Abstract
Interleukin-8 (IL-8), a C-X-C chemokine bound to endothelium proteoglycans, initiates the activation and selective recruitment of leukocytes at inflammatory foci. We demonstrate that human lactoferrin, an antimicrobial lipopolysaccharide (LPS)-binding protein, decreases both IL-8 mRNA and protein expression induced by the complex Escherichia coli 055:B5 LPS/sCD14 in human umbilical vein endothelial cells. The use of recombinant lactoferrins mutated in the LPS-binding sites indicates that this inhibitory effect is mediated by an interaction of lactoferrin with LPS and CD14s that suppresses the endotoxin biological activity. Furthermore, since dimeric IL-8 and lactoferrin are both proteoglycan-binding molecules, the competition between these proteins for heparin binding was investigated. Lactoferrin strongly inhibited the interaction of radiolabeled IL-8 to immobilized heparin, whereas a lactoferrin variant lacking the amino acid residues essential for heparin binding was not inhibitory. Moreover, this process is specific, since serum transferrin, a glycoprotein whose structure is close to that of lactoferrin, did not prevent the interaction of IL-8 with heparin. These results suggest that the anti-inflammatory properties of lactoferrin during septicemia are related, at least in part, to the regulation of IL-8 production and also to the ability of lactoferrin to compete with chemokines for their binding to proteoglycans.
During the inflammatory process, the vascular endothelium expresses various chemoattractant and cell adhesion molecules that result in the selective recruitment of leukocytes to inflammatory foci (8, 34). In that process, the selectins and their ligands first initiate the rolling of leukocytes. Then chemokines are expressed that stimulate integrins responsible for the strong adherence of cells to endothelium (10). Interleukin-8 (IL-8), a potent C-X-C chemokine, activates LFA-1 integrins (LFA-1) on neutrophils (3, 10) and also binds, as a dimer and with a low affinity, to heparin and heparan sulfate molecules (16, 20, 40). Once firmly attached, the cells are directed by a chemoattractant gradient to transmigrate into tissue affected by injury or infection (38). The cell surface proteoglycans increase the local concentration of IL-8 that, in turn, regulates the activation of neutrophils through specific interactions with a G protein coupled receptor (30). Thus, interactions between IL-8 and proteoglycans present on the endothelium or in the extracellular matrix drive the formation of haptotactic chemokine gradients to the inflammatory sites. This critical step in the innate immune response involves the concerted action of adhesion molecules and chemokines.
When highly expressed, IL-8 may have pathophysiological consequences for the organism. Various exogenous stimuli, such as lipopolysaccharides (LPS) and proinflammatory cytokines (tumor necrosis factor alpha, IL-1), induce the secretion of IL-8 from many cells, including endothelial cells (15, 36). During septic shock, LPS strongly activate the endothelial cells and promote leukocyte infiltration and microvascular thrombosis. This contributes to the pathogenesis of disseminated intravascular inflammation, leading to severe damage of endothelium (9). Endotoxin stimulation of endothelial cells is mediated by soluble CD14 (sCD14), a specific LPS receptor present in serum (2, 14). sCD14 binds to LPS with a high affinity and interacts with a signaling molecule, the Toll-4-like receptor (7, 12). At low endotoxin levels, a serum acute protein called the LPS-binding protein (LBP) catalyzes the transfer of LPS monomers from aggregates to CD14 and enhances the sensitivity of cells to LPS (13).
Lactoferrin (Lf), an iron-binding glycoprotein found in exocrine secretions of mammals and released from granules of neutrophils during inflammation (28), modulates the endotoxin activation of cells in vivo and in vitro (5). Lf protects against sublethal doses of LPS in mice (26, 44) and in germfree piglets (21). Moreover, human Lf (hLf) binds specifically and with a high affinity to the lipid A regions of LPS (1, 11), sCD14, and the sCD14/LPS complex (4). Two N-terminal basic clusters of hLf, residues 1 to 5 and 28 to 34, are responsible for the binding to anionic molecules, such as LPS (11, 41), heparin, or cell-surface heparan sulfates (22, 27, 43). Recently interactions between Lf, LPS, and the LPS/CD14s complex were demonstrated that impede the expression of two adhesion molecules, E-selectin and the intercellular adhesion molecule 1 (ICAM-1), an integrin ligand, on human umbilical vascular endothelial cells (HUVEC) (4). These observations indicate that Lf may down-regulate the adherence of leukocytes to endothelial cells. We hypothesized that hLf may also inhibit the expression and the function of chemokines, thus limiting not only the binding of leukocytes to the endothelium but also their migration to inflamed tissues.
In this paper the effect of hLf on the expression of IL-8 induced by LPS in endothelial cells and its binding to proteoglycans was investigated. We first studied the inhibitory effect of hLf on the expression of IL-8 in LPS-activated HUVEC. Then we determined whether hLf could compete with IL-8 for binding to glycosaminoglycans. Finally, an insight in the role of domain N-I of hLf in the inhibition of IL-8 production was gained with mutated recombinant hLfs or hLf variants.
MATERIALS AND METHODS
Materials.
RPMI 1640 medium, obtained from Gibco-BRL (Eragny, France), was supplemented with 10% fetal calf serum (FCS), which was purchased from Dominique Dutscher S.A. (Brumath, France), and with 2 mM l-glutamine from Gibco-BRL. Endothelial cell growth medium SFM, endothelial cell growth supplement (heparin, epidermal growth factor basic fibroblast growth factor (bFGF), hydrocortisone, gentamicin, and amphotericin B), and FCS (10%) were from PromoCell (Heidelberg, Germany). SP-Sepharose fast flow and PD10 G-25 columns were from Pharmacia (Uppsala, Sweden). The apyrogen water was from Cooper (Melun, France). Dulbecco's phosphate-buffered saline (PBS), human serum transferrin, bovine serum albumin (BSA), collagenase, gelatin, heparin-BSA (H0403), heparin Grade IA from porcine intestinal mucosal (H3149), and LPS 055:B5 from Escherichia coli were purchased from Sigma Chemical Co. (St. Louis, Mo.). Recombinant human IL-8 expressed in E. coli was from Sigma Chemical Co., and radiolabeled 125I-IL-8 was from Amersham (specific activity, 2,000 Ci/mmol). Recombinant human sCD14 was purchased from Biometec (Greifswald, Germany). sCD14 was obtained from serum-free culture supernatant of CHO cells transfected with human sCD14 cDNA cloned into pPOL-DHFR expression vector. Human IL-8/Nap-1 module set (BSM204MST) was from Bender Medsystems Diagnostic (Vienna, Austria).
All chemicals used were of the highest analytical grade, and LPS contamination was evaluated with the Limulus amoebocyte lysate assay kit (QCL1000; BioWhittaker, Walkersville, Md.).
Endothelial cell culture.
Endothelial cells (HUVEC) were derived from human umbilical vein, according to a method previously described (17). Briefly, after treatment of the umbilical vein with 0.2% (wt/vol) collagenase in 37°C prewarmed RPMI for 30 min, HUVEC were collected by centrifugation (600 × g for 15 min). Cells were resuspended in endothelial cell growth medium SFM and were cultured in gelatin-coated 35-mm-diameter tissue culture wells at 37°C and 5% CO2. They were collected after trypsinization and then were cultured in gelatin-coated 96- or 6-well flat-bottomed culture plates until confluency. Only cells of the third and fourth passages were used. Viability was over 96% as determined by trypan blue dye exclusion.
Preparation of nhLf and hLf(−3N).
Native hLf (nhLf) was purified from fresh human milk (provided by the milk bank of “Jeanne de Flandres” Hospital, Lille, France) by cation exchange chromatography and was iron saturated, as previously reported (29, 39). Homogeneity of the protein was checked by sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis (SDS-PAGE). Since the binding of LPS to Lf was abrogated by NaCl concentrations of greater than 0.4 M (41), 50 mg of purified nhLf was injected on a 7- by 1-cm SP-Sepharose fast flow column equilibrated in 0.1 M NaCl and then washed with 70 ml of 0.5 M NaCl. Native hLf was eluted with 2 M NaCl and desalted on a PD10 G25 column equilibrated in 0.1 M NaCl. All buffers were prepared with pyrogen-free water. Mild tryptic digestion of nhLf gave the N-terminal deletion of the first three amino acids residues, 1GRR3, as reported previously (22), and was designated hLf(−3N) (residues 4 to 692). The LPS contamination of both nhLf and hLf(−3N) was less than 50 pg of endotoxin/mg of protein, as estimated by the Limulus amoebocyte lysate assay (QCL1000; BioWhittaker, Walkersville, Md.).
Expression and purification of recombinant and mutated hLfs.
The expression and purification of various hLf mutants were performed as previously described (23, 35). A full-length 2.3-kbp cDNA coding for hLf was obtained from a human mammary gland cDNA library (Clontech, Palo Alto, Calif.). Three human recombinant hLf (rhLf) variants were obtained by site-directed mutagenesis of the cDNA-coding hLf sequence by using the Sculptor in vitro mutagenesis system kit (Amersham International, Buckinghamshire, United Kingdom). Nonmodified rhLf; G4R-rhLf, a mutated rhLf in which sequence 1GRRRR5 was deleted; EGS-rhLf, an rhLf whose sequence 28RKVRGPP34 was replaced by EGS (the 365 to 367 C-terminal counterpart of sequence 28 to 34); and G4R-EGS-rhLf, a rhLf with both G4R and EGS mutations, were produced in a baculovirus expression system and were purified as previously reported (23). The purity of the rhLf mutants was checked by SDS-7.5% PAGE. The N-terminal amino acid sequence was checked by the Edman degradation procedure by using an Applied Biosystem 477 protein sequencer.
Activation of endothelial cells by LPS in the presence of hLf.
HUVEC were seeded into 6-well plates for studying IL-8 mRNA expression or into 96-well plates for assaying the IL-8 production. HUVEC grown to confluence were washed twice and incubated in RPMI-FCS in the presence of 10 to 1,000 ng of E. coli 055:B5 LPS/ml. The effect of hLf on IL-8 mRNA expression by HUVEC was investigated in the presence of 50 μg of nhLf/ml. To study the effect of hLf on IL-8 production, 5- to 150-μg/ml concentrations of either nhLf, rhLf, hLf(−3N), or mutated rhLfs (EGS-rhLf, G4R-rhLf, G4R-EGS-rhLf) were incubated with the cells. These Lf concentrations are those encountered at the inflammatory sites (6, 25). Before incubation with cells, nhLf and its variants were preincubated for 30 min at room temperature with LPS. Controls were performed without LPS and hLf or with hLf alone. After 5 h of incubation at 37°C and 5% CO2, the cells were washed immediately prior to RNA extraction. Prior to IL-8 protein assays, confluent cells were incubated at 37°C with RPMI-FCS for 18 h. Viability was over 96% as determined by trypan blue dye exclusion. The supernatants were collected and centrifuged prior to quantitation of IL-8 by enzyme-linked immunosorbent assay (ELISA).
In some experiments, 2 μg of purified recombinant sCD14/ml was preincubated for 30 min at room temperature with LPS or with LPS and nhLf and then added for 5 h with cells at 37°C in RPMI without FCS. Controls were performed with sCD14 alone and with sCD14 in the presence of nhLf. The effect of nhLf on the production of IL-8 induced by sCD14-LPS was investigated in conditions similar to those described above.
RNA extraction from LPS-induced endothelial cells and RT-PCR.
Extraction of total RNA from HUVEC was performed with the Total Quick RNA Cells and Tissues kit (Talent srl, Trieste, Italy) according to the manufacturer's instructions. Five micrograms of each total RNA preparation was reverse transcribed (RT) into first-strand cDNA by using oligo(dT) primers (Stratagene) and 20 U of Moloney murine leukemia virus reverse transcriptase (Promega). One twentieth of the mixture was then amplified by PCR with a primer pair (Cybergène, Saint-Malo, France) designed for the specific detection of human IL-8: 5′-CCCAAATTTATCAAAGAACT-3′ (sense primer) and 5′-AATTTAACCAGGAATCTTGT-3′ (antisense primer). ICAM-2 was used as a reference, since it was found to be constitutively expressed in HUVEC cells (31). First-strand sequence amplification was performed with Tfl polymerase (Promega) and the following steps: an initial denaturation at 94°C for 5 min, multiple cycles (25 and 35 cycles for IL-8 and ICAM-2, respectively) consisting of denaturation at 94°C for 5 min, annealing at the optimal temperature (55 and 60°C for IL-8 and ICAM-2, respectively) for 1 min, and a primer extension at 74°C for 1 min, followed by a final extension step at 74°C for 1 min. PCR assays were performed in triplicate. Twenty out of 25 μl of each PCR was loaded on a 1% agarose gel stained with 0.5 mg of ethidium bromide/ml. The gel was then analyzed by computerized densitometric imaging with the Bio-Rad GelDoc analysis system and Quantity One software, version 4.1.0 (Bio-Rad, Milano, Italy). Amplification products were subcloned in TA cloning vector (Invitrogen BV, Leek, The Netherlands) and were sequenced to confirm the specificity of the PCR products. The results were expressed as the ratio of the fluorescence intensities of IL-8 and ICAM-2 PCR products.
Quantitation of IL-8 release by ELISA.
HUVEC plated into gelatin-coated 96-well plates and grown to confluence were stimulated by LPS as described above. Cell culture supernatants were diluted fourfold, and 100 μl was processed for IL-8 quantification by sandwich ELISA according to the manufacturer's instructions (Bender Medsystems Diagnostic). Briefly, microtiter plates were coated overnight at 4°C with 100 μl of (5 μg/ml) anti-IL-8 monoclonal antibody diluted in PBS, washed with PBS-0.05% (vol/vol) Tween 20, and blocked for 2 h with 200 μl of PBS-0.05% Tween containing 0.5% (wt/vol) BSA. Supernatants diluted in PBS buffer-0.05% Tween-0.1% Triton X-100 were then transferred to the wells. After 2 h of incubation at room temperature with a second peroxidase-conjugated anti-IL-8 polyclonal antibody (100 μl of a 1/6,500 dilution), the wells were washed and detection was performed with o-phenylenediamine-dihydrochloride (150 μl) for 20 min at room temperature. The reaction was stopped with 50 μl of 2 M H2SO4 per well, and the absorbance at 490 nm was measured on a microplate reader. IL-8 concentrations in the cell culture supernatant were quantitated in comparison with a standard curve generated with recombinant human IL-8.
The number of unstimulated cells present in each well was estimated by assaying the protein contents according to Lowry's method (24).
Competitive inhibition of the IL-8 binding to immobilized heparin.
Microtiter plates (Immobilon) were coated for 24 h at 4°C with 100 μl of (10 μg/ml) heparin-BSA in PBS. After being washed with PBS, wells were blocked with 200 μl of 1% BSA in PBS for 2 h at room temperature. The solution was discarded and plates were washed three times with PBS. The binding of IL-8 to immobilized heparin-BSA was assessed in the presence of 0.5 nM 125I-IL-8 and 5 nM unlabeled chemokine by using binding buffer (PBS [pH 7.4] containing 5 mM MgCl2, 1 mM CaCl2, and 0.5% BSA) for 4 h at 20°C. As previously reported (16, 20), the addition of unlabeled IL-8 in the nanomolar range increases the binding of radiolabeled IL-8 to heparin by forming chemokine multimers. Inhibition of 125I-IL-8 binding to immobilized heparin-BSA was assessed with increasing concentrations of heparin, human serum transferrin, nhLf, rhLf, hLf (−3N), or mutated rhLfs (EGS-rhLf, G4R-rhLf, G4R-EGS-rhLf). Under similar conditions, 125I-IL-8 binding to heparin-free tubes coated with BSA was estimated and deduced from the binding to heparin-BSA. The results were expressed as a percentage of the total IL-8 binding to heparin. IC50 was determined with the equation B/Bmax = 1/1(1 + [L]/IC50). IC50 represents the amount of unlabeled competitor required to inhibit the binding of IL-8 ligand by 50%. B and Bmax are the amounts of IL-8 bound to heparin, in the presence or absence of competitor, respectively, and [L] is the concentration of the competing ligand.
Statistical analysis.
Data are presented as the means ± standard errors for the indicated number of independent experiments. Statistical significance was analyzed with a Student's t test for unpaired data. Values of P < 0.05 were considered to be significant.
RESULTS
Effect of hLf on the expression of IL-8 by LPS-stimulated endothelial cells.
In order to determine if hLf may modulate the expression of IL-8 by LPS-stimulated endothelial cells, we studied the IL-8 mRNA expression and protein production by HUVEC cells in the presence of various amounts of LPS and nhLf.
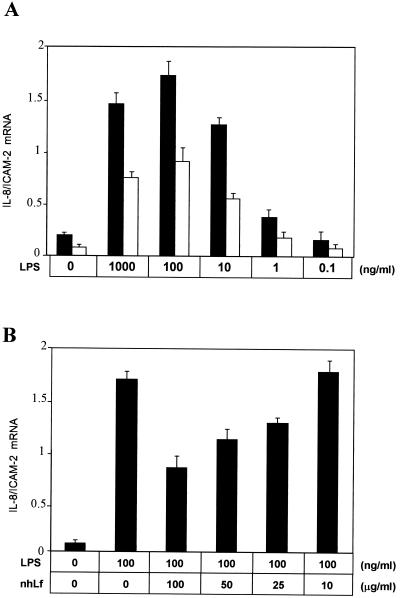
First, the expression of IL-8 mRNA was assessed by RT-PCR (Fig. 1). The IL-8 mRNA expression by HUVEC was clearly dependent on the LPS concentration (0.1 to 1,000 ng/ml) and was maximal when cells were incubated with 100 ng of LPS/ml. The preincubation of LPS with 50 μg of nhLf/ml induced a twofold decrease of IL-8 mRNA expression (Fig. 1A). This phenomenon was observed at any LPS concentration used for the activation of cells. The inhibition was nhLf dose-dependent and reached 53% with 100 ng of LPS/ml in the presence of 100 μg of nhLf/ml (Fig. 1B).
FIG. 1.
Effect of hLf on IL-8 mRNA expression in LPS-stimulated HUVEC. (A) Endothelial cells were incubated for 5 h with various concentrations of LPS in 10% FCS-containing medium alone (▪) or in the presence (□) of 50 μg of nhLf/ml. (B) Cells were exposed to 100 ng of LPS/ml with 10% FCS for 5 h in the presence of increasing concentrations of nhLf. Total IL-8 and ICAM-2 mRNAs were then extracted and RT-PCR was performed as described in Materials and Methods. ICAM-2 was used as a constitutively expressed marker in HUVEC, and the ratios of the fluorescence intensities of IL-8 and ICAM-2 PCR products are shown. Data presented are means ± standard errors of four separate experiments.
Second, the production of IL-8 by LPS-stimulated HUVEC in the presence of nhLf was assessed by ELISA. As shown in Fig. 2A, the exposure of cells to LPS in the presence of 10% FCS resulted in a dose-dependent increase in IL-8 secretion, whereas nhLf alone had no effect. Similar to findings of a previous study (15), we detected about three- and fourfold higher production of IL-8 with 10 and 100 ng of LPS/ml, respectively, compared to that of unstimulated cells. The IL-8 secretion induced by endotoxin was significantly decreased in the presence of nhLf; 32% ± 3% and 51% ± 5% inhibitions were measured when cells were incubated in the presence of 100 μg of nhLf/ml and induced with 10 and 100 ng of LPS/ml, respectively. Furthermore, hLf blocked the secretion of IL-8 in a concentration-dependent manner (Fig. 2B). Up to 54% ± 3% inhibition was measured with 100 ng of LPS/ml in the presence of 150 μg of nhLf/ml. These findings demonstrate that Lf interferes with both endotoxin-induced IL-8 mRNA expression and production by endothelial cells.
FIG. 2.
Effect of hLf on IL-8 production induced in LPS-activated endothelial cells in the presence of serum. (A) HUVEC were incubated for 5 h with 10 or 100 ng of LPS/ml and 10% FCS in the absence (▪) or in the presence of 50 (□) or 100 μg (░⃞) of nhLf/ml. After being washed, cells were maintained for 18 h at 37°C and the amounts of IL-8 in the supernatants were assayed by ELISA technique. (B) HUVEC were incubated for 5 h with 100 ng of LPS/ml and 10% FCS in the presence of increasing nhLf concentrations, from 0 to 150 μg/ml. The results are expressed as percentages of the total IL-8 production obtained with 100 ng of LPS/ml alone and after subtraction of the basal IL-8 production of unstimulated cells. Data presented are means ± standard errors of four separate experiments.
Importance of the N-terminal domain of hLf in the inhibition of IL-8 production by LPS-induced endothelial cells.
The N-terminal domain I of hLf and, more precisely, the sequences involving amino acid residues 1GRRRR5 and 28RKVRGPP34, have been identified as LPS-binding sites; thus, we investigated by ELISA whether the hLf mutated in these two N-terminal basic clusters could modify the LPS-induced IL-8 production. As illustrated in Fig. 3, IL-8 secretion was decreased by 43% ± 3% in the presence of 100 μg of rhLf/ml. In contrast, neither rhLf changed between residues 28 to 34 (EGS-rhLf) nor G4R-EGS-rhLf lacking residues 1 to 5 and changed at residues 28 to 34 were able to prevent the IL-8 production in HUVEC. No more than 6% ± 6% inhibition was detected in the presence of 100-μg/ml concentrations of these mutants. Moreover, no clear inhibitory effect was measured with 50 μg of G4R-rhLf/ml, and the addition of 100 μg of the protein/ml resulted in a twofold less inhibition (18% ± 4%) than that of rhLf. The deletion of only the first three residues from hLf [hLf(−3N)] partially modified the IL-8 production of cells. About 15% ± 3% inhibition was obtained with 50 μg of hLf(−3N)/ml and up to 30% ± 5% with 100 μg of the protein. Nonmutated rhLf exhibited inhibitory effects similar to those of nhLf. These experiments indicate that the two basic N-terminal clusters involving both residues 1 to 5 and 28 to 34 of hLf are essential for the inhibitory effect of hLf on the LPS-stimulated IL-8 production.
FIG. 3.
Effect of hLf variants on the secretion of IL-8 by LPS-activated HUVEC. Cells were incubated for 5 h with 100 ng of LPS/ml and 10% FCS in the absence (░⃞) or in the presence of variants of 50 (▪) or 100 μg (□) of hLf, hLf(−3N), G4R-rhLf, EGS-rhLf, or G4R-EGS-rhLf, as described in Materials and Methods. Controls were performed with each hLf variant in the absence of LPS (data not shown). The results are expressed as percentages of the total IL-8 production induced by 100 ng of LPS/ml and after subtraction of the basal IL-8 production of unstimulated cells. Data presented are means ± standard errors of four separate experiments.
Inhibition by hLf of the IL-8 production induced by the sCD14-LPS complex in endothelial cells.
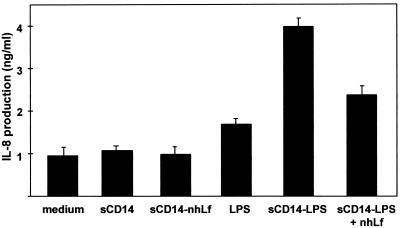
Serum sCD14 mediates the binding of LPS to endothelial cells (14). Elsewhere, interactions between Lf and either sCD14 or the sCD14-LPS complex were recently described (4). These interactions may be relevant to the inhibiting properties of hLf on IL-8 production by LPS-stimulated HUVEC. Thus, we studied the production of IL-8 induced by the sCD14-LPS complex in the presence of various concentrations of nhLf. As shown in Fig. 4, the production of IL-8 was threefold increased in the presence of complexed sCD14 and LPS compared to that of the controls with medium, sCD14, sCD14 with nhLf, or LPS alone. Inhibition of IL-8 production was dependent on the nhLf concentration (data not shown). About 46% ± 3% inhibition was detected at 200 μg of nhLf/ml (Fig. 4). These results show that the inhibition effect of hLf is related to its interaction with the sCD14-LPS complex.
FIG. 4.
Effect of hLf on IL-8 production induced in LPS-activated endothelial cells in the presence of recombinant sCD14. HUVEC were activated with 100 ng of LPS/ml preincubated for 30 min at room temperature with 2 μg of human recombinant sCD14/ml in the absence or in the presence of 200 μg of nhLf/ml. After 5 h of activation, cells were washed and incubated with RPMI-FCS for 18 h at 37°C. The supernatants were collected and processed for IL-8 quantification by ELISA. Data presented are means ± standard errors of three separate experiments.
Inhibition of IL-8 binding to immobilized heparin by hLf.
Cell surface proteoglycans of endothelial cells are required for presenting highly diffusible inflammatory chemokines to leukocytes (16, 20, 40). IL-8 homodimers bind to heparan sulfate and heparin. Since hLf interacts with proteoglycans (22, 27, 43), we hypothesized that hLf may interfere with the presentation of IL-8 on glycosaminoglycans. To check this hypothesis, the binding of radiolabeled IL-8 to immobilized heparin-BSA was analyzed in the presence of increasing concentrations of nhLf (Fig. 5A). Human serum transferrin and soluble heparin were used as controls (Fig. 5A). Both nhLf and soluble heparin were able to strongly compete with 125I-IL-8 for the binding to immobilized heparin-BSA. As little as 10 μg of nhLf/ml yielded 58% ± 3% inhibition. Lf was a more effective competitor than the soluble heparin molecule. In contrast, serum transferrin, whose structure is highly homologous to that of hLf, did not prevent the 125I-IL-8 binding to heparin-BSA.
FIG. 5.
Inhibition of IL-8 binding to immobilized heparin by hLf, hLf variants, serum transferrin, and soluble heparin. Heparin-BSA was immobilized on microtiter plates, and the binding of [125I]IL-8, in the presence of increasing concentrations of competitors, was determined as described in Materials and Methods. Competitors shown in panel A are soluble heparin (▪), human serum transferrin (▴), and nhLf (•); competitors shown in panel B are rhLf (•), hLf(−3N) (▪), G4R-rhLf (○), EGS-rhLf (▴), and G4R-EGS-rhLf (□). The results are expressed as percentages of the total IL-8 bound to heparin. Data presented are means ± standard errors of four separate experiments.
Importance of the N-terminal domain of hLf for the inhibition of the IL-8 binding to immobilized heparin.
We investigated the role of hLf basic residues 1 to 4 and 28 to 34 in the inhibition of radiolabeled IL-8 binding to proteoglycans (Fig. 5B). Both sequences were reported to act as cationic cradles for heparin binding (27, 43). Increasing concentrations of N-terminally modified hLf variants were used in competitive binding experiments to immobilized heparin. About 10 μg of rhLf/ml and 35 μg of EGS-rhLf/ml reduced the amount of 125I-IL-8 bound to heparin by 50%. Maximal inhibition (around 70%) was gained from 100-μg/ml concentrations of any of the two proteins. Competition between 125I-IL-8 and G4R-rhLf or Lf(−3N) for heparin-BSA binding was less effective than that obtained with rhLf. The IC50 values calculated for G4R-rhLf and Lf(−3N) were 72 and 88 μg/ml, respectively. Very low inhibition (9% ± 4%) was detected with G4R-EGS-rhLf. These results demonstrate the ability of Lf, through its glycosaminoglycan-binding site, to compete with IL-8 for binding to heparin.
DISCUSSION
Endothelial cells express chemokines that initiate the activation and recruitment of circulating leukocytes at inflammatory tissue sites. Nevertheless, during septicemia chemokines participate in a series of cellular events that severely damage the endothelium and surrounding tissues (9, 30). Anti-inflammatory therapies aiming to regulate the chemokine responses may thus prevent those septic shock-associated events. Lactoferrin, a glycoprotein released from neutrophils during inflammation and present in most secretions, exhibits anti-inflammatory properties (5). In particular, the protective effect of exogenous Lf against endotoxin lethal shock in various animals was previously reported (19, 21, 26, 44). Recently, we brought evidence that Lf strongly interacts with LPS, sCD14, or the sCD14-LPS complex, thus lowering the expression of adhesion molecules, E-selectin, and ICAM-1 on endothelial cells (4). However, its effect on the expression and function of chemokines in the leukocyte recruitment process was not investigated.
We first investigated the effect of hLf on the IL-8 expression induced in LPS-activated endothelial cells. In the present report, we provide evidence that hLf decreases the IL-8 expression by LPS-stimulated HUVEC at both transcriptional and translational levels. Our results obtained by activating cells with LPS in the presence of recombinant sCD14 suggest that interactions between Lf and LPS or the sCD14-LPS complex are responsible for that inhibitory effect. The experiments with hLf variants modified at residues 2RRRR5 and 28RKVRGPP34, which participate in the recognition of LPS (11, 41), strongly support this assertion. Mutations in both sequences totally abrogated the inhibitory effect of Lf. Furthermore, removal of the first three N-terminal amino acid residues (Lf-3N), which are not essential for the binding to LPS (11), had a lesser influence on the inhibition properties of Lf. From these results we determined that hLf released from neutrophils during infection or used as a therapeutic agent may down-regulate the expression of endothelial IL-8 and the recruitment of immune cells during inflammation. Since IL-8 is a key molecule which triggers neutrophils degranulation (18) and subsequent release of Lf, a reciprocal negative feedback regulation of both molecules is likely under physiological conditions. In contrast to the results of Shinoda et al. (37), who reported that Lf stimulates the release of IL-8 from neutrophils, hLf alone has no effect on IL-8 production by HUVEC. This difference could be explained either by the presence of LPS contaminants in the Lf fractions used in their experiments or by different Lf binding sites on HUVEC and neutrophilic cells. Furthermore, these authors have not investigated the activity of Lf on LPS-activated cells. A previous study by Wang et al. (42) demonstrates that iron-free hLf decreases the oxidative burst induced by LPS in neutrophils. This result may be supported by the hypothesis that Lf could also down-regulate the IL-8 production induced by LPS in neutrophils.
In a second step we investigated whether Lf, a glycosaminoglycan-binding molecule (27, 32, 43), could compete with IL-8 for its binding to proteoglycans. Endothelial glycosaminoglycans may act as storage sites for chemokines. Hence, their presentation to leukocyte-expressed chemokine receptors may be facilitated (30). IL-8 binds to heparin with a moderate affinity (Kd of 0.39 to 2.63 μM) (20) while the affinity between Lf and heparin molecules is significantly higher (15 to 124 nM) (27, 32).
Our results show that Lf, at concentrations encountered at the inflammatory sites (6, 25), strongly inhibits the binding of IL-8 to heparin. This inhibition was specific, since serum transferrin, another heparin-binding member of the transferrin family (33), did not compete with IL-8 for heparin binding. Further evidence of such a competition was brought by some experiments with mutated Lf. We show that alterations of only one out of the two N-terminal basic stretches, 1GRRR4 and 28KKVRGPP34, do not significantly alter the competitive binding properties of Lf. In contrast, alterations at both sites abrogate these properties. These results are supported by the requirement of residues 1GRRRR5 and 28KKVR31 to form a cationic cradle for heparin binding (27, 43). Moreover, we previously demonstrated that R2 to R4 but not R5 contribute to the binding of hLf to the heparan sulfate molecule present at the surface of Jurkat lymphocytic cells (22). These observations in total strongly suggest that the heparin-binding features of Lf are responsible for its ability to compete with IL-8. The heparin structural motifs recognized by Lf and those interacting with IL-8 have been characterized previously (16, 20, 40). About five N- and O-sulfated saccharide units are required for the optimal binding of one IL-8 monomer (20). Furthermore, the 2-O-and 6-O-disulfated disaccharides structure, and more precisely the di-O-sulfated disaccharides unit -IdceA(2-OSO3)-GlcNSO3(6-OSO3), promotes the binding of IL-8 (40). Concerning Lf, octosaccharides were the smallest heparin oligosaccharides showing significant binding, and the 2-O-, 6-O-, and N-sulfate groups were of equal importance for Lf binding. Consequently, the fact that both Lf and IL-8 may interact with common sulfate groups on heparin and the fact that Lf binds to heparin with a higher affinity than IL-8 probably account for the displacement of chemokine from heparin in the presence of Lf.
In conclusion, our results demonstrate that the anti-inflammatory activity of Lf is relevant, at least in part, to its ability to inhibit both expression and presentation of IL-8 on endothelial cells, therefore regulating the recruitment of leukocytes at inflammatory sites. Recently, Lf was shown to prevent gut mucosa damages in mice during LPS endotoxemia (19). Our findings shed light on one of the mechanisms that explain the protective effect of Lf against the septic shock and subsequent tissue damages. An optimal effect is seen when exogenous Lf is administered to animals prior to provoked septicemia (44) and may be explained by the coating of endothelium proteoglycans with Lf, thus impeding further IL-8 presentation. The demonstration that Lf inhibits IL-8 expression and presentation by cells opens the way to investigating the role of Lf in septic shock and in other pathologies.
Acknowledgments
This work was supported in part by the Université des Sciences et Technologies de Lille, the Centre National de la Recherche Scientifique (UMR n°8576; director, J. C. Michalski).
We are grateful to M. Sénéchal, M. Benaïssa, and A. Clermont for their skillful technical assistance.
Editor: R. N. Moore
REFERENCES
- 1.Appelmelk, B. J., Y. Q. An, M. Geerts, B. G. Thijs, H. A. de Boer, D. M. MacLaren, J. de Graaff, and J. H. Nuijens. 1994. Lactoferrin is a lipid A-binding protein. Infect. Immun. 62:2628-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arditi, M., J. Zhou, R. Dorio, G. W. Rong, S. M. Goyert, and K. S. Kim. 1993. Endotoxin-mediated endothelial cell injury and activation: role of soluble CD14. Infect. Immun. 61:3149-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggiolini, M., A. Wales, and S. L. Kunkel. 1989. Neutrophil-activation peptide 1/IL-8, a novel cytokine that activates neutrophils. J. Clin. Investig. 84:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baveye, S., E. Elass, D. G. Fernig, C. Blanquart, J. Mazurier, and D. Legrand. 2000. Human lactoferrin interacts with soluble CD14 and inhibits the expression of endothelial adhesion molecules, E-selectin, and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 68:6519-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baveye, S., E. Elass, J. Mazurier, G. Spik, and D. Legrand. 1999. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin. Chem. Lab. Med. 37:281-286. [DOI] [PubMed] [Google Scholar]
- 6.Bennett, R. M., and C. Mohla. 1976. A solid-phase radioimmunoassay for the measurement of lactoferrin in human plasma: variations with age, sex and disease. J. Lab. Clin. Med. 88:156-166. [PubMed] [Google Scholar]
- 7.Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. [DOI] [PubMed] [Google Scholar]
- 8.Bevilacqua, M. P., J. S. Pober, D. L. Mendrick, R. S. Cotran, and M. A. Gimbrone. 1987. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc. Natl. Acad. Sci. USA 84:9238-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bone, R. C. 1991. The pathogenesis of sepsis. Ann. Intern. Med. 115:457-469. [DOI] [PubMed] [Google Scholar]
- 10.Detmers, P. A., S. K. Lo, I. Olsen-Egbert, R. Walz, M. Baggiolini, and Z. A. Cohn. 1990. Neutrophil-activating protein 1/IL-8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J. Exp. Med. 171:1155-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elass-Rochard, E., A. Roseanu, D. Legrand, M. Trif, V. Salmon, C. Motas, J. Montreuil, and G. Spik. 1995. Lactoferrin-lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli O55:B5 lipopolysaccharide. Biochem. J. 312:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faure, E., O. Equils, P. A. Sieling, L. Thomas, F. X. Zhang, C. J. Kirschning, N. Polentarutti, M. Muzio, and M. Arditi. 2000. Bacterial lipopolysaccharide activates NF-kappa B through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 275:11058-11063. [DOI] [PubMed] [Google Scholar]
- 13.Hailman, E., H. S. Lichenstein, M. M. Wurfel, D. S. Miller, D. A. Johnson, M. Kelley, L. A. Busse, M. M. Zukowski, and S. D. Wright. 1994. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J. Exp. Med. 179:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haziot, A., G. W. Rong, J. Silver, and S. M. Goyert. 1993. Recombinant soluble CD14 mediates the activation of endothelial cells by lipopolysaccharide. J. Immunol. 151:1500-1507. [PubMed] [Google Scholar]
- 15.Hipperstiel, S., S. Soeth, B. Kellas, O. Fuhmann, J. Seybold, M. Krull, C. V. Eichel-Streiber, M. Goebeler, S. Ludwig, and N. Suttoup. 2000. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood 95:3044-3051. [PubMed] [Google Scholar]
- 16.Hoogewerf, A. J., G. S. V. Kurschert, A. E. I. Proudfoot, F. Borlat, I. Clark-Lewis, C. A. Power, and T. N. C. Wells. 1997. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry 36:13570-13578. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 52:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juffrie, M., G. M. Van der Meer, C. E. Hack, K. Haasnoot, A. Sutaryo, A. J. Veerman, and L. G. Thijs. 2000. Inflammatory mediators in dengue virus infection in children: interleukin-8 and its relationship to neutrophil degranulation. Infect. Immun. 68:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruzel, M. L., Y. Harari, C. Y. Chen, and G. A. Castro. 2000. Lactoferrin protects gut mucosal integrity during endotoxemia induced by lipopolysaccharide in mice. Inflammation 24:33-44. [DOI] [PubMed] [Google Scholar]
- 20.Kuschert, G. S. V., F. Coulin, C. A. Power, A. E. I. Proudfoot, R. E. Hubbard, A. J. Hoogewerf, and T. N. C. Wells. 1999. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry 38:12959-12968. [DOI] [PubMed] [Google Scholar]
- 21.Lee, W. J., J. L. Farmer, M. Hilty, and Y. B. Kim. 1998. The protective effects of lactoferrin feeding against endotoxin lethal shock in germfree piglets. Infect. Immun. 66:1421-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legrand, D., P. H. Van Berkel, V. Salmon, H. A. Van Veen, M. C. Slomianny, J. H. Nuijens, and G. Spik. 1997. The N-terminal Arg2, Arg3 and Arg4 of human lactoferrin interact with sulphated molecules but not with the receptor present on Jurkat human lymphoblastic T-cells. Biochem. J. 327:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legrand, D., V. Salmon, B. Coddeville, M. Bena&ıuml;ssa, Y. Plancke, and G. Spik. 1995. Structural determination of two-linked glycans isolated from recombinant human lactoferrin expressed in BHK cells. FEBS Lett. 365:57-60. [DOI] [PubMed] [Google Scholar]
- 24.Lowry, D., N. J. Rosebrough, A. L. Fahr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biochem. 193:265-275. [PubMed] [Google Scholar]
- 25.Maacks, S., H. Z. Yuan, and W. G. Wood. 1989. Development and evaluation of luminescence based sandwich assay for plasma lactoferrin as a marker for sepsis and bacterial infections in paediatric medicine. J. Biolumin. Chemilumin. 3:2211-2226. [Google Scholar]
- 26.Machnicki, M., M. Zimecki, and T. Zagulski. 1993. Lactoferrin regulates the release of tumor necrosis factor alpha and interleukin-6 in vivo. Int. J. Exp. Pathol. 74:433-439. [PMC free article] [PubMed] [Google Scholar]
- 27.Mann, D. M., E. Romm, and M. Migliorini. 1994. Delineation of the glycosaminoglycan-binding site in the human inflammatory response protein lactoferrin. J. Biol. Chem. 269:23661-23667. [PubMed] [Google Scholar]
- 28.Masson, P. L., J. F. Heremans, and E. Schonne. 1969. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J. Exp. Med. 130:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazurier, J., and G. Spik. 1980. Comparative study of the iron-binding properties of human transferrins. I. Complete and sequential iron saturation and desaturation of the lactotransferrin. Biochim. Biophys. Acta 629:399-408. [DOI] [PubMed] [Google Scholar]
- 30.Murdoch, C., and A. Finn. 2000. Chemokine receptors and their role in inflammation and infections diseases. Blood 95:3032-3043. [PubMed] [Google Scholar]
- 31.Nortamo, P., R. Li, R. Renkonen, T. Timonen, J. Prieto, M. Pattaroyo, and C. G. Gahmberg. 1991. The expression of human intercellular adhesion molecule-2 is refractory to inflammatory cytokines. Eur. J. Immunol. 21:2629-2632. [DOI] [PubMed] [Google Scholar]
- 32.Pejler, G. 1996. Lactoferrin regulates the activity of heparin-proteoglycans bound mast cell chymase: characterization of the binding of heparin to lactoferrin. Biochem. J. 320:897-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regoeczi, E., P. A. Chindemi, and W. L. Hu. 1994. Interaction of transferrin and its iron-binding fragments with heparin. Biochem. J. 299:819-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rot, A. 1993. Neutrophil attractant/activation protein-1 (interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. Eur. J. Immunol. 23:303-306. [DOI] [PubMed] [Google Scholar]
- 35.Salmon, V., D. Legrand, B. Georges, M. C. Slomianny, B. Coddeville, and G. Spik. 1997. Characterization of human lactoferrin produced in the baculovirus expression system. Protein Exp. Purif. 9:203-210. [DOI] [PubMed] [Google Scholar]
- 36.Schroder, J. M., and E. Christophers. 1989. Secretion of novel and homologous neutrophil activating peptides by LPS stimulated human endothelial cells. J. Immunol. 142:244-251. [PubMed] [Google Scholar]
- 37.Shinoda, I., M. Takasa, Y. Fukuwatari, S. Shimamura, M. Koller, and W. Konig. 1996. Effects of lactoferrin and lactoferricin on the release of IL-8 from human polymorphonuclear leukocytes. Biosci. Biotechnol. Biochem. 60:521-523. [DOI] [PubMed] [Google Scholar]
- 38.Smith, W. B., J. R. Gamble, I. Clark-Lewis, and M. A. Vades. 1991. IL8 induces neutrophil transendothelial migration. Immunology 72:65-72. [PMC free article] [PubMed] [Google Scholar]
- 39.Spik, G., G. Strecker, B. Fournet, S. Bouquelet, J. Montreuil, L. Dorland, H. Van Halbeek, and J. F. Vliegenthart. 1982. Primary structure of the glycans from human lactotransferrin. Eur. J. Biochem. 121:413-419. [DOI] [PubMed] [Google Scholar]
- 40.Spillmann, D., D. Witt, and U. Lindahl. 1998. Defining the IL-8-binding domain of heparan sulfate. J. Biol. Chem. 273:158487-158493. [DOI] [PubMed] [Google Scholar]
- 41.Van Berkel, P. H., M. E. Geerts, H. A. Van Veen, M. Mericskay, H. A. de Boer, and J. H. Nuijens. 1997. N-terminal stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem. J. 328:145-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, D., K. M. Pabst, Y. Aida, and J. C. Mathison. 1995. Lipopolysaccharide-inactivating activity of neutrophils is due to lactoferrin. J. Leukoc. Biol. 57:865-874. [DOI] [PubMed] [Google Scholar]
- 43.Wu, H. F., D. M. Monroe, and F. C. Church. 1995. Characterization of the glycosaminoglycan-binding region of lactoferrin. Arch. Biochem. Biophys. 317:85-92. [DOI] [PubMed] [Google Scholar]
- 44.Zagulski, T., P. Lipinski, A. Zagulska, S. Broniek, and Z. Jarzabek. 1989. Lactoferrin can protect mice against a lethal dose of Escherichia coli in experimental infection in vivo. Br. J. Exp. Pathol. 70:697-704. [PMC free article] [PubMed] [Google Scholar]