Abstract
The local intrapulmonary role of tumor necrosis factor alpha (TNF-α) in a protective host response during acute and chronic infection with Mycobacterium tuberculosis is incompletely understood. To directly assess its role in the intrapulmonary immune response, we compared the responses of transgenic mice with a local pulmonary blockade of TNF-α (SPCTNFRIIFc mice) to mice with globally inhibited TNF-α (TNFRKO mice) and mice with normal immune systems (control mice). Consistent with previous reports, 100% of TNFRKO mice died by 28 days after aerosol infection, and these mice had markedly increased numbers of bacteria and widespread tissue necrosis in their lungs compared to controls. The median survival time of the SPCTNFRIIFc mice was 142 days, and 75% died by 180 days. Even though the numbers of bacteria in the lungs of the SPCTNFRIIFc mice were marginally increased compared to controls, these mice had a persistent neutrophilic inflammatory response and increased expression of proinflammatory cytokines (interleukin-1α/β [IL-1α/β], IL-18, gamma interferon, IL-6, and macrophage migration inhibitory factor) and chemokines (eotaxin, macrophage inflammatory protein 1α/β, gamma interferon-inducible protein 10, macrophage chemotaxic protein 1, and TCA-3) in their lungs. These studies with the SPCTNFRIIFc mice provide direct evidence for the local importance of TNF-α in the proper regulation of host defense to M. tuberculosis. The studies also suggest that when the local actions of TNF-α are selectively impaired in the lungs, tissue destruction and death ensue, at least in part, due to persistent expression of proinflammatory mediators that would normally be downregulated.
Cytokines, including gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-12 (IL-12) have been implicated in a protective immune response to Mycobacterium tuberculosis. The role of TNF-α during mycobacterial infection is complex, mediating both protection and tissue damage (4, 29). Several animal studies have shown that the absence of TNF-α leads to overwhelming infection, shortened survival, and altered tissue pathology, with formation of abnormal granulomas, indicating that TNF-α may be critical for the orderly recruitment of cells to areas of infection (1, 3, 4, 9, 13). Patients with rheumatoid arthritis or inflammatory bowel disease who receive recombinant anti-TNF antibodies have increased rates of tuberculosis, particularly extrapulmonary forms (12). While these studies collectively underscore the importance of TNF-α in an effective host response, they do not examine the local role of TNF-α in the lung tissue where both primary and chronic infection occurs.
Although the extrapulmonary immune response is important for eventual control of infection, animal studies support the view that the local pulmonary host response is a critical part of the immune response to M. tuberculosis. The lungs of mice infected with M. tuberculosis develop persistent infection and support the growth of the largest number of organisms regardless of the route of inoculation (5, 9, 21). Only adoptive transfer of CD8+ T cells is protective following high-dose aerosol infection, while adoptive transfer of CD4+ T cells is protective following low-dose aerosol or intravenous infection (22). Additionally, aerosol infection of normal mice with ∼500 M. tuberculosis bacilli results in 100% mortality at 180 days, while intravenous infection with 105 organisms causes no mortality even though both routes of infection deposit the same number of organisms in the lungs (20). Human patients with pulmonary tuberculosis have a lymphocytic alveolitis that consists primarily of activated αβ T-cell receptor-positive T cells, which localize preferentially to the lungs (26). Experimental evidence also suggests that the pulmonary host response is compartmentalized from the response outside of the lungs (14, 19), providing a rationale to examine the lung-specific host response.
To directly examine the role of TNF-α in the lung host response, we generated and characterized transgenic mice with local intrapulmonary inhibition of TNF-α (SPCTNFRIIFc mice) (27). These transgenic mice express a fusion protein of the type II TNF receptor and the Fc portion of immunoglobulin G exclusively in the lungs. In the SPCTNFRIIFc mice, the biologic effects of low to moderate concentrations of TNF-α are completely inhibited, whereas higher concentrations are inhibited by ∼50% by the soluble inhibitor (sTNFRIIFc) present in lung tissue. Although a small amount of the sTNFRIIFc protein is present in the extrapulmonary circulation, there is no inhibition of the effects of TNF-α outside of the lungs. The studies presented here compared the response of the SPCTNFRIIFc mice to those of mice with both systemic and intrapulmonary inhibition of TNF-α (mice with disrupted genes for the type I and II TNF-α receptors [i.e., TNFRKO mice]) and control mice to investigate the relative importance of TNF-α in the local lung host defense after aerosol infection with M. tuberculosis.
MATERIALS AND METHODS
Mice.
Mice with a disrupted gene for the type I TNF receptor and mice with disrupted genes for both type I and type II TNF receptors were obtained from Immunex (TNFRKO mice) (24). Mice expressing a soluble inhibitor of TNF exclusively in the lung (SPCTNFRIIFc mice) were generated in our laboratory (27). The SPCTNFRIIFc mice used in these experiments had been back-crossed eight generations to C57BL/6 mice. Control mice used in all experiments were either C57BL/6 mice obtained from Jackson Laboratories (Bar Harbor, Maine) or littermate transgene-negative controls for the SPCTNFRIIFc mice. Preliminary experiments demonstrated no differences in responses between the C57BL/6 and littermate control mice or between the type I TNF receptor- and type I and II TNF receptor-deficient mice (data not shown). All mice were housed in the biohazard level three animal facility of the University of Washington under specific-pathogen-free conditions and given a standard diet. All components of the experiments with animals were approved by the Animal Care Committee at the University of Washington and comply with federal guidelines.
Bacteria.
M. tuberculosis (Erdman) was initially grown from a seed stock (ATCC 35801; American Type Culture Collection, Manassas, Va.) to mid-log phase in modified Proskauer-Beckett media (30). The organisms were passed once in mice, and a single CFU was selected from lung tissue, inoculated in modified Proskauer-Beckett medium, and grown to mid-log phase, and individual 1-ml aliquots of organisms were frozen at −80°C. Bacteria were thawed and briefly sonicated in a water bath sonicator to remove clumps prior to infection.
Infection.
Mice of different genotypes were placed in wire cages within an aerosol chamber that has been previously described (17). An aliquot of frozen mycobacteria was thawed, sonicated for 90 s to disrupt any clumps of bacteria and administered via Salter nebulizers (Salter Labs, Arvin, Calif.) for 30 min. A portion of each aliquot was serially diluted and plated on 7H10 media to ensure the uniformity of the initial inoculum for each experiment. Conditions to deliver different numbers of organisms to mice were determined in preliminary studies (data not shown). In each experiment, several mice were sacrificed at 24 h, and the average number of organisms deposited in the lungs of mice was determined on whole lung homogenates. Two different infectious inoculum sizes (low, ∼50 to 100 organisms/mouse; moderate, ∼200 organisms/mouse) were used so that the SPCTNFRIIFc mice could be evaluated under conditions in which disease proceeded at different tempos, either rapidly progressive or indolent. Additionally, the lower inoculum allowed us to compare the pulmonary immune responses more effectively, as time to death was delayed.
Mycobacterial cultivation and enumeration.
Organs were harvested at various time points during the experiments and homogenized in phosphate-buffered saline (PBS) with 0.05% Nonidet P-40 (NP-40; Sigma, St. Louis, Mo.) by using a Biohomogenizer (Biospec Products, Bartlesville, Okla.). Serial dilutions of the organ homogenates were performed in PBS with 0.05% NP-40, plated in duplicate on Middlebrook 7H10 media (Difco, Detroit, Mich.), incubated at 37°C, and examined for growth at 3 to 4 weeks.
Isolation of lymphocytes.
Spleens or intrathoracic lymph nodes were aseptically removed and placed in serum-free HL-1 medium (BioWhittaker) on ice at the various indicated time points during the experiments. Lymphocytes were isolated from spleens or thoracic lymph nodes and processed into single cell suspensions as previously described (2). The number of viable cells was determined by using trypan blue exclusion.
Cell culture and mycobacterial antigens.
Mycobacterial antigens were provided by Corixa Corporation (Seattle, Wash.) or by John Belise (Colorado State University, Colorado Springs, through NIH NIAID contract N01 AI-75320). Optimal concentrations of the various stimuli were determined in pilot experiments (data not shown). Pooled lymphocytes from either spleen or regional lymph nodes were plated in triplicate at a final concentration of 2.5 × 106 cells/ml, and cells were stimulated with an optimal concentration of monoclonal antibody to CD3 (α-CD3 at a 1:10 dilution of 145.2C11 hybridoma cell culture supernatant), mycobacterial culture filtrate protein (CFP [Corixa], at a 1:10 dilution), or recombinant 85b antigen at 10 μg/ml for splenocytes and at 25 μg/ml for lymphocytes from thoracic lymph nodes. Cell culture supernatants were harvested at 24 and 72 h and frozen at −80°C for cytokine determination.
Cytokine analysis.
IFN-γ, TNF-α, IL-10, and IL-4 levels were determined on cell culture supernatants by using Genzyme immunoassay kits (Genzyme, Boston, Mass.). The levels of detection in the assays for each of the cytokines were as follows: IFN-γ (25 pg/ml), TNF-α (30 pg/ml), IL-10 (30 pg/ml), and IL-4 (15 pg/ml). TNF-α levels were determined on supernatants harvested at either 24 or 72 h, and all other cytokine levels were determined on supernatants harvested at 72 h. Cytokine concentrations were determined by comparison to recombinant standards by using Delta One software (BioMetallics, Inc.).
Histology.
The right caudal lobe of the lung of selected animals of each genotype was harvested and inflated with methyl Carnoys (60% methanol, 30% chloroform, 10% glacial acetic acid) and fixed overnight. Organs were embedded in paraffin, sectioned, and stained with hematoxylin and eosin or a modified Fite stain (16). A veterinary pathologist reviewed two to four lung sections from individual mice in a manner blinded to genotype and time from infection. The percentage of tissue with inflammation was determined by inspection and expressed in quartiles: ≤25% involvement, 26 to 50% involvement, 51 to 75% involvement, and ≥75% involvement. The relative severity of tissue necrosis was determined, and a score of 1 to 4 was assigned, with 1 representing scant necrosis and 4 representing severe necrosis. Finally, the relative amounts of either mononuclear or polymorphonuclear cells in the perivascular region and parenchymal inflammatory foci were determined and assigned a score of 0 to 4, with a score of 0 representing absence of cells and a score of 4 representing maximal cell accumulation relative to other tissue sections. The results from individual mice were averaged at each time point after infection. The final results were from pooled data from three infections (∼100 organisms/mouse) with at least eight mice/genotype/time point.
RNA analysis.
RNA was isolated and purified from the left lung and liver of mice at the indicated time points after infection by using phenol-guanidinium extraction (Trisolv; Gibco-BRL, Rockville, Md.). Samples (20 μg) were analyzed by using the Riboquant Multiprobe RNase Protection Assay System (PharMingen, San Diego, Calif.). The RNase protection assay was performed according to the manufacturer's protocol with the templates MCK2b (specific for IL-12 p35 and p40, IL-10, IL-1α, IL-1β, IL-1ra, IL-18, IL-6, IFN-γ, and MIF) and MCK5 (specific for lymphotaxin, RANTES, eotaxin, macrophage migration inhibition factor [MIF], macrophage inflammatory protein 1α [MIP-1α], MIP-1β, MIP-2, gamma interferon-inducible protein 10 [IP-10], macrophage chemotaxic protein 1 [MCP-1], and TCA-3). Internal standards for RNA quantity (GAPDH [glyceraldehyde-3-phosphate dehydrogenase] and L32) and quality (yeast tRNA) were included for each assay, as were samples from uninfected control mice. Radiographs of the RNase protection assay were analyzed by densitometry, and values were normalized initially to internal standards to compensate for loading inequality. Preliminary experiments comparing the cytokine and chemokine expression levels from uninfected TNFRKO, SPCTNFRIIFc and control mice demonstrated undetectable expression of all messages except internal standards for RNA quantity. Undetectable levels were defined as equivalent to the background of the assay and were <10% of the lowest detectable band of infected mice at each time point. All data are expressed as a percentage of infected control mice for that time point.
Statistics.
Statistical analysis by Kaplan-Meier, analysis of variance (ANOVA), or Student's t test was performed when appropriate by using Statview (Abacus, Cary, N.C.), SPSS 10 (SPSS, Chicago, Ill.), or Excel (Microsoft, Redmond, Wash.). A P value of ≤0.05 was considered significant.
RESULTS
Survival.
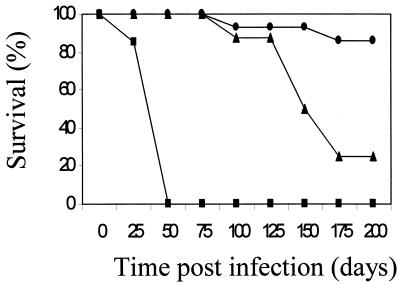
To explore the local importance of TNF-α in the lung, we first compared the survival of SPCTNFRIIFc mice to those of TNFRKO and control mice (Fig. 1). As previously reported (3, 9), 100% of the TNFRKO mice died with a median survival time of 28 days after aerosol infection with a low inoculum, whereas only 10% of control mice died (P < 0.01 versus controls). SPCTNFRIIFc mice were also more susceptible than controls (P = 0.03), but the time to first death (80 days), the overall survival at 180 days (25%), and the median survival time (142 days) were less severely compromised than for TNFRKO mice (survival P < 0.01 versus TNFRKO mice). Similarly, when mice were infected with a moderate inoculum, the mortality in the SPCTNFRIIFc mice (40%) was less than that of the TNFRKO mice (100%) but greater than that of the controls (0%) at 6 weeks, when the experiment was terminated.
FIG. 1.
Decreased survival of SPCTNFRIIFc mice after aerosol infection with M. tuberculosis. SPCTNFRIIFc (▴), TNFRKO (▪), and control (•) mice were infected with a low inoculum (50 to 100 organisms/mouse) of M. tuberculosis Erdman via the aerosol route and monitored for 200 days. The TNFRKO mice showed the greatest mortality (100% [P < 0.01 versus SPCTNFRIIFc and controls]), while the SPCTNFRIIFc mice showed increased mortality but to a lesser degree (P = 0.03 versus controls). Each genotype of mice had at least 10 mice/group per experiment. The experiment was repeated twice, with similar results each time.
Mycobacterial growth.
To determine whether intrapulmonary inhibition of TNF-α significantly inhibited the host's ability to control mycobacterial replication, the number of mycobacteria in the lungs and liver was determined at various time points after infection. After aerosol infection with a low inoculum, TNFRKO mice had higher numbers of mycobacteria in the lungs (Table 1) and in the liver (data not shown) at 3 and 4 weeks, after which time they succumbed as reported previously (9, 13). In contrast, the SPCTNFRIIFc and control mice had similar numbers of organisms in the lungs until 4 weeks after infection. Thereafter, the SPCTNFRIIFc mice had modestly elevated numbers (∼0.5 log10 CFU) of mycobacteria (P < 0.01 at 4 weeks and P = 0.09 at 6 weeks versus controls). A similar pattern of increased mycobacterial growth was seen after infection with a moderate inoculum (SPCTNFRIIFc mice [7.3 ± 0.1 log10 mycobacterial CFU/lung] versus control mice [6.6 ± 0.2 log10 mycobacterial CFU/lung; P < 0.05] at 6 weeks after infection). In contrast to the TNFRKO mice, there was no increase in mycobacterial growth outside of the lung in the SPCTNFRIIFc mice.
TABLE 1.
Mycobacterial growth in lung tissue of SPCTNFRIIFc mice after aerosol infection with M. tuberculosis
| Mouse type (n) | Mycobacterial CFU (mean log10 ± SD)a at (wk postinfection):
|
|||
|---|---|---|---|---|
| 2 wk | 3 wk | 4 wk | 6 wk | |
| TNFRKO (10) | 6.08 ± 0.28 | 7.45 ± 0.40b | 8.18 ± 0.79b | ND |
| SPCTNFRIIFcd (15) | 6.1 ± 0.74 | 6.9 ± 0.87 | 7.0 ± 0.45c | 6.3 ± 0.76 |
| Control (15) | 5.90 ± 0.57 | 6.5 ± 0.62 | 6.5 ± 0.33 | 5.7 ± 0.67 |
Values are the results of three separate experiments with TNFRKO, SPCTNFRIIFc, and control mice. ND, not determined.
Significantly different (P < 0.001) versus control mice.
Significantly different (P < 0.01) versus control mice.
There was no significant difference between SPCTNFRIIFc and TNFRKO mice.
Lung pathology.
In addition to its role in the containment of mycobacterial growth, TNF-α is a critical cytokine for the development of a granulomatous inflammatory response to M. tuberculosis infection (3). Granuloma formation is felt to represent a successful host response to infection, and failure to develop organized granulomas has been reported with global inhibition of TNF-α. To determine whether local inhibition of TNF-α within the lung resulted in abnormal granuloma formation and contributed to the early deaths of the SPCTNFRIIFc mice, lung sections were analyzed for the character and degree of inflammation following aerosol infection.
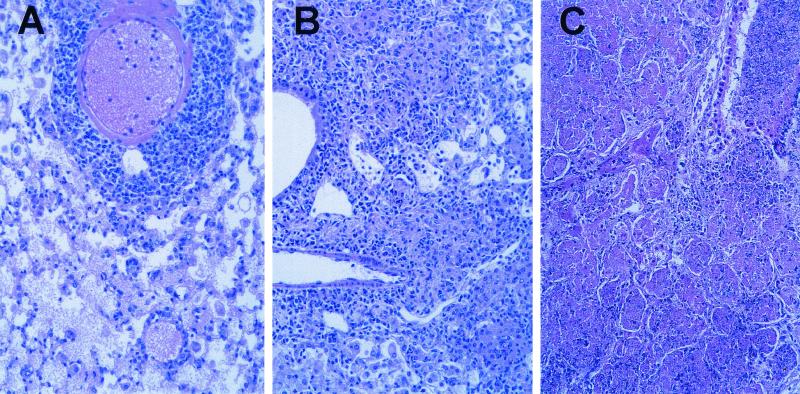
At 4 weeks after infection with a moderate inoculum, the lungs of both control and SPCTNFRIIFc mice (Fig. 2A and B) were totally involved with an inflammatory process that replaced 50 to 70% of the alveolar space with densely packed inflammatory cells. The SPCTNFRIIFc mice had moderately increased numbers of neutrophils throughout the parenchyma, and the inflammatory infiltrate was less organized than in the control mice. There was a moderate amount of necrosis seen in the lungs of the SPCTNFRIIFc mice. In contrast, the lungs of TNFRKO mice (Fig. 2C) were characterized by widespread inflammatory infiltrates and necrosis of essentially the entire lung. In the TNFRKO mice, the organized lymphocytic infiltrate seen in the SPCTNFRIIFc and the control mice was absent.
FIG. 2.
Increased pulmonary pathology in SPCTNFRIIFc mice after aerosol infection with M. tuberculosis. Control (A), SPCTNFRIIFc (B), and TNFRKO (C) mice were infected via the aerosol route with a moderate inoculum (∼200 organisms/mouse) of M. tuberculosis. The lungs were examined 4 weeks after infection, and sections were stained with hematoxylin and eosin. Magnification, ×20.
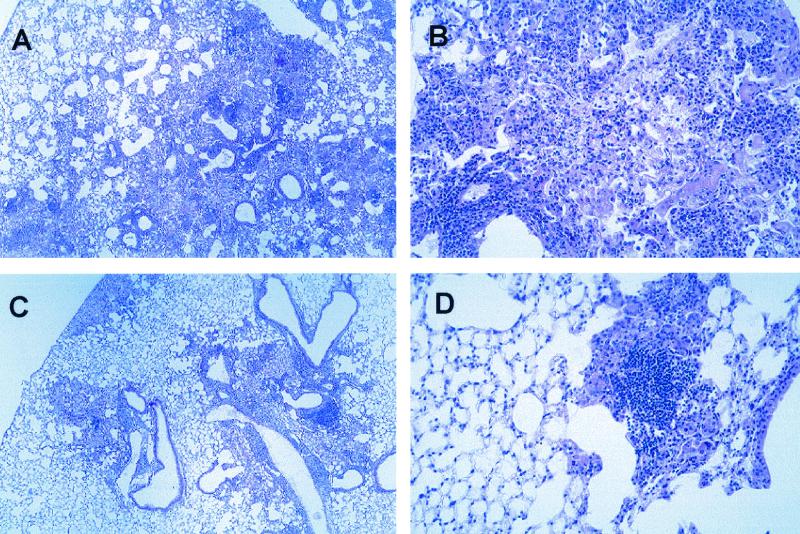
To better characterize the early inflammatory response that did not result in death in the SPCTNFRIIFc mice, we analyzed histologic sections of their lungs after infection with a low inoculum. Systematic analysis of multiple tissue sections revealed a pattern of persistent neutrophilic inflammation and tissue necrosis (Table 2). TNFRKO mice had more severe necrosis and lung inflammation than either SPCTNFRIIFc or control mice, conditions characterized by larger numbers of polymorphonuclear lymphocytes in the parenchymal inflammatory foci, a pattern that has been described by others (3). The differences between the SPCTNFRIIFc mice and control mice were most striking at 3 and 4 weeks after infection. By 3 weeks, the SPCTNFRIIFc mice had more tissue necrosis, although the total amount of inflammation in the lungs was similar to that of controls. This difference persisted at 4 weeks, at which time the parenchymal inflammatory foci had more neutrophils, similar to the pathological features seen in the TNFRKO mice, but not as severe. By 6 weeks, the lungs of SPCTNFRIIFc and control mice were similar. At 24 weeks after infection, a divergence in morphologic features became apparent (Fig. 3). The lung lesions in the control mice were consolidated into distinct multifocal granulomas separated by essentially normal parenchyma. In contrast, the granulomatous inflammatory lesions in SPCTNFRIIFc mice were diffuse to multifocal-confluent, with much of the intervening parenchyma demonstrating alveolar thickening, accumulation of macrophages, and occasional protein-rich edema fluid. Focal necrosis was also more common in SPCTNFRIIFc mice. Occasional cholesterol clefts, multinucleated giant cells (Langerhan's type), and scant numbers of acid-fast bacteria were evident in both groups at this time.
TABLE 2.
Histologic characterization of lung inflammation in SPCTNFRIIFc mice after infection with M. tuberculosis
| Wk | Mouse genotyped | % Lung involvement | Severity of necrosis (mean scoree ± SD) | Severity of lung inflammation (mean scoree ± SD)
|
||
|---|---|---|---|---|---|---|
| Mononuclear inflammation in perivascular region | Polymorphonuclear infiltrate in parenchymal focus | Mononuclear infiltrate in parenchymal focus | ||||
| 2 | TNFRKO | 25-50 | 2.22 ± 0.67a | 2.11 ± 0.6 | 3 ± 0.87a | 2 ± 0.5 |
| SPCTNFRIIFc | 25-30 | 1.36 ± 0.84b | 1.5 ± 0.52b | 1.64 ± 0.93b | 1.79 ± 0.8 | |
| Control | 25-35 | 1.5 ± 0.71 | 1.89 ± 0.6 | 2 ± 0.71 | 2.11 ± 0.6 | |
| 3 | TNFRKO | 50-60 | 3.5 ± 0.53a | 2 ± 0.53a | 2.13 ± 0.99a | 2.13 ± 0.99a |
| SPCTNFRIIFc | 25-50 | 1.82 ± 0.87b | 2.64 ± 0.67b | 1.73 ± 1.01 | 2.82 ± 0.6 | |
| Control | 50-60 | 1 ± 0c | 2.64 ± 0.5 | 1.27 ± 0.79 | 2.91 ± 0.54 | |
| 4 | SPCTNFRIIFc | 50-70 | 1.1 ± 0.32a | 2.8 ± 0.63 | 1.1 ± 0.74a | 3 ± 0 |
| Control | 50-60 | 0.75 ± 0.27c | 2.63 ± 0.52 | 0.25 ± 0.46c | 3 ± 0 | |
| 6 | SPCTNFRIIFc | 50-70 | 0.93 ± 0.18 | 2.88 ± 0.52 | 0.63 ± 0.52 | 2.88 ± 0.35 |
| Control | 50-60 | 1 ± 0 | 2.93 ± 0.19 | 0.86 ± 0.38 | 3 ± 0 | |
Significantly different (P < 0.05) for TNFRKO mice versus control mice.
Significantly different (P < 0.05) for SPCTNFRIIFc mice versus TNFRKO mice.
Significantly different (P < 0.05) for SPCTNFRIIFc mice versus control mice.
Each group had at least eight mice/time point.
See Materials and Methods for a detailed description.
FIG. 3.
Impaired granuloma development in SPCTNFRIIFc mice during chronic infection with M. tuberculosis. SPCTNFRIIFc and control mice were infected via the aerosol route with a low inoculum (∼50 to 100 organisms/mouse) of M. tuberculosis. Lung sections were examined 24 weeks after infection and stained with hematoxylin and eosin. Representative lung sections from SPCTNFRIIFc mice (A and B) and control mice (C and D) are shown. Magnifications: A and C, ×4; B and D, ×20.
Lung cytokine and chemokine expression.
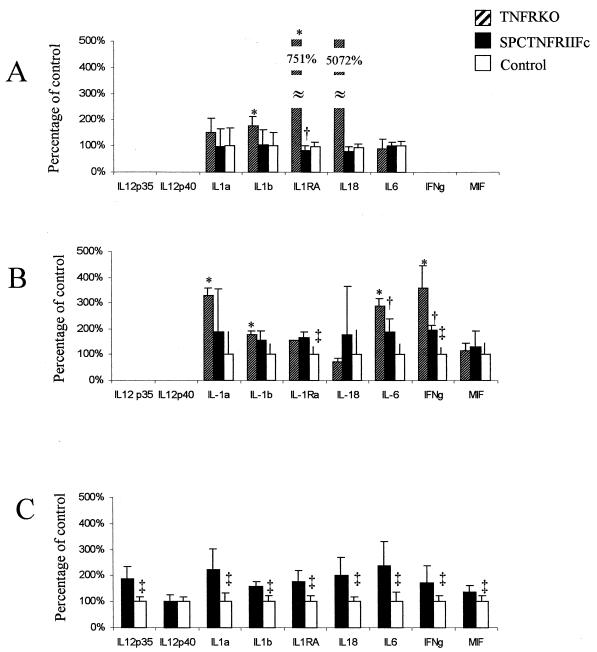
We next examined the cytokines expressed in infected lung tissue to determine whether altered cytokine expression within the lungs could explain the exaggerated pathology. At each time point we found significant differences in cytokine mRNA expression in lung tissues of the SPCTNFRIIFc and TNFRKO mice compared to those of control mice (Fig. 4). The SPCTNFRIIFc and TNFRKO mice had a similar phenotype, although the rate of increase in cytokine levels was lower in the SPCTNFRIIFc mice, perhaps reflecting incomplete and local TNF-α blockade in the SPCTNFRIIFc mice (27). At 4 weeks after infection, the SPCTNFRIIFc mice had a pattern of globally increased cytokine mRNA in the lungs compared to the controls.
FIG. 4.
Dysregulated cytokine production in the lungs of SPCTNFRIIFc mice after aerosol infection with M. tuberculosis. Total lung RNA was collected, and cytokine gene expression was analyzed by RNase protection assay of 20 μg of mRNA at 2 weeks (A), 3 weeks (B), and 4 weeks (C) after infection. Four animals were analyzed per group: TNFRKO, SPCTNFRIIFc, and control mice. Densitometry was performed on each specimen, and gene expression was normalized to internal standards (GADPH and L32) and then compared to the expression in control mice. Preliminary experiments comparing the cytokine and chemokine expression levels from uninfected TNFRKO, SPCTNFRIIFc, and control mice demonstrated undetectable expression of all messages except for internal standards for RNA quantity. Cytokine values are percentages of expression in infected control mice, and error bars indicate the standard deviations. Significant differences (P < 0.05) between TNFRKO and control mice are indicated by asterisks (∗), between TNFRKO and SPCTNFRIIFc mice by daggers (†), and between SPCTNFRIIFc and control mice by double daggers (‡).
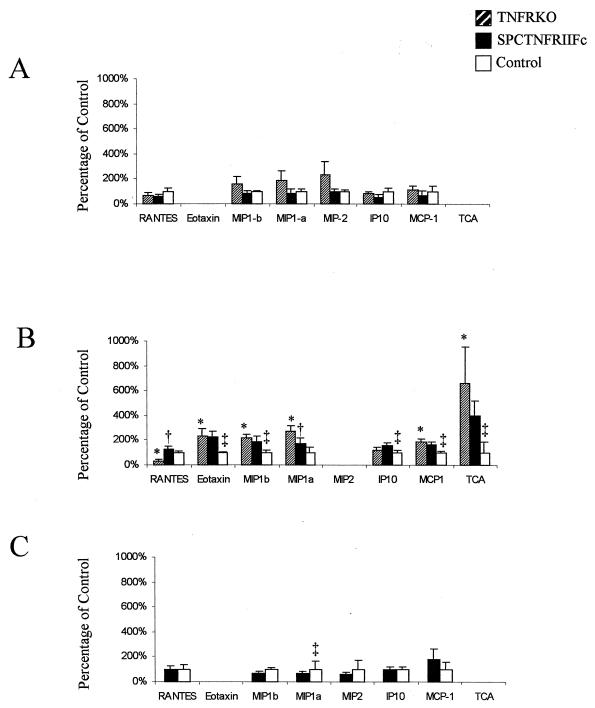
Chemokines coordinate the trafficking of cells to sites of inflammation and are elaborated during the course of mycobacterial infection (25, 31). To further explore the mechanisms for the exaggerated lung pathology, we examined the expression of chemokines in lung tissue (Fig. 5). By 3 weeks after infection, both the SPCTNFRIIFc and TNFRKO mice had increased expression of chemokines, including chemokines that attract neutrophils (TCA-3). In contrast, the TNFRKO mice had decreased expression of RANTES, which was not evident in the SPCTNFRIIFc mice. At 4 weeks after infection, differences between the SPCTNFRIIFc and control mice were no longer observed, even though there were clear differences in lung pathology at that time point.
FIG. 5.
Altered chemokine expression in the lungs of SPCTNFRIIFc mice after aerosol infection with M. tuberculosis. Lung chemokine gene expression was determined by RNase protection assay of 20 μg of whole lung RNA from four animals per group (TNFRKO, SPCTNFRIIFc, and control mice) at 2 weeks (A), 3 weeks (B), and 4 weeks (C) after infection. Densitometry was performed on each specimen, and gene expression was normalized to internal standards (GADPH and L32) and then compared to the expression in control mice. Preliminary experiments comparing cytokine and chemokine expression levels from uninfected TNFRKO, SPCTNFRIIFc, and control mice demonstrated undetectable expression of all messages except for internal standards for RNA quantity. Chemokine values are expressed as percentages of expression in infected control mice, and error bars represent the standard deviations. Significant differences (P < 0.05) between TNFRKO and control mice are indicated by asterisks (∗), between TNFRKO and SPCTNFRIIFc mice by daggers (†), and between SPCTNFRIIFc and control mice by double daggers (‡).
Antigen-specific immunity.
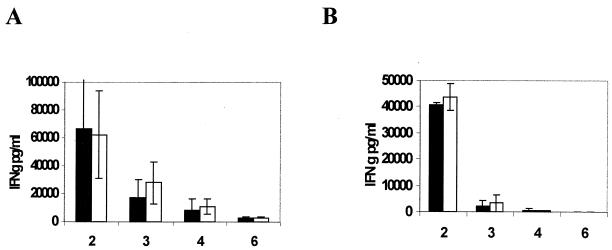
TNF-α has been shown to be important in the development of a protective adaptive immune response to certain bacterial and fungal pathogens (10). It acts to facilitate the development of delayed-type hypersensitivity and to enhance clonal expansion and differentiation of activated CD4+ T cells (23). Whether TNF-α has such a role in the protective immune response to M. tuberculosis is not known. A diminished or altered antigen-specific immune response could explain the pathological change seen in the lungs of mice lacking TNF-α. However, using lymphocytes isolated from intrathoracic lymph nodes, we found no difference in the antigen-specific immune response to either CFP or 85b, as measured by IFN-γ production after antigen stimulation (Fig. 6). Similarly, there was no difference in IL-10, IL-4, or TNF-α production (data not shown). We obtained similar results with splenocytes.
FIG. 6.
Cytokine production in response to mycobacterial antigens by regional pulmonary lymphocytes in SPCTNFRIIFc mice. Lymphocytes were isolated from thoracic lymph nodes of SPCTNFRIIFc (solid bars) or control (open bars) mice 2, 3, 4, or 6 weeks after infection with a low inoculum (ca. 100 organisms/mouse) of M. tuberculosis. Lymphocytes were stimulated with either mycobacterial CFP (A) or recombinant mycobacterial antigen 85b (B). We collected cell culture supernatants at 72 h and determined IFN-γ levels by enzyme-linked immunosorbent assay. The means ± the standard deviations from three separate experiments are shown. No significant difference was noted between the two groups (P > 0.1).
DISCUSSION
The SPCTNFRIIFc mice provided a unique tool with which to explore the role of TNF-α in the lung-specific immune response to infection with M. tuberculosis. From the studies described here, we conclude that inhibition of TNF-α activity solely in the lungs impairs the host response to this infection. This indicates that the presence of TNF-α outside of the lungs cannot compensate for an intrapulmonary defect in TNF-α activity and that TNF-α plays an important local role in control of this infection. These findings complement those from studies done by Bekker et al. (4). They used a gain-of-function approach in which TNFRKO mice, pretreated by aerosol inoculation with recombinant BCG that had been engineered to secrete TNF-α showed improved survival after an aerosol challenge with virulent M. tuberculosis.
The results in SPCTNFRIIFc mice also demonstrated that the development of pulmonary injury when TNF-α activity is blocked is more closely linked to dysregulation of the cytokine-chemokine network than to the increase in numbers of bacteria in the lungs. It has not been possible to make this distinction in TNFRKO and TNF-α knockout mice (3, 9), in which the disease progresses rapidly and severe tissue inflammation occurs in concert with markedly greater numbers of bacteria in the lungs. This is also the case in IFN-γ knockout mice (5, 8). In contrast, the less-acute tempo in SPCTNFRIIFc mice permitted the course of the infection and disease to be analyzed longitudinally after aerosol infection with M. tuberculosis. Strikingly, even though the numbers of bacteria were increased only slightly in the lungs of SPCTNFRIIFc mice, they failed to develop a focused granulomatous response and showed a persistent neutrophilic and confluent inflammatory response, which ultimately was associated with their early death. Interestingly, IL-6, IL-12, and IL-18 knockout mice have a phenotype that is the inverse of that seen in SPCTNFRIIFc mice: the numbers of mycobacteria in the lungs of these mice are increased substantially compared to controls, but tissue necrosis is not (6, 15, 28). This supports the view that inflammation is a complex process and that bacterial numbers are only one determinant that influences this process.
The increased inflammation in the lungs of SPCTNFRIIFc mice after infection with M. tuberculosis suggested that more inflammatory cells were recruited into their lungs than into controls. Consistent with this, the expression of TCA-3, a chemokine that attracts neutrophils and monocytes and facilitates the exocytosis of elastase and lysozyme, was increased in SPCTNFRIIFc compared to controls (7). The expression of mRNA for chemokines that attract mononuclear cells (e.g., MIP-1α, MIP-1β, and MCP-1) and proinflammatory cytokines, including IL-1, IL-6, IL-18, IFN-γ, and MIF, was increased and may have contributed to the greater and more persistent inflammation in these mice. This pattern of increased chemokine and cytokine mRNA was also true in TNFRKO mice. These findings contrast with the lower mRNA levels of TNF-α, IL-6, and IL-10 found in the lungs when thalidomide is used to block the effects of TNF-α (18). It is also possible, although not studied here, that once recruited, inflammatory cells persisted for a longer time in the lungs of SPCTNFRIIFc (and TNFRKO) mice. In vitro stimulation with TNF-α and either M. tuberculosis or purified protein derivative results in neutrophil apoptosis, an effect not produced by IL-1 (11). Thus, programmed cell death may be a mechanism for clearing neutrophils from sites of inflammation during mycobacterial infection, and when TNF-α activity is blocked, neutrophils may persist and promote tissue damage.
In summary, we conclude that TNF-α plays an important and complex local role in the lungs in response to infection with M. tuberculosis. In addition to its role in facilitating bacterial clearance, it appears to be an important modulator of lung inflammation. Inhibition of TNF-α activity in the lungs early in the course of the infection leads to persistent inflammation and immune activation out of proportion to the increase in mycobacterial tissue burden, with resultant exaggerated pathology and lung injury.
Acknowledgments
This work was supported by grants NIH-AIO1468 and NIH-HL64550 from the American Lung Association (S.S.) and grant NIH-HD18184 (C.B.W.).
REFERENCES
- 1.Adams, L. B., C. M. Mason, J. K. Kolls, D. Scollard, J. L. Krahenbuhl, and S. Nelson. 1995. Exacerbation of acute and chronic murine tuberculosis by administration of a tumor necrosis factor receptor-expressing adenovirus. J. Infect. Dis. 171:400-405. [DOI] [PubMed] [Google Scholar]
- 2.Alaniz, R., S. Thomas, M. Perez-Melgosa, K. Mueller, A. Farr, R. Plamiter, and C. Wilson. 1999. Doamine beta-hydroxylase deficiency impairs cellular immunity. Proc. Natl. Acad. Sci. USA 96:2274-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 4.Bekker, L. G., A. L. Moreira, A. Bergtold, S. Freeman, B. Ryffel, and G. Kaplan. 2000. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect. Immun. 68:6954-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper, A., D. K. Dalton, T. A. Stewart, J. Griffin, D. Russell, and I. Orme. 1993. Disseminated tuberculosis in interferon γ gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper, A., J. Margram, J. Ferrante, and I. Orme. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devi, S., J. Laning, Y. Luo, and M. Dorf. 1995. Biologic activities of the β-chemokine TCA-3 on neutrophils and macrophages. J. Immunol. 154:5376-5383. [PubMed] [Google Scholar]
- 8.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 10.Huffnagle, G., G. Toews, M. Burdick, M. Boyd, K. McAllister, R. McDonald, S. Kunkel, and R. Strieter. 1996. Afferent phase production of TNF-α is required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 157:4529-4536. [PubMed] [Google Scholar]
- 11.Kasahara, K., I. Sato, K. Ogura, H. Takeuchi, K. Kobyashi, and M. Adachi. 1998. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J. Infect. Dis. 178:127-137. [DOI] [PubMed] [Google Scholar]
- 12.Keane, J., S. Gershon, R. P. Wise, E. Mirabile-Levens, J. Kasznica, W. D. Schwieterman, J. N. Siegel, and M. M. Braun. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098-1104. [DOI] [PubMed] [Google Scholar]
- 13.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 14.Kolls, J. K., S. Nelson, and W. Summer. 1993. Recombinant cytokines and pulmonary host defense. Am. J. Med. Sci. 306:330-335. [DOI] [PubMed] [Google Scholar]
- 15.Ladel, C., C. Blum, A. Dreher, K. Reifenberg, M. Kopf, and S. Kaufmann. 1997. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect. Immun. 65:4843-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luna, L. (ed.). 1968. Manual of histologic staining methods of the Armed Forces Institute of Pathology. McGraw-Hill Blakiston Division, New York, N.Y.
- 17.Martin, T., L. Altman, and O. Alvares. 1983. The effects of severe protein-calorie malnutrition on antibacterial defense mechanisms of the rat lung. Am. Rev. Respir. Dis. 128:1013-1019. [DOI] [PubMed] [Google Scholar]
- 18.Moreira, A. L., L. Tsenova-Berkova, J. Wang, P. Laochumroonvorapong, S. Freeman, V. H. Freedman, and G. Kaplan. 1997. Effect of cytokine modulation by thalidomide on the granulomatous response in murine tuberculosis. Tuberc. Lung Dis. 78:47-55. [DOI] [PubMed] [Google Scholar]
- 19.Nelson, S., G. Bagby, and B. Bainton. 1989. Compartmentalization of intraalveolar and systemic lipopolysaccharide-induced tumor necrosis factor and the pulmonary inflammatory response. J. Infect. Dis. 159:189-194. [DOI] [PubMed] [Google Scholar]
- 20.North, R. 1995. Mycobacterium tuberculosis is strikingly more virulent for mice when given via the aerosol than via the intravenous route. J. Infect. Dis. 172:1550-1553. [DOI] [PubMed] [Google Scholar]
- 21.Orme, I. M., and F. Collins. 1988. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J. Immunol. 140:3589-3593. [PubMed] [Google Scholar]
- 22.Orme, I. M., and F. Collins. 1983. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. J. Exp. Med. 158:74-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pape, K., A. Khoruts, A. Mondino, and M. Jenkins. 1997. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J. Immunol. 159:591-598. [PubMed] [Google Scholar]
- 24.Peshon, J., D. Dauphine, K. Stocking, M. Glaccum, C. Otten, C. Willis, K. Charrier, P. Morrissey, C. Ware, and K. Mohler. 1998. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160:943-952. [PubMed] [Google Scholar]
- 25.Rhoades, E. R., A. M. Cooper, and I. M. Orme. 1995. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect. Immun. 63:3871-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwander, S., M. Torres, E. Sada, C. Carranza, E. Ramos, M. Tary-Lehmann, R. Wallis, J. Sierra, and E. Rich. 1998. Enhanced responses to Mycobacterium tuberculosis antigens by human alveolar lymphocytes during active pulmonary tuberculosis. J. Infect. Dis. 178:1434-1445. [DOI] [PubMed] [Google Scholar]
- 27.Smith, S., S. Skerrett, E. Chi, M. Jonas, K. Mohler, and C. Wilson. 1998. The locus of tumor necrosis factor-α action in lung inflammation. Am. J. Respir. Cell Mol. Biol. 19:881-891. [DOI] [PubMed] [Google Scholar]
- 28.Sugawara, I., H. Yamada, H. Kaneko, S. Mizuno, K. Takeda, and S. Akira. 1999. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 67:2585-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsenova, L., A. Bergtold, V. H. Freedman, R. A. Young, and G. Kaplan. 1999. Tumor necrosis factor alpha is a determinant of pathogenesis and disease progression in mycobacterial infection in the central nervous system. Proc. Natl. Acad. Sci. USA 96:5657-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wayne, L. 1994. Cultivation of Mycobacterium tuberculosis for research purposes, p. 389-415. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection and control. ASM Press, Washington, DC.
- 31.Zlotnik, A., J. Morales, and J. A. Hedrick. 1999. Recent advances in chemokines and chemokine receptors. Crit. Rev. Immunol. 19:1-47. [PubMed] [Google Scholar]