Abstract
Dendritic cells ignite adaptive immunity by priming naïve T lymphocytes. Human monocyte-derived dendritic cells (MDDCs) infected with Toxoplasma gondii induce T-lymphocyte gamma interferon production and may thus activate T. gondii-specific immunity. However, we now demonstrate that T. gondii-infected MDDCs are poor at activating T lymphocytes and are unable to induce specific cytotoxic T lymphocytes. On the other hand, MDDCs acquiring nonviable T. gondii antigens directly, or indirectly through captured apoptotic or necrotic cell bodies, induce potent T-lymphocyte activation. T lymphocytes exposed to infected MDDCs are significantly impaired in upregulation of CD69 and CD28, are refractory to activation, and die through contact-dependent apoptosis mediated by an as-yet-unidentified mechanism not requiring Fas, tumor necrosis factor-related apoptosis-inducing ligand, leukocyte function antigen 1, intercellular adhesion molecule 1, tumor necrosis factor alpha, interleukin 10, alpha interferon, gamma interferon, prostaglandins, or reactive nitrogen intermediates. Bystander T lymphocytes that were neither infected nor apoptotic were refractory to activation, suggesting global dysfunction. Immunosuppression and T-lymphocyte unresponsiveness and apoptosis are typical of acute T. gondii infection. Our data suggest that infected dendritic cells contribute to these processes. On the other hand, host cells infected with T. gondii are resistant to multiple inducers of apoptosis. Thus, regulation of host cell and bystander cell apoptosis by viable T. gondii may be significant components of a strategy to evade immunity and enhance intracellular parasite survival.
Toxoplasma gondii is an obligate, intracellular protozoan parasite in the order Coccidia (29). Humans become infected through ingestion of food contaminated with tissue cysts or oocysts (39). In immunocompetent hosts, infection is usually benign owing to a rapid, effective cellular immune response (42, 43). However, persons with defective cellular immunity are at risk for severe sequelae including death (3, 31, 44). For human immunodeficiency virus-infected persons, cerebral toxoplasmic encephalitis is an important cause of morbidity and mortality (28, 34, 40).
Obligate intracellular parasites must infect their hosts without killing them for optimal parasite survival. In this regard, acute T. gondii infection in immunocompetent hosts is initially associated with induction of transient immunosuppression (22, 46, 49, 53), followed by induction of protective immunity (reviewed in reference 54). Encystation and clinical latency ensue. This early immunosuppression may be a parasite-mediated mechanism for facilitating establishment of a permanent infection (54) or limiting potentially fatal host immunopathology (16, 50). Global T-lymphocyte inactivation (19), gamma interferon (IFN-γ)-induced Fas-dependent T-lymphocyte apoptosis (27), and other factors (4-7, 16, 20, 25, 54) contribute to immunosuppression in acute T. gondii infection.
Dendritic cells are generally regarded as helping to ignite adaptive immunity (1). However, encounters between T lymphocytes and dendritic cells infected with various pathogens are not always favorable to the host. For example, measles virus infects dendritic cells, inducing apoptosis in them and the T lymphocytes contacting them (15). Plasmodium-infected erythrocytes (51) or Trypanosoma infection of dendritic cells (52) inhibits functional dendritic cell maturation. Human immunodeficiency virus infection is facilitated by contact between infected Langerhans dendritic cells and T lymphocytes (33). We thus undertook the present studies to examine the potential for MDDCs infected with viable T. gondii to induce specific immunity or contribute to immunosuppression.
MATERIALS AND METHODS
Human subjects.
Healthy volunteers gave informed, written consent, and the Institutional Review Board of Baylor University Medical Center approved these studies. Serum antibodies against T. gondii were measured by a commercial laboratory. Hereafter, the terms seropositive and seronegative refer to the presence and absence, respectively, of T. gondii-specific serum antibodies.
T. gondii.
T. gondii strain RH was originally obtained from Elmer Pfeffercorn (Dartmouth University, Hanover, N.H.), and strain Me49 was obtained from Randolph Berens and Edward Krug (University of Colorado, Denver, Colo.). CTK11 tachyzoites expressing recombinant thymidine kinase, rendering them sensitive to ganciclovir (38), and A2 tachyzoites (2) were both originally derived from strain RH. All tachyzoites were passaged in human foreskin fibroblasts as described previously (10). Cells were tested periodically for Mycoplasma infection by PCR (Molecular Probes, Eugene, Oreg.) and by staining with Hoechst 33342 dye (Hoechst Chemicals, Frankfurt, Germany) and were negative. Heat-inactivated tachyzoites were prepared by heating extracellular tachyzoites to 56°C for 2 h. Soluble T. gondii antigen was prepared by subjecting extracellular tachyzoites to seven rapid freeze-thaw cycles (10).
Production of MDDCs and responder T lymphocytes.
Peripheral blood mononuclear cells were obtained by phlebotomy into sterile, heparin-containing glass vials (Becton Dickinson, Franklin Lakes, N.J.) and purified by Ficoll-Hypaque (Amersham Pharmacia Biotech AB, Uppsala, Sweden) density centrifugation. Peripheral blood mononuclear cells were adhered to plastic plates (Costar, Corning, N.Y.) for 2 h in medium RPMI 1640 (GIBCO BRL, Grand Island, N.Y.) supplemented with heat-inactivated fetal bovine serum (GIBCO), 10 mM HEPES buffer, 2 mM l-glutamine, and antibiotics. Nonadherent cells were gently rinsed away and frozen for later use as responder cells. Where noted, CD3+ T lymphocytes were obtained from peripheral blood mononuclear cells in ≥98% purity by depleting CD14-, CD16-, CD19-, and CD56-expressing cells by using antibody-coated microbeads (Miltenyi Biotech, Auburn, Calif.) according to the manufacturer's directions.
MDDCs were produced by growing adherent peripheral blood mononuclear cells in medium containing 100 ng of human granulocyte-macrophage colony-stimulating factor (R&D Systems, Minneapolis, Minn.) per ml plus 5 ng of human interleukin 4 (IL-4) (R&D) per ml and used on days 5 to 7 of culture (57). Fresh cytokines were replaced every 2 or 3 days. High-purity MDDCs were obtained by isolating CD1a+ cells from MDDC culture by flow cytometry (≥99% CD1a+ cells).
T. gondii infections.
Viable tachyzoites were obtained by lysis of fibroblasts with forced passage through a 27-gauge needle and used within 2 h of collection. Tachyzoites were enumerated with a hemacytometer using trypan blue dye. Host cells were infected with tachyzoites for 14 to 18 h at a multiplicity of infection (MOI) of 10 unless otherwise noted prior to addition to T lymphocytes. Fixed MDDCs were prepared 14 to 18 h after infection by incubation in 0.1% paraformaldehyde (Sigma, St. Louis, Mo.) at ambient temperature for 1 h, followed by thorough washing. To interfere with the function of tachyzoite surface antigens, tachyzoites were incubated for 1 h with murine monoclonal anti-SAG-1 or -SAG-2 antibodies or F(ab) fragments of anti-Toxoplasma polyclonal antiserum (8) prior to infection, which occurred in the continuous presence of additional antibody or F(ab) fragments (final concentration, 10 μg/ml). Ganciclovir (Roche Research Centre, Hertfordshire, United Kingdom) was added to MDDCs 4 h after infection with CTK11 tachyzoites at the concentrations indicated and was maintained when MDDCs were cocultured with T lymphocytes.
To estimate the intensity of T. gondii infection, cytospin preparations of cells were stained with Giemsa stain and examined by light microscopy using a 40× lens objective. At least 200 cells per condition were counted. To quantify T. gondii tachyzoite replication, 1 μCi of [3H]methyluracil (New England Nuclear, Cambridge, Mass.) was added to infected cells in 96-well plastic microtiter plates (Falcon, Oxnard, Calif.) 16 h before harvest onto filter mats (Wallac, Gaithersburg, Md.) and analyzed on a Microbeta Trilux liquid scintillation and luminescence counter (Wallac, Turku, Finland). Proliferation assays were performed in triplicate, and the means ± the standard errors of the means (SEMs) are reported.
T-lymphocyte proliferation and cytotoxic T-lymphocyte induction.
To induce T-lymphocyte proliferation, peripheral blood mononuclear cells or purified CD3+ T lymphocytes were incubated with various numbers of autologous MDDCs (infected or uninfected) in a 200-μl total volume of medium in triplicate in 96-well round-bottomed plastic tissue culture plates. Ten nanograms of Staphylococcus aureus enterotoxin B (superantigen; Sigma) per ml or 1 μg of phytohemagglutinin (Sigma) per ml was added to MDDCs where indicated. The anti-CD3 antibody 12F6 (11) was used at a final concentration of 0.05 μg/ml. Allogeneic mixed lymphocyte reactions were performed using bead-purified CD4+ CD45RA+ T lymphocytes as described previously (57). A 50 μM concentration of N-methylmalonic acid (Sigma), 10−4 M indomethacin (Sigma), or neutralizing antibodies to IFN-γ or IFN-α (R&D) was added where indicated at the initiation of coculture of MDDCs with T lymphocytes and maintained throughout the entire culture period.
To quantify T-lymphocyte proliferation, 1 μCi of [3H]methylthymidine (New England Nuclear) was added to cultures during the final 16 h of a 5-day (T. gondii antigens, allogeneic mixed lymphocyte reaction, tetanus toxoid antigens) or 3-day (all other conditions) incubation, except additionally noted, and harvested and detected as described above for [3H]methyluracil. The means ± the SEMs of triplicate determinations are reported.
Transwell experiments were performed using 24-well plastic plates (Corning, Inc., Corning, N.Y.) with 5-μm-pore-size inserts (Neuro Probe, Inc., Gaithersburg, Md.). Thirty thousand infected MDDCs were added to the lower chamber, and 100,000 peripheral blood mononuclear cells or 70,000 CD3+ T lymphocytes were added to the upper chamber.
Polyclonal, T. gondii-specific T-lymphocyte cultures were produced as previously described (57). Cytotoxicity was determined after 10 days of culture in the absence of exogenous IL-2, using T. gondii-infected, 51Cr-labeled Epstein-Barr virus-transformed B lymphocytes as target cells as described previously (10, 35, 37).
Cell enumeration, viability, and apoptosis detection.
Peripheral blood mononuclear cells and autologous MDDCs were incubated in six-well plates under culture conditions identical to those of the proliferation assays for 3,000 infected MDDCs/well in 96-well plates. Aliquots of cells were removed periodically, and total numbers and viability were determined with a hemacytometer and trypan blue dye. Apoptosis was assessed by using a combination of 7-AAD dye (Sigma) and antibody to annexin V (BD PharMingen, San Diego, Calif.), and analyzed by fluorescence-activated cell sorter (FACS) as described below, gating on CD3+ T lymphocytes. In preliminary experiments, we examined Giemsa-stained, cytocentrifuged cells by light microscopy to evaluate for apoptosis by nuclear morphology. Light microscopic determinations were in good agreement with FACS determinations. Thus, all further work used FACS alone to quantify apoptosis. Murine monoclonal blocking antibodies against tumor necrosis factor-related apoptosis-inducing ligand (1 μg/ml; R& D), Fas (clone NOK-1, 10 μg/ml; PharMingen), intercellular adhesion molecule 1 (10 μg/ml; R&D), and leukocyte function antigen 1 (25 μg/ml; R&D) were added 30 to 120 min prior to coculture.
Supernatants from infected MDDCs and from T lymphocytes exposed to infected MDDCs.
Supernatants from infected MDDCs were obtained 16 to 18 h after infection and from CD3+ T lymphocytes after 3 days of coculture with infected MDDCs, centrifuged to remove cells, and frozen at −86°C until use. Cytokine measurements were determined with commercial enzyme-linked immunosorbent assay kits (R&D) according to the manufacturer's suggestions. Production of reactive nitrogen intermediates was assessed by using Griess Reagent (Sigma) as suggested by the manufacturer. Dilutions of supernatants were added at the outset of culture.
Production of necrotic or apoptotic monocytes bearing T. gondii antigens.
As a source of cell-associated T. gondii antigens for capture by MDDCs, autologous monocytes were obtained from peripheral blood mononuclear cells by plastic adherence and incubated with 10 heat-inactivated tachyzoites/cell for 16 h. Monocyte necrosis was induced by heat shock (56°C, 2 h), and apoptosis was induced by incubation with 20 μM beauvericin (Sigma) for 3 h (32). Apoptosis or necrosis was confirmed by flow cytometry (see below) using a combination of anti-annexin V antibody and propidium iodide (Sigma). One cell equivalent of necrotic or apoptotic monocyte/MDDC was incubated with MDDCs for 2 h, extracellular debris was removed by centrifugation, and these MDDCs were resuspended in fresh medium prior to coculture with autologous peripheral blood mononuclear cells for an additional 5 days.
Flow cytometry.
MDDCs were stained with antibodies 16 to 18 h after infection, and T lymphocytes were stained after 1 to 3 days of coculture with MDDCs as indicated, using murine monoclonal anti-human antibodies followed by fixation with paraformaldehyde. Antibodies were purchased from commercial sources: Biosource International, Camarillo, Calif. (CD1a), Becton Dickinson (CD3, CD4, CD8, CD28, CD69, CD80, human leukocyte antigen [DR]), Caltag, Burlingame, Calif. (CD14), BD PharMingen (CD40, CD86, CD95, major histocompatibility complex [MHC] class I), and Immunotech, Miami, Fla. (CD25, CD54, CD83). Flow cytometric data were acquired on a FACSCalibur (Becton Dickinson, Mountain View, Calif.) and analyzed with CellQuest software (Becton Dickinson) with 5,000 or more events evaluated for each condition. Analysis gates were set by using appropriate isotype controls, and dead cells were excluded based on light scatter characteristics.
Statistical analysis.
Differences between means of continuous variables were assessed by using a two-sided Student's t test assuming equal variances. Differences between discrete variables were assessed by two-tailed χ2 distribution. Correlations were determined by least-squares linear regression. A P value of <0.05 was considered significant.
RESULTS
T. gondii infection induces upregulation of MDDC activation markers and de novo CD83 expression.
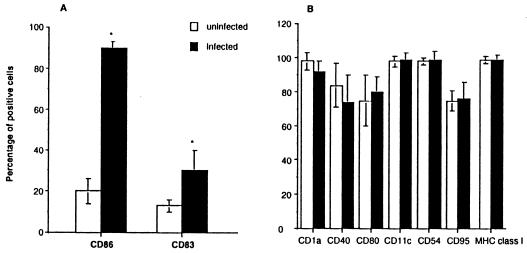
Day 5 to day 7 immature MDDC cultures were comprised of ≥95% CD1a+ cells and expressed a DRlo CD86lo CD83−/lo phenotype. By 16 h following T. gondii infection at an MOI of 10, 90% ± 5% of MDDC were parasitized as evidenced by Giemsa stain. Infection of immature MDDCs with live T. gondii tachyzoites rapidly induced a partially matured phenotype as evidenced by a significant increase in CD86 expression and de novo expression of the dendritic cell-specific maturation marker CD83 (56) (Fig. 1A). Greater than 95% of MDDC expressed DR whether infected or not. However, the mean fluorescence intensity (expressed in arbitrary units) (+SEM) of DR expression increased from 60 ± 4 to 130 ± 12 (n = 10; P < 0.01) following infection. Other surface markers including CD1a, CD11c, CD40, CD54, CD80, CD95, and major histocompatibility complex class I were not significantly altered by infection (Fig. 1B). As MDDC CD40 and CD80 were reported to increase following T. gondii infection (48), we also measured the mean relative fluorescence intensities of these markers, but found no significant change (CD40 mean relative fluorescence intensity, 14.6 ± 5.2 versus 15.5 ± 3.1, and CD80 mean fluorescence intensity, 29.5 ± 5.9 versus 33.4 ± 6.4 in infected versus uninfected MDDCs, respectively; n = 10; P > 0.70 for both). Neither heat-killed T. gondii tachyzoites nor soluble fibroblast extracts significantly affected the expression of the above markers on dendritic cells, suggesting that viable parasites mediated the observed changes and demonstrating that contaminating endotoxin and residual fibroblast antigens did not activate or mature dendritic cells in these cultures.
FIG. 1.
T. gondii infection induces a partially mature MDDC phenotype. (A) CD86 and CD83 expressions were significantly increased after infection, and the mean relative fluorescence intensity of DR was significantly increased (see text). (B) Expression of other markers was not affected. Immature MDDCs were infected at an MOI of 10 and evaluated after 16 h. FACS analyses are of 10 individual experiments from 10 separate subjects, with the means ± SEMs shown. ∗, P < 0.01.
T. gondii-infected MDDCs do not induce significant T. gondii-specific T-lymphocyte proliferation and suppress superantigen-induced T-lymphocyte activation.
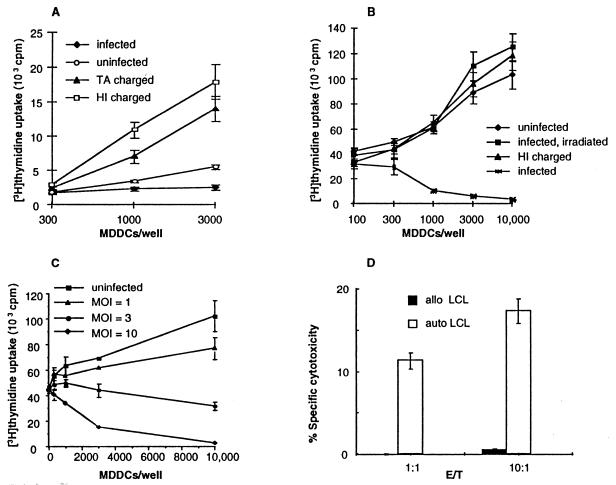
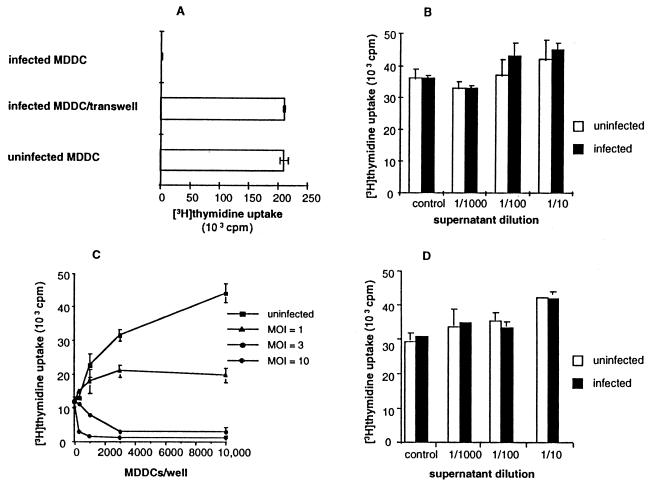
Mature dendritic cells are potent activators of T lymphocytes (1), and peripheral blood mononuclear cells from seropositive donors proliferate in response to T. gondii antigens (10, 35, 37). However, T. gondii-infected MDDCs did not effect significant proliferation of autologous peripheral blood mononuclear cells from seropositive subjects, whereas MDDCs charged with whole, heat-inactivated tachyzoites or soluble T. gondii antigens did (Fig. 2A). Uninfected MDDCs presenting superantigen effected significant peripheral blood mononuclear cell proliferation as expected. However, when superantigen was presented by T. gondii-infected MDDCs, peripheral blood mononuclear cell proliferation was inhibited (Fig. 2B). MDDCs charged with heat-inactivated tachyzoites did not inhibit superantigen-induced peripheral blood mononuclear cell proliferation (Fig. 2B). There was a direct correlation between the intensity of MDDC infection and reduction of superantigen-induced peripheral blood mononuclear cell proliferation (Fig. 2C). T. gondii-infected MDDCs inhibited superantigen-mediated T-lymphocyte activation from both seropositive and seronegative subjects equally, and thus both seropositive and seronegative subjects were used for all additional studies, unless otherwise noted.
FIG. 2.
MDDCs infected with live T. gondii tachyzoites are defective in activating T lymphocytes. (A) T. gondii-specific T-lymphocyte activation. MDDCs infected with live tachyzoites (MOI = 10) fail to induce T-lymphocyte proliferation from seropositive subjects (P < 0.01 versus heat-inactivated [HI] tachyzoites or T. gondii Ag [TA], whereas MDDCs pulsed with 1 μg of nonviable soluble T. gondii Ag per ml or with 10 whole, heat-inactivated tachyzoites/MDDC elicited potent T-lymphocyte proliferation. (B) Killed or irradiated tachyzoites do not inhibit superantigen-mediated T-lymphocyte activation. MDDCs were pulsed with three heat inactivated tachyzoites/MDDC, or infected at an MOI of 3, or infected and irradiated with 20,000 rads from a γ source 16 h after infection. (C) Infected MDDCs inhibit superantigen-induced T-lymphocyte proliferation in a dose-dependent fashion. Peripheral blood mononuclear cells were incubated with autologous MDDCs plus 10 ng of superantigen per ml for 3 days. (D) MDDCs charged with heat-killed tachyzoites elicit T. gondii-specific cytotoxic T lymphocytes. Peripheral blood mononuclear cells from a seropositive subject were cultured with one charged MDDC per 100 peripheral blood mononuclear cells for 10 days. Cytotoxicity was assessed by 51Cr release following a 4-h incubation with autologous (auto) or allogeneic (allo) Epstein-Barr virus-transformed B cells (LCL) infected with T. gondii. The data in each panel reflect one experiment representative of five or more, except for panel D, which is representative of three experiments.
Infected MDDCs did not generate cytotoxic T lymphocytes, owing to the destruction of cultures by replicating tachyzoites. However, MDDCs loaded with heat-killed tachyzoites were potent inducers of T. gondii-specific cytotoxic T lymphocytes (Fig. 2D).
T. gondii-infected MDDCs inhibit T-cell activation induced by a variety of stimuli.
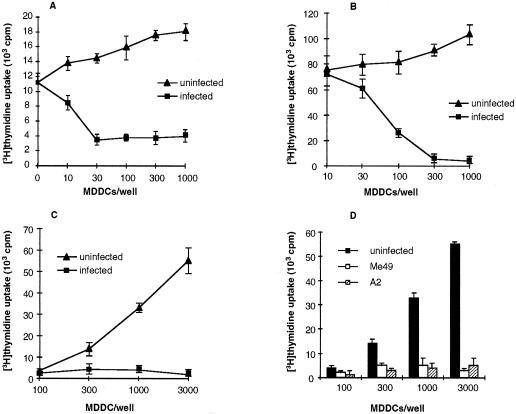
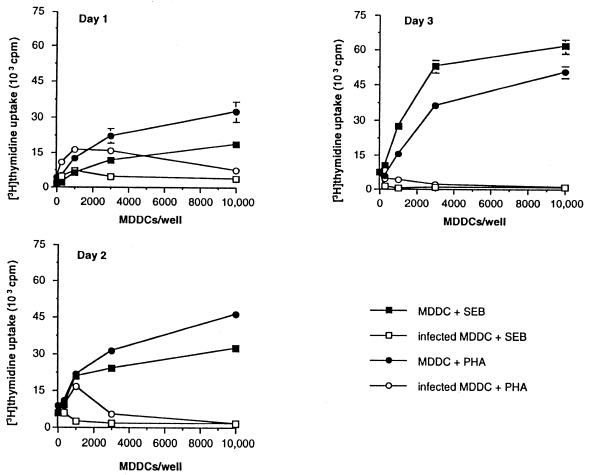
The preceding experiments suggested a profound defect in T-lymphocyte activation in response to T. gondii antigen and superantigen-induced activation. To determine whether T-lymphocyte activation in response to additional, distinct stimuli was impaired, we next attempted to induce T-lymphocyte activation with infected MDDCs presenting the recall antigen tetanus toxoid (Fig. 3A), the anti-CD3 antibody 12F6 (Fig. 3B), and allogeneic major histocompatibility antigens in a mixed-lymphocyte reaction (Fig. 3C). In each case, T. gondii-infected MDDCs significantly suppressed T-lymphocyte activation. T. gondii strains Me49 and A2 are avirulent for some mouse strains (2). However, MDDCs infected with either strain also induced potent T-lymphocyte immunosuppression (Fig. 3D). Thus, MDDCs infected with either virulent or avirulent strains of T. gondii inhibited T-lymphocyte activation in response to a wide variety of stimuli.
FIG. 3.
T. gondii-infected MDDCs inhibit T-lymphocyte proliferation in response to a variety of stimuli. MDDCs were infected with T. gondii strain RH (MOI = 3) and then incubated with naïve, autologous CD4+ T lymphocytes and 4 limiting flocculation units (LFU) of tetanus toxoid per ml (A) or 0.05 μg of anti-CD3 antibody per ml (B), or added to allogeneic T lymphocytes (C). (D) MDDCs infected with either A2 or Me49 T. gondii tachyzoites (MOI = 3) were defective in eliciting an allogeneic mixed lymphocyte response. Similar results with Me49- and A2-infected MDDCs were obtained with phytohemagglutinin and superantigen activation. Data shown are mean values of triplicate determinations, representative of more than five experiments for each condition, except for panel D (three experiments).
Blockade of T. gondii attachment ligands reverses immunosuppression, although this correlated with reduced dendritic cell infection.
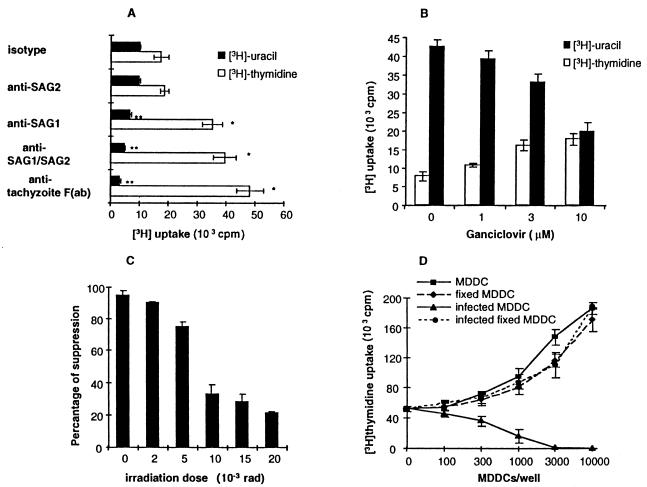
SAG-1 and SAG-2 are tachyzoite attachment ligands that contribute to immunosuppression mediated by T. gondii-infected monocytes (8). MDDCs infected with T. gondii in the presence of monoclonal anti-SAG-1 or polyclonal anti-Toxoplasma antiserum F(ab) significantly reversed immunosuppression as measured by [3H]methylthymidine incorporation into T lymphocytes (Fig. 4A), suggesting that T. gondii attachment ligands might be involved in dendritic cell-mediated immunosuppression. As SAG proteins are involved in tachyzoite host cell entry (18), it was important to exclude decreased MDDC infection in reducing immunosuppression. Anti-SAG-1 monoclonal antibodies or polyclonal anti-Toxoplasma antiserum F(ab) reduced [3H]methyluracil uptake (Fig. 4A) and decreased MDDC parasitism by analysis of Giemsa-stained cytospin specimens, which directly correlated with reversed immunosuppression. Uracil is not metabolized by mammalian cells, and its incorporation correlates with tachyzoite replication (26). Thus, reduced immunosuppression appeared to be a consequence of reduced dendritic cell parasitism.
FIG. 4.
Immunosuppression mediated by T. gondii-infected MDDCs correlates directly with parasite burden and parasite replication. (A) Blockade of T. gondii attachment ligands reverses immunosuppression mediated by infected MDDCs. However, this reversal relates to reduced MDDC parasitism. MDDCs infected with tachyzoites incubated with α-SAG-1, or anti-tachyzoite F(ab) were less immunosuppressive than those infected with control antibody. Tachyzoites were incubated with antibodies shown, and then used to infect MDDCs at an MOI of 10. Superantigen-mediated activation of autologous T lymphocytes with these MDDCs was determined 3 days later. ∗ and ∗∗, P < 0.05 compared to isotype control. (B) Reduced parasite burden following infection was accomplished by using CTK11 mutant tachyzoites that are sensitive to ganciclovir (38). MDDCs were infected at an MOI of 10. Infected MDDCs were treated with ganciclovir 4 h after infection and incubated with autologous T lymphocytes in a 1:100 ratio with 10 ng of superantigen per ml in the continuous presence of drug starting 16 h after infection. Radiolabel incorporation was determined at the end of the 3-day coculture. Uracil uptake quantifies parasite replication (26). Black bars show [3H]uracil uptake by tachyzoites, and white bars show [3H]thymidine uptake by T lymphocytes. (C) Gamma irradiation of infected MDDCs 16 h following infection (MOI = 10) reduces immunosuppression. MDDCs were infected at an MOI of 10 and irradiated 16 h after infection. Superantigen-mediated activation of autologous T lymphocytes was determined as described in Materials and Methods. Percentage of suppression was calculated as follows: [3H]methylthymidine uptake of T cells exposed to infected MDDC divided by [3H]methylthymidine uptake of T cells exposed to uninfected MDDC, multiplied by 100%. (D) Paraformaldehyde-fixed, infected MDDCs are not immunosuppressive. Fixed MDDCs were prepared 14 to 18 h after infection by incubation in 0.1% paraformaldehyde at ambient temperature for 1 h, followed by thorough washing. Superantigen-mediated activation of autologous T lymphocytes was determined as described for panel B. One experiment representative of four to six is shown for panels A to D.
Active parasite entry into the host cell is not sufficient to induce immunosuppression, but parasite replication is required.
Antibody-coated tachyzoites may be captured by phagocytosis (13), whereas infection by live T. gondii tachyzoites is an active, parasite-mediated process (21) followed by parasite replication within the host cell. Killed tachyzoites or soluble antigens are acquired through phagocytosis or pinocytosis and obviously do not replicate. To examine further the consequences of infection and replication of live tachyzoites inside cells, MDDCs were infected with live CTK11 mutant T. gondii tachyzoites (sensitive to ganciclovir treatment) and then treated with ganciclovir to inhibit intracellular tachyzoite proliferation, allowing infection through normal active processes prior to inhibition of intracellular replication. Ganciclovir treatment following infection reduced or abolished MDDC-mediated immunosuppression in a dose-dependent fashion, which inversely correlated with tachyzoite replication as measured by [3H]methyluracil uptake (correlation coefficient, −0.864; P < 0.01), suggesting a requirement for replicating tachyzoites to mediate immunosuppression (Fig. 4B). Ganciclovir treatment had no significant effect on T-cell proliferation up to 3 μM in the absence of infected MDDCs. Above that concentration, ganciclovir was slightly toxic to T cells and reduced their viability. Nonetheless, the reduction in tachyzoite replication effected by ganciclovir more than offset this toxicity, resulting in a net increase in T-cell proliferation in the presence of T. gondii-infected MDDCs (Fig. 4B).
Gamma irradiation of T. gondii-infected MDDCs reversed immunosuppression in a dose-dependent fashion (Fig. 2B and 4C), which inversely correlated with reduced parasite replication. Paraformaldehyde fixation reversed immunosuppression mediated by infected MDDCs (Fig. 4D). These data demonstrated that active parasite entry into cells was insufficient to induce immunosuppression and confirmed the direct dose-dependent relationship between tachyzoite replication and immunosuppression.
T. gondii-infected MDDCs require cell-to-cell contact to mediate immunosuppression, which does not involve bystander cell participation.
Superantigen-induced proliferation was equivalent when peripheral blood mononuclear cells exposed across a semipermeable membrane to T. gondii-infected, highly purified MDDCs were used and when peripheral blood mononuclear cells in cell-to-cell contact with uninfected, highly purified MDDCs were used (Fig. 5A). In confirmatory experiments, supernatants from infected, bead-purified MDDCs (≥99% CD1a+ cells) did not inhibit superantigen-induced peripheral blood mononuclear cell proliferation (Fig. 5B). Addition of indomethacin or N-methylmalonic acid had no effect on immunosuppression mediated by T. gondii-infected MDDCs (not shown).
FIG. 5.
Direct cell-to-cell contact is required for immunosuppression mediated by T. gondii-infected MDDCs. Bystander cells do not appear to contribute to this process. (A) Peripheral blood mononuclear cells (100,000 in the upper chamber) exposed to infected MDDCs (MOI = 10; 30,000 in the lower chamber) across a semipermeable membrane of a transwell proliferated normally in response to superantigen, but not when the peripheral blood mononuclear cells and infected MDDCs were in direct contact. (B) Supernatants from purified CD1a+ MDDCs infected with T. gondii do not inhibit superantigen-mediated T-lymphocyte proliferation. Supernatant was added to a fresh, uninfected coculture. (C) T. gondii-infected, purified MDDCs (>99% CD1a+ cells) suppressed superantigen-induced proliferation of purified CD3+ T lymphocytes (>98% CD3+ cells). These experiments were set up as for Fig. 2C. (D) Supernatants from purified CD1a+ MDDCs infected with T. gondii and cocultured with T lymphocytes do not inhibit superantigen-mediated T-lymphocyte proliferation. Supernatants collected after a 3-day coculture were added to fresh, uninfected cocultures. One experiment representative of three to six is shown for all panels.
In direct cell-to-cell contact, infected MDDCs may mediate T-lymphocyte suppression directly, or they may do so indirectly by acting on bystander cells. T. gondii-infected monocytes and macrophages are immunosuppressive through a soluble mediator (7, 8). To deplete contaminating monocytes and/or macrophages, we obtained bead-purified MDDCs (≥99% CD1a+ cells) and T lymphocytes (>98% CD3+ cells). Infected but not uninfected highly purified MDDCs inhibited superantigen-mediated highly purified CD3+ T-lymphocyte proliferation. The intensity of MDDC infection directly correlated with the degree of immunosuppression induced (Fig. 5C). Supernatants from highly purified CD3+ T lymphocytes incubated with infected MDDCs did not inhibit superantigen-mediated T-lymphocyte activation (Fig. 5D). These data established that immunosuppression was not mediated by indirect effects of contaminating cells or soluble factors and was due directly to an effect of the infected MDDC on the T lymphocyte.
Immunosuppression does not correlate with T-lymphocyte infection.
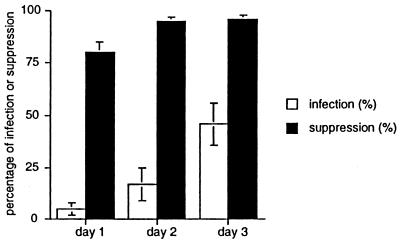
Immunosuppression via direct cell-to-cell contact may be due to infection of responder T lymphocytes. Examination of Giemsa-stained cytocentrifuged specimens of T lymphocytes cocultured with infected MDDCs demonstrated that infection of T lymphocytes increased throughout the culture period, from undetectable at the outset to <5% after 1 day, to a maximum of approximately 46% by 3 days of culture (Fig. 6). Nonetheless, MDDCs were defective in induced superantigen-mediated T-lymphocyte proliferation even when significant numbers of viable, apparently uninfected T lymphocytes remained in culture (Fig. 6 and 7). Further, immunosuppression peaked well in advance of maximum T-lymphocyte infection (Fig. 6 and 7). Significantly reduced T-lymphocyte proliferation was observed on day 1, when only approximately 5% of T lymphocytes were infected (Fig. 6 and 7), suggesting a generalized state of T-cell unresponsiveness, as has been reported for acutely infected mice (24). We attempted to recover viable, uninfected T lymphocytes from cultures for additional studies using a recombinant tachyzoite expressing green fluorescent protein (47) (gift of Boris Striepen, Washington University, St. Louis, Mo.), but this method was too insensitive.
FIG. 6.
T-lymphocyte immunosuppression induced by T. gondii-infected MDDCs precedes detectable T-lymphocyte infection. MDDCs were infected with T. gondii at an MOI of 10, and infection was determined by inspection of Giemsa-stained cytospin preparations with light microscopy. At least 200 T lymphocytes per condition were analyzed. Percentage suppression was determined by dividing [3H]methylthymidine incorporation induced by T. gondii-infected MDDCs by [3H]methylthymidine incorporation induced by uninfected MDDCs, both bearing superantigen, and subtracting from 100%. Incorporation experiments were set up as described in the text using 10,000 MDDCs and 70,000 CD3+ T lymphocytes/well, and harvested on the days of culture shown. Means ± SEMs of three independent experiments using three separate subjects are reported.
FIG. 7.
Immunosuppression mediated by T. gondii-infected MDDCs appears as early as after 1 day of coculture, when only a minority of T lymphocytes are infected, and is maximal around day 2. MDDCs were infected with T. gondii at an MOI of 10 and cocultured with purified CD3+ T lymphocytes 16 h later with 10 ng of superantigen (SEB) per ml or 1 μg of phytohemagglutinin (PHA) per ml. Means ± SEMs of three independent experiments using three separate subjects are reported. See also Fig. 6.
T lymphocytes contacting T. gondii-infected MDDCs are impaired in upregulation of activation and costimulatory molecules.
To explore the concept of a basic defect in T-lymphocyte activation further, we examined surface markers correlating with T-lymphocyte activation by flow cytometry. After 2 days of culture with infected MDDCs, around the time of maximal immunosuppression, upregulation of the early activation marker CD69 and the T-lymphocyte costimulatory molecule CD28 on T lymphocytes was significantly lower than that of the control (Table 1), whereas expressions of CD25, CD3, CD4, and CD8 were unaffected. T lymphocytes exposed to infected MDDCs were uniformly low in CD69 and CD28 expression, with no bimodal distribution, suggesting that both infected and uninfected T lymphocytes were impaired in the upregulation of these molecules. As fewer than 10% of T lymphocytes in these cultures were parasitized at the time of FACS analysis, we did not evaluate the effect of T. gondii infection of T lymphocytes on their expression of CD28 or CD69.
TABLE 1.
T lymphocytes contacting T. gondii-infected MDDCs do not upregulate CD28 or CD69 appropriatelya
| Mean ± SEM of cell surface marker expressiona (% positive cells) in culture with:
|
|||
|---|---|---|---|
| No MDDCs | Uninfected MDDCs | Infected MDDC | |
| CD69 | 20 ± 8 | 52 ± 4 | 17 ± 1b |
| CD28 | 75 ± 5 | 86 ± 3 | 72 ± 4c |
MDDCs were infected with T. gondii at an MOI of 10 and cocultured with bead-purified T lymphocytes (>98% CD3+ cells) for 48 h. Values were determined by flow cytometry and are from four independent experiments using different subjects for each.
P < 0.01 compared to uninfected MDDCs. There was no significant difference compared to no MDDCs.
P < 0.05 compared to uninfected MDDCs. There was no significant difference compared to no MDDCs.
T lymphocytes contacting infected MDDCs die by apoptosis.
To determine whether T. gondii-infected MDDCs induced T-lymphocyte apoptosis, purified CD3+ T lymphocytes plus infected, autologous MDDCs were cocultured in 6-well plates in the presence or absence of superantigen, under conditions identical to those of the 96-well plates. T-lymphocyte viability and numbers were significantly reduced compared to those of cultures maintained with uninfected MDDCs. Further, the percentage of apoptotic T lymphocytes in cultures with infected MDDCs was significantly higher than that found with uninfected MDDCs (Table 2). Infected MDDCs cultured with T lymphocytes from seronegative individuals in the absence of a general T-lymphocyte activation signal nonetheless underwent significant apoptosis. Further, infection of T lymphocytes with T. gondii at an MOI of 10 in the absence of MDDCs did not significantly reduce lymphocyte numbers or decrease their viability over a 3-day period, the length of culture with superantigen. Thus, early apoptosis helps explain reduced T-lymphocyte activation.
TABLE 2.
CD3+ T lymphocytes cultured with T. gondii-infected, autologous MDDCs undergo apoptosis that increases beyond 24 ha
| Antigen-presenting cell | Apoptotic cellsb (mean % ± SD)
|
|
|---|---|---|
| 24 h | 48 h | |
| MDDC | 6.9 ± 2.0 | 11.3 ± 3.5 |
| MDDC/T. gondii | 25.3c ± 8.7 | 37.7c,d ± 3.2 |
| MDDC + SEB | 21.7 ± 4.0 | 20.0 ± 4.6 |
| MDDC/T. gondii + SEB | 36.7c ± 8.1 | 40.3c ± 4.2 |
| MDDC + PHA | 25.3 ± 4.5 | 25.0 ± 3.6 |
| MDDC/T. gondii + PHA | 40.7c ± 6.7 | 41.3c ± 4.2 |
MDDCs were infected with T. gondii at an MOI of 10 and added to autologous, bead-purified T lymphocytes (≥98% CD3+ cells) the next day together with 10 ng of superantigen (SEB) per ml or 1μg of phytohemagglutinin (PHA) per ml as indicated.
Apoptosis was determined by flow cytometry using 7-AAD and annexin V as described in the text; values are from three independent experiments.
P < 0.05 compared to same time point with uninfected MDDCs.
P < 0.05 compared to 24-h time point with infected MDDCs.
IFN-γ induces Fas-dependent apoptosis of CD4+ and CD8+ intraepithelial gut T lymphocytes in mice following oral infection with T. gondii (27). As IFN-γ was generated in cultures of T lymphocytes exposed to T. gondii-infected MDDCs (mean ± SEM, 2,926 ± 369 pg/ml at 72 h; n = 3), we examined CD95 (Fas) expression on T. gondii-infected MDDCs, but it was not increased (Fig. 1B). Further, anti-Fas blocking antibodies altered apoptosis induction by <3% at 24 and 48 h (P > 0.8), and anti-IFN-γ neutralizing antibodies did not reverse immunosuppression (not shown). In addition, T lymphocytes and infected MDDCs cultured in the presence of saturating concentrations of blocking or neutralizing antibodies to tumor necrosis factor-related apoptosis-inducing ligand, intercellular adhesion molecule 1, leukocyte function antigen 1, and tumor necrosis factor alpha exhibited similar percentages of apoptotic T lymphocytes (P > 0.6 for all). Thus, the putative factor inducing T-lymphocyte apoptosis may not be a molecule commonly known to induce apoptosis.
Nonviable T. gondii antigen acquired by MDDCs through capture of apoptotic or necrotic antigen-bearing cells is immunogenic.
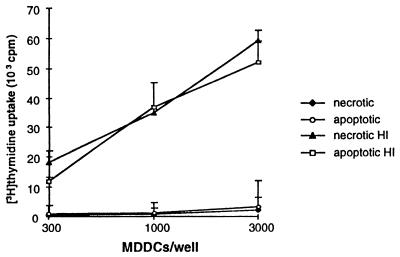
As infection significantly impaired MDDC-mediated T-lymphocyte activation, we determined whether phagocytic cells bearing nonviable tachyzoites served as a source of immunogenic antigens. MDDCs loaded with either apoptotic or necrotic monocyte bodies bearing T. gondii antigens induced significant autologous T-lymphocyte activation from seropositive subjects, whereas apoptotic or necrotic autologous monocytes not loaded with antigen effected no significant proliferation (Fig. 8). Monocytes infected with live tachyzoites and then induced to become apoptotic were unable to donate immunogenic antigens to MDDCs due to the continued presence of viable, replicating tachyzoites that destroyed the culture.
FIG. 8.
MDDCs acquiring T. gondii antigens from necrotic or apoptotic monocytes induce T. gondii-specific T-lymphocyte proliferation. Monocytes were incubated with heat-inactivated (HI) T. gondii tachyzoites and induced to become necrotic (heat shock) or apoptotic (beauvericin treatment). Cell bodies were loaded onto autologous MDDCs, which were then incubated with autologous CD3+ T lymphocytes at a 1:10 ratio for 5 days, after which time incorporated [3H]methylthymidine incorporation was measured. Means ± SEMs of one experiment representative of four are shown. Necrotic HI, autologous monocytes loaded with heat-inactivated T. gondii tachyzoites and induced to become necrotic, etc. Necrosis versus apoptosis was confirmed by FACS for 7-AAD and propidium iodide as described in Materials and Methods.
DISCUSSION
With T. gondii used as a model pathogen in mice, it was shown that dendritic cells are potentially the earliest producers of IL-12 igniting parasite-specific immunity and IFN-γ secretion (45). However, this study used injection of nonviable T. gondii antigens, leaving undefined the role played by viable tachyzoites in induction of specific immunity. Human MDDCs infected with T. gondii do not secrete significant IL-12 unless CD40 is engaged (41), although significant T-lymphocyte IFN-γ is induced (48). MDDCs bearing nonviable T. gondii antigens were less efficient in inducing T-lymphocyte IFN-γ secretion (48). Encounters between immune cells and dendritic cells bearing viable pathogens do not always favor induction of adaptive immunity (15, 33, 51, 52). We were thus interested in examining the presentation of viable T. gondii tachyzoites by human dendritic cells in the generation of T. gondii-specific immunity.
We now present a body of data demonstrating that T. gondii-infected human MDDCs are poor at activating T. gondii-specific lymphocyte proliferation, inhibit superantigen-mediated T-lymphocyte proliferation, appear to induce a state of general T-lymphocyte unresponsiveness, and are potent inducers of contact-dependent T-lymphocyte apoptosis.
Infection of MDDCs with T. gondii rapidly induced a partially mature dendritic cell phenotype with upregulation of CD86, de novo expression of CD83, and enhanced DR expression, confirming a recent report (48). However, in contrast to results presented in that report, T. gondii infection here did not upregulate CD80 or CD40 in dendritic cells. CD54 was also not increased, suggesting incomplete induction of dendritic cell maturation. We used T. gondii tachyzoites and MDDCs similar to those employed by Subauste and Wessendarp (48). Differences in experimental results may thus relate to the MOI, which was lower in the previous report than in our studies.
T. gondii strain Me49 is relatively avirulent for mice compared to strain RH. A2, a mutated RH strain, is avirulent (2). MDDCs infected with either Me49 or A2 tachyzoites induced a level of immunosuppression comparable to that of MDDCs infected with strain RH, demonstrating that immunosuppression was not strain specific and did not correlate with tachyzoite virulence for mice.
Inhibition of intracellular tachyzoite replication and reduction of dendritic cell parasitism reduced immunosuppression mediated by infected MDDCs in a dose-dependent fashion, suggesting a dependence on tachyzoite replication, contrasting with immunosuppression mediated by T. gondii-infected monocytes, which are immunosuppressive when bearing viable, nonreplicating tachyzoites (7). Interference with T. gondii attachment ligands did reverse MDDC-mediated immunosuppression as reported with infected monocytes (8). However, reversal of immunosuppression was proportional to reduced dendritic cell parasitism and may simply reflect the lower parasite burden in treated MDDCs. Thus, our data do not support an independent contribution of tachyzoite attachment ligands to MDDC-mediated immunosuppression.
Fixed, infected MDDCs were efficiently activated T lymphocytes from T. gondii-seropositive individuals, suggesting that T. gondii antigens were processed and presented in infected dendritic cells and that lack of T-lymphocyte activation was not due to a generic defect in antigen processing or presentation. This observation is consistent with a previous report (35).
By contrast, MDDCs acquiring nonviable T. gondii antigens directly or indirectly through acquisition of necrotic or apoptotic antigen-bearing cells induced potent T. gondii-specific T-lymphocyte proliferation. Thus, cross-priming, long the domain primarily of tumor immunologists, may have particular relevance to pathogens that infect dendritic cells, especially if such infection induces immunosuppression. At a low MOI, T. gondii-infected MDDCs induced T-lymphocyte activation, although significantly less efficiently than that induced by uninfected MDDCs. However, we propose that infected dendritic cells are unlikely to contribute to adaptive immunity as they induce local T-lymphocyte dysfunction, even in uninfected, bystander lymphocytes.
T. gondii-infected human monocytes induce IFN-γ-dependent immunosuppression of T lymphocytes that also involves additional soluble mediators other than IL-10, transforming growth factor β, and nitric oxide (4, 7). We excluded inhibition of T-lymphocyte activation by trace soluble mediators known to mediate immunosuppression during T. gondii infection, including reactive nitrogen intermediates, prostaglandins, IFN-α, and IFN-γ (4-6, 23, 55). It is therefore unlikely that soluble factors mediate immunosuppression induced by T. gondii-infected MDDCs.
Superantigen-mediated activation of highly purified CD3+ T lymphocytes was significantly impaired in the presence of T. gondii-infected highly purified CD1a+ MDDCs. We therefore conclude that the mechanism of T-lymphocyte immunosuppression mediated by T. gondii-infected MDDCs is distinct from that mediated by T. gondii-infected monocytes or macrophages and further that secondary effects of bystander cells are not involved.
Early after contact with infected MDDCs, T lymphocytes were significantly impaired in their capacity to respond to activation signals when only a minority were infected, suggesting a global defect in T-lymphocyte activation, as has been described for T. gondii-infected mice (24) and humans (53). We determined that T lymphocytes exposed to T. gondii-infected MDDCs underwent apoptosis, which increased between 24 and 48 h of coculture. On the other hand, apoptosis induced by MDDCs bearing the mitogen phytohemagglutinin or superantigen was maximal by 24 h, suggesting that apoptotic death induced by T. gondii-infected MDDCs is mechanistically distinct. We previously demonstrated that nonviable T. gondii antigens are T-lymphocyte mitogens but do not act as superantigens for humans (36), as has been proposed to occur with mice (12). As nonviable T. gondii antigens are not immunosuppressive and do not induce T-lymphocyte apoptosis (not shown), a mitogenic effect of T. gondii likely does not explain apoptosis. The additional apoptosis induced between 24 and 48 h by T. gondii-infected MDDCs may be explained by slow induction of a death ligand on infected MDDCs, slow upregulation of a death receptor on T lymphocytes, or general T-lymphocyte unresponsiveness, in which apoptosis is a secondary phenomenon. These are not mutually exclusive scenarios and are subjects of ongoing investigations. Induction of T-lymphocyte apoptosis has previously been observed in vivo in intraepithelial gut T lymphocytes of mice acutely infected with T. gondii (27).
T lymphocytes exposed to infected MDDCs upregulated CD69 and CD28 poorly compared to T lymphocytes exposed to uninfected MDDCs, consistent with observations in T. gondii-infected mice (24). Thus, activation-induced death likely does not explain T-lymphocyte apoptosis here. The finding of a uniform decrease in CD28 and CD69 expression, without a bimodal distribution, is consistent with a global T-lymphocyte defect, rather than a defect specific to apoptotic or infected cells, although the nature of that defect remains unknown at present.
Apoptosis of intraepithelial lymphocytes in mice infected orally with T. gondii is mediated by Fas ligation and depends on IFN-γ (27). However, in our experiments neutralization of IFN-γ did not reverse immunosuppression, and blockade of Fas did not inhibit T-lymphocyte apoptosis. Nonetheless, apoptosis was contact dependent and did not depend on soluble mediators. Blockade of other potential mediators on the dendritic cell or T-lymphocyte membrane, including tumor necrosis factor-related apoptosis-inducing ligand (9, 14), membrane-bound tumor necrosis factor alpha (30), leukocyte function antigen 1 (17), and intercellular adhesion molecule 1 (17), did not prevent apoptosis induction. Thus, if a dendritic cell ligand induces apoptosis, it may not be one commonly known to mediate this function.
Superantigen-induced T-lymphocyte thymidine incorporation was reduced up to 85% after 1 day of coculture with T. gondii-infected MDDCs, when only 5% of T lymphocytes were infected and only one-quarter were apoptotic. After 2 days of coculture, there was essentially no T-lymphocyte proliferation in cultures exposed to infected MDDCs in which less than half of lymphocytes were found to be infected or apoptotic by our detection methods. Thus, although apoptosis contributes to defective T-lymphocyte activation following exposure to T. gondii-infected MDDCs, additional factors likely also contribute. Occult apoptosis could explain these results, but we favor induction of global T-lymphocyte dysfunction, analogous to observations for mice as described below.
Mice acutely infected with T. gondii have significant defects in T-lymphocyte function. T-lymphocyte IL-2 production is defective, and exogenous IL-2 fails to reverse dysfunction (20). Defective nuclear factor-AT signaling is one potential mechanism for T. gondii-induced T-lymphocyte dysfunction (19). However, neither exogenous IL-7, which activates nuclear factor-AT, nor exogenous IL-2 reversed T-lymphocyte unresponsiveness in our cultures (S. Wei and T. Curiel, unpublished observations). This apparently global defect in T-lymphocyte function induced by T. gondii-infected MDDCs likely contributes to the immunosuppression of acute toxoplasmosis and requires further investigation. Its relative contribution to immunosuppression induced by infected monocytes or macrophages, which are more abundant at foci of infection, remains to be defined.
We previously demonstrated that T. gondii-infected cells, including both CD4+ and CD8+ T lymphocytes, were resistant to multiple inducers of apoptosis (32). In the present studies, MDDCs infected with T. gondii were potent inducers of T-lymphocyte apoptosis, which is an additional mechanism for immunosuppression. Thus, T. gondii can either protect from or induce apoptosis, depending on its cellular context. Regulation of apoptosis may be part of the immunopathogenesis of T. gondii infection and deserves further study.
Acknowledgments
Thanks to Elizabeth Kraus for invaluable assistance with flow cytometry, and to Viven L. Campbell of Roche Research Centre, Hertfordshire, United Kingdom, for the gift of ganciclovir. Green fluorescent protein-expressing tachyzoites were the gift of Boris Striepen.
Funding was provided by the NIH (grants R21AI44322 and R01AI39379) and the Baylor Endowment (T.J.C.).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 2.Berens, R. L., E. C. Krug, P. B. Nash, and T. J. Curiel. 1998. Selection and characterization of Toxoplasma gondii mutants resistant to artemisinin. J. Infect. Dis. 177:1128-1131. [DOI] [PubMed] [Google Scholar]
- 3.Brinkman, K., S. Debast, R. Sauerwein, F. Ooyman, J. Hiel, and J. Raemaekers. 1998. Toxoplasma retinitis/encephalitis 9 months after allogeneic bone marrow transplantation. Bone Marrow Transplant. 21:635-636. [DOI] [PubMed] [Google Scholar]
- 4.Candolfi, E., C. A. Hunter, and J. S. Remington. 1994. Mitogen- and antigen-specific proliferation of T cells in murine toxoplasmosis is inhibited by reactive nitrogen intermediates. Infect. Immun. 62:1995-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Candolfi, E., C. A. Hunter, and J. S. Remington. 1995. Roles of gamma interferon and other cytokines in suppression of the spleen cell proliferative response to concanavalin A and toxoplasma antigen during acute toxoplasmosis. Infect. Immun. 63:751-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Candolfi, E., O. Villard, M. Thouvenin, and T. T. Kien. 1996. Role of nitric oxide-induced immune suppression in toxoplasmosis during pregnancy and in infection by a virulent strain of Toxoplasma gondii. Curr. Top. Microbiol. Immunol. 219:141-154. [DOI] [PubMed] [Google Scholar]
- 7.Channon, J. Y., and L. H. Kasper. 1996. Toxoplasma gondii-induced immune suppression by human peripheral blood monocytes: role of gamma interferon. Infect. Immun. 64:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Channon, J. Y., E. I. Suh, R. M. Seguin, and L. H. Kasper. 1999. Attachment ligands of viable Toxoplasma gondii induce soluble immunosuppressive factors in human monocytes. Infect. Immun. 67:2547-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke, P., S. M. Meintzer, S. Gibson, C. Widmann, T. P. Garrington, G. L. Johnson, and K. L. Tyler. 2000. Reovirus-induced apoptosis is mediated by TRAIL. J. Virol. 74:8135-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curiel, T. J., E. C. Krug, M. B. Purner, P. Poignard, and R. L. Berens. 1993. Cloned human CD4+ cytotoxic T lymphocytes specific for Toxoplasma gondii lyse tachyzoite-infected target cells. J. Immunol. 151:2024-2031. [PubMed] [Google Scholar]
- 11.Curiel, T. J., J. T. Wong, P. F. Gorczyca, R. T. Schooley, and B. D. Walker. 1993. CD4+ human immunodeficiency virus type 1 (HIV-1) envelope-specific cytotoxic T lymphocytes derived from the peripheral blood cells of an HIV-1-infected individual. AIDS Res. Hum. Retrovir. 9:61-68. [DOI] [PubMed] [Google Scholar]
- 12.Denkers, E. Y., P. Caspar, and A. Sher. 1994. Toxoplasma gondii possesses a superantigen activity that selectively expands murine T cell receptor V beta 5-bearing CD8+ lymphocytes. J. Exp. Med. 180:985-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadul, C. E., J. Y. Channon, and L. H. Kasper. 1995. Survival of immunoglobulin G-opsonized Toxoplasma gondii in nonadherent human monocytes. Infect. Immun. 63:4290-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanger, N. A., C. R. Maliszewski, K. Schooley, and T. S. Griffith. 1999. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J. Exp. Med. 190:1155-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 17.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 18.Grimwood, J., and J. E. Smith. 1996. Toxoplasma gondii: the role of parasite surface and secreted proteins in host cell invasion. Int. J. Parasitol. 26:169-173. [DOI] [PubMed] [Google Scholar]
- 19.Haque, S., H. Dumon, A. Haque, and L. H. Kasper. 1998. Alteration of intracellular calcium flux and impairment of nuclear factor-AT translocation in T cells during acute Toxoplasma gondii infection in mice. J. Immunol. 161:6812-6818. [PubMed] [Google Scholar]
- 20.Haque, S., I. Khan, A. Haque, and L. Kasper. 1994. Impairment of the cellular immune response in acute murine toxoplasmosis: regulation of interleukin 2 production and macrophage-mediated inhibitory effects. Infect. Immun. 62:2908-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joiner, K. 1991. Cell attachment and entry by Toxoplasma gondii. Behring Inst. Mitt. 1991:20-26. [PubMed] [Google Scholar]
- 22.Jones, T. C. 1981. Interactions between murine macrophages and obligate intracellular protozoa. Am. J. Pathol. 102:127-132. [PMC free article] [PubMed] [Google Scholar]
- 23.Kalinski, P., J. H. Schuitemaker, C. M. Hilkens, and M. L. Kapsenberg. 1998. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J. Immunol. 161:2804-2809. [PubMed] [Google Scholar]
- 24.Khan, I. A., T. Matsuura, and L. H. Kasper. 1996. Activation-mediated CD4+ T cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int. Immunol. 8:887-896. [DOI] [PubMed] [Google Scholar]
- 25.Khan, I. A., T. Matsuura, and L. H. Kasper. 1995. IL-10 mediates immunosuppression following primary infection with Toxoplasma gondii in mice. Parasite Immunol. 17:185-195. [DOI] [PubMed] [Google Scholar]
- 26.Krug, E. C., J. J. Marr, and R. L. Berens. 1989. Purine metabolism in Toxoplasma gondii. J. Biol. Chem. 264:10601-10607. [PubMed] [Google Scholar]
- 27.Liesenfeld, O., J. C. Kosek, and Y. Suzuki. 1997. Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii. Infect. Immun. 65:4682-4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luft, B. J., and J. S. Remington. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect Dis. 15:211-222. [DOI] [PubMed] [Google Scholar]
- 29.Miller, N. L., J. K. Frenkel, and J. P. Dubey. 1972. Oral infections with Toxoplasma cysts and oocysts in felines, other mammals, and in birds. J. Parasitol. 58:928-937. [PubMed] [Google Scholar]
- 30.Monastra, G., A. Cabrelle, A. Zambon, A. Rosato, B. Macino, D. Collavo, and P. Zanovello. 1996. Membrane form of TNF alpha induces both cell lysis and apoptosis in susceptible target cells. Cell. Immunol. 171:102-110. [DOI] [PubMed] [Google Scholar]
- 31.Montoya, J. G., and J. S. Remington. 1996. Toxoplasmic chorioretinitis in the setting of acute acquired toxoplasmosis. Clin. Infect Dis. 23:277-282. [DOI] [PubMed] [Google Scholar]
- 32.Nash, P. B., M. B. Purner, R. P. Leon, P. Clarke, R. C. Duke, and T. J. Curiel. 1998. Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. J. Immunol. 160:1824-1830. [PubMed] [Google Scholar]
- 33.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, L. Hoffman, S. Gezelter, G. Schuler, P. U. Cameron, and R. M. Steinman. 1995. Dendritic cell-T cell conjugates that migrate from normal human skin are an explosive site of infection for HIV-1. Adv. Exp. Med. Biol. 378:457-460. [DOI] [PubMed] [Google Scholar]
- 34.Porter, S. B., and M. A. Sande. 1992. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N. Engl. J. Med. 327:1643-1648. [DOI] [PubMed] [Google Scholar]
- 35.Purner, M. B., R. L. Berens, P. B. Nash, A. van Linden, E. Ross, C. Kruse, E. C. Krug, and T. J. Curiel. 1996. CD4-mediated and CD8-mediated cytotoxic and proliferative immune responses to Toxoplasma gondii in seropositive humans. Infect. Immun. 64:4330-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purner, M. B., R. L. Berens, S. Tomavo, L. Lecordier, M. F. Cesbron-Delauw, B. L. Kotzin, and T. J. Curiel. 1998. Stimulation of human T lymphocytes obtained from Toxoplasma gondii-seronegative persons by proteins derived from T. gondii. J. Infect. Dis. 177:746-753. [DOI] [PubMed] [Google Scholar]
- 37.Purner, M. B., E. C. Krug, P. Nash, D. R. Cook, R. L. Berens, and T. J. Curiel. 1995. Cross-reactivity of human Toxoplasma-specific T cells: implications for development of a potential immunotherapeutic or vaccine. J. Infect. Dis. 171:984-991. [DOI] [PubMed] [Google Scholar]
- 38.Radke, J. R., and M. W. White. 1998. A cell cycle model for the tachyzoite of Toxoplasma gondii using the Herpes simplex virus thymidine kinase. Mol. Biochem. Parasitol. 94:237-247. [DOI] [PubMed] [Google Scholar]
- 39.Remington, J. S., and E. N. Cavanaugh. 1965. Isolation of the encysted form of Toxoplasma gondii from human skeletal muscle and brain. N. Engl. J. Med. 273:1308-1310. [DOI] [PubMed] [Google Scholar]
- 40.Renold, C., A. Sugar, J. P. Chave, L. Perrin, J. Delavelle, G. Pizzolato, P. Burkhard, V. Gabriel, and B. Hirschel. 1992. Toxoplasma encephalitis in patients with the acquired immunodeficiency syndrome. Medicine (Baltimore) 71:224-239. [DOI] [PubMed] [Google Scholar]
- 41.Seguin, R., and L. H. Kasper. 1999. Sensitized lymphocytes and CD40 ligation augment interleukin-12 production by human dendritic cells in response to Toxoplasma gondii. J. Infect. Dis. 179:467-474. [DOI] [PubMed] [Google Scholar]
- 42.Sher, A., E. Y. Denkers, and R. T. Gazzinelli. 1995. Induction and regulation of host cell-mediated immunity by Toxoplasma gondii. Ciba Found. Symp. 195:95-104. [DOI] [PubMed] [Google Scholar]
- 43.Sher, A., and C. Reis e Sousa. 1998. Ignition of the type 1 response to intracellular infection by dendritic cell-derived interleukin-12. Eur. Cytokine Netw. 9:65-68. [PubMed] [Google Scholar]
- 44.Singh, N., T. Gayowski, and I. R. Marino. 1996. Toxoplasma gondii pneumonitis in a liver transplant recipient: implications for diagnosis. Liver Transpl. Surg. 2:299-300. [DOI] [PubMed] [Google Scholar]
- 45.Sousa, C. R., S. Hieny, T. Scharton-Kersten, D. Jankovic, H. Charest, R. N. Germain, and A. Sher. 1997. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strickland, G. T., and P. C. Sayles. 1977. Depressed antibody responses to a thymus-dependent antigen in toxoplasmosis. Infect. Immun. 15:184-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Striepen, B., C. Y. He, M. Matrajt, D. Soldati, and D. S. Roos. 1998. Expression, selection, and organellar targeting of the green fluorescent protein in Toxoplasma gondii. Mol. Biochem. Parasitol. 92:325-338. [DOI] [PubMed] [Google Scholar]
- 48.Subauste, C. S., and M. Wessendarp. 2000. Human dendritic cells discriminate between viable and killed Toxoplasma gondii tachyzoites: dendritic cell activation after infection with viable parasites results in CD28 and CD40 ligand signaling that controls IL-12-dependent and -independent T cell production of IFN-gamma. J. Immunol. 165:1498-1505. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki, Y., and A. Kobayashi. 1985. Macrophage-mediated suppression of immune responses in Toxoplasma-infected mice. II. Both H-2-linked and -nonlinked control of induction of suppressor macrophages. Cell. Immunol. 91:375-384. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki, Y., A. Sher, G. Yap, D. Park, L. E. Neyer, O. Liesenfeld, M. Fort, H. Kang, and E. Gufwoli. 2000. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 164:5375-5382. [DOI] [PubMed] [Google Scholar]
- 51.Urban, B. C., D. J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73-77. [DOI] [PubMed] [Google Scholar]
- 52.Van Overtvelt, L., N. Vanderheyde, V. Verhasselt, J. Ismaili, L. De Vos, M. Goldman, F. Willems, and B. Vray. 1999. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: inhibition of cytokines, HLA-DR, and costimulatory molecules. Infect. Immun. 67:4033-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yano, A., K. Norose, K. Yamashita, F. Aosai, K. Sugane, K. Segawa, and S. Hayashi. 1987. Immune response to Toxoplasma gondii-analysis of suppressor T cells in a patient with symptomatic acute toxoplasmosis. J. Parasitol. 73:954-961. [PubMed] [Google Scholar]
- 54.Yap, G. S., and A. Sher. 1999. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology 201:240-247. [DOI] [PubMed] [Google Scholar]
- 55.Zagury, D., A. Lachgar, V. Chams, L. S. Fall, J. Bernard, J. F. Zagury, B. Bizzini, A. Gringeri, E. Santagostino, J. Rappaport, M. Feldman, A. Burny, and R. C. Gallo. 1998. Interferon alpha and Tat involvement in the immunosuppression of uninfected T cells and C-C chemokine decline in AIDS. Proc. Natl. Acad. Sci. USA 95:3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou, L. J., and T. F. Tedder. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. USA 93:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou, W., J. Borvak, F. Marches, S. Wei, P. Galanaud, D. Emilie, and T. J. Curiel. 2000. Macrophage-derived dendritic cells have strong Th1-polarizing potential mediated by beta-chemokines rather than IL-12. J. Immunol. 165:4388-4396. [DOI] [PubMed] [Google Scholar]