Abstract
Brucella is a facultative intracellular parasite that causes brucellosis in animals and humans. The protective immune response against Brucella involves both humoral and cell-mediated immunity. In previous studies, we demonstrated that the T-dominant Brucella antigens bacterioferritin (BFR) and P39 administered either as CpG adjuvant recombinant proteins or as naked-DNA plasmids induced a specific Th1-biased immune response in mice. In order to improve the protection conferred by the BFR and P39 vaccines and to evaluate the additive role of antilipopolysaccharide (anti-LPS) antibodies, we used live attenuated Yersinia enterocolitica serotypes O:3 and O:9 as delivery vectors for naked-DNA plasmids encoding these BFR and P39 antigens. Following two intragastric immunizations in BALB/c mice, the Yersinia vectors harboring a DNA vaccine encoding BFR or P39 induced antigen-specific serum immunoglobulin and Th1-type responses (both lymphocyte proliferation and gamma interferon production) among splenocytes. Moreover, as expected, antibodies recognizing Brucella abortus 544 lipopolysaccharide were detected in O:9-immunized mice but not in O:3-treated animals. Animals immunized with O:9 organisms carrying pCI or with O:9 organisms alone were found to be significantly resistant to infection by B. abortus 544. Our data demonstrated that pCI plasmids encoding BFR or P39 and delivered with live attenuated strains of Yersinia O:3 or O:9 can trigger Th1-type responses. The fact than only O:9 vectors induced a highly significant protective immunity against B. abortus 544 infection pointed out the crucial role of anti-LPS antibodies in protection. The best protection was conferred by a serotype O:9 strain carrying pCIP39, confirming the importance of the P39 T-cell antigen in this mechanism.
Brucella abortus, a gram-negative bacterium, is pathogenic for cattle, causing abortion in pregnant females due to colonization of the placenta and sterility in males, and causes a debilitating fever (undulant fever) in humans (1, 60). Brucellosis still constitutes an important health problem in many developing countries and in some developed areas of the world (8). Vaccination is an essential element in the control of brucellosis. The vaccinal B. abortus strain 19 (S19) has been used successfully for eradication of brucellosis in cattle (47). However, as this vaccine is virulent for humans and is responsible for abortion in pregnant animals (5, 48), alternative vaccinal approaches are needed.
The bacterioferritin (BFR) and P39 antigens of Brucella were previously identified as T-dominant antigens (16, 17). Recently, we demonstrated that both BFR and P39 antigens elicited a strong Th1-dominated immune response in mice, using either the purified recombinant proteins with CpG oligodeoxynucleotide adjuvant (3) or naked-DNA vectors (pCIP39 or pCIBFR) encoding these antigens (3a). Whatever the candidate vaccine, only the P39 antigen was able to protect against B. abortus 544 challenge. Nevertheless, the protection achieved was not comparable in quality or in duration to the protection conferred by the S19 live vaccine.
A probable explanation for this difference could be that the live smooth Brucella strain S19 elicits not only protective cell-mediated immunity (CMI) against a panel of Brucella T-cell epitopes but also humoral responses mostly against the lipopolysaccharide (LPS) O chain. These antibodies were demonstrated to be partially protective (4, 45, 70). So, in order to improve protection, we tested an approach by which a live bacterial vector delivers the selected Brucella antigens.
In such delivery vectors, the foreign genes may be present under the control of procaryotic promoters, enabling bacteria to express the heterologous protein and to deliver it to the immune system of the host (26, 27, 30). Alternatively, the live vector may deliver a naked-DNA vaccine plasmid in which the gene encoding the foreign antigen is under the control of a eucaryotic promoter (11, 18, 19, 55). We preferred the latter approach, assuming that intracellular antigen expression and plasmid-borne CpG motifs would drive a Th1-oriented immune response, including major histocompatibility complex class I-specific CD8+ cells, which are the keystones of the protective immune response against Brucella.
Several attenuated live bacterial vectors, such as Salmonella enterica serovar Typhimurium, Listeria monocytogenes, and Shigella flexneri, have already been shown to be able to deliver naked-DNA vaccine (13, 20, 33, 51, 58, 59).
We were interested in the use of Yersinia enterocolitica as a delivery vector for several reasons. First, Yersinia organisms are able to persist in tissues for several days after immunization, and they replicate the naked-DNA vaccine during their division, thereby increasing the amount of plasmidic DNA. Finally and more importantly, structural and immunological studies revealed that B. abortus and Y. enterocolitica serotype O:9 share common epitopes on their LPS O chains (A and CY epitopes) (52). This is not the case for Y. enterocolitica O:3, which has an antigenically unrelated LPS (2). Animals infected with serotype O:9 organisms but not with O:3 organisms produced antibodies cross-reacting with Brucella smooth LPS (S-LPS) in diagnostic tests (25, 67). In addition, it has been demonstrated that monoclonal antibodies specific for these epitopes were protective against B. abortus infection in passive transfer experiments (12, 45, 41). This will allow us to test the relative role of anti-LPS antibodies by using serotype O:3 and O:9 strains as delivery vectors for the naked-DNA vaccines.
We report here the use of attenuated strains of Y. enterocolitica serotypes O:3 and O:9 to deliver eucaryotic expression vectors (pCIBFR and pCIP39) in BALB/c mice. We analyzed the immune response (humoral and cellular) against BFR and P39 and against B. abortus extracts and compared the elicited protection against a B. abortus 544 challenge to that elicited by the live vaccinal S19 strain.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. abortus 544 was obtained from J.-M. Verger (Institut National de la Recherche Agronomique, Pathologie Infectieuse et Immunologie, Nouzilly, France), and B. abortus S19 and the Y. enterocolitica serotype O:3 and O:9 strains harboring virulence plasmid pYV+ (wild types) were obtained from J. Godfroid (Centre d'Etude et de Recherche Vétérinaire et Agrochimique, Brussels, Belgium). Brucella organisms were grown in, per liter, 10 g of yeast extract-10 g of Tryptone-5 g of NaCl at 37°C. Yersinia strains were generally grown on tryptic soy agar (Difco) at 28°C. The plasmid-cured strains (pYV−) was prepared by three sequential platings on tryptic soy broth (TSB) at 37°C. The loss of the plasmid was ascertained both by Congo red colony staining (6) and by loss of YopD expression (see below). Y. enterocolitica pYV− strains were used to derive DNA vaccine plasmid transformants. The pCI expression vectors encoding BFR (pCIBFR) and P39 (pCIP39) were described previously (3). Plasmids pCIBFR and pCIP39 were electroporated into attenuated Yersinia O:3 and O:9 strains. None of the Brucella-encoded antigens were expressed in the Yersinia vector, as demonstrated by Western blotting (data not shown). When necessary, the following antibiotics were included in the media (concentrations in micrograms per milliliter): nalidixic acid (25) and ampicillin (100) for Yersinia strains without pCI plasmids and ampicillin (600) for strains with pCI plasmids. For the immunization experiments, overnight cultures of pYV− Yersinia strains were diluted 100 times in TSB medium and grown at 28°C to an optical density at 600 nm of 0.4 to 0.6. Bacterial concentrations for immunization were quantified by plating serial dilutions on tryptic soy agar plates and counting CFU after incubation for 24 to 48 h at 28°C.
Isolation of Yersinia strains from spleens and PP of mice.
In order to examine the colonization and the persistence of the attenuated live delivery vectors, mice received a gastric administration of attenuated Y. enterocolitica (pYV−) with or without a pCI plasmid (1 × 108 to 3 × 108 bacteria per mouse) (see below). Three mice from both groups were killed at 2, 7, and 21 days postinfection, and their spleens were removed and homogenized in 1 ml of sterile phosphate-buffered saline (PBS) containing 0.1% Triton-100. Four centimeters of the ileum was cut upstream of the ileocecal junction. For simplicity, this organ was referred to as Peyer's patches (PP), and it was thoroughly washed with PBS before being homogenized in 5 ml of sterile PBS containing 0.1% Triton-100. The CFU were counted by plating serial dilutions onto Tryptic soy agar plates with an appropriate antibiotic selection.
Immunization and challenge.
In the first experiment, 10 groups of female BALB/c mice (Iffa Credo, Brussels, Belgium) 6 to 7 weeks old were immunized via an intragastric (i.g.) route as follows: mice were deprived of water and food for 4 h and were then inoculated i.g., by using a gavage needle, with 30 μl of 10% sodium bicarbonate buffer (NaHCO3) to neutralize stomach acidity (36). Thirty minutes later, they received 3 × 108 organisms of recombinant attenuated Y. enterocolitica O:9 or O:3 strains harboring one of the eucaryotic expression vectors (pCIBFR or pCIP39) in a 100-μl volume of PBS. Two groups of mice received a mixture (at twice the individual dose) of either O:3-O:9 or O:3(pCIP39)-O:9. Control groups of mice were immunized i.g. with the same doses of O:9 or O:3 strains harboring the empty pCI plasmid (host-vector controls) or not (host controls). Mice received two immunizations at 14-day intervals. Serum samples from all mice were obtained 1 day prior to the first immunization and then every 2 weeks for up to 2 weeks after the second injection and stored at −20°C for use in enzyme-linked immunosorbent assay (ELISA) and Western blot analyses (see below). Splenocytes for the study of proliferation activity or cytokine release were recovered 2 weeks after the second immunization from four mice of each group. For protection studies, mice were challenged intraperitoneally (i.p.) at 2 or 8 weeks after the last immunization with 5 × 104 CFU of B. abortus 544. As a positive control, a group of mice were injected i.p. with 5 × 104 CFU of Brucella S19 4 or 8 weeks before the challenge, and another group was injected i.g. with 1 × 109 CFU of S19 4 weeks before the challenge. Another group injected with PBS alone was used as a negative control. Four and eight weeks after the challenge infection, the mice were killed, bacteria from their spleens were recovered and plated, and CFU were counted.
Measurement of levels of serum antibody against Yersinia and the specific antigen.
Nunc-Immuno Maxisorp plates (Nunc, Roskilde, Denmark) were used in all ELISA experiments for measurement of levels of antibody against BFR, P39, and Yersinia O:3 and O:9 and B. abortus 544 extracts as described previously (3). Recombinant BFR or P39 (50 μl; 1 mg/ml) or bacterial extracts (50 μl; 3 mg/ml [Yersinia or B. abortus 544]) were used as the coating antigen. Dilutions of mouse sera were assayed for antibody titer by using goat-anti mouse immunoglobulin G1 (IgG1) and IgG2a (Amersham). 3,3′,5,5′-tetramethylbenzidine in citrate-phosphate buffer (pH 5) was used as the substrate. The absorbance of each well was measured at 450 to 630 nm. Antibody titers were estimated by end point dilutions for mouse sera, with the signal being considered positive for values greater than three times the values for naive animals.
In vitro stimulation of spleen cells.
Antigen-specific proliferative responses against BFR and P39 proteins were measured in splenocytes from immunized mice 14 days postimmunization (p.i.). Erythrocytes were lysed by the addition of 5 ml of Gey's solution per spleen and incubated on ice for 10 min. The cells were then washed twice, resuspended in RPMI 1640 medium (Gibco, BRL) supplemented with 5% heat-inactivated fetal bovine serum, and resuspended in RPMI 1640 cell culture medium supplemented with 2 mM l-glutamine, 10 mM HEPES, 2 mM pyruvate, 5 × 10−5 M 2-mercaptoethanol, 10 μg of streptomycin per ml, 100 U of penicillin per ml, and 10% fetal bovine serum. A mixed acridine orange (0.0003% [wt/vol])-ethidium bromide (0.001% [wt/vol]) stain was used to determine the percentage of viable cells in the preparation. The cells, at a final concentration of 2 × 106 per ml, were incubated with BFR, P39, or a whole-cell lysate of the O:3 or O:9 strain in quadruplicate in a 96-well round-bottomed plate. The mitogen concanavalin A (ConA) (3 μg/ml; Sigma, St. Louis, Mo.) was included in the assay as a positive control. Equivalent volumes of medium alone provided the negative control for the test. The plates were incubated for 72 h at 37°C in a CO2 incubator, and the cell cultures were pulsed with [3H]thymidine (0.5 μCi per well; ICN, Brussels, Belgium) 18 h prior to harvesting the cells. Thymidine incorporation was measured by liquid scintillation (using a Betaplate counter). The values (in counts per minute) are means and standard errors of the means for each quadruplicate assay of cells.
Cytokine analysis.
Gamma interferon (IFN-γ) and interleukin-5 (IL-5) were measured by a commercially available kit (Pharmingen, San Diego, Calif.), according to the manufacturer's instructions. Spleen cells from vaccinated and control mice were collected and cultured (4 days at 37°C and 5% CO2 as described above). Culture supernatants were used for cytokine analyses. The lower detection limit of the IFN-γ ELISA was 40 pg/ml, whereas the detection limit of the IL-5 ELISA was 15 pg/ml.
Western blotting.
Four micrograms of total lysates of bacteria, 0.1 μg of the recombinant proteins, or 15 μl of culture supernatants of the attenuated (pYV−) O:3 or O:9 strains or from the wild-type strain (see below) were loaded on a sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-PAGE) gel and analyzed by immunoblotting. Protein concentrations were first quantified by a bicinchoninic acid assay. Samples were boiled for 10 min prior to SDS-PAGE separation. They were then transferred onto nitrocellulose by electroblotting. Specific proteins were visualized on the membranes by using a rabbit anti-BFR polyclonal antibody (3), a mouse monoclonal antibody directed against P39 protein (40), or an anti-YopD monoclonal antibody.
Expression of YopD.
Under certain in vitro conditions, virulent Yersinia strains (harboring the pYV plasmid) secrete Yop proteins into the medium (38). To confirm the cure of the pYV virulence plasmid in the Yersinia strain used as a vector, we compared YopD production from the cured and the wild-type Yersinia strains. For YopD production, 15 ml of an overnight culture at 28°C in TSB (Difco) containing 0.3% (wt/vol) yeast extract (Gibco, BRL), and the appropriate antibiotics were pelleted; then the bacteria were grown in 15 ml of TSB-MOX medium (TSB containing 20 mM MgCl2, 20 mM Na2C2O4, and 0.4% galactose). The cultures were shaken for 2 h at 28°C and then shifted for 3 h at 37°C. The supernatant was then harvested and treated for SDS-PAGE and Western blotting.
Statistics.
For the protection experiments, two-way analysis of variance (vaccine by time) and pairwise comparisons between vaccine for the same level of time (Scheffe test at an alpha level of 0.05) have been obtained by the ANOVA procedure of the SAS software (SAS Institute, Cary, N.C.).
RESULTS
Persistence of attenuated Yersinia in the spleen and PP after i.g. immunization.
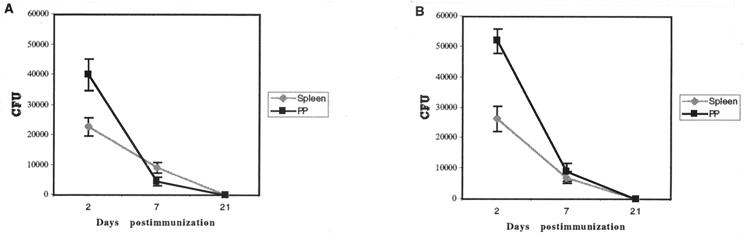
In order to assess the use of Yersinia as a live vector for vaccination, we analyzed the persistence of the bacteria in vivo. BALB/c mice were inoculated once i.g. with attenuated O:9 alone (nine mice) or O:9(pCIP39) (nine mice). On days 2, 7, and 21 after inoculation, three mice from each group were killed and the numbers of bacteria present in the spleen and PP were determined by CFU counts (Fig. 1). Whatever the strain used, on day 2 after inoculation, the number of CFU in the spleen was almost twice as low as in PP. On day 7, the number of CFU had decreased (by a factor of 5 in the PP and 2 in the spleen) compared to day 2. On day 21, no Yersinia organisms were detected in PP or in the spleen. O:3 had a similar pattern of persistence (data not shown).
FIG. 1.
Time course of persistence of bacteria in spleen and PP. Groups of nine mice were immunized i.g. with 1 × 108 to 3 × 108 CFU of Y. enterocolitica O:9(pCIP39) (A) or O:9 (B) and sacrificed after 2, 7, or 21 days (three mice at each time). The spleen and PP were recovered, homogenized, and plated on agar plates. Data are means ± standard deviations (n = 3).
Expression of BFR and P39 from pCI after i.g. immunization with pYV− Y. enterocolitica.
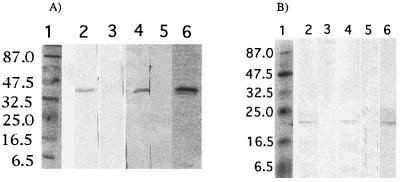
In order to assess the ability of Yersinia to deliver a plasmid DNA vaccine and of the two plasmid-encoded antigens to be expressed in the host, we monitored by Western blotting the antibody response against the relevant antigens. Plasmids pCIBFR and pCIP39 were electrotransformed into O:3 and O:9 (pYV−) and these strains were administered twice i.g. to mice (n = 4) at 2-week intervals. Sera collected 14 days postinoculation were pooled and analyzed by Western blot on recombinant BFR or P39. As shown in Fig. 2, a characteristic band at ∼39 kDa corresponding to the P39 protein was revealed with sera from mice immunized with O:3(pCIP39) or O:9(pCIP39) (Fig. 2A, lanes 2 and 4) and not in the lanes probed with sera from control mice (lanes 3 and 5). Similarly, a characteristic band at ∼20 kDa corresponding to the BFR protein was revealed with sera from mice immunized with O:3(pCIBFR) or O:9(pCIBFR) (Fig. 2B, lanes 2 and 4) but was absent in mice receiving O:3 or O:9 without pCIBFR (Fig. 2B, lanes 3 and 5). Monoclonal anti-P39 antibody (Fig. 2A, lane 6) and polyclonal anti-BFR antibody (Fig. 2B, lane 6) were used as positive controls.
FIG. 2.
Western blot of sera from recombinant-Yersinia-immunized mice. Recombinant BFR (A) and P39 (B) were subjected to SDS-PAGE and then transferred to nitrocellulose, which was subsequently probed with sera from immunized mice (four mice per group). (A) Lanes 2, 3, 4, and 5 were probed with sera from mice immunized with O:3(pCIP39), O:3 alone, O:9(pCIP39), and O:9 alone, respectively. Lane 6 was probed with anti-P39 monoclonal antibody as a control. (B) Lanes 2, 3, 4, and 5 were probed with sera from mice immunized with O:3(pCIBFR), O:3 alone, O:9(pCIBFR), and O:9 alone, respectively. Lane 6 was probed with rabbit anti-BFR polyclonal antibody as a control. Lanes 1 contain prestained molecular markers. Sizes (in kilodaltons) are indicated on the left.
Humoral immune response against recombinant proteins.
The levels of IgG1 and IgG2a antibody responses (Table 1) against the recombinant proteins (BFR and P39) and the whole-cell bacterial extract (B. abortus 544, O:3, or O:9) were tested in serum 14 days after the second i.g. administration of O:3 or O:9 transformed with pCI, pCIBFR, or pCIP39 or of untransformed bacteria. All mice inoculated with O:3 and O:9 produced high titer of antibody against O:3 and O:9, respectively, whatever the harbored plasmid. These titers were similar when sera of mice immunized with O:9 (with or without plasmid) were tested against B. abortus 544. In contrast, mice immunized with O:3 (with or without plasmid) did not develop any detectable humoral response against B. abortus 544. Recombinant P39-specific antibodies (IgG1 and IgG2a) were detected in sera of all mice immunized i.g. with the two attenuated Yersinia strains carrying pCIP39. By contrast, sera from O:3(pCIBFR) and O:9(pCIBFR) groups had a weak IgG2a response but no detectable IgG1 response against the purified recombinant BFR. There were no detectable specific anti-BFR or anti-P39 antibodies in the sera of mice which received the attenuated Yersinia strains carrying no plasmid or the control vector pCI. Strains carrying pCIBFR did not induce P39-specific antibodies, and strains carrying pCIP39 did not induce BFR-specific antibodies. There was no significant difference between the levels of antibodies induced by O:3(pCIBFR or pCIP39) and O:9(pCIBFR or pCIP39) against the respective recombinant protein (BFR or P39). In all sera except those from mice receiving O:9(pCI), the level of IgG2a was equal to or higher than the IgG1 titers.
TABLE 1.
IgG responses in mice immunized with live attenuated Yersinia strainsa
| Antibody | Treatment group | Log10 titer of antibody tob:
|
||||
|---|---|---|---|---|---|---|
| BFR | P39 | 544 | O:3 | O:9 | ||
| IgG1 | O:3 | — | — | — | 2.9 ± 0.02 | ND |
| O:3(pCI) | — | — | — | 2.9 ± 0.09 | ND | |
| O:3(pCIBFR) | — | — | — | 2.6 ± 0.02 | ND | |
| O:3(pCIP39) | — | 2.0 ± 0.04 | — | 2.9 ± 0.04 | ND | |
| O:9 | — | — | 2.3 ± 0.04 | ND | 3.2 ± 0.12 | |
| O:9(pCI) | — | — | 2.6 ± 0.1 | ND | 2.9 ± 0.09 | |
| O:9(pCIBFR) | — | — | 2.0 ± 0.04 | ND | 2.3 ± 0.12 | |
| O:9(pCIP39) | — | 2.0 ± 0.04 | 2.0 ± 0.04 | ND | 2.3 ± 0.04 | |
| IgG2a | O:3 | — | — | — | 2.9 ± 0.07 | ND |
| O:3(pCI) | — | — | — | 2.9 ± 0.07 | ND | |
| O:3(pCIBFR) | 1.7 ± 0.02 | — | — | 2.9 ± 0.04 | ND | |
| O:3(pCIP39) | — | 2.3 ± 0.07 | — | 3.2 ± 0.07 | ND | |
| O:9 | — | — | 2.3 ± 0.02 | ND | 3.2 ± 0.09 | |
| O:9(pCI) | — | — | 2.3 ± 0.09 | ND | 2.9 ± 0.05 | |
| O:9(pCIBFR) | 1.7 ± 0.0 | — | 2.3 ± 0.05 | ND | 2.6 ± 0.09 | |
| O:9(pCIP39) | — | 2.6 ± 0.06 | 2.3 ± 0.04 | ND | 2.9 ± 0.07 | |
Groups (n = 4) were immunized i.g., twice at 14-day intervals.
ND, not determined. —, not detectable.
Cellular immune response against recombinant proteins.
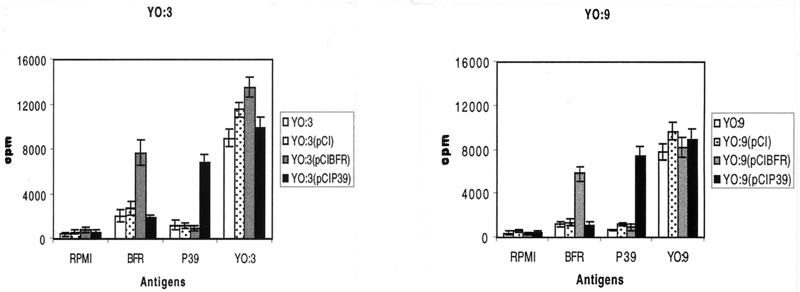
The ability of the Yersinia vaccines to induce a cell-mediated immune response was determined by assaying the proliferation of spleen cells in vitro and the production IFN-γ after stimulation with BFR, P39, or bacterial extracts (Yersinia or B. abortus 544). Upon BFR and P39 antigenic stimulation, splenocytes isolated from mice immunized with O:3(pCIBFR) and O:3(pCIP39) showed proliferative responses almost equivalent to those of splenocytes from mice immunized with O:9(pCIBFR) and O:9(pCIP39), respectively (Fig. 3). In contrast, splenocytes from control mice [O:3, O:3(pCI), O:9, and O:9(pCI)] exhibited little or no proliferative response to recombinant BFR and P39. In all groups, a strong proliferative response was observed upon stimulation with the respective Y. enterocolitica O:3 or O:9 extracts (Fig. 3) or with the mitogen ConA (data not shown). A response to Brucella lysate was also evidenced, but at a lower level (data not shown).
FIG. 3.
Proliferative T-cell responses among splenocytes recovered from BALB/c mice. The assays were performed 14 days after two i.g. inoculations with attenuated Y. enterocolitica serotype O:3 or O:9 harboring pCI, pCIBFR, or pCIP39 or with Yersinia strains alone. Splenic cells were stimulated in quadruplicate with 10 μg of attenuated O:3 or O:9 lysate or the recombinant proteins BFR or P39 per ml or were not stimulated (RPMI). Data are means ± standard deviations of quadruplicate measurements from four mice.
Table 2 shows the IFN-γ response upon stimulation with the different antigens. Supernatants of splenocytes stimulated with O:3 or O:9 extracts or with the recombinant proteins (BFR and P39) were collected and used in cytokine assays. Determination of IFN-γ levels revealed that splenocytes from mice immunized i.g. (Table 2) with O:3(pCIBFR), O:9(pCIBFR), O:3(pCIP39), or O:9(pCIP39) produced significant quantities of IFN-γ when stimulated with the relevant recombinant proteins (BFR and P39) compared to the control groups [O:3, O:9, O:3(pCI), or O:9(pCI)]. Splenocytes from all mice stimulated with ConA (as a control) produced high levels of IFN-γ (Table 2). IL-5 was not detected in the supernatants of recombinant-protein-stimulated splenocyte cultures derived from any Yersinia-immunized mice (data not shown). However, IL-5 was detected in the supernatants of ConA-stimulated splenocytes from all mice, but only at low levels (from 62 to 170 pg/ml).
TABLE 2.
IFN-γ production in spleen cell cultures from i.g. immunized mice
| Antigen | IFN-γ concn (pg/ml) in cells from mice receivinga:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| O:3 | O:3(pCI) | O:3(pCIBFR) | O:3(pCIP39) | O:9 | O:9(pCI) | O:9(pCIBFR) | O:9(pCIP39) | |
| RPMI | — | — | — | — | — | — | — | — |
| BFR | 125 | 125 | 1,700 | — | 62.5 | 75 | 1,970 | — |
| P39 | — | — | — | 1,900 | 75 | — | — | 2,100 |
| O:3 | 2,200 | 2,200 | 1,900 | 2,000 | ND | ND | ND | ND |
| O:9 | ND | ND | ND | ND | 1,800 | 1,980 | 2,200 | 2,000 |
| ConA | 3,200 | 3,300 | 1,980 | 2,100 | 2,200 | 2,450 | 2,300 | 2,500 |
Splenocytes were recovered from groups of four mice 14 days after two inoculations. Levels of IFN-γ in culture supernatants from triplicate wells were measured after 92 h of incubation. ND, not determined; —, below the detection limit of the assay (40 pg/ml).
Efficacy of live Y. enterocolitica vectors in generating protective immunity against a B. abortus 544 challenge.
Mice immunized twice i.g. with O:3, O:3(pCI), O:3(pCIBFR), O:3(pCIP39), O:9, O:9(pCI), O:9(pCIBFR), or O:9(pCIP39) were challenged i.p. with 5 × 104 CFU of B. abortus 544 at 2 or 8 weeks p.i. Protection was assessed by testing the reduction of splenic Brucella CFU 4 and 8 weeks postchallenge. All of the immunizing bacteria (O:9 with or without plasmid) which gave high titers of antibodies to B. abortus 544 in the serum 14 days after inoculation provided a significant level of protection against B. abortus 544 compared to the mock-vaccinated group 20 (Table 3). Protection in the animals that were challenged 2 weeks postvaccination (groups 5 to 10) ranged from 1.43 to 2.22 (log10 units) at 4 weeks p.i. and from 1.15 to 1.97 at 8 weeks p.i. This slight decrease in protection at 8 weeks p.i. was not evidenced when the challenge was given 8 weeks postvaccination (groups 14 to 16). In the latter case, the protection ranged from 1.26 to 1.75 (log10 units) at 4 weeks p.i. and from 1.64 to 1.92 at 8 weeks p.i.
TABLE 3.
Protection against a B. abortus 544 challenge provided to BALB/c mice by vaccination with live O:3, O:9, or S19 strainsa
| Treatment group (n = 3 or 4) | Vaccine injection route | Vaccine | Vaccination challenge delay (wk) | Log10 brucellae in spleen (mean ± SD) at time (wk) postchallenge
|
Log units of protection at wk
|
||
|---|---|---|---|---|---|---|---|
| 4 | 8 | 4 | 8 | ||||
| 1 | i.g. | O:3 | 2 | 4.32 ± 0.17 | 3.58 ± 0.13 | 0.13 | 0.09 |
| 2 | i.g. | O:3(pCI) | 2 | 4.43 ± 0.14 | 3.55 ± 0.02 | 0.02 | 0.12 |
| 3 | i.g. | O:3(pCIBFR) | 2 | 3.96 ± 0.12 | 2.95 ± 0.16 | 0.49 | 0.72 |
| 4 | i.g. | O:3(pCIP39) | 2 | 3.76 ± 0.20 | 2.80 ± 0.11 | 0.69 | 0.87b,c |
| 5 | i.g. | O:9 | 2 | 3.02 ± 0.16 | 2.52 ± 0.16 | 1.43b,c | 1.15b,c |
| 6 | i.g. | O:9(pCI) | 2 | 3.15 ± 0.19 | 2.44 ± 0.13 | 1.3b,c | 1.23b |
| 7 | i.g. | O:9(pCIBFR) | 2 | 2.81 ± 0.45 | 2.04 ± 0.10 | 1.64b | 1.63b |
| 8 | i.g. | O:9(pCIP39) | 2 | 2.03 ± 0.25 | 1.7 | 2.22b | 1.97b |
| 9 | i.g. | O:3-O:9 | 2 | 2.92 ± 0.07 | 2.40 ± 0.12 | 1.53b,c | 1.27b |
| 10 | i.g. | O:3(pCIP39)-O:9 | 2 | 2.35 ± 0.02 | 1.7 | 2.1b | 1.97b |
| 11 | i.g. | O:3 | 8 | 4.27 ± 0.13 | 3.61 ± 0.02 | 0.18 | 0.06 |
| 12 | i.g. | O:3(pCIBFR) | 8 | 4.15 ± 0.07 | 3.42 ± 0.04 | 0.30 | 0.25 |
| 13 | i.g. | O:3(pCIP39) | 8 | 4.03 ± 0.17 | 3.26 ± 0.03 | 0.42 | 0.41 |
| 14 | i.g. | O:9 | 8 | 3.19 ± 0.04 | 2.03 ± 0.16 | 1.26b,c | 1.64b |
| 15 | i.g. | O:9(pCIBFR) | 8 | 3.08 ± 0.04 | 1.97 ± 0.18 | 1.37b,c | 1.7b |
| 16 | i.g. | O:9(pCIP39) | 8 | 2.7 ± 0.25 | 1.72 ± 0.14 | 1.75b | 1.92b |
| 17 | i.g. | B19 | 4 | 3.67 ± 0.19 | 2.77 ± 0.22 | 0.73b,c | 0.9b,c |
| 18 | i.p. | B19 | 4 | 2.15 ± 0.15 | 1.7 | 2.3b | 1.97b |
| 19 | i.p. | B19 | 8 | 1.85 ± 0.15 | 1.7 | 2.6b | 1.97b |
| 20 | i.p. | PBS | 4.45 ± 0.13 | 3.67 ± 0.08 | |||
Groups 1 through 16 were vaccinated with 1 × 108 to 3 × 108 CFU, group 17 was vaccinated with 1 × 109 CFU, and groups 18 and 19 were vaccinated with 5 × 104 CFU. All groups were challenged i.p. with 5 × 104 CFU of B. abortus 544.
Statistically different from the unvaccinated control (P = 0.05).
Statistically different from the B19 i.p. vaccinated control (P = 0.05).
Mice inoculated with O:3 bacteria with or without pCI plasmids (groups 1, 2, and 11), which failed to develop antibodies against B. abortus 544 in the serum at day 14 after the second immunization, were not protected. With regard to the additional protection conferred by the plasmid-encoded antigens, results clearly show that O:9(pCIP39) (groups 8 and 16) induced a better protection that O:9 alone (groups 5 and 14) or O:9(pCI) (group 6). Except for group 4 at 8 weeks postchallenge, results obtained for O:3(pCIP39) were not significantly different from those for O:3 alone (groups 1 and 11) or O:3(pCI) (group 2). Whatever the serotype of the vector or the vaccination-to-challenge interval, the pCIBFR plasmid is not statistically different from the empty respective vector.
To confirm the additive effect of the immune response against the plasmid-encoded P39 antigen and of the antibodies cross-reactive against Brucella S-LPS in protection, we performed the following control experiment.
We immunized two groups of mice with either O:3 plus O:9 or O:3(pCIP39) plus O:9 twice i.g. at a 14-day interval; these animals were then challenged with B. abortus 544 2 weeks after the last immunization. Mice immunized with O:3(pCIP39)-O:9 had a higher level of protection than mice immunized with O:3-O:9 at weeks 4 and 8 p.i. while having almost the same level of protection as mice immunized with O:9(pCIP39).
As positive control of protection, mice were immunized either i.g. or i.p. with B. abortus S19 at 1 × 109 or 5 × 104 CFU, respectively. All animals were significantly protected compared to the nonvaccinated group (group 20); however, i.g. immunization (group 17) gave a significantly lower protection than i.p. immunization (groups 18 and 19). All O:9-vaccinated animals (groups 5 to 10 and 14 to 16) showed a better protection than the mice treated i.g. with S19. Nevertheless, only mice receiving both pCIP39 and O:9 (groups 8, 10, and 16) consistently demonstrated a protection level almost comparable to that in the group vaccinated i.p. with S19.
DISCUSSION
Several reports have shown that DNA vaccination engenders long-lived humoral and cellular immune responses in vivo against viruses, bacteria, or parasites in a variety of animal models (21, 39, 64). DNA vaccine provides prolonged antigen expression, leading to amplification of immune response and induces memory responses against infectious agents (29, 63). Moreover, endogenous expression of antigen from DNA introduced into host cells leads to processed peptides presented with the major histocompatibility complex class I, able to induce cytotoxic T lymphocytes (21, 31). This type of vaccine is capable of eliciting the strong CMI that is required for control of infection by many intracellular agents (28, 37, 61). While this kind of immune response has been shown to be of paramount importance against Brucella (4, 24, 50), the antibody response, mostly directed against S-LPS, has also often been considered crucial (23, 53).
This complementarity between humoral anti S-LPS response and the CMI response is one of the explanations we favored to explain why, in a previous experiment, candidate subunit vaccines based on Brucella T-dominant antigens (P39 and BFR) were not as effective as the live attenuated vaccinal strain S19 in protecting mice against a B. abortus 544 challenge (3, 3a).
In order to test this hypothesis and with a view to improving the protective efficacy of our candidate vaccine, we planned to deliver the naked-DNA vaccine encoding P39 or BFR using a carefully chosen live bacterial vector.
The use of attenuated intracellular bacteria (S. enterica serovar Typhimurium, Shigella flexneri, and L. monocytogenes) as delivery vehicles to target DNA vaccine to professional antigen-presenting cells has been recently described (14, 57). This approach combines the advantages of naked-DNA vaccination with those of live vaccines. Such live vaccines carry their own immunostimulatory motifs, are able to replicate in the host (and thus also replicate the plasmid they harbor), and can elicit immune responses not only against the plasmid-encoded foreign antigen but also against the bacterial carrier itself (22, 42, 62). The latter point was the basis on which we selected Y. enterocolitica O:9 as a vehicle for our candidate DNA vaccines. In fact, the antigenic relationship between O:9 S-LPS and A-dominant B. abortus S-LPS is close (both S-LPSs being built mostly from α1-2-linked 4-6-dideoxy-4-formamido-α-d-mannopyranosyl units). While Y. enterocolitica O9 is pathogenic only for humans and rodents, not for sheep or cattle (34, 69), it is well known that antibodies from O:9-infected animals are highly cross-reactive against Brucella S-LPS (25, 69). In addition, this bacterium can easily be transformed with eucaryotic expression vectors containing origins of replication that are functional in Escherichia coli, such as pCI. Nevertheless, pathogenic Yersinia spp. remain mainly extracellular, avoiding phagocytosis by means of a type III secretion system encoded by its virulence plasmid (pYV) (7, 9, 10).
This is a potential limitation to the efficient targeting of DNA vaccine to host antigen-presenting cells. To avoid this drawback we took the precaution of curing the Y. enterocolitica O:3 and O:9 strains from their virulence plasmid, giving the respective pYV minus strain. As ascertained by Western blotting with antibodies against YopD protein (a type III effector) and by Congo red staining of colonies, the cure was effective. These pYV-cured strains are also known to be harmless even in susceptible species (7), which should allow their controlled field use as vaccinal carriers.
Both the pYV− O:9 and O:3 strains were still able to gain access to systemic lymphoid organs (spleen) in addition to mucosa-associated lymphoid tissues, where they predominate, and were detectable for 2 to 3 weeks after i.g. administration (Fig. 1).
On this preliminary basis, we started to evaluate whether recombinant attenuated Y. enterocolitica O:3 and O:9 harboring eucaryotic expression vectors could induce a specific systemic humoral immune response after i.g. administration. We found that mice immunized with the attenuated (pYV−) Y. enterocolitica O:3 or O:9 strain transformed with pCIBFR or pCIP39 effectively delivered plasmids into mouse cells, as shown by the humoral immune response against these two antigens. As illustrated by Western blotting (Fig. 2), both Yersinia strains were able to induce antibodies against the plasmid-encoded Brucella antigens (P39 and BFR). No antigen-specific responses were observed for control bacteria which contained empty pCI plasmid or no plasmid.
To our knowledge this is the first description of the use of a bacterium with a noninvasive phenotype to deliver a DNA vaccine.
As measured by ELISA (Table 1), the mice immunized with O:9 strains (with or without plasmids) produced antibodies against total B. abortus 544 extracts whereas the animals immunized with O:3 strains failed to do so. This is consistent with the cross-reactive antibodies being mostly directed towards the shared S-LPS epitopes between O:9 and B. abortus 544, as expected.
The above data prompted us to analyze the type of humoral and cellular immune responses induced by the candidate vaccines. Assays for IgG1 and IgG2a levels were used to determine whether the humoral response was inclined toward the Th2 or Th1 pattern, respectively. It can be seen that Y. enterocolitica O:3 or O:9 administered i.g. was effective in inducing specific IgGs, indicating that systemic immunity was induced. Mice immunized with Y. enterocolitica(pCIP39) developed higher titers of serum antibody against P39 than the animals immunized with Y. enterocolitica(pCIBFR) against BFR. Except for the mice immunized with Y. enterocolitica(pCIBFR), which failed to develop anti-BFR IgG1 antibodies, most of the mice developed specific IgG1 and IgG2a antibodies (Table 1). When analyzed on whole bacterial extracts, the IgG1 titers paralleled the IgG2a levels but the IgG1 titers were lower than the IgG2a titers when tested on P39. All these data are reminiscent of the previous data obtained with these antigens as recombinant proteins or as DNA vaccine (3, 3a). The IFN-γ-induced expression of immunoglobulins of the IgG2a isotype is widely recognized as a characteristic of a Th1-type immune response (46). The results in Table 2 and Fig. 3 demonstrate that the O:3 and O:9 strains bearing the recombinant plasmids (pCIBFR and pCIP39) were able to elicit antigen-specific T cells, as judged by the proliferation assay and the assay for IFN-γ (in the absence of detectable IL-5). We showed that the live attenuated Yersinia strains used as delivery vectors for DNA vaccine induced a Th1 response, as it was observed in the naked-DNA immunization experiments with the same plasmids (3).
Experiments were then carried out to determine whether the recombinant Y. enterocolitica O:9 or O:3 with pCIBFR or pCIP39 could protect BALB/c mice in a challenge with B. abortus 544. To determine also whether these vaccinal strains elicit long-lasting immunity and subsequent protection, we compared a challenge given 2 or 8 weeks after the last i.g. immunization. Whatever the immunization-to-challenge interval, O:9 strains (even without plasmid) elicited far better protection against B. abortus 544 infection than O:3 strains. This result correlates with the level of antibodies detected in ELISA against B. abortus 544 extracts. The two Yersinia strains being mostly different at the level of the LPS O-chain structure, these findings suggest that the observed protection was dependent on antibody response against LPS of O:9, which was cross-reactive against B. abortus 544 S-LPS.
While empty O:3 strains failed to protect, O:3 strains harboring pCIBFR and pCIB39 showed low but significant protection. This effect was most apparent when the challenge was given 2 weeks after the immunization. The same additional effect of the recombinant plasmids was observed when O:9 strains were used as vehicles. Although the two recombinant plasmid vaccines induced BFR- or P39-specific Th1 immune responses, in all cases, mice immunized with pCIP39 exhibited a higher degree of protective immunity against B. abortus 544 infection those receiving the equivalent pCIBFR strains. This also agreed with the results obtained with intramuscularly administered naked DNA (3a).
The level of protection achieved (at either 4 or 8 weeks postchallenge) when both O:9 and pCIP39 were present in the vaccinal preparation was comparable to the protection conferred by the S19 live vaccine administered i.p. when the challenge was given 2 weeks after the immunization. With a challenge given 8 weeks after the last immunization the protection level measured at 4 weeks p.i. was generally inferior to those demonstrated when the challenge was given 2 weeks after the immunization and to the protection conferred by the S19 strain. In contrast, at 8 weeks postchallenge, both O:9(pCIBFR) and O:9(pCIP39) give a protection similar to that observed with a challenge given 2 weeks after the immunization with the same strain. In addition, under these conditions, O:9(pCIP39) gives a protection almost equal to that provided by the S19 vaccinal strain.
While Yersinia organisms were previously used successfully as carriers for protective antigens encoded on procaryotic expression plasmids (34, 54, 65), this is the first description of their promise as vehicles for DNA vaccines given orally. Although systemic immunity has been shown to be protective against brucellosis, whether mucosal immune responses can also be effective is currently not known (12). Nevertheless, the use of the i.g. route of immunization described in this paper could offer an opportunity to test this hypothesis. It is in fact well known that antigens given orally reach M cells, which play an important role in the initiation of both mucosal and systemic immune responses (32, 35, 49). This was not evaluated in the present study, because the only currently used mouse model of challenge uses a systemic (i.p.) route which bypasses the digestive tract and needs systemic immune effectors. Nevertheless, the recently described (43) nasal infection route will be of great value in validating our oral vaccine.
The last point which needs to be stressed concerns the discrimination between vaccinated and infected animals, which is very important in the brucellosis field. Because the usual serological tests use Brucella S-LPS as the diagnostic antigen, the use of a live Yersinia O:9 vector inducing LPS cross-reactive antibodies could seem paradoxical. Nevertheless, there could be two ways to avoid this problem. The first is to use CMI (either skin testing [56] or in vitro IFN-γ production [68]) as a diagnostic tool with a brucellin extract prepared from a Brucella strain devoid of the p39 gene as the antigen. The other is based on the existence of S-LPS epitopes which are strictly specific for Brucella S-LPS (66, 67) and as such not borne by the O:9 vector. The so-called C epitope or a peptidic mimic (15, 44) of it could be used as surrogate diagnostic antigen, allowing the specific identification of Brucella-infected animals. Both approaches are under investigation in our laboratory.
In summary, this study provided direct evidence that recombinant pYV− O:9 harboring DNA vaccines engenders vigorous specific Th1 T-cell responses and specific serum IgG responses against the encoded antigens and the LPS of the bacterial vector, which were able to protect BALB/c mice against a B. abortus 544 infection. This observation confirms that efficient protection against Brucella requires the action of both humoral and cellular immune mechanisms (4, 28).
Whether the pCIP39 administered in this system will prove to be a efficient vaccine against brucellosis will wait until it can be tested in a pregnant sheep model.
Acknowledgments
We thank G. Houbeau for his help with the mouse model and E. Depiereux for statistical analysis.
Ayman Al-Mariri holds a fellowship from the Atomic Energy Commission of Syria (AECS). This work was supported by the Commission of the European Communities, contract QLK2-CT-1999-00014.
Editor: J. T. Barbieri
REFERENCES
- 1.Aleixo, M. J., M. L. Ferreira, and F. Antunes. 1999. Brucellosis. Acta Med. Port. 12:323-330. (In Portuguese.) [PubMed] [Google Scholar]
- 2.Al-Hendy, A., P. Toivanen, and M. Skurnik. 1992. Lipopolysaccharide O side chain of Yersinia enterocolitica O:3 is an essential virulence factor in an orally infected murine model. Infect. Immun. 60:870-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mariri, A., A. Tibor, P. Mertens, X. De Bolle, P. Michel, J. Godefroid, K. Walravens, and J.-J. Letesson. 2001. Protection of BALB/c mice against challenge with B. abortus 544 by vaccination with BFR or P39 recombinant proteins adjuvanted with CpG ODN. Infect. Immun 69:4816-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Al-Mariri, A., A. Tibor, P. Mertens, X. De Bolle, P. Michel, J. Godefroid, K. Walravens, and J.-J. Letesson. 2001. Induction of immune response in BALB/c mice with a DNA vaccine encoding bacterioferritin or P39 of Brucella spp. Infect. Immun. 69:6264-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araya, L. N., P. H. Elzer, G. E. Rowe, F. M. Enright, and A. J. Winter. 1989. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J. Immunol. 143:3330-3337. [PubMed] [Google Scholar]
- 5.Beckett, F. W., and S. C. MacDiarmid. 1985. The effect of reduced-dose Brucella abortus strain 19 vaccination in accredited dairy herds. Br. Vet. J. 141:507-514. [DOI] [PubMed] [Google Scholar]
- 6.Bhaduri, S., C. Turner-Jones, and R. V. Lachica. 1991. Convenient agarose medium for simultaneous determination of the low-calcium response and Congo red binding by virulent strains of Yersinia enterocolitica. J. Clin. Microbiol. 29:2341-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleves, S., and G. R. Cornelis. 2000. How to survive in the host: the Yersinia lesson. Microbes Infect. 2:1451-1460. [DOI] [PubMed] [Google Scholar]
- 8.Boschiroli, M. L., V. Foulongne, and D. O'Callaghan. 2001. Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 4:58-64. [DOI] [PubMed] [Google Scholar]
- 9.Bottone, E. J. 1999. Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. 1:323-333. [DOI] [PubMed] [Google Scholar]
- 10.Boyd, A. P., N. Grosdent, S. Totemeyer, C. Geuijen, S. Bleves, M. Iriarte, I. Lambermont, J. N. Octave, and G. R. Cornelis. 2000. Yersinia enterocolitica can deliver Yop proteins into a wide range of cell types: development of a delivery system for heterologous proteins. Eur. J. Cell Biol. 79:659-671. [DOI] [PubMed] [Google Scholar]
- 11.Chen, S. C., D. H. Jones, E. F. Fynan, G. H. Farrar, J. C. Clegg, H. B. Greenberg, and J. E. Herrmann. 1998. Protective immunity induced by oral immunization with a rotavirus DNA vaccine encapsulated in microparticles. J. Virol. 72:5757-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cloeckaert, A., I. Jacques, P. de Wergifosse, G. Dubray, and J. N. Limet. 1992. Protection against Brucella melitensis or Brucella abortus in mice with immunoglobulin G (IgG), IgA, and IgM monoclonal antibodies specific for a common epitope shared by the Brucella A and M smooth lipopolysaccharides. Infect. Immun. 60:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darji, A., C. A. Guzman, B. Gerstel, P. Wachholz, K. N. Timmis, J. Wehland, T. Chakraborty, and S. Weiss. 1997. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell 91:765-775. [DOI] [PubMed] [Google Scholar]
- 14.Darji, A., S. zur Lage, A. I. Garbe, T. Chakraborty, and S. Weiss. 2000. Oral delivery of DNA vaccines using attenuated Salmonella typhimurium as carrier. FEMS Immunol. Med. Microbiol. 27:341-349. [DOI] [PubMed] [Google Scholar]
- 15.De Bolle, X., T. Laurent, A. Tibor, F. Godfroid, V. Weynants, J. J. Letesson, and P. Mertens. 1999. Antigenic properties of peptidic mimics for epitopes of the lipopolysaccharide from Brucella. J. Mol. Biol. 294:181-191. [DOI] [PubMed] [Google Scholar]
- 16.Denoël, P. A., T. K. Vo, A. Tibor, V. E. Weynants, J. M. Trunde, G. Dubray, J. N. Limet, and J. J. Letesson. 1997. Characterization, occurrence, and molecular cloning of a 39-kilodalton Brucella abortus cytoplasmic protein immunodominant in cattle. Infect. Immun. 65:495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denoël, P. A., T. K. Vo, V. E. Weynants, A. Tibor, D. Gilson, M. S. Zygmunt, J. N. Limet, and J. J. Letesson. 1997. Identification of the major T-cell antigens present in the Brucella melitensis B115 protein preparation, Brucellergene OCB. J. Med. Microbiol. 46:801-806. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich, G., A. Bubert, I. Gentschev, Z. Sokolovic, A. Simm, A. Catic, S. H. Kaufmann, J. Hess, A. A. Szalay, and W. Goebel. 1998. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat. Biotechnol. 16:181-185. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich, G., I. Gentschev, J. Hess, J. B. Ulmer, S. H. Kaufmann, and W. Goebel. 1999. Delivery of DNA vaccines by attenuated intracellular bacteria. Immunol. Today 20:251-325. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich, G., A. Kolb-Maurer, S. Spreng, M. Schartl, W. Goebel, and I. Gentschev. 2001. Gram-positive and Gram-negative bacteria as carrier systems for DNA vaccines. Vaccine 19:2506-2512. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 22.Drabner, B., and C. A. Guzman. 2001. Elicitation of predictable immune responses by using live bacterial vectors. Biomol. Eng. 17:75-82. [DOI] [PubMed] [Google Scholar]
- 23.Elzer, P. H., R. H. Jacobson, K. H. Nielsen, J. T. Douglas, and A. J. Winter. 1994. BALB/c mice infected with Brucella abortus express protracted polyclonal responses of both IgG2a and IgG3 isotypes. Immunol. Lett. 42:145-150. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes, D. M., X. Jiang, J. H. Jung, and C. L. Baldwin. 1996. Comparison of T cell cytokines in resistant and susceptible mice infected with virulent Brucella abortus strain 2308. FEMS Immunol. Med. Microbiol. 16:193-203. [DOI] [PubMed] [Google Scholar]
- 25.Garin-Bastuji, B., N. Hummel, G. Gerbier, C. Cau, R. Pouillot, M. Da Costa, and J. J. Fontaine. 1999. Nonspecific serological reactions in the diagnosis of bovine brucellosis: experimental oral infection of cattle with repeated doses of Yersinia enterocolitica O:9. Vet. Microbiol. 66:223-233. [DOI] [PubMed] [Google Scholar]
- 26.Gentschev, I., G. Dietrich, S. Spreng, A. Kolb-Maurer, V. Brinkmann, L. Grode, J. Hess, S. H. Kaufmann, and W. Goebel. 2001. Recombinant attenuated bacteria for the delivery of subunit vaccines. Vaccine 19:2621-2628. [DOI] [PubMed] [Google Scholar]
- 27.Gentschev, I., G. Dietrich, S. Spreng, A. Kolb-Maurer, J. Daniels, J. Hess, S. H. Kaufmann, and W. Goebel. 2000. Delivery of protein antigens and DNA by virulence-attenuated strains of Salmonella typhimurium and Listeria monocytogenes. J. Biotechnol. 83:19-26. [DOI] [PubMed] [Google Scholar]
- 28.Golding, B., D. E. Scott, O. Scharf, L. Huang, M. Zaitseva, C. Lapham, N. Eller, and H. Golding. 2001. Immunity and protection against Brucella abortus. Microbes Infect. 3:43-48. [DOI] [PubMed] [Google Scholar]
- 29.Gregoriadis, G. 1998. Genetic vaccines: strategies for optimization. Pharm. Res. 15:661-670. [DOI] [PubMed] [Google Scholar]
- 30.Grillot-Courvalin, C., S. Goussard, and P. Courvalin. 1999. Bacteria as gene delivery vectors for mammalian cells. Curr. Opin. Biotechnol. 10:477-481. [DOI] [PubMed] [Google Scholar]
- 31.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 32.Hathaway, L. J., and J. P. Kraehenbuhl. 2000. The role of M cells in mucosal immunity. Cell. Mol. Life Sci. 57:323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hess, J., I. Gentschev, D. Miko, M. Welzel, C. Ladel, W. Goebel, and S. H. Kaufmann. 1996. Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection against listeriosis. Proc. Natl. Acad. Sci. USA 93:1458-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Igwe, E. I., H. Russmann, A. Roggenkamp, A. Noll, I. B. Autenrieth, and J. Heesemann. 1999. Rational live oral carrier vaccine design by mutating virulence-associated genes of Yersinia enterocolitica. Infect. Immun. 67:5500-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiserlian, D., and N. Etchart. 1999. Entry sites for oral vaccines and drugs: a role for M cells, enterocytes and dendritic cells? Semin. Immunol. 11:217-224. [DOI] [PubMed] [Google Scholar]
- 36.Kantele, A., M. Hakkinen, Z. Moldoveanu, A. Lu, E. Savilahti, R. D. Alvarez, S. Michalek, and J. Mestecky. 1998. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect. Immun. 66:5630-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufmann, S. H., C. H. Ladel, and I. E. Flesch. 1995. T cells and cytokines in intracellular bacterial infections: experiences with Mycobacterium bovis BCG. Ciba Found. Symp. 195:123-132. [DOI] [PubMed] [Google Scholar]
- 38.Koster, M., W. Bitter, H. de Cock, A. Allaoui, G. R. Cornelis, and J. Tommassen. 1997. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol. Microbiol. 26:789-797. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan, B. R. 2000. Current status of DNA vaccines in veterinary medicine. Adv. Drug Delivery Rev. 43:3-11. [DOI] [PubMed] [Google Scholar]
- 40.Letesson, J. J., A. Tibor, G. van Eynde, V. Wansard, V. Weynants, P. Denoël, and E. Saman. 1997. Humoral immune responses of Brucella-infected cattle, sheep, and goats to eight purified recombinant Brucella proteins in an indirect enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 4:556-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Limet, J. N., N. Bosseray, B. Garin-Bastuji, G. Dubray, and M. Plommet. 1989. Humoral immunity in mice mediated by monoclonal antibodies against the A and M antigens of Brucella. J. Med. Microbiol. 30:37-43. [DOI] [PubMed] [Google Scholar]
- 42.Medina, E., and C. A. Guzman. 2000. Modulation of immune responses following antigen administration by mucosal route. FEMS Immunol. Med. Microbiol. 27:305-311. [DOI] [PubMed] [Google Scholar]
- 43.Mense, M. G., L. L. Van De Verg, A. K. Bhattacharjee, J. L. Garrett, J. A. Hart, L. E. Lindler, T. L. Hadfield, and D. L. Hoover. 2001. Bacteriologic and histologic features in mice after intranasal inoculation of Brucella melitensis. Am. J. Vet. Res. 62:398-405. [DOI] [PubMed] [Google Scholar]
- 44.Mertens, P., W. D., T. Laurent, N. Dedschrevel, J.-J. Letesson, and X. Debolle. 2001. Selection of phage displayed peptides recognized by monoclonal antibodies directed against the lipopolysaccharide of Brucella. Int. Rev. Immunol. 20:181-199. [DOI] [PubMed] [Google Scholar]
- 45.Montaraz, J. A., A. J. Winter, D. M. Hunter, B. A. Sowa, A. M. Wu, and L. G. Adams. 1986. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect. Immun. 51:961-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 47.Nicoletti, P. 1990. Vaccination against Brucella. Adv. Biotechnol. Process. 13:147-168. [PubMed] [Google Scholar]
- 48.Nicoletti, P., J. Ring, B. Boysen, and J. Buczek. 1986. Illness in a veterinary student following accidental inoculation of Brucella abortus strain 19. J. Am. Coll. Health 34:236-237. [DOI] [PubMed] [Google Scholar]
- 49.Niedergang, F., and J. P. Kraehenbuhl. 2000. Much ado about M cells. Trends Cell Biol. 10:137-141. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira, S. C., and G. A. Splitter. 1995. CD8+ type 1 CD44hi CD45RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur. J. Immunol. 25:2551-2557. [DOI] [PubMed] [Google Scholar]
- 51.Paglia, P., E. Medina, I. Arioli, C. A. Guzman, and M. P. Colombo. 1998. Gene transfer in dendritic cells, induced by oral DNA vaccination with Salmonella typhimurium, results in protective immunity against a murine fibrosarcoma. Blood 92:3172-3176. [PubMed] [Google Scholar]
- 52.Palmer, D. A., and J. T. Douglas. 1989. Analysis of Brucella lipopolysaccharide with specific and cross-reacting monoclonal antibodies. J. Clin. Microbiol. 27:2331-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plommet, M., and A. M. Plommet. 1983. Immune serum-mediated effects on brucellosis evolution in mice. Infect. Immun. 41:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pouwels, P. H., R. J. Leer, M. Shaw, M. J. Heijne den Bak-Glashouwer, F. D. Tielen, E. Smit, B. Martinez, J. Jore, and P. L. Conway. 1998. Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes. Int. J. Food Microbiol. 41:155-167. [DOI] [PubMed] [Google Scholar]
- 55.Russell-Jones, G. J. 2000. Oral vaccine delivery. J. Control. Release 65:49-54. [DOI] [PubMed] [Google Scholar]
- 56.Saegerman, C., T. K. Vo, L. De Waele, D. Gilson, A. Bastin, G. Dubray, P. Flanagan, J. N. Limet, J. J. Letesson, and J. Godfroid. 1999. Diagnosis of bovine brucellosis by skin test: conditions for the test and evaluation of its performance. Vet. Rec. 145:214-218. [DOI] [PubMed] [Google Scholar]
- 57.Shata, M. T., L. Stevceva, S. Agwale, G. K. Lewis, and D. M. Hone. 2000. Recent advances with recombinant bacterial vaccine vectors. Mol. Med. Today 6:66-71. [DOI] [PubMed] [Google Scholar]
- 58.Sizemore, D. R., A. A. Branstrom, and J. C. Sadoff. 1997. Attenuated bacteria as a DNA delivery vehicle for DNA-mediated immunization. Vaccine 15:804-807. [DOI] [PubMed] [Google Scholar]
- 59.Sizemore, D. R., A. A. Branstrom, and J. C. Sadoff. 1995. Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science 270:299-302. [DOI] [PubMed] [Google Scholar]
- 60.Smith, L. D., and T. A. Ficht. 1990. Pathogenesis of Brucella. Crit. Rev. Microbiol. 17:209-230. [DOI] [PubMed] [Google Scholar]
- 61.Splitter, G., S. Oliveira, M. Carey, C. Miller, J. Ko, and J. Covert. 1996. T lymphocyte mediated protection against facultative intracellular bacteria. Vet. Immunol. Immunopathol. 54:309-319. [DOI] [PubMed] [Google Scholar]
- 62.Staats, H. F., R. J. Jackson, M. Marinaro, I. Takahashi, H. Kiyono, and J. R. McGhee. 1994. Mucosal immunity to infection with implications for vaccine development. Curr. Opin. Immunol. 6:572-583. [DOI] [PubMed] [Google Scholar]
- 63.Talwar, G. P., M. Diwan, F. Razvi, and R. Malhotra. 1999. The impact of new technologies on vaccines. Natl. Med. J. India 12:274-280. [PubMed] [Google Scholar]
- 64.Ulmer, J. B., J. C. Sadoff, and M. A. Liu. 1996. DNA vaccines. Curr. Opin. Immunol. 8:531-536. [DOI] [PubMed] [Google Scholar]
- 65.Van Damme, M., M. P. Sory, T. Biot, J. P. Vaerman, and G. R. Cornelis. 1992. Oral immunization against cholera toxin with a live Yersinia enterocolitica carrier in mice. Gastroenterology 103:520-531. [DOI] [PubMed] [Google Scholar]
- 66.Weynants, V., D. Gilson, A. Cloeckaert, P. A. Denoël, A. Tibor, P. Thiange, J. N. Limet, and J. J. Letesson. 1996. Characterization of a monoclonal antibody specific for Brucella smooth lipopolysaccharide and development of a competitive enzyme-linked immunosorbent assay to improve the serological diagnosis of brucellosis. Clin. Diagn. Lab. Immunol. 3:309-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weynants, V., D. Gilson, A. Cloeckaert, A. Tibor, P. A. Denoël, F. Godfroid, J. N. Limet, and J. J. Letesson. 1997. Characterization of smooth lipopolysaccharides and O polysaccharides of Brucella species by competition binding assays with monoclonal antibodies. Infect. Immun. 65:1939-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weynants, V., J. Godfroid, B. Limbourg, C. Saegerman, and J. J. Letesson. 1995. Specific bovine brucellosis diagnosis based on in vitro antigen-specific gamma interferon production. J. Clin. Microbiol. 33:706-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weynants, V., A. Tibor, P. A. Denoel, C. Saegerman, J. Godfroid, P. Thiange, and J. J. Letesson. 1996. Infection of cattle with Yersinia enterocolitica O:9 a cause of the false positive serological reactions in bovine brucellosis diagnostic tests. Vet. Microbiol. 48:101-112. [DOI] [PubMed] [Google Scholar]
- 70.Winter, A. J., J. R. Duncan, C. G. Santisteban, J. T. Douglas, and L. G. Adams. 1989. Capacity of passively administered antibody to prevent establishment of Brucella abortus infection in mice. Infect. Immun. 57:3438-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]