Abstract
The bacterial pathogen Citrobacter rodentium belongs to a family of gastrointestinal pathogens that includes enteropathogenic and enterohemorrhagic Escherichia coli and is the causative agent of transmissible colonic hyperplasia in mice. The molecular mechanisms used by these pathogens to colonize host epithelial surfaces and form attaching and effacing (A/E) lesions have undergone intense study. In contrast, little is known about the host's immune response to these infections and its importance in tissue pathology and bacterial clearance. To address these issues, wild-type mice and mice lacking T and B lymphocytes (RAG1 knockout [KO]) were infected with C. rodentium. By day 10 postinfection (p.i.), both wild-type and RAG1 KO mice developed colitis and crypt hyperplasia, and these responses became more exaggerated in wild-type mice over the next 2 weeks, as they cleared the infection. By day 24 p.i., bacterial clearance was complete, and the colitis had subsided; however, crypt heights remained increased. In contrast, inflammatory and crypt hyperplastic responses in the RAG1 KO mice were transient, subsiding after 2 weeks. By day 24 p.i., RAG1 KO mice showed no signs of bacterial clearance and infection was often fatal. Surprisingly, despite remaining heavily infected, tissues from RAG1 KO mice surviving the acute colitis showed few signs of disease. These results thus emphasize the important contribution of the host immune response during infection by A/E bacterial pathogens. While T and/or B lymphocytes are essential for host defense against C. rodentium, they also mediate much of the tissue pathology and disease symptoms that occur during infection.
Enteropathogenic and enterohemorrhagic Escherichia coli (EPEC and EHEC, respectively) are among the most important causative agents of diarrhea worldwide (12, 17, 20, 48, 58), belonging to a family of related pathogenic bacteria that infect a wide range of animal species, including mice and humans (25, 38, 42). Intestinal biopsy samples and tissue culture studies have revealed that these classically noninvasive pathogens attach to the surface of epithelial cells, forming lesions distinguished by the effacement of the epithelial cell surface and the production of pedestal-like structures beneath the adherent bacteria (24, 48). The formation of these attaching and effacing (A/E) lesions has been shown to correlate with the ability to cause diarrheal disease (1, 48, 53). As a result, much work has been done to characterize the bacterial virulence mechanisms responsible for their formation. Recent studies have shown that these pathogens share a homologous DNA region called the LEE (for locus of enterocyte effacement), a pathogenicity island required for A/E lesion formation (42, 43, 48). The LEE region encodes a type III secretion apparatus; several virulence factors, including the secreted proteins EspA, EspB and EspD; an outer membrane adhesin termed intimin; and the translocated intimin receptor, Tir (25, 34, 58). These proteins have proven to be critical for EPEC and other A/E pathogens to intimately attach to host cells (17, 25, 35), as well as to cause disease (1, 36, 41, 44, 49).
Despite the recent elucidation of the mechanisms used by A/E pathogens to infect their hosts, there is now a growing realization that studying bacterial effectors in isolation may be insufficient to fully understand the mechanisms underlying the disease and tissue damage caused by these infections. While A/E lesions no doubt contribute to the pathology associated with EPEC and EHEC infections (48, 58), it is likely that the host immune response underlies much of the disease symptomatology (48, 53, 55). However, this hypothesis remains unproven and is based primarily on histological analysis of infected tissues, showing that EPEC infection leads to a strong inflammatory response within the gastrointestinal tract (18, 30, 32). Studies of peripheral blood lymphocytes (53) and convalescent-phase sera (37) also indicate that the host immune system is strongly activated during infection, recognizing and producing antibodies against bacterial surface proteins and secreted virulence factors. Unfortunately, determining the relative contribution of the host immune response to disease symptoms and pathology, as well as to the eventual clearance of these infections, poses significant difficulties, since EPEC and EHEC only cause disease in humans (16, 22, 58).
An alternative approach to address such issues is to study the immune mechanisms that are activated during infection by veterinary A/E pathogens. Bacteria capable of causing A/E lesions have been isolated from infected rabbits, dogs, cats, calves, pigs, and mice (1-3, 7, 11, 42). Not only do these bacterial pathogens possess an array of LEE-encoded virulence factors similar to those found in both EPEC and EHEC (42), but they also cause disease symptoms in their respective hosts similar to those seen in human infections (8, 11, 39, 48). Among these pathogens is Citrobacter rodentium, the causative agent of transmissible colonic hyperplasia in mice (7-9, 39, 52). Our laboratory and others (29, 39, 40) have begun to use this model to investigate the role of the host immune system in the tissue pathology that occurs during infection by A/E lesion-causing bacteria, as well as to elucidate the mechanisms of host defense in response to infection. Recent studies by Higgins et al. provided an initial characterization of the colonic inflammatory response that occurs in mice infected by C. rodentium (29). Infiltrating T cells and macrophages were associated with increased expression of Th1 cytokines, including gamma interferon (IFN-γ). During infection, the colon undergoes a massive crypt hyperplasia that appears dependent on the host immune system, since the hyperplastic response did not occur in IFN-γ receptor-deficient mice (28). Despite this dependence, the cellular source for the increased production of IFN-γ was not determined. Furthermore, the relative contributions of innate versus acquired immunity to the tissue pathology as well as to the eventual clearance of the infection have yet to be addressed.
To address these questions, we infected RAG1 knockout (KO) mice with C. rodentium. These mice have undergone a targeted deletion of the recombination-activating gene 1 and are unable to perform V(D)J recombination of immunoglobulin and T-cell receptor genes. As a result, they lack both mature T and B lymphocytes and thus are deficient in developing acquired immunity (45, 46). Our results demonstrate that while innate immunity is sufficient to cause acute colitis and crypt hyperplasia in response to infection, T and/or B lymphocytes are required for the prolonged inflammatory and crypt hyperplastic responses seen in wild-type mice. Furthermore, this is the first study to show that T and/or B lymphocytes are required by the host to clear a C. rodentium infection.
MATERIALS AND METHODS
Mice.
Three- to 4-week-old wild-type (C57BL/6) mice and the congenic RAG1-deficient (RAG1 KO) mice (45) (backcrossed 10 generations onto the C57BL/6 background) were obtained from Jackson Laboratories (Bar Harbor, Maine). Mice were kept in sterilized, filter-topped cages, handled in tissue culture hoods, and fed autoclaved food and water under specific-pathogen-free conditions at our animal facilities. Sentinel animals were routinely tested for common pathogens. The protocols employed were in direct accordance with guidelines drafted by the University of British Columbia's Animal Care Committee and the Canadian Council on the Use of Laboratory Animals.
Immune cell reconstitution of RAG1 KO mice.
The immune system of RAG1 KO mice was reconstituted with splenic populations of T and B lymphocytes by routine techniques (57). In brief, wild-type immunocompetent mice were euthanized, and their spleens were aseptically removed. Spleens were placed in RPMI medium with 10% fetal bovine serum, cut into small pieces, and then forced through mesh, generating a single-cell suspension. Cells were incubated in tissue culture dishes at 37°C for 2 h. Nonadherent cells were then spun down over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) for 20 min at 1,500 rpm (Beckman GS-6R centrifuge). The buffy coat of mononuclear cells was collected, washed with RPMI medium, pelleted, and resuspended in phosphate-buffered saline (PBS). Cells were then counted and analyzed for viability by trypan blue exclusion. Recipient RAG1 KO mice were then injected via the tail vein with 2 × 108 viable mononuclear cells. Mice were left for 3 to 4 weeks and then tested for the success of reconstitution by staining the spleens of selected mice for the presence of T lymphocytes by using the marker CD3 and for B lymphocytes by using the marker B220.
Bacterial strains and infection of mice.
In all experiments, mice were orally inoculated with wild-type C. rodentium (formerly Citrobacter freundii biotype 4280), strain DBS100 (52). For inoculations, bacteria were grown overnight in Luria broth. Mice were infected by oral gavage with 0.1 ml of Luria broth containing approximately 2.5 × 108 CFU of C. rodentium. To minimize differences between infections, both wild-type and RAG1 KO mice were infected with the same bacterial preparation. Mice were killed at various time points postinfection (p.i.) and tissues were prepared for histological analysis, viable bacterial counts, or RNA isolation as described below. To control for the greater age of the immune cell-reconstituted RAG1 KO mice, they were infected along with and compared to age-matched (2 month old) unreconstituted RAG1 KO and wild-type mice.
Survival and body weight measurement.
To assess the systemic effects of C. rodentium infection on the host, the survival of infected mice and changes in their body weight were measured over the course of infection. Mice were weighed just prior to infection, as well as at specified intervals until day 24 p.i. Mice were monitored throughout the infection, and any that showed extreme distress or became moribund were euthanized. Survival and body weight data are from a representative experiment out of three experiments showing similar results. Body weight data are presented as the mean of the percent start weight of 10 mice at each time point, while survival data are presented as the percentage of the initial 10 mice still surviving at each time point.
Tissue collection.
Over the course of the infection, mice were euthanized, and following careful dissection, the first 4 cm of each colon, beginning at the anal verge, was collected. Fecal pellets were removed before the colonic tissue was weighed. Tissues were then placed in 10% neutral buffered formalin (NBF) (Sigma) for histological analysis, or the colon and pellets were collected in PBS (pH 7.4) and kept on ice before being processed for viable bacterial counts.
Bacterial counts.
Colonic tissues plus fecal pellets were homogenized at low speed with a Kinematica tissue homogenizer (Brinkmann). Homogenates were then serially diluted and plated onto MacConkey agar plates (selective for gram-negative organisms). Bacterial colonies were enumerated the following day. C. rodentium colonies were easily distinguished from colonies derived from commensal flora by their size and distinctive appearance (pink center with white rim) (51, 52). The validity of this approach was verified by PCR analysis for LEE genes.
Histology.
Full-thickness colonic tissues were fixed in 10% NBF. Sections (3 μm) were cut and stained with hematoxylin and eosin. Photomicrographs were taken with a Zeiss camera. Crypt heights were measured by micrometry by an observer blinded to the experimental condition, with 10 measurements being taken in the distal colon for each mouse. Only well-oriented crypts were measured.
Immunofluorescence staining.
In brief, colonic tissues were rinsed with ice-cold PBS, embedded in optimal cutting template compound (Sakura Finetech), frozen with isopentane (Sigma) and liquid N2, and stored at −70°C. Serial sections were cut at a thickness of 6 μm and fixed in ice-cold acetone for 10 min. Tissues were blocked with 1% bovine serum albumin. For detection of C. rodentium, rabbit polyclonal antisera against E. coli lipopolysaccharide (LPS; E. coli Poly 8 [Biotec Laboratories, England]) was used. For immunofluorescence labeling, Alexa568-conjugated goat anti-rabbit was used as the secondary antibody. F-actin was stained with Alexa488-conjugated phalloidin. In addition, 4′,6′-diamidino-2-phenylindole (DAPI) (1 μg/ml) (Sigma) was used to stain host cell DNA. Coverslips were mounted in Mowiol (Aldrich) and viewed at 350, 488, and 594 nm on a Zeiss Axiophot epifluoresence microscope.
RNA extraction and semiquantitative RT-PCR.
Immediately following dissection, colonic tissues were transferred to TRIzol reagent (Gibco BRL), frozen in liquid N2, and stored at −70°C until required. Prior to mRNA extraction, tissues were pooled from three mice for each group. RNA was purified according to the manufacturer's instructions. Total RNA was treated with DNase I (Ambion) to remove any contaminating DNA. DNase I was removed with DNase inactivation reagent (Ambion) according to the manufacturer's instructions. cDNA was synthesized with Superscript II reverse transcriptase (RT; Gibco BRL) by using 3 μg of DNase-treated RNA and the oligo(dT) primer LK030 [5′-CGAGCTGCGTCGACAGGC (T)17 −3′]. A 1/40 aliquot of the cDNA reaction mixture was subject to PCR analysis according to the manufacturer's instructions for AmpliTaq Gold DNA polymerase (Perkin-Elmer). For IFN-γ, the oligonucleotides mIFN-2F (5′-CACGGCACAGTCATTGAAAG-3′) and mIFN-2R (5′-GACCTGTGGGTTGTTGACCT-3′) were used with cycling conditions of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min, for a total of 35 cycles. As a control for a constitutively expressed gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was amplified with the oligonucleotides CR127 (5′-AGAACATCATCCCTGCATCC-3′) and CR128 (5′-CTGGGATGGAAATTGTGAGG-3′) with the following cycling conditions: 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min, for a total of 22 cycles. PCR products were separated by agarose gel electrophoresis.
Data presentation and statistical analysis.
Crypt height and colonic weight results are expressed as the mean value ± 1 standard error of three independent experiments, while survival, body weight, and bacterial count data are presented as the mean value ± 1 standard error from one representative infection (out of three independent experiments). Statistical significance was calculated with the nonparametric Mann-Whitney test. Multiple comparisons were performed with the Neuman-Keuls multiple comparison test. P < 0.05 was considered significant.
RESULTS
Macroscopic appearance and body weight loss during C. rodentium infection.
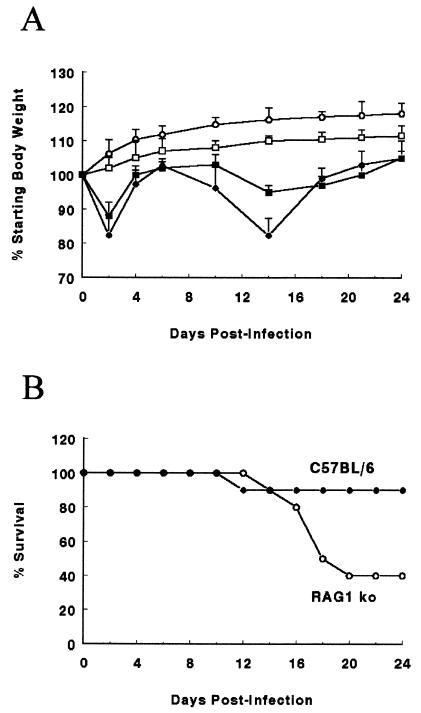
To assess the systemic effects of C. rodentium infection and the resulting host response, changes in body weight (Fig. 1A) and macroscopic appearance of the infected mice were assessed. While uninfected mice of both strains steadily gained weight, infection of immunocompetent wild-type mice and RAG1 KO mice led to a similar early drop in body weight in both strains by day 2 p.i., likely attributable to the acute effects of LPS from the inoculated bacteria (Fig. 1A). By day 4 p.i., body weight had rebounded and it slowly increased in both mouse strains over the next several days. By day 10, body weight again fell in the wild-type mice. Weight loss at these later stages of infection (days 10 to 14) was significant, with wild-type mice losing as much as 20% of their original weight (Fig. 1A). This phase of the infection was accompanied by changes in the behavior and appearance of infected mice, including reduced activity, ruffled fur, loose stool, and perianal fecal staining. A drop in weight also occurred in the RAG1 KO mice, but began later (day 14 p.i.) and was less severe than that seen in the wild-type group, suggesting that the acquired immune response is at least partially responsible for this later phase of weight loss (Fig. 1A).
FIG. 1.
(A) Both wild-type (solid circles) and RAG1 KO mice (solid squares) lose body weight during the first 24 days of a C. rodentium infection, compared to uninfected wild-type (open circles) and RAG1 KO (open squares) mice. Weight data from one representative experiment out of three are shown. Error bars represent standard errors. Each data point represents average weight data pooled from 10 mice and is expressed as the percentage of the initial body weight. Infected wild-type mice weighed significantly less than uninfected mice from day 2 until day 24 p.i. Infected RAG1 KO mice weighed less than uninfected mice on day 2 and days 14 to 21 p.i. ∗, P < 0.05. (B) Survival of wild-type mice (solid circles) is greater than that of RAG1 KO mice (open circles) over the first 24 days of a C. rodentium infection. Survival data from one representative experiment out of three are shown. Each data point represents the percentage of surviving mice from an initial population of 10 mice.
RAG1 KO mice suffer greater mortality during C. rodentium infection.
We also assessed the severity of the infection by plotting the survival of infected mice (Fig. 1B). Despite the relatively large weight loss seen in the wild-type mice, infection-induced mortality was rare (0 to 20%) in these mice, occurring between days 10 and 14 p.i. if at all (Fig. 1B). However mortality was much higher in the RAG1 KO mice, ranging between 40 and 70%, and occurred later during the infection, predominantly between days 14 and 18 p.i. Following day 18, the surviving wild-type and RAG1 KO mice began to regain weight, and both their body weight and appearance continued to improve until the end of the experiment (day 24 p.i.). Thus, the presence of T and/or B lymphocytes significantly reduce mortality during infection.
Colonic pathology: macroscopic and histologic changes in the colon during infection.
During the course of the infection, mice of both strains were killed, and their colons were examined for any alterations in macroscopic and microscopic appearance caused by the infection. As well, changes in colonic weight were assessed as an indirect measure of epithelial hyperplasia, mucosal inflammation, and hyperemia (common features of C. rodentium infection). The observations outlined below focus only on those features considered of relevance to this particular study. More detailed descriptions of the colonic pathology caused by C. rodentium infection have been published elsewhere (7, 29, 39).
Acute histologic changes are similar in infected wild-type and RAG1 KO mice.
There was little change in the appearance or weight of colonic tissues over the first 6 days of infection in either wild-type or RAG1 KO mice. No significant increases in colonic crypt heights or signs of inflammation were observed, despite the frequent presence of bacteria adhering to epithelial cells at the apical surface of crypts at this time point. Signs of infection were more evident by day 10 p.i. While overt diarrhea is not frequently seen during C. rodentium infection, infected colons were filled with large amounts of fluid and contained few stool pellets. In addition, colonic tissues from both strains showed evidence of macroscopic thickening (not shown). At day 10 p.i., the average colon weight had increased by almost 60% in the RAG1 KO mice and over 120% in the wild-type mice (Table 1). The larger increase in tissue weight in the wild-type mice was probably attributable to the greater edematous response seen in this strain. Large numbers of bacteria were also seen in close association with enterocytes at the apical surface of crypts. C. rodentium was also seen at the base of some crypts; however, this population was found in large aggregates with few bacteria observed in close association with host cells.
TABLE 1.
Colon weights of the mice in this studya
| Strain | Mean colon wt (mg) at:
|
||||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 6 | Day 10 | Day 14 | Day 18 | Day 21 | Day 24 | |
| Wild type | 101 ± 5 | 126 ± 5 | 233 ± 9b | 229 ± 6b | 175 ± 4b | 159 ± 4b | 155 ± 3b |
| RAG1 KO | 97 ± 4 | 119 ± 5 | 151 ± 4bc | 171 ± 5bc | 130 ± 3bc | 125 ± 4bc | 123 ± 3bc |
C. rodentium infection results in greater colon weights in wild-type than RAG1 KO mice. Values represent the mean colon weight ± 1 standard error of three independent experiments, each with groups of four to five mice per time point.
Significantly increased colon weight compared to uninfected mice of the same strain (P < 0.05).
Significantly lower colon weight compared to wild-type mice at the same time point p.i. (P < 0.05).
Inflammatory and immune cell infiltrate differ between wild-type and RAG1 KO mice.
Colonic inflammation was also a significant feature in both strains by day 10 p.i. and thereafter; however, the compositions of the infiltrating cells were quite different in wild-type and RAG1 KO mice. In wild-type mice, the colonic mucosa was infiltrated by large numbers of lymphocytes, other mononuclear cells, and granulocytes (Fig. 2E). In contrast, the infiltrate in the RAG1 KO mice was predominantly granulocytic (Fig. 2F). Infection also led to the formation of large mucosal lymphoid follicles in the colons of wild-type mice (Fig. 2H). Similar to the Peyer's patches of the small bowel, the colon also contains organized lymphoid follicles; however, they are normally quite small. The unusually large lymphoid tissues seen in the colon during C. rodentium infection (Fig. 2H) are probably enlarged or hypertrophic colonic patches (15) and were not seen in the colons of RAG1 KO mice.
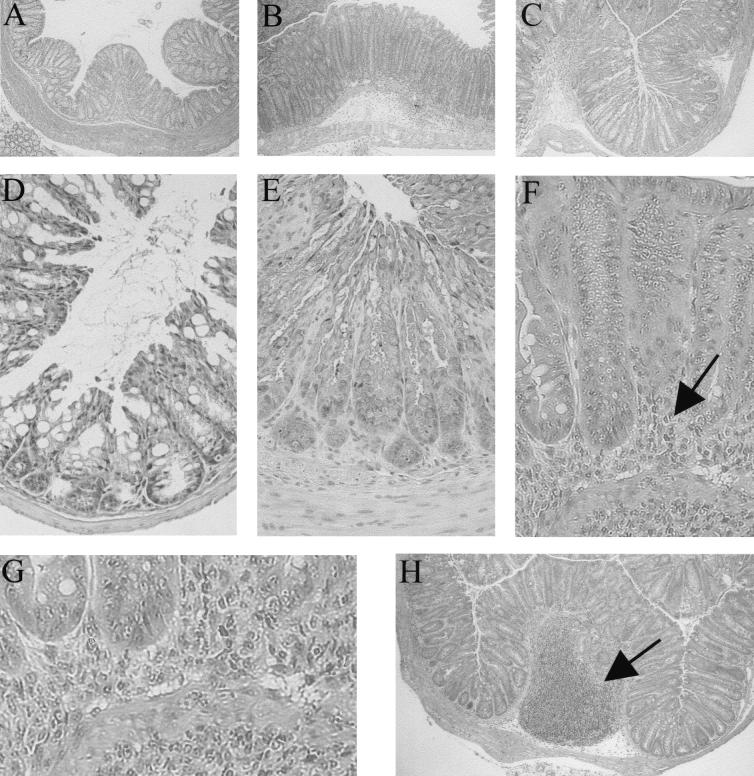
FIG. 2.
Histology showing the colonic crypt hyperplasia and inflammation that occur during the course of C. rodentium infection in both wild-type and RAG1 KO mice. Control colonic tissue taken from a wild-type mouse (A), showing the normal appearance of the colon and basal crypt heights. Uninfected RAG1 KO tissues were similar in size and appearance. By day 10, lengthening of the crypts and significant infiltration of inflammatory cells were observed in both wild-type (B) and RAG1 KO (C) mice. The original magnification for panels A to C was ×40. Under higher magnification, (originally ×200), the dramatic increase in crypt heights and crypt cellularity can be more clearly seen when comparing an uninfected wild-type colon (D) to tissues taken from day 10 p.i. wild-type (E) and RAG1 KO (F) mice. Note the massive inflammatory infiltrate in the RAG1 KO tissue (arrows). The infiltrate was comprised predominantly of neutrophils, as seen by enlarging the submucosal region (G). A common observation during infection of wild-type mice was the presence of enlarged or hypertrophic colonic patches as shown in panel H (original magnification, ×40).
Colonic pathology is attenuated in RAG1 KO at later time points of infection.
At the early stages of the infection (day 10 p.i.), both wild-type and RAG1 KO mice showed similar degrees of mucosal hyperplasia, with crypt heights increasing from 150 μm to 250 μm (Fig. 3). Similarly the host inflammatory response and increased mucosal hyperplasia continued in both mouse strains on day 14 p.i. Crypt heights increased to levels twice that of uninfected controls (Fig. 3), while bacterial attachment to colonocytes remained evident in the colons of both mouse strains (not shown). While epithelial cells that had been sloughed from the mucosa into the lumen were rarely seen in uninfected tissues, numerous extruded epithelial cells, many still with bacteria attached, were seen within the colonic lumen of both wild-type and RAG1 KO mice during infection.
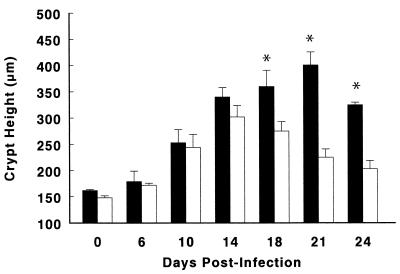
FIG. 3.
Both wild-type (solid bars) and RAG1 KO (open bars) mice undergo significant increases in colonic crypt heights over the first 24 days of a C. rodentium infection. However, the mean crypt heights (in micrometers) in the colons of wild-type mice were significantly greater than those in RAG1 KO mice on days 18, 21, and 24 p.i., at the later stages of infection. The data represent the mean of three independent experiments in which each group contained five mice. ∗, P < 0.05. Error bars represent standard errors.
Despite the similarity in the degree of crypt hyperplasia and inflammation seen in the early phase of the infection, the responses in the two mouse strains began to diverge after day 14. While the colons of wild-type mice at days 18 and 21 p.i. were still thickened and contained little, if any, stool, tissues removed from RAG1 KO mice had almost returned to their preinfected state, showing little evidence of thickening and stool pellets that appeared almost normal (not shown). These observations were supported by tissue weight and crypt height measurements, with wild-type tissues weighing more (Table 1) and having significantly longer crypts than those removed from RAG1 KO mice from day 18 p.i. onwards (Fig. 3).
Histologically, inflammation was still much in evidence in the mucosa of wild-type mice, despite a reduced bacterial presence at both the tips and at the base of crypts. In contrast, few inflammatory cells or signs of inflammatory damage could be found in the colons of RAG1 KO mice, even though numerous bacteria could still be found attached to mature enterocytes (not shown). These observations remained valid for the RAG1 KO mice until the completion of the experiment. As for wild-type mice, although their colons were still thicker than before infection, they had regained much of their normal appearance by day 24 p.i. At this time, the excessive fluid found in the colonic lumen had disappeared, while stool pellets had regained their normal consistency and appearance. While the elongated crypt heights as well as the massive inflammatory response had receded by day 24 p.i., there still remained evidence of mild inflammation, and histologically the mucosal tissues were still greatly enlarged in size compared to uninfected control tissues.
IFN-γ expression in the infected colon increases only in wild-type mice.
Previous studies have suggested that IFN-γ is critical for the development of epithelial hyperplasia in response to C. rodentium (28). Not only was it found to be highly expressed in the colon during infection, but IFN-γ receptor KO mice did not develop mucosal hyperplasia (28, 29). Additional studies have identified the importance of IFN-γ in inducing colonic hyperplasia during infection by other pathogens (4). Our observation of crypt heights increasing only transiently in RAG1 KO mice raised the question of whether IFN-γ was expressed in the colons of RAG1 KO mice during infection and how its expression compared to that in wild-type mice. Because RAG1 KO mice lack T lymphocytes, the only other well-described source of IFN-γ in RAG1 KO mice is natural killer (NK) cells (4, 6, 33, 56).
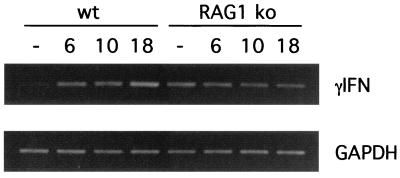
As assessed by RT-PCR, very little IFN-γ was expressed in the colons of uninfected wild-type mice (Fig. 4). Infection resulted in increased expression on days 6 (6-fold) and 10 (9-fold) p.i., and expression continued to increase at day 18 p.i. until levels were more than 15-fold higher than before infection, as assessed by densitometric analysis. Interestingly, increased IFN-γ expression mirrored the same time course as the crypt hyperplasia seen in wild-type mice. In contrast, although colonic tissues from uninfected RAG1 KO mice had much higher expression of IFN-γ mRNA than was found in wild-type mice, mRNA transcript levels for IFN-γ in infected RAG1 KO mice actually decreased below preinfected levels. Densitometry analysis revealed that IFN-γ mRNA expression decreased to 61% of uninfected levels at day 6, 37% at day 10, and only 30% at day 18 p.i.
FIG. 4.
Colonic tissues taken from wild-type (wt) and RAG1 KO mice express IFN-γ mRNA during C. rodentium infection, but only wild-type mice showed increased expression during infection. RT-PCR for IFN-γ shows expression significantly increases in the tissues of wild-type mice, from undetectable levels in uninfected mice to a progressive elevation in expression seen on days 6, 10, and 18 p.i. In contrast, as the infection progressed, IFN-γ mRNA expression progressively decreased in the colons of RAG1 KO mice at each of days 6, 10, and 18 p.i. GAPDH was used as the housekeeping gene control.
Bacterial clearance occurs in wild-type but not RAG1 KO mice.
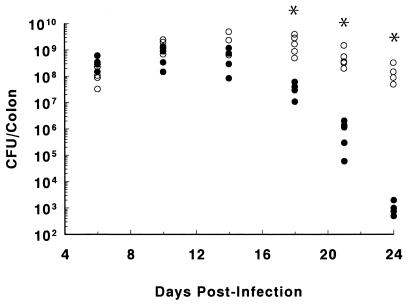
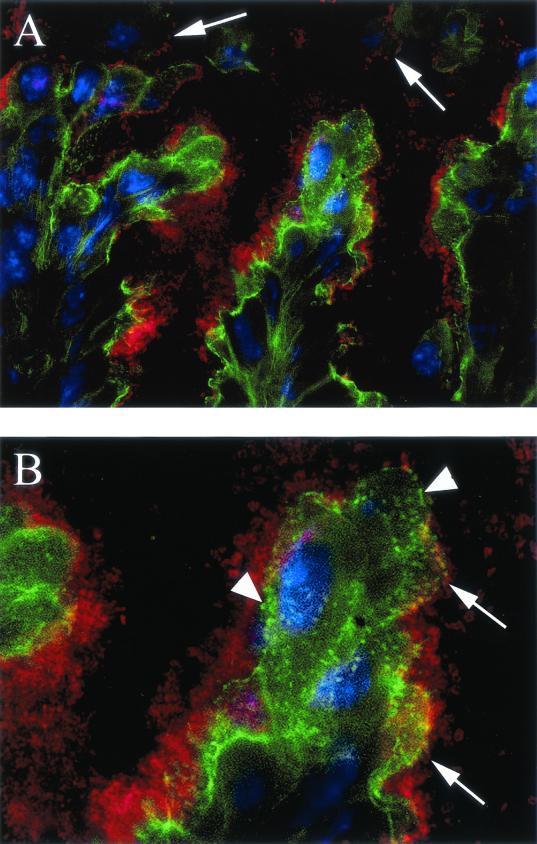
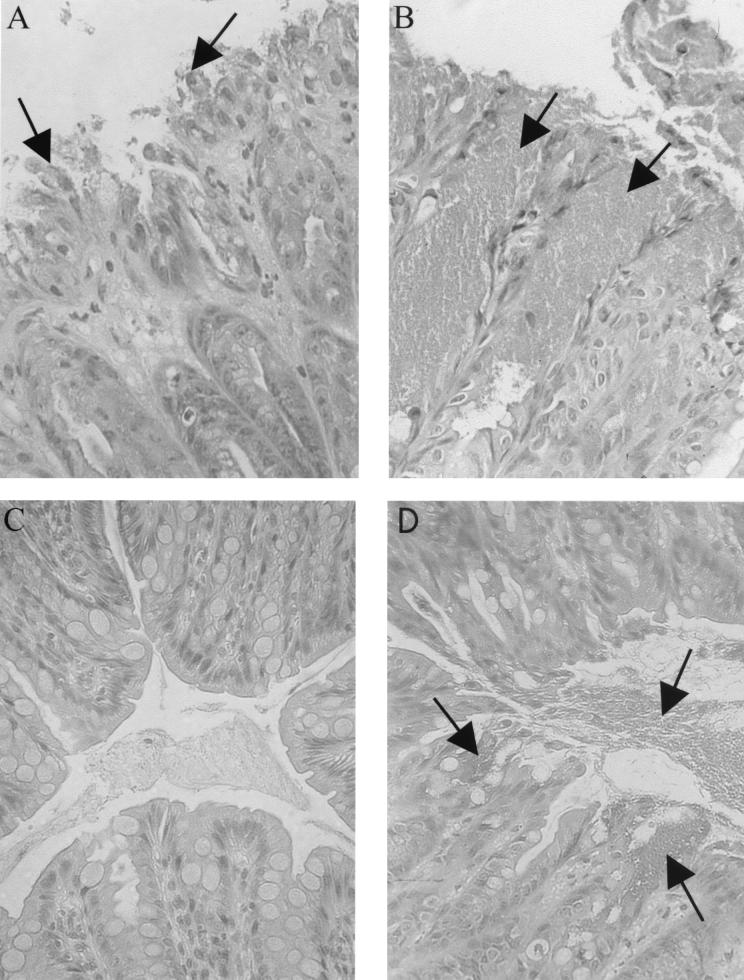
While histological evidence pointed to marked differences in the ability of wild-type and RAG1 KO mice to clear C. rodentium from the large bowel, such observations were qualitative only. We therefore compared the courses of bacterial colonization and clearance in wild-type and RAG1 KO mice by homogenizing whole colons and plating the homogenate on selective media in serial dilutions. While only a few thousand bacteria were identified by day 4 p.i. (not shown), by day 6, the number of bacteria had dramatically increased (Fig. 5), with approximately similar numbers recovered from the two mouse strains. Bacterial numbers peaked in both strains at day 10 p.i. (mean ± standard error of 7.9 × 108 ± 2.4 × 108 in wild-type mice and 1.5 × 109 ± 0.3 × 109 in RAG1 KO mice). By day 18 p.i., the number of C. rodentium cells had begun to drop in the wild-type mice and continued to fall thereafter. By day 21 p.i., bacterial levels were almost 1,000-fold lower than at day 10 p.i. By day 24, bacterial numbers in the colons of wild-type mice had decreased to less than a few thousand viable bacteria. The identities of several colonies isolated from day 24 p.i. wild-type mice were confirmed as C. rodentium by PCR (not shown). Surprisingly, a small population (few hundred to a few thousand) of C. rodentium was cultured from the colons of wild-type mice until at least day 60 p.i. (W. Deng, unpublished observation). Despite the dramatic clearance of C. rodentium from wild-type mice after day 14 p.i., there was no significant decrease in bacterial numbers recovered from RAG1 KO mice over the same time frame. By day 24 p.i., RAG1 KO mice remained heavily colonized at levels ranging between 1.5 × 107 and 9 × 108 CFU. To confirm the prolonged colonization in the RAG1 KO mice, we examined tissues from infected mice by immunofluorescence and normal-light microscopy under high magnification. By immunofluorescence, we were able to detect numerous bacteria that stained positively as C. rodentium coating the surface of the colonic epithelium (Fig 6A) at day 10 p.i. At higher magnification, we were also able to detect the presence of F-actin-rich projections (putatively identified as pedestals) beneath many of the bacteria (Fig. 6B). The presence of numerous bacteria on the mucosal surface and within the lumen of wild-type (Fig. 7A) and RAG1 KO mouse tissues at day 10 p.i. (Fig. 7B) was also confirmed by light microscopy. However, by day 24 p.i., no adherent bacteria were evident in the colons of wild-type mice (Fig. 7C). In contrast, numerous C. rodentium organisms were still seen attached to tissues taken from RAG1 KO mice at day 24 p.i. (Fig. 7D).
FIG. 5.
Following oral infection, both wild-type and RAG1 KO mice were heavily colonized by C. rodentium. The numbers of bacteria recovered from the colons of individual infected wild-type mice (solid circles) and RAG1 KO mice (open circles) are presented on days 6, 10, 14, 18, 21, and 24 p.i. Each group contained four to five mice. The asterisk denotes the recovery of significantly more bacteria (mean value) recovered from RAG1 KO mice than from wild-type mice at days 18, 21, and 24 p.i. ∗, P < 0.05.
FIG. 6.
Immunofluorescence staining showing the presence of C. rodentium on the lumenal surface of the colons of wild-type mice (day 10 p.i.). Staining for C. rodentium LPS is seen in red, and host cell F-actin is seen in green, while host cell nuclei are blue. In panel A, the colonic lumen is at the top, and the mucosa is at the bottom (original magnification, ×630). Note the jagged appearance of the intestinal epithelium with projections jutting into the lumen, a common feature of the colonic hyperplasia seen during C. rodentium infection. Adherent bacteria (red) are seen coating the surface of most epithelial cells. In addition, several epithelial cells, some with bacteria still adhering (see arrows) are seen being sloughed into the colonic lumen. Note that the F-actin staining is reduced on the sloughed cells. An enlarged view of the infected epithelium is seen in panel B. Note the numerous bacteria covering the epithelial surface. While many appear adherent (arrows), others appear to form a thick coating on the epithelial surface. As well, F-actin rearrangements seen as areas of intense green (arrowheads) are found across the surface of the infected cells. Where the bacteria and the rearrangements are on the same plane, the rearrangements are found underneath many of the adhering bacteria. As such, we believe these to be A/E lesions.
FIG. 7.
Histology showing the presence of C. rodentium on the lumenal surface of the colons of both wild-type and RAG1 KO mice during infection. Note the ragged appearance of the epithelial surface and the epithelial cells sloughing into the lumen on day 10 p.i. in a wild-type mouse (A). Many of the cells being sloughed are still covered with adherent bacteria (see arrows). While similar observations were made in RAG1 KO tissues (B), the crypts in RAG1 KO tissues were almost always filled with bacteria (arrows). By day 24 p.i., wild-type mice had cleared the infection, with no C. rodentium found on the superficial surface of the colon (C), while at the same time point, RAG1 KO mice remained heavily infected with large numbers of C. rodentium cells coating the mucosal surface and in the colonic lumen (arrows) (D) Magnification for panels A to D, ×400.
Immune cell reconstitution restores colonic pathology during infection.
To better characterize the role of the host immune system during C. rodentium infection, we reconstituted the immune system of RAG1 KO mice by using splenic nonadherent mononuclear cells. Splenic cell reconstitution is known to restore many but not all T- and B-cell populations (60). Due to time constraints, instead of testing the effects of full reconstitution of the murine immune system, because that can take up to 6 months, we decided to look at reconstitution after 1 month. Immune cell reconstitution was confirmed by immunostaining the spleens of the recipient RAG1 KO mice for T cells (anti-CD3) and B cells (anti-B220). Both B and T cells were found to be present in the spleens of reconstituted mice, but at roughly half the numbers in wild-type mice (not shown).
Preliminary infection studies found that while reconstituted RAG1 KO mice survived a few days longer than unreconstituted RAG1 KO mice, they were unable to fully clear the infection, and most eventually died before day 21 p.i. We therefore chose to compare the reconstituted mice (n = 7) with age-matched wild-type (n = 8) and unreconstituted RAG1 KO (n = 6) mice at day 18 p.i. At this point, pathology was greater in the reconstituted mice, with colon weights (236 ± 25 mg) significantly (P < 0.05) increased over that seen in unreconstituted RAG1 KO mice (140 ± 33 mg) and similar to that seen in wild-type C57BL/6 mice (212 ± 16 mg). A similar increase in crypt heights was seen in the reconstituted mice (340 ± 41 μm) compared to unreconstituted RAG1 KO (265 ± 35 μm) and wild-type (315 ±49 μm) mice. In contrast to the substantial effect of immune reconstitution on tissue pathology, the effect on bacterial clearance was less dramatic. While unreconstituted RAG1 KO mice had mean bacterial colony counts of 1.1 × 109 ± 0.2 × 109 CFU by day 18 p.i., C57BL/6 mice had only 3.4 × 106 ± 0.9 × 106 CFU. At the same time, the number of bacteria in the reconstituted RAG1 KO mice was significantly (P < 0.05) reduced to 2.9 × 108 ± 0.9 × 108 CFU (ranging from 4.5 × 106 to 6.4 × 108 CFU), but not to the levels seen in immunocompetent wild-type mice.
DISCUSSION
Because it is the only bacterial pathogen that causes A/E lesions in laboratory mice, C. rodentium has been exploited for almost a decade as a model for the in vivo pathogenesis of EPEC and EHEC (7, 8, 21, 29, 51). Like these classically noninvasive human pathogens, C. rodentium attachment leads to the formation of characteristic actin-rich pedestals on infected cells (27, 39, 49). While it is clear that A/E lesions are a pathological feature associated with all three bacteria, due to ethical concerns, there are few studies examining other forms of mucosal injury in patients infected with either EPEC or EHEC. There have been occasional reports examining the intestinal injury that occurs in children suffering from chronic diarrhea caused by EPEC infection (18, 19, 30). These studies support the observations made with animal models (29, 55) that infection provokes an intense inflammatory response by the host, leading to histological changes in the bowel. During C. rodentium infection, the most obvious pathological change is the massive crypt hyperplasia that results from increased proliferation of crypt stem cells (7, 39). Interestingly, the majority of the biopsies taken from pediatric patients infected with EPEC showed evidence of crypt hyperplasia (18, 19, 30, 32).
In recent years, C. rodentium and other animal models of EPEC infection, including the rabbit pathogens REPEC and RDEC-1, have also proven useful in evaluating the roles of secreted EPEC proteins including EspA, EspB, and Tir in the disease process (1, 41, 49). In contrast, elucidation of the host side of these host-pathogen interactions has proven more difficult, requiring an animal model possessing both well-characterized genetics and a variety of immunological tools available for study. With these requirements in mind, the mouse model of C. rodentium infection has many advantages over other A/E pathogen models. Higgins et al. recently demonstrated that the mucosal hyperplasia that develops in response to C. rodentium was dependent on the host immune response, since it did not occur in mice lacking the receptor for IFN-γ (28). While IFN-γ signaling was found to be essential, the cell types mediating the crypt hyperplasia were not determined. This has relevance not only to this model, but also to models of spontaneous colitis in which similar pathologies are seen, but the mechanisms underlying these events are still poorly understood. In addition, the question of what cell types are required to clear a C. rodentium infection from its infected host has not been addressed.
In the present study, we have characterized the course and immune dependence of the crypt hyperplasia and bacterial clearance that occur in the colons of young mice infected with the A/E bacterial pathogen C. rodentium. We first demonstrate that infection of wild-type mice leads to a sustained and significant inflammatory response and increases in colonic crypt heights. While acute colitis and crypt hyperplasia were initially also seen in RAG1 KO mice, the responses in wild-type and RAG1 KO mice diverged after the first 2 weeks p.i., with crypt heights in the immunodeficient mice returning to almost preinfected levels at later stages of infection. Studies examining a variety of bacterial pathogens have found that the impact of T and B lymphocytes in host defense tends to increase as these bacterial infections progress (6, 56). Thus, the gradual increase in tissue pathology and bacterial clearance seen in wild-type mice was consistent with the activation of acquired immunity during infection. However, the transient nature of the host response to C. rodentium infection seen in the RAG1 KO mice was surprising, because infection of RAG1 KO mice by other bacterial pathogens usually leads to prolonged inflammatory responses (31, 56, 61). Whether the attenuation of the host response in the RAG1 KO mice is host or bacterial driven is uncertain. However, it is clear from this study that while innate immunity can mediate acute responses, T and/or B lymphocytes mediate most of the tissue pathology and inflammation, particularly in the later stages of the C. rodentium infection. This was confirmed by the ability of T- and B-lymphocyte reconstitution to increase the crypt hyperplasia and colon weights of RAG1 KO mice to wild-type levels. Second, although infection led to colitis in both wild-type and RAG1 KO mice, significant increases in colonic IFN-γ expression levels were detected only in infected wild-type mice. Thus despite a temporal correlation between crypt hyperplasia and increased IFN-γ during infection of wild-type mice in this and previous studies (29), the demonstration of crypt hyperplasia in RAG1 KO mice in the absence of increased IFN-γ levels suggests a more complex regulation of crypt hyperplasia than previously thought. This has important implications in understanding the colonic hyperplasia seen in this model as well as in other models of murine colitis. Finally, we demonstrate that although wild-type mice cleared the infection in approximately 3 weeks, RAG1 KO mice were unable to do so. As a result, C. rodentium infection in RAG1 KO mice was often lethal, and those mice that survived the infection remained heavily infected. The inability of splenic T- and B-cell reconstituted RAG1 KO mice to fully clear the infection likely indicates a role for immune cell populations that are not restored by this protocol. These include B-cell populations in the peritoneal cavity (60) as well as some T-cell populations. Perhaps not surprisingly, these results indicate that it is easier for the immune system to cause pathology than it is to clear an infection.
Infection led to a significant inflammatory response in both mouse strains, but the makeup of the inflammatory infiltrate differed between immunocompetent and immunodeficient mouse strains. While wild-type mice responded with lymphocytes and other mononuclear cells, as well as granulocytic neutrophils, RAG1 KO mice responded primarily with a granulocytic infiltration of the colonic mucosa. Interestingly, while infection led to changes in IFN-γ transcription levels in both mouse strains, the changes were in opposite directions. Similar to studies by Higgins et al. (29), IFN-γ mRNA in the wild-type mice increased many fold from preinfected levels that were barely detectable with the PCR cycling conditions employed here. In contrast, despite having higher basal levels of expression, IFN-γ mRNA levels in the RAG1 KO mice dropped below uninfected levels during infection. In the absence of T lymphocytes, the source of the IFN-γ in the colonic mucosa of the RAG1 KO mice can almost certainly be attributed to NK cells (6, 33, 56). The exaggerated IFN-γ expression found in uninfected RAG1 KO mice was not totally unexpected, because NK cells and the innate immune system in general are often more activated in immunodeficient mice than in wild-type strains (10). However, the basis for the decrease in IFN-γ levels seen in infected RAG1 KO mice is unclear. Such a reduction in expression may reflect the presence of fewer NK cells in the mucosa at the later stages of infection. Unfortunately, despite the numerous T cells that are seen in the mucosa during infection of wild-type mice 29; data not shown), there are no specific markers for NK cells that would unequivocally identify their presence or change in numbers in these tissue sections.
These primitive lymphocytes have also been previously linked to crypt hyperplasia and IFN-γ production in other infection models (4). In these studies, IFN-γ expression increased in concert with elevated crypt heights, even in immunodeficient mice lacking acquired immunity. Furthermore, depletion of IFN-γ prevented crypt hyperplasia (4), implying a possible direct connection between IFN-γ expression and epithelial cell proliferation. These results are obviously different from ours. While the mechanisms involved may be model specific, in our studies, despite a decrease in IFN-γ expression, we still saw significant crypt hyperplasia in RAG1 KO mice. Taken together with the observation that IFN-γ treatment of epithelial cells in culture typically inhibits cell proliferation (50), these results suggest that any requirement for IFN-γ in crypt hyperplasia is probably indirect. Most likely, the actions of IFN-γ in inducing crypt hyperplasia involve the activation of macrophages and stromal cells, increasing the production of growth factors that directly induce crypt cell proliferation (29). Since interleukin-12 has been shown to be important in inducing the production of IFN-γ, future studies should address the expression of this cytokine as well.
While colonic hyperplasia is the hallmark of C. rodentium infection, both the underlying mechanisms and the benefits to the host of this response are unclear. Normally, the number of epithelial cells found in colonic crypts is tightly regulated. Undifferentiated colonocytes develop from stem cells at the base of the crypts, differentiating into mature epithelial cells as they migrate up the length of the crypt over 4 to 5 days (54), until they are ultimately shed into the lumen. With each cell that is sloughed, a new epithelial cell is generated at the base of the crypt, ensuring that crypt heights remain constant. This homeostatic mechanism is disrupted during C. rodentium infection. As previous studies have shown, the resulting increases in crypt heights are the by-product of increased crypt cell proliferation (7, 39). Aside from increased crypt heights, the increased proliferation results in more rapid sloughing of epithelial cells into the lumen. Histological and immunofluorescence results from this study show large numbers of epithelial cells being sloughed into the lumen, many with bacteria still attached. This end result may be of value in host resistance. Interestingly, a similar exfoliation of infected epithelial cells has been described in the bladders of mice following infection by uropathogenic E. coli (47), while increased crypt cell proliferation is a well-described feature of infection by the large bowel parasite Trichuris muris (4, 5, 26). Despite the similarities of these responses against such diverse pathogens, the relative importance of epithelial cell sloughing to the eventual clearance of C. rodentium infection is uncertain. As seen by immunofluoresence staining and light microscopy, many of the C. rodentium cells are not actually attached to the colonic epithelium, but instead are found in the lumen of the crypts. These bacteria would not be affected by epithelial cell sloughing and are probably responsible for the infection of newly arisen epithelial cells as these immature epithelial cells migrate up the length of the crypt.
Although crypt hyperplasia and the sloughing of infected cells may contribute to bacterial clearance, it is unlikely that they represent the primary mechanisms used by the host to clear A/E bacterial pathogens from the gastrointestinal tract. The inability of RAG1 KO mice to clear the infection implicates the acquired immune system in this role. Probably both cellular and humoral immunity are involved in clearance of C. rodentium from the host, based on human studies showing that EPEC and EHEC infections generate strong cellular as well as humoral immune responses, as assessed through human volunteer studies (53) and by examining sera from convalescent patients (37). While antibody responses were not examined, Higgins et al. did demonstrate that the host immune response to C. rodentium infection is particularly intense because infected colonic tissues expressed substantial levels of a number of proinflammatory and immunomodulatory cytokines (29). While cytokine production could promote antibacterial activity by macrophages and neutrophils (6), it has also been postulated that antibodies generated against virulence factors, including Tir and intimin, could limit the ability of bacteria to attach to epithelial cells (59) or aid in phagocytosis of the bacteria, an event that EPEC actively counteracts (13, 23). It seems likely that host defense against C. rodentium infection will prove to be a complex process, involving a number of host factors activated through acquired immunity. The effectiveness of this response is demonstrated not only by the clearance of the initial infection, but also by the observation that mice that recover from the infection are strongly protected against future challenge 39; unpublished observations).
Surprisingly, despite the massive inflammatory response and the rapid clearance of bacteria from wild-type mice, a small number of C. rodentium cells were still found in the colon long after the host response and tissue pathology had ended. While it is possible that these bacteria are no longer virulent, they still possessed the LEE pathogenicity island as assessed by PCR (not shown). Alternatively, their numbers may have no longer been a sufficient stimulus to continue triggering a host response. In contrast to the wild-type mice, the prolonged bacterial colonization found in the RAG1 KO mice was of much greater magnitude. Despite an acute inflammatory response, the number of bacteria found in the RAG1 KO mice did not significantly decrease during the infection and C. rodentium attached to the mucosal surface could still be easily seen at the late stages of infection. Their inability to clear the infection resulted in substantial mortality in the RAG1 KO mice during the third week of infection; however, the cause of death is unclear. Although infected RAG1 KO mice did develop significant colonic inflammation, it appeared to be no worse than that seen in wild-type mice. In fact, despite their massive and prolonged colonization by C. rodentium, the inflammatory response gradually subsided in the small number of RAG1 KO mice that survived past day 18 p.i., such that they exhibited few overt signs of disease at these later stages of infection. Alternatively, rather than tissue pathology or inflammation being the cause of their increased mortality, bacterial translocation from the gut may be involved. Studies examining this hypothesis are currently under way.
In conclusion, this study demonstrates that C. rodentium infection results in prolonged colonic crypt hyperplasia in immunocompetent mice. This hyperplasia can be divided into two phases. The first is an acute response that also occurs in RAG1 KO mice and is thus independent of either T or B lymphocytes. The second, more prolonged component of the response is only seen in wild-type mice, indicating it involves acquired immunity. Such an acquired immune response is essential for clearance of the infection by the host. These data thus provide important clues about the cell types that are involved in the host response against C. rodentium and how their involvement changes over the course of the infection. Moreover, they emphasize the role of host factors in mediating the disease. While the ability to cause A/E lesions is undoubtedly essential for virulence, these and other studies suggest that the contributions of host-dependent factors are probably more important to the disease symptomatology observed during A/E bacterial infections. Understanding what host cells are involved in the infection, aside from the epithelial cells that the bacteria adhere to, will not only help us understand how these noninvasive pathogens cause disease, but also how the host eventually clears the infection. The potential for this model to elucidate such areas of interest is greatly increased with the recent sequencing of the C. rodentium LEE pathogenicity island (14). The ability to manipulate the genetics on both sides of the pathogen-host relationship will provide an enormous boost to improving our understanding of these infectious diseases.
Acknowledgments
We thank Valerie Templeman for technical assistance.
This work was supported by an operating grant from the Canadian Institutes for Health Research (CIHR). B.A.V. is supported by a Canadian Digestive Diseases Institute/Medical Research Council of Canada (MRC) postdoctoral fellowship and is an honorary Izaak Walton Killam fellow. W.D. was supported by an MRC postdoctoral fellowship. B.B.F. is a Howard Hughes International Research Scholar and a CIHR Distinguished Investigator.
Editor: A. D. O'Brien
REFERENCES
- 1.Abe, A., U. Heczko, R. G. Hegele, and B. B. Finlay. 1998. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J. Exp. Med. 188:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agin, T. S., J. R. Cantey, E. C. Boedeker, and M. K. Wolf. 1996. Characterization of the eaeA gene from rabbit enteropathogenic Escherichia coli strain RDEC-1 and comparison to other eaeA genes from bacteria that cause attaching-effacing lesions. FEMS Microbiol. Lett. 144:249-258. [DOI] [PubMed] [Google Scholar]
- 3.An, H., J. M. Fairbrother, C. Desautels, T. Mabrouk, D. Dugourd, H. Dezfulian, and J. Harel. 2000. Presence of the LEE (locus of enterocyte effacement) in pig attaching and effacing Escherichia coli and characterization of eae, espA, espB and espD genes of PEPEC (pig EPEC) strain 1390. Microb. Pathog. 28:291-300. [DOI] [PubMed] [Google Scholar]
- 4.Artis, D., C. S. Potten, K. J. Else, F. D. Finkelman, and R. K. Grencis. 1999. Trichuris muris: host intestinal epithelial cell hyperproliferation during chronic infection is regulated by interferon-gamma. Exp. Parasitol. 92:144-153. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft, A. J., and R. K. Grencis. 1998. Th1 and Th2 cells and immunity to intestinal helminths. Chem. Immunol. 71:192-208. [DOI] [PubMed] [Google Scholar]
- 6.Bancroft, G. J., and J. P. Kelly. 1994. Macrophage activation and innate resistance to infection in SCID mice. Immunobiology 191:424-431. [DOI] [PubMed] [Google Scholar]
- 7.Barthold, S. W., G. L. Coleman, R. O. Jacoby, E. M. Livstone, and A. M. Jonas. 1978. Transmissible murine colonic hyperplasia. Vet. Pathol. 15:223-236. [DOI] [PubMed] [Google Scholar]
- 8.Barthold, S. W., G. L. Coleman, P. N. Bhatt, G. W. Osbaldiston, and A. M. Jonas. 1976. The etiology of transmissible murine colonic hyperplasia. Lab. Anim. Sci. 26:889-894. [PubMed] [Google Scholar]
- 9.Barthold, S. W., G. W. Osbaldiston, and A. M. Jonas. 1977. Dietary, bacterial, and host genetic interactions in the pathogenesis of transmissible murine colonic hyperplasia. Lab. Anim. Sci. 27:938-945. [PubMed] [Google Scholar]
- 10.Budzynski, W., and C. Radzikowski. 1994. Cytotoxic cells in immunodeficient athymic mice. Immunopharmacol. Immunotoxicol. 16:319-346. [DOI] [PubMed] [Google Scholar]
- 11.Cantey, J. R., and R. K. Blake. 1977. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J. Infect. Dis. 135:454-462. [DOI] [PubMed] [Google Scholar]
- 12.Celli, J., W. Deng, and B. B. Finlay. 2000. Enteropathogenic Escherichia coli (EPEC) attachment to epithelial cells: exploiting the host cell cytoskeleton from the outside. Cell Microbiol. 2:1-9. [DOI] [PubMed] [Google Scholar]
- 13.Celli, J., M. Olivier, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J. 20:1245-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng, W., Y. Li, B. A. Vallance, and B. B. Finlay. 2001. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 69:6323-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohi, T., K. Fujihashi, P. D. Rennert, K. Iwatani, H. Kiyono, and J. R. McGhee. 1999. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. J. Exp Med. 189:1169-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnenberg, M. S., J. B. Kaper, and B. B. Finlay. 1997. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 5:109-114. [DOI] [PubMed] [Google Scholar]
- 17.Donnenberg, M. S., and T. S. Whittam. 2001. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J. Clin. Investig. 107:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagundes Neto, U., V. Ferreira, F. R. Patricio, V. L. Mostaco, and L. R. Trabulsi. 1989. Protracted diarrhea: the importance of the enteropathogenic E. coli (EPEC) strains and Salmonella in its genesis. J. Pediatr. Gastroenterol. Nutr. 8:207-211. [PubMed] [Google Scholar]
- 19.Fagundes Neto, U., I. Scaletsky, L. G. Schmitz, and E. Freymuller. 1995. Serotypes of classical enteropathogenic Escherichia coli that produce changes in the small bowel ultrastructure and invasion of HeLa cells. Rev. Asoc. Med. Bras. 41:318-324. [PubMed] [Google Scholar]
- 20.Finlay, B. B., S. Ruschkowski, B. Kenny, M. Stein, D. J. Reinscheid, M. A. Stein, and I. Rosenshine. 1996. Enteropathogenic E. coli exploitation of host epithelial cells. Ann. N. Y. Acad. Sci. 797:26-31. [DOI] [PubMed] [Google Scholar]
- 21.Frankel, G., A. D. Phillips, M. Novakova, H. Field, D. C. A. Candy, D. B. Schauer, G. Douce, and G. Dougan. 1996. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect. Immun. 64:5315-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 23.Goosney, D. L., J. Celli, B. Kenny, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli inhibits phagocytosis. Infect. Immun. 67:490-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goosney, D. L., M. de Grado, and B. B. Finlay. 1999. Putting E. coli on a pedestal: a unique system to study signal transduction and the actin cytoskeleton. Trends Cell Biol. 9:11-14. [DOI] [PubMed] [Google Scholar]
- 25.Goosney, D. L., S. Gruenheid, and B. B. Finlay. 2000. Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu. Rev. Cell Dev. Biol. 16:173-189. [DOI] [PubMed] [Google Scholar]
- 26.Grencis, R. K. 1993. Cytokine-mediated regulation of intestinal helminth infections: the Trichuris muris model. Ann. Trop. Med. Parasitol. 87:643-647. [DOI] [PubMed] [Google Scholar]
- 27.Hartland, E. L., V. Huter, L. M. Higgins, N. S. Goncalves, G. Dougan, A. D. Phillips, T. T. MacDonald, and G. Frankel. 2000. Expression of intimin γ from enterohemorrhagic Escherichia coli in Citrobacter rodentium. Infect. Immun. 68:4637-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins, L. M., G. Frankel, I. Connerton, N. S. Goncalves, G. Dougan, and T. T. MacDonald. 1999. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285:588-591. [DOI] [PubMed] [Google Scholar]
- 29.Higgins, L. M., G. Frankel, G. Douce, G. Dougan, and T. T. MacDonald. 1999. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 67:3031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill, S. M., A. D. Phillips, and J. A. Walker-Smith. 1991. Enteropathogenic Escherichia coli and life threatening chronic diarrhoea. Gut 32:154-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izadjoo, M. J., Y. Polotsky, M. G. Mense, A. K. Bhattacharjee, C. M. Paranavitana, T. L. Hadfield, and D. L. Hoover. 2000. Impaired control of Brucella melitensis infection in Rag1-deficient mice. Infect. Immun. 68:5314-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallas, M. R., F. R. Patricio, and U. Fagundes Neto. 1995. Morphometrics of the small intestine in children with diarrhea due to classical enteropathogenic Escherichia coli and to environmental asymptomatic enteropathy. Rev. Asoc. Med. Bras. 41:162-166. [PubMed] [Google Scholar]
- 33.Kawakami, K., Y. Koguchi, M. H. Qureshi, A. Miyazato, S. Yara, Y. Kinjo, Y. Iwakura, K. Takeda, S. Akira, M. Kurimoto, and A. Saito. 2000. IL-18 contributes to host resistance against infection with Cryptococcus neoformans in mice with defective IL-12 synthesis through induction of IFN-gamma production by NK cells. J. Immunol. 165:941-947. [DOI] [PubMed] [Google Scholar]
- 34.Kenny, B., R. DeVinney, M. Stein, D. Reinscheid, E. Frey, and B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 35.Kenny, B., L. C. Lai, B. B. Finlay, and M. S. Donnenberg. 1996. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol. Microbiol. 20:313-323. [DOI] [PubMed] [Google Scholar]
- 36.Krejany, E. O., T. H. Grant, V. Bennett-Wood, L. M. Adams, and R. M. Robins-Browne. 2000. Contribution of plasmid-encoded fimbriae and intimin to capacity of rabbit-specific enteropathogenic Escherichia coli to attach to and colonize rabbit intestine. Infect. Immun. 68:6472-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, Y., E. Frey, A. M. Mackenzie, and B. B. Finlay. 2000. Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect. Immun. 68:5090-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luperchio, S. A., J. V. Newman, C. A. Dangler, M. D. Schrenzel, D. J. Brenner, A. G. Steigerwalt, and D. B. Schauer. 2000. Citrobacter rodentium, the causative agent of transmissible murine colonic hyperplasia, exhibits clonality: synonymy of C. rodentium and mouse-pathogenic Escherichia coli. J. Clin. Microbiol. 38:4343-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luperchio, S. A., and D. B. Schauer. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3:333-340. [DOI] [PubMed] [Google Scholar]
- 40.Malstrom, C., and S. James. 1998. Inhibition of murine splenic and mucosal lymphocyte function by enteric bacterial products. Infect. Immun. 66:3120-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marches, O., J. P. Nougayrede, S. Boullier, J. Mainil, G. Charlier, I. Raymond, P. Pohl, M. Boury, J. De Rycke, A. Milon, and E. Oswald. 2000. Role of Tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect. Immun. 68:2171-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23:399-407. [DOI] [PubMed] [Google Scholar]
- 44.Milon, A., E. Oswald, and J. De Rycke. 1999. Rabbit EPEC: a model for the study of enteropathogenic Escherichia coli. Vet Res. 30:203-219. [PubMed] [Google Scholar]
- 45.Mombaerts, P. 1995. Lymphocyte development and function in T-cell receptor and RAG-1 mutant mice. Int. Rev. Immunol. 13:43-63. [DOI] [PubMed] [Google Scholar]
- 46.Mombaerts, P., J. Iacomini, R. S. Johnson, K. Herrup, S. Tonegawa, and V. E. Papaioannou. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869-877. [DOI] [PubMed] [Google Scholar]
- 47.Mulvey, M. A., J. D. Schilling, J. J. Martinez, and S. J. Hultgren. 2000. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. USA 97:8829-8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newman, J. V., B. A. Zabel, S. S. Jha, and D. B. Schauer. 1999. Citrobacter rodentium espB is necessary for signal transduction and for infection of laboratory mice. Infect. Immun. 67:6019-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruemmele, F. M., C. Gurbindo, A. M. Mansour, R. Marchand, E. Levy, and E. G. Seidman. 1998. Effects of interferon gamma on growth, apoptosis, and MHC class II expression of immature rat intestinal crypt (IEC-6) cells. J. Cell Physiol. 176:120-126. [DOI] [PubMed] [Google Scholar]
- 51.Schauer, D. B. 1994. Murine colonic hyperplasia, p. 197-208. In V. L. Miller, J. B. Kaper, D. A. Portnoy, and R. R. Isberg (ed.), Molecular genetics of bacterial pathogenesis. American Society for Microbiology, Washington, D.C.
- 52.Schauer, D. B., and S. Falkow. 1993. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect. Immun. 61:4654-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tacket, C. O., M. B. Sztein, G. Losonsky, A. Abe, B. B. Finlay, B. P. McNamara, G. T. Fantry, S. P. James, J. P. Nataro, M. M. Levine, and M. S. Donnenberg. 2000. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect. Immun. 68:3689-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsubouchi, S. 1981. Kinetic analysis of epithelial cell migration in the mouse descending colon. Am. J. Anat. 161:239-246. [DOI] [PubMed] [Google Scholar]
- 55.Tzipori, S., R. Gibson, and J. Montanaro. 1989. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infect. Immun. 57:1142-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Unanue, E. R. 1997. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 158:11-25. [DOI] [PubMed] [Google Scholar]
- 57.Vallance, B. A., K. Croitoru, and S. M. Collins. 1998. T lymphocyte-dependent and -independent intestinal smooth muscle dysfunction in the T. spiralis-infected mouse. Am. J. Physiol. 275:G1157-G1165. [DOI] [PubMed] [Google Scholar]
- 58.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wizemann, T. M., J. E. Adamou, and S. Langermann. 1999. Adhesins as targets for vaccine development. Emerg. Infect. Dis. 5:395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang, Y. G., E. deGoma, R. Barth, J. J. Sergio, and M. Sykes. 1998. B-cell reconstitution and xenoreactive anti-pig natural antibody production in severe combined immunodeficient mice reconstituted with immunocompetent B cells from varying sources. Transplantation 66:89-95. [DOI] [PubMed] [Google Scholar]
- 61.Young, V. B., C. A. Dangler, J. G. Fox, and D. B. Schauer. 2000. Chronic atrophic gastritis in SCID mice experimentally infected with Campylobacter fetus. Infect. Immun. 68:2110-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]