres. 2000. Leptospirosis in Latin America. Infect. Dis. Clin. N. Am. 14:23-39. [DOI] [PubMed] [Google Scholar]
- 25.Macedo-Santos, R. T., E. E. Sakata, P. H. Yasuda, A. Wakamatsu, C. T. Kanamura, I. Candelori, C. B. Pestana, and V. A. F. Alves. 1989. Glicoproteína de Leptospira interrogans sorogrupo icterohaemorrhagiae: distribuição em fígado e rim de cobaias experimentalmente infectadas. Rev. Inst. Med. Trop. S. Paulo 31:235-241. [PubMed] [Google Scholar]
- 26.Magaldi, A. J., P. H. Yasuda, L. H. Kudo, A. C. Seguro, and A. S. Rocha. 1992. Renal involvement in leptospirosis: a pathophysiologic study. Nephron 62:332-339. [DOI] [PubMed] [Google Scholar]
- 27.Marakhova, I. I., A. A. Vereninov, T. A. Vinogradova, and F. V. Toropova. 1998. Cyclosporin A inhibits long-term activation of Na+,K+ pump in phytohemagglutinin-stimulated human lymphocytes. Membr. Cell Biol. 12:363-374. [PubMed] [Google Scholar]
- 28.Marzio, R., J. Mauël, and S. Betz-Corradin. 1999. CD69 and regulation of the immune function. Immunopharmacol. Immunotoxicol. 21:565-582. [DOI] [PubMed] [Google Scholar]
- 29.Nicodemo, A. C., N. Medeiros, G. Del Negro, and V. Amato Neto. 1989. Alterações hematológicas na leptospirose. Rev. Inst. Med. Trop. S. Paulo 31:71-79. [DOI] [PubMed] [Google Scholar]
- 30.Olej, B., L. de La Rocque, F. P. Castilho, I. F. Mediano, M. M. Campos, and V. M. Rumjanek. 1994. Effect of ouabain on lymphokine-activated killer cells. Int. J. Immunopharmacol. 16:769-774. [DOI] [PubMed] [Google Scholar]
- 31.Olej, B., N. F. dos Santos, L. Leal, and V. M. Rumjanek. 1998. Ouabain induces apoptosis on PHA-activated lymphocytes. Biosci. Rep. 18:1-7. [DOI] [PubMed] [Google Scholar]
- 32.Oravec, C., and E. Kmety. 1978. Antilymphoid activity of leptospiral exoproducts. Neoplasma 25:565-569. [PubMed] [Google Scholar]
- 33.Pereira, M. M., J. Andrade, M. D. Lacerda, N. M. Batoreu, R. S. Marchesvsky, and R. Ribeiro dos Santos. 1997. Demonstration of leptospiral antigens on tissues using monoclonal antibodies and avidin-biotin peroxidase staining. Exp. Toxicol. Pathol. 49:505-511. [DOI] [PubMed] [Google Scholar]
- 34.Radolf, J. D., L. L. Arndt, D. R. Akins, L. L. Curetty, M. E. Levi, Y. Shen, L. S. Davis, and M. V. Norgard. 1995. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 154:2866-2877. [PubMed] [Google Scholar]
- 35.Salomao, R., O. Rigato, A. C. Pignatari, M. A. Freudenberg, and C. Galanos. 1999. Bloodstream infections: epidemiology, pathophysiology and therapeutic perspectives. Infection 27:1-11. [DOI] [PubMed] [Google Scholar]
- 36.Seguro, A. C., A. V. Lomar, and A. S. Rocha. 1990. Acute renal failure of leptospirosis: nonoliguric and hypokalemic forms. Nephron 55:146-151. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu, T., E. Matsusaka, K. Takayanagi, T. Masuzawa, Y. Iwamoto, T. Morita, I. Mifuchi, and Y. Yanagihara. 1987. Biological activities of lipopolysaccharide-like substance (LLS) extracted from Leptospira interrogans serovar canicola strain Moulton. Microbiol. Immunol. 31:727-735. [DOI] [PubMed] [Google Scholar]
- 38.Slifka, M. K., and J. L. Whitton. 2000. Clinical implications of dysregulated cytokine production. J. Mol. Med. 78:74-80. [DOI] [PubMed] [Google Scholar]
- 39.Tajiki, M. H., and R. Salomão. 1996. Association of plasma levels of tumor necrosis factor α with severity of disease and mortality among patients with leptospirosis. Clin. Infect. Dis. 23:1177-1178. [DOI] [PubMed] [Google Scholar]
- 40.Tajiki, M. H., A. S. Nakama, and R. Salomão. 1997. The ratio of plasma levels of IL-10/TNF-α and its relationship to disease severity and survival in patients with leptospirosis. Braz. J. Infect. Dis. 1:138-141. [PubMed] [Google Scholar]
- 41.van der Poll, T., and S. J. H. van Deventer. 1999. Cytokines and anticytokines in the pathogenesis of sepsis. Infect. Dis. Clin. N. Am. 13:413-426. [DOI] [PubMed] [Google Scholar]
- 42.Vassali, P. 1992. The pathophysiology of tumor necrosis factors. Annu. Rev. Immunol. 10:411-452. [DOI] [PubMed] [Google Scholar]
- 43.Vihn, T., B. Adler, and S. Faine. 1986. Glycolipoprotein cytotoxin from Leptospira interrogans serovar copenhageni. J. Gen. Microbiol. 132:111-123. [DOI] [PubMed] [Google Scholar]
- 44.Volina, E. G., L. F. Levina, and G. L. Soboleva. 1986. Phospholipase activity and virulence of pathogenic Leptospirae. J. Hyg. Epidemiol. Microbiol. Immunol. 2:163-169. [PubMed] [Google Scholar]
- 45.Wooten, R. M., T. B. Morrison, J. H. Weis, S. D. Wright, R. Thieringer, and J. J. Weiss. 1998. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 160:5485-5492. [PubMed] [Google Scholar]
- 46.Yanagihara, Y., T. Taniyama, H. Misaki, Y. Suzuki, M. Matsumoto, and I. Mifuchi. 1984. Phospholipases of Leptospira. I. Presence of phospholipase A1 and lysophospholipase in Leptospira biflexa. Microbiol. Immunol. 28:747-756. [DOI] [PubMed] [Google Scholar]
- 47.Younes-Ibrahim, M., P. Burth, M. V. C. Faria, B. Buffin-Meyer, S. Marsy, C. Barlet-Bas, L. Cheval, and A. Doucet. 1995. Inhibition of Na,K-ATPase by endotoxin extracted from Leptospira interrogans: a possible mechanism for the physiopathology of leptospirosis. C. R. Acad. Sci. 318:619-625. [PubMed] [Google Scholar]