Abstract
Cryptosporidium parvum is a protozoan parasite that infects intestinal epithelial cells and induces inflammation of the intestine. To better understand the inflammatory process occurring during cryptosporidiosis, we investigated in this study the kinetics of chemokine expression in the mucosa of mice by quantitative reverse transcription-PCR. Our results demonstrate that among the chemokine mRNAs studied, gamma interferon (IFN-γ)-inducible protein 10 (IP-10), monokine induced by IFN-γ (MIG), i-TAC, lymphotactin, macrophage inflammatory protein 1β (MIP-1β), and RANTES mRNAs were strongly up-regulated in infected neonate mice, which correlated with the immunofluorescence staining results showing T-cell and macrophage infiltration in the mucosa. Our in vitro data showed that intestinal epithelial cells infected by C. parvum or stimulated by the proinflammatory cytokines (IFN-γ, interleukin-1β, and tumor necrosis factor alpha) produce a pattern of chemokine secretion similar to that observed in vivo, suggesting that these cells may take part in the initial production of chemokines. In order to identify the chemokines responsible for the recruitment of the inflammatory cells leading to a protective immune response, we compared the patterns of chemokine expression in a healing neonate mouse model and a nonhealing IFN-γ knockout (GKO) mouse model of cryptosporidiosis. In the absence of IFN-γ, the chemokine response was altered for IP-10, MIG, i-TAC, RANTES, and MIP-1β mRNAs, while the three ELR C-X-C chemokine mRNAs studied (lipopolysaccharide-induced C-X-C chemokine, MIP-2α, and KC mRNAs) were strongly overexpressed. These results are consistent with the neutrophil recruitment observed in the lamina propria of GKO mice at day 9 postinfection but are not consistent with the hypothesis that these cells play an important role in the resolution of the infection. On the contrary, the altered response of chemokines responsible for the recruitment of macrophages and T cells in GKO mice suggests that these two populations may be critical in the development of a protective immune response.
Cryptosporidium parvum is a protozoan parasite that causes enteric infections and diarrhea in humans and animals. While the disease is self-limiting in immunocompetent hosts (13), diarrhea persists in young and immunodeficient hosts (e.g., patients with AIDS) and can lead to death (18). C. parvum infection is characterized by mucosal injury and villus atrophy with infiltration of the lamina propria by inflammatory cells.
Protection against this parasite has been largely associated with the cell-mediated immune response and the production of gamma interferon (IFN-γ) (10, 25, 26, 33). We recently found that C57BL/6J neonate mice deficient in IFN-γ (GKO mice) died from the infection, whereas wild-type neonate mice were able to clear the infection in 3 weeks (21). In the absence of IFN-γ, expression of tumor necrosis factor alpha (TNF-α) mRNA and expression of the mRNAs of Th1-type cytokines in the infected mucosa were dramatically altered, suggesting that these molecules play an important role in the resolution of cryptosporidiosis.
Recently, there has been much interest in the chemokines, a novel class of inflammatory mediators. After activation, a range of different cell types produce these low-molecular-weight proteins which are responsible for attraction and activation of leukocytes at the site of inflammation. Based on the positions of their cysteine residues, these proteins can be divided into four families. The two major families are the C-C and C-X-C chemokine families; the latter can be further divided into two classes based on the presence of the glutamate-leucine-arginine (ELR) motif preceding the CXC sequence. The ELR-containing C-X-C chemokines, like interleukin-8 (IL-8), have stimulatory and chemotactic activities on neutrophils, whereas the chemokines that do not contain ELR act mainly on lymphocytes. C-C chemokines attract monocytes, eosinophils, and lymphocytes. To date, two other families, each consisting of a single member, have been described; these are the C-X3-C chemokine family, containing fractalkine, and the C chemokine family, containing lymphotactin. Fractalkine is a soluble or membranous chemokine that is chemotactic for mononuclear cells and lymphocytes, whereas lymphotactin preferentially attracts T lymphocytes.
Epithelial cells of the intestinal mucosa surface form a barrier that separates the host's internal milieu from the external environment. Mucosal surfaces have a highly specialized immune system, and intestinal epithelial cells (IEC) produce an array of cytokines and chemokines in response to different enteric pathogens (9, 19, 30) or locally released cytokines (15, 28, 32). We have previously shown that C. parvum infection of human IEC in vitro induces polarized secretion of C-X-C chemokine IL-8 and GRO-α (22). Taken together, these observations suggest that IEC play an important role in initiation of the mucosal immune response.
In this study, to further investigate initiation of the mucosal immune response, we analyzed for the first time expression of the mRNAs of several members of the four chemokine families in the ileum of C. parvum-infected C57BL/6J neonates. To further assess the contribution of the IEC in the production of chemokines observed in vivo, we measured in vitro the chemokine response of these cells to C. parvum infection and to cytokines that are produced during neonate cryptosporidiosis, like IFN-γ, TNF-α, and IL-1β. Finally, to determine which chemokines could be involved in protection, we compared the chemokine responses in a healing mouse model and a nonhealing mouse model (wild-type neonate mice and GKO mice, respectively).
MATERIALS AND METHODS
Mice.
C57BL/6J-Ifgtml and wild-type mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Dams with 1- to 2-day-old litters were housed individually in cages under pathogen-free conditions. When GKO neonates were grown with their mothers, none survived more than 5 days postinfection (p.i.) because the dams became infected and did not feed their young. One-day-old GKO neonates were therefore cross-fostered to normal C57BL/6J mothers. Food and water were available ad libitum. For reverse transcription (RT)-PCR analysis, 3-day-old GKO or wild-type neonates and 6- to 7-week-old adult GKO mice were inoculated with 106 oocysts of C. parvum by the oral route. Neonate mice were killed 2, 4, 9, or 15 days p.i., and the ilea were removed. Controls were killed at identical ages. In order to study the immune response in the upper layer of the infected mucosa, the ilea were not crushed; instead, they were split lengthwise and shaken in TRI-zol (Gibco-BRL Life Technologies, Cergy Pontoise, France).
Parasite.
Oocysts of the C. parvum CpINRA/PRZ isolate were initially purified from the feces of an infected child (6) and were maintained by regular passage in newborn calves. Fecal samples were stored in 2.5% potassium dichromate at 4°C until they were used. C. parvum oocysts, isolated from feces by filtration and diethyl ether sedimentation, were treated with 1.25% sodium hypochlorite, washed, and stored at 4°C in phosphate-buffered saline (pH 7.4) containing 50 U of penicillin G per ml and 0.25 mg of amphotericin B per ml. Oocysts were less than 2 months old when they were used as inocula.
C. parvum oocyst determination.
The level of infection in individual neonatal mice was assessed by determining the number of oocysts in the intestinal contents. Since infection is not always distributed homogeneously along the intestine, the whole intestines were removed from neonatal mice. They were individually homogenized in 1 ml of water with an Ultra-turax, and oocyst quantification was performed in Sheather's solution with a Thoma cell.
Immunofluorescence.
Pieces of ileum were snap frozen in liquid nitrogen. Seven-micrometer-thick frozen sections were fixed with an acetone gradient and incubated with the primary rat monoclonal antibodies for 2 h. After several washes, sections were incubated with a monoclonal anti-rat biotin conjugate (Sigma, Saint Quentin Fallavier, France) for 1 h at room temperature. These sections were washed and incubated with ExtrAvidine fluorescein isothiocyanate conjugate (Sigma) for 20 min. Finally, the sections were slightly counterstained with Evans Blue before microscopic examination. The monoclonal antibodies used were A3-1(F4/80) (macrophages and monocytes; Serotec, Oxford, United Kingdom), KT3 (CD3; Serotec, Oxford, United Kingdom), and 7/4 (neutrophils; Serotec, Oxford, United Kingdom).
RNA extraction.
After 5 min of incubation in TRI-zol, the ilea were removed and the TRI-zol solutions were centrifuged for 5 min at 8,000 × g to eliminate any debris. The supernatants were stored at −70°C until further processing. RNAs were extracted according to the manufacturer's instructions and were quantified by using optical density at 260 nm. RNA quality was estimated by agarose gel electrophoresis with a gel containing ethidium bromide.
Cell culture and infection protocols.
Murine cell line ICcl2 was kindly provided by A. Vandewalle (7). This immortalized cell line is from the crypt of the small intestine of mice and was maintained at 37°C in the presence of 5% CO2 in growth medium containing Dulbecco modified Eagle medium and Ham's F12 medium at a ratio of 1:1 (vol/vol), 60 nM sodium selenate, 5 μg of transferrin per ml, 2 mM glutamine, 5 μg of insulin per ml, 50 nM dexamethasone, 5 nM triiodothyronine, 10 ng of epidermal growth factor per ml, 20 mM d-glucose, 2% fetal calf serum, and 20 mM HEPES (pH 7.4). Cells were also cultured in the top and bottom compartments of Transwell chambers. Parasites were added at a 5:1 oocyst/cell ratio and incubated for 24 h at 37°C. As controls, cells were incubated with medium alone and with oocysts that had been heated for 2 h at 60°C at the same ratio. RNA extraction was performed as described above. ICcl2 cells were incubated for 5 h with recombinant IL-1β, IFN-γ, and TNF-α (Pharmingen, San Diego, Calif.) at a final concentration of 10 ng/ml.
RNA standards for quantitative RT-PCR.
To quantify the levels of mRNAs for the genes of interest, plasmids coding for truncated mRNA templates (standards) were constructed as described previously (23). Briefly, in vitro transcription of these plasmids yielded RNAs that had primer sites identical to those that amplify the target RNAs. However the distances between specific 5′ and 3′ primer sites and, therefore, the sizes of the PCR amplification products differed from those of the standard and target RNAs. A corresponding PCR product was obtained by using the composite primer and the downstream primer with RNAs extracted 9 days p.i. from the ileum of a C. parvum-infected neonate mouse as the template. The amplified fragment was cloned into the pGEMeasy plasmid (PROMEGA, Lyon, France). This procedure was performed for each of the 10 plasmid constructs. Finally, to provide a poly(A) tail at the 3′ end of the coding sequence, two complementary oligonucleotides (5′-TCGACA20AAGCTTC-3′ and 5′-TCGAGAAGCCTTT20G-3′) were inserted at the SalI site of the plasmid. To generate standard RNAs, plasmids were linearized for in vitro transcription with T7 RNA polymerase under conditions recommended by the supplier (Eurogentec, Angers, France).
Oligonucleotide primers for PCR amplification.
The sequences of the chemokine oligonucleotide primers used for PCR amplification and the sizes of the predicted PCR products obtained with target and standard RNAs are shown in Table 1. Primers were designed based on previously published sequences and were obtained from Eurobio (Les Ulis, France). The sequences of the primers used for amplification of the C. parvum gene were 5′-GAATGGTTAATTGACACCGGTAGTGT-3′ and 5′-AACGCCGTTTGGTGCCTTACCTTCG-3′ (accession number AA224668), and the predicted PCR products were 229 and 318 bp long for the target and the standard, respectively. When genomic sequences were available in the databases, we selected primers that amplify fragments from cDNA that are distinguishable on the basis of size from fragments amplified from genomic DNA or primers that span exon-exon boundaries and, therefore, do not amplify genomic DNA.
TABLE 1.
Primer sequences and PCR product sizes
| Chemokine family | Chemokine | Accession no. | 5′ Primer | 3′ Primer | Target size (bp) | Standard size (bp) |
|---|---|---|---|---|---|---|
| C | Ltn | U15607 | 5′-ATGAGACTTCTCCTCCTGACTTT-3′ | 5′-ACCCAGTCAGGGTTACTGCTG-3′ | 343 | 256 |
| C-C | MCP-1 | M19681-2 | 5′-TGCAGGTCCCTGTCATGCTTC-3′ | 5′-AAGTGCTTGAGGTGGTTGTGGA-3′ | 411 | 307 |
| MIP-1β | X62502 | 5′-GCGTGTCTGCCCTCTCTCTC-3′ | 5′-GCAAGGACGCTTCTCAGTGAG-3′ | 349 | 261 | |
| RANTES | U02298 | 5′-TCTCTGCAGCTGCCCTCACC-3′ | 5′-TGGGAGTAGGGGATTACTGAGT-3′ | 367 | 276 | |
| C-X-C | IP-10 | L07417 | 5′-ATGAACCCAAGTGCTGCCGTC-3′ | 5′-TGGAGAGACAGGCTCTCTGCT-3′ | 355 | 264 |
| i-TAC | AF167354 | 5′-CCATAGCCCTGGCTGCGATC-3′ | 5′-CCAAGACAGCAGAGGGTCCG-3′ | 379 | 283 | |
| MIG | M34815 | 5′-TGAAGTCCGCTGTTCTTTTCCT-3′ | 5′-TTATGTAGTCTTCCTTGAACGACG-3′ | 380 | 284 | |
| KC | NM008176 | 5′-CACCTCAAGAACATCCAGAGCT-3′ | 5′-CAAGCAGAACTGAACTACCATCG-3′ | 354 | 272 | |
| LIX | U27267 | 5′-CCTCCAGCTCCGCAGCTCC-3′ | 5′-CACTGCGAGTGCATTCCGCTT-3′ | 369 | 276 | |
| MIP-2α | NM009140 | 5′-CTGCCGGCTCCTCAGTGCTG-3′ | 5′-CAGGTACGATCCAGGCTTCCC-3′ | 383 | 283 | |
| C-X3-C | Fractalkine | U92565 | 5′-GCCTGGCAACACAGTCCACC-3′ | 5′-TCACACTGGCACCAGGACGTA-3′ | 357 | 265 |
Quantification of chemokine and β-actin mRNA levels.
Quantitative RT-PCR were performed as described by Jung et al. (19). Briefly, serial dilutions of known quantities of standard RNA molecules were mixed with 1 μg of total cellular RNA in a total volume of 20 μl, and the RNAs were reverse transcribed at 37°C. Two microliters of the reaction mixture was used in a PCR. The sizes of the PCR amplification products for standard and target RNAs differed by 25 to 30%, and the products could be easily separated on a 2% agarose gel and visualized by ethidium bromide staining. Band intensities were quantified by densitometry (Scion image; 2000 Scion Corporation, Frederick, Md.). The band intensity ratios of the PCR products obtained with the standard RNA and the target RNA were plotted against the starting number of standard RNA molecules by using a double logarithmic scale. When the ratio of the band intensities was 1, the number of target RNA molecules was equivalent to the number of standard RNA molecules. Data were expressed as the number of target mRNA molecules per microgram of total sample RNA. For every RNA sample, a set of serial 10-fold dilutions of the standard was used for the reaction in order to determine the range in which the gene was expressed. After this, six serial threefold dilutions of the standard surrounding the estimated value were used. The quantitative RT-PCR was sensitive to 103 mRNA molecules/μg of total RNA.
RESULTS
Course of C. parvum infection in neonate mice.
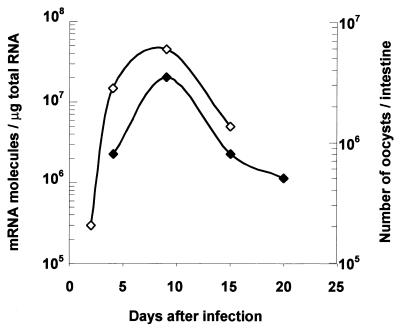
To estimate the level of infection by the cryptosporidial parasite in the intestines of neonate mice, we used two different techniques, a quantitative RT-PCR technique and enumeration of oocysts in the intestines of neonate mice. The RT-PCR technique is based on amplification of a selected gene of C. parvum which exhibits homology to the 1PE1 gene of Eimeria acervulina (accession number 224668). Amplification of this gene was performed with RNA extracted from the ileum tissue of neonates, where the parasite resides. In contrast, enumeration of oocysts in Sheather's solution with a hemacytometer was performed by using the entire intestines of neonates (contents and tissue). The kinetics of infection had similar profiles regardless of the technique used (Fig. 1). The level of infection increased, reaching a maximum at day 9 p.i., and then decreased. The RT-PCR technique had the following three major advantages: (i) this technique was more sensitive since we could detect the presence of the parasite in the ilea of adult mice, whereas enumeration in Sheather's solution detected no oocysts (data not shown); (ii) we could determine the level of infection before oocyst excretion began, as early as 2 days p.i. in neonate mice; and (iii) this technique allowed us to save animals since we could study the chemokine response with the RT-PCR and in parallel evaluate the infection level with the same RNA sample without killing other neonates.
FIG. 1.
Kinetics of C. parvum infection in the C57BL/6J neonate mouse model. The course of infection was determined by enumeration of oocysts in the intestine (⧫) (n = 5) and by quantitative RT-PCR (◊) performed with a pool of RNA extracted from the ilea of six mice, as described in Materials and Methods.
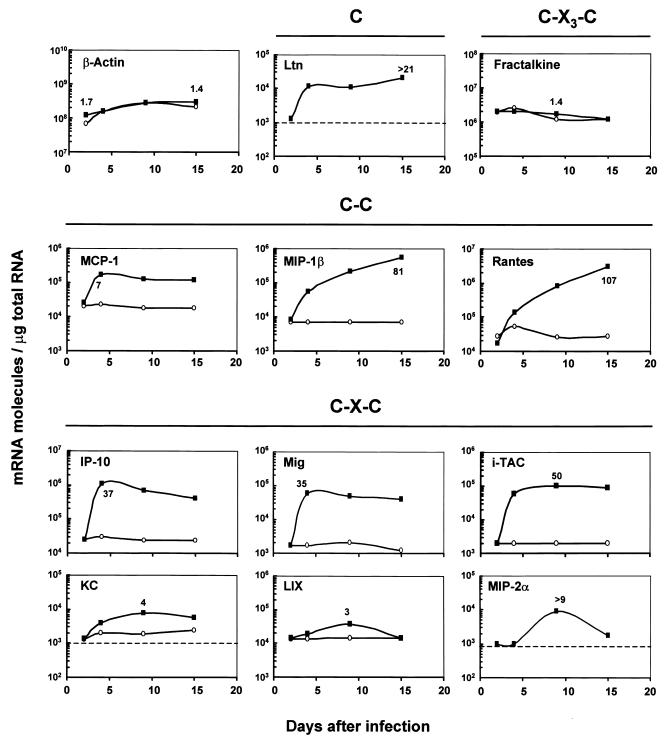
C, C-C, and C-X-C chemokine mRNA expression, but not C-X3-C chemokine mRNA expression, was increased in neonate mice infected by C. parvum.
We first assessed the kinetics of chemokine mRNA expression in the ilea of C57BL/6J neonate mice after infection by C. parvum at days 2, 4, 9, and 15 p.i. (Fig. 2). The fractalkine mRNA (C-X3-C chemokine) levels were not affected by the infection. The levels of IFN-γ-inducible protein 10 (IP-10), monokine induced by IFN-γ (MIG), and i-TAC, the non-ELR C-X-C chemokines dependent on IFN-γ, and the levels of monocyte chemoattractant protein 1 (MCP-1) and lymphotactin were much higher on day 4 p.i. and remained high on days 9 and 15 p.i. During infection, the C-C chemokines macrophage inflammatory protein 1β (MIP-1β) and RANTES were the most up-regulated chemokines studied (81- and 107-fold, respectively). The time course data showed that the levels of MIP-1β and RANTES mRNAs continued to increase from 4 to 15 days p.i., whereas the infection started to decrease after 10 days p.i. In contrast, the three ELR C-X-C chemokines studied, lipopolysaccharide-induced C-X-C chemokine (LIX), KC, and MIP-2α, exhibited only modest up-regulation at day 9 p.i. (four-, three-, and ninefold, respectively).
FIG. 2.
Kinetics of chemokine expression in the ilea of neonate mice infected by C. parvum. Three-day-old neonates were inoculated with 106 oocysts of C. parvum. RNAs were extracted from infected (▪) and uninfected age-matched control (○) neonates as described in Materials and Methods. For each time point, pools of total RNA extracted from the ilea of five to seven neonates were combined. Levels of chemokine and β-actin mRNAs were determined by quantitative RT-PCR performed with internal standards. The values are the numbers of mRNA transcripts per microgram of total RNA. The lower limit of detection of the quantitative RT-PCR method used is 103 mRNA molecules/μg of total RNA and is indicated by dashed lines on some of the graphs. The highest ratio of mRNA level in infected mice to mRNA level in control mice is indicated in every panel. The results are representative of the results of two experiments. Ltn, lymphotactin.
Increased C-C and C-X-C chemokine expression in response to C. parvum infection of murine IEC (ICcl2 cells).
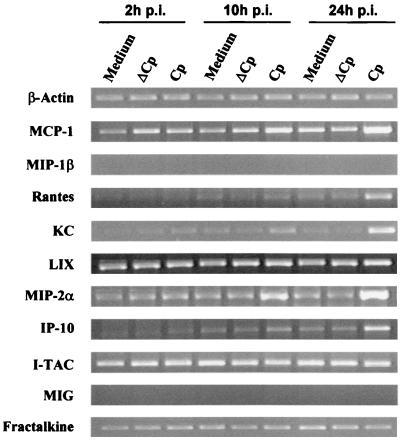
In vivo, C. parvum infects only IEC. In order to investigate which chemokines are up-regulated by infected host cells without environmental cytokines, murine IEC (ICcl2 cells) were infected in vitro with C. parvum. This murine IEC line had never previously been shown to support C. parvum infection. We initially compared the levels of infection of these cells and the human HCT-8 cell line, which among all the cell lines tested to date sustains the most efficient infection (35). When an oocyst/cell ratio of 2.5 was used, 52% of the ICcl2 cells allowed parasite development after 24 h, whereas 90% of the HCT-8 cells were infected. In order to study the chemokine response of ICcl2 cells to C. parvum infection, a higher inoculation dose was used (five oocysts/cell). Total RNA was extracted at different times after infection (2, 10, and 24 h p.i.), and expression of chemokine mRNAs was assessed by RT-PCR (Fig. 3). The mRNAs of fractalkine and KC were constitutively expressed at high levels in noninfected ICcl2 cells, and PCR amplification required only 28 and 21 cycles, respectively. On the other hand, expression of MIG and MIP-1β mRNAs was not detected even after 35 cycles of amplification. This was not due to a problem of amplification, since we used cDNA from neonates that were infected for 9 days as a positive control for the reaction in every experiment. C. parvum infection of murine IEC resulted in up-regulation of the C-C chemokines RANTES and MCP-1 at 24 h p.i. and up-regulation of the C-X-C chemokines MIP-2α and KC. Our data showed that the level of IP-10 mRNA also increased in C. parvum-infected IEC. For KC, MIP-2α, and MCP-1, increased mRNA levels were observed as early as 10 h p.i. It should be noted that increased chemokine expression required infection with viable C. parvum oocysts, since a mock infection performed with heat-killed C. parvum oocysts did not change the level of chemokine expression. The chemokine response 24 h after C. parvum infection was studied with another murine epithelial cell line, Mode-K (36). The chemokine responses were similar for the two murine epithelial cell lines except for the responses of RANTES and MCP-1; the levels of the mRNAs of these chemokines did not vary during Mode-K cell infection (data not shown).
FIG. 3.
Chemokine mRNA expression after C. parvum infection of ICcl2 cells. Confluent monolayers of ICcl2 cells on six-well plates were infected with oocysts at a ratio of five oocysts per cell for 24 h (Cp). Control cells were cultured with medium alone or with heat-inactivated oocysts (ΔCp), as described in Materials and Methods. Chemokine mRNA levels were determined by qualitative RT-PCR. For most chemokines 35 amplification cycles were used; the exceptions were fractalkine (28 cycles), KC (21 cycles), and β-actin (21 cycles). Similar results were obtained in three repeated experiments.
Mediators released from infected lysed ICcl2 cells are not responsible for increased chemokine expression.
We asked whether increased chemokine expression after C. parvum infection of ICcl2 cells was due to the infection itself or to the release of mediators by the lysed cells. We have previously shown that C. parvum infection in vitro results in apoptosis of infected cells (24, 27). For these experiments, ICcl2 cells were cultured in the top and bottom compartments of Transwell chambers. Cells in the bottom compartments were infected with C. parvum, and the total RNAs of cells in the top compartments were extracted 24 h after infection and used for chemokine expression analysis. There was no increase in mRNA expression in the noninfected cells grown in the top compartments of Transwell chambers (data not shown). This result suggests that the chemokine up-regulation observed in infected cultures is derived directly from C. parvum-infected cells and not from bystander uninfected cells that were activated by mediators released from the infected or lysed cells.
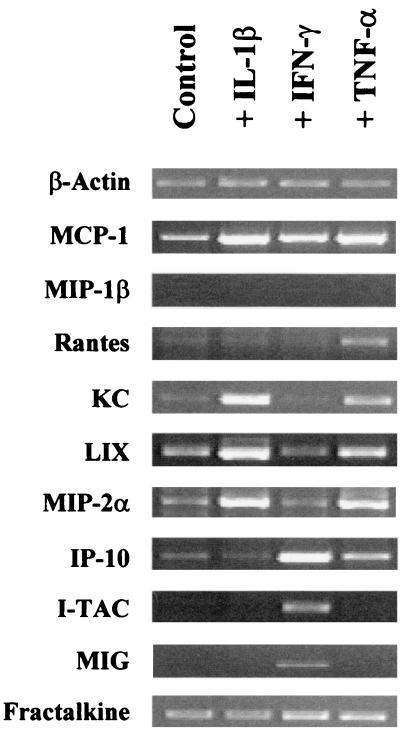
Effect of proinflammatory cytokines on chemokine expression by ICcl2 cells.
We have recently shown that C. parvum infection of neonates induces strong up-regulation of IFN-γ mRNA and, to a lesser extent, up-regulation of TNF-α and IL-1β mRNAs (21). In GKO neonates, although IL-1β is still overexpressed after infection, TNF-α mRNA is not. In order to further understand the inflammatory process mediated by the chemokine response observed in vivo after C. parvum infection, we investigated the effects of these different proinflammatory cytokines on chemokine expression by the murine IEC line (Fig. 4). Murine IEC line ICcl2 overexpressed i-TAC and MIG mRNAs only after IFN-γ stimulation, whereas the IP-10 mRNA level increased after IFN-γ treatment and, to a lesser extent, after TNF-α treatment. TNF-α or IL-1β increased the levels of mRNAs of the C-X-C chemokines KC, LIX, and MIP-2α expressed by cell line ICcl2. This murine cell line did not express MIP-1β mRNA even after cytokine treatment. Although TNF-α, IFN-γ, or IL-1β induced up-regulation of the MCP-1 mRNA level, only TNF-α allowed overexpression of RANTES mRNA by the murine IEC. Finally, the fractalkine (C-X3-C chemokine) mRNA level did not vary irrespective of cytokine treatment.
FIG. 4.
Chemokine mRNA expression by ICcl2 cells treated with IL-1β, TNF-α, and IFN-γ. Confluent monolayers of ICcl2 cells grown in six-well plates were incubated with IL-1β (10 ng/ml), TNF-α (10 ng/ml), and IFN-γ (10 ng/ml) for 5 h. Control cells were cultured with medium alone. Chemokine mRNA expression was determined by qualitative RT-PCR. For most chemokines 35 amplification cycles were used; the exceptions were fractalkine (28 cycles), KC (21 cycles), and β-actin (21 cycles). The data are representative of the data obtained in three experiments.
Comparison of the ileal chemokine responses in C. parvum-infected wild-type and GKO mice.
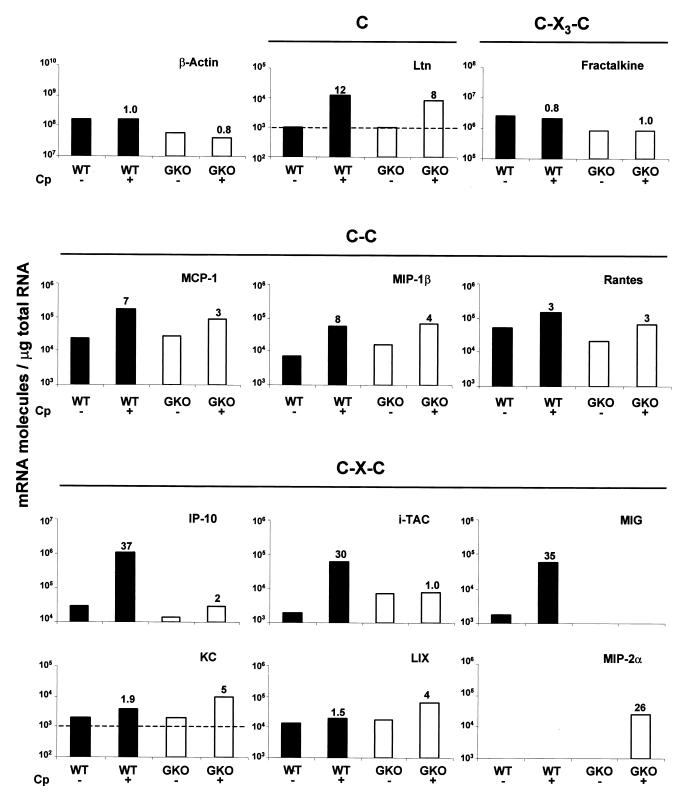
In adult IFN-γ-deficient mice, C. parvum infection results in acute inflammation of the intestine, which does not allow protection of the mice. In GKO neonates, cryptosporidiosis results in death as soon as 6 or 7 days p.i. (21). In order to identify chemokines that are dependent on IFN-γ during cryptosporidiosis and that allow recruitment of inflammatory cells, which could lead to protection, we compared chemokine mRNA expression in wild-type neonates and chemokine mRNA expression in GKO neonates at the beginning of infection, when the immune response was just initiated. The IP-10, i-TAC, and MIG chemokines, which are dependent on IFN-γ production, were not overexpressed after C. parvum infection in GKO neonate mice (Fig. 5). Overexpression of the mRNAs for the C-X-C chemokines KC, LIX, and especially MIP-2α was greater in GKO neonates than in wild-type neonates. Conversely, the overexpression of the CC chemokines MCP-1, MIP-1β, and RANTES observed in the GKO neonates was weaker than or similar to that in the wild-type neonates. In conclusion, as we did not observe strong overexpression of chemokines in GKO neonates at 4 days p.i., except for the early highly regulated chemokines in wild-type neonates, such as IP-10, MIG, and i-TAC, it was difficult to compare the chemokine responses in wild-type and GKO neonates. Moreover, as described previously, the GKO neonate mouse model could not be used at day 9 p.i. (21). We therefore chose to compare the chemokine responses in wild-type neonates and GKO adult mice at day 9 p.i. (Table 2), when the level of infection was maximal. In the absence of IFN-γ, as in GKO neonates, IP-10, MIG, and i-TAC overexpression was impaired. Moreover, expression of MIP-1β mRNA and expression of RANTES mRNA were weak despite the high level of infection observed in GKO adult mice. In contrast, there was strong up-regulation of the C-X-C chemokines MIP-2α, KC, and LIX at day 9 p.i. (588-, 54-, and 1,111-fold, respectively).
FIG. 5.
Chemokine mRNA response during C. parvum infection in wild-type (WT) (solid bars) and GKO (open bars) neonates at day 4 p.i. Three-day-old neonates were inoculated with 106 oocysts of C. parvum (Cp). Pools of total RNA extracted from the ilea of seven neonates were combined. Levels of chemokine and β-actin mRNAs were determined by quantitative RT-PCR performed with internal standards. The values are the numbers of mRNA transcripts per microgram of total RNA. The lower limit of detection of the quantitative RT-PCR method used is 103 mRNA molecules/μg of total RNA and is indicated by dashed lines on some of the graphs. The ratios of mRNA levels for infected mice (+) to mRNA levels for control mice (−) are indicated in every panel. The results are representative of the results of two experiments. Ltn, lymphotactin.
TABLE 2.
Chemokine mRNA responses in infected wild-type neonates and GKO adult mice after 9 days of infectiona
| Chemokine | Wild-type neonate mice
|
GKO adult mice
|
||||
|---|---|---|---|---|---|---|
| No. of transcripts/μga
|
Infected/control ratio | No. of transcripts/μg
|
Infected/control ratio | |||
| Control | Infected | Control | Infected | |||
| β-Actin | 2.7 × 108 | 2.7 × 108 | 1.0 | 4.0 × 107 | 2.5 × 107 | 0.6 |
| Lymphotactin | 1.0 × 103 | 1.1 × 104 | 11.0 | 1.1 × 103 | 7.0 × 103 | 6.4 |
| MCP-1 | 1.8 × 104 | 1.3 × 105 | 7.2 | 6.2 × 103 | 1.2 × 105 | 19.0 |
| MIP-1β | 7.0 × 103 | 2.1 × 105 | 30.0 | 7.5 × 104 | 7.0 × 105 | 9.3 |
| RANTES | 2.6 × 104 | 8.0 × 105 | 30.8 | 1.0 × 106 | 1.8 × 106 | 1.8 |
| KC | 1.9 × 103 | 7.6 × 103 | 4.0 | 3.3 × 103 | 1.8 × 105 | 54.5 |
| LIX | 1.4 × 104 | 3.7 × 104 | 2.6 | 2.7 × 103 | 3.0 × 106 | 1,111.0 |
| MIP-2α | <103 | 9.0 × 103 | NCb | 3.4 × 103 | 2.0 × 106 | 588.0 |
| IP-10 | 2.4 × 104 | 7.0 × 105 | 29.2 | 3.3 × 104 | 1.0 × 104 | 0.3 |
| MIG | 2.0 × 103 | 5.0 × 104 | 25.0 | 6.0 × 103 | 1.0 × 103 | 0.2 |
| i-TAC | 2.0 × 103 | 1.0 × 105 | 50.0 | 1.7 × 104 | 1.4 × 104 | 0.8 |
| Fractalkine | 1.2 × 106 | 1.7 × 106 | 1.4 | 7.0 × 105 | 9.0 × 105 | 1.3 |
Each RT-PCR was performed with a pool of total RNA extracted from the ilea of five to seven animals. The values are the numbers of chemokine transcripts per microgram of total RNA; 103 transcripts/μg of RNA was considered the lower limit of detection.
NC, value could not be calculated.
Characterization of the immune cells recruited after C. parvum infection.
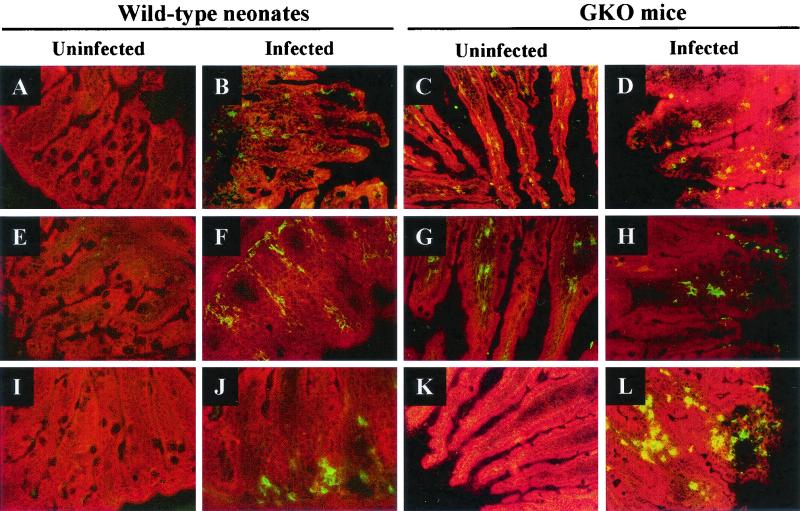
In wild-type neonates infected for 9 days, the inflammatory response was accompanied by recruitment in the lamina propria of CD3+ T cells, macrophages, and neutrophils, and neutrophils were preferentially located at the base of the villi (Fig. 6B, F, and J). None of these cell populations was detected in the uninfected age-matched control neonates (Fig. 6A, E, and I). While no neutrophils were detected in uninfected GKO adult mice (Fig. 6K), strong infiltration of the lamina propria by neutrophils was observed 9 days p.i. (Fig. 6L). In contrast, T cells and macrophages were present in uninfected GKO mice (Fig. 6C and G), but no further recruitment was observed at 9 days p.i. (Fig. 6D and H).
FIG. 6.
Ileum histopathology of control and C. parvum-infected wild-type neonates and GKO mice. (A, E, and I) Control wild-type neonates; (C, G, and K) control GKO mice; (B, F, and J) wild-type neonates infected for 9 days; (D, H, and L) GKO mice infected for 9 days. (A to D) Immunofluorescence staining with anti-CD3; (E to H) immunofluorescence staining with anti-F4/80 (macrophage); (I to L) immunofluorescence staining with anti-neutrophils. All immunofluorescence-labeled samples were counterstained with Evans Blue. Magnification, ×200.
DISCUSSION
Intestinal C. parvum infection is often associated with infiltration of inflammatory cells in the lamina propria, which underlies the epithelium, where the parasite resides. The mechanisms by which C. parvum infection results in recruitment of these cells and the role of IFN-γ in the subepithelial inflammation which leads to protection are still not well understood. Chemokines are low-molecular-weight proteins that have pleiotropic effects on the recruitment and activation of leukocytes at sites of inflammation. Analysis of the pattern of chemokine expression in the mucosa should help explain the attraction of different leukocyte subsets during cryptosporidiosis. Therefore, in this study we assessed the intestinal chemokine response during murine cryptosporidiosis in the presence or absence of IFN-γ.
IEC can respond to infection or injury and serve as an early signal in immune and inflammatory reactions. We have previously reported that human epithelial cells respond to C. parvum infection by secreting two C-X-C chemokines, IL-8 and GRO-α (22). The present study performed with a murine epithelial cell line confirmed this result by demonstrating that up-regulation of KC and MIP-2α mRNA levels occurs and extended the findings by demonstrating that C. parvum infection induces direct overexpression of IP-10, MCP-1, and RANTES mRNAs by murine epithelial cells. Infection of epithelial cells by other pathogens, like Toxoplasma gondii and rotaviruses, can also induce the release of these chemokines (8, 29). The overexpression of multiple chemokine mRNAs observed in IEC after C. parvum infection could be related to the transcription factor NF-κB. It is well known that NF-κB plays a central role in the regulation of a variety of genes involved in host innate immunity, including the genes for various chemokines, such as IP-10, RANTES, MCP-1, MIP-1β, and MIP-2α. For these chemokines, regulation by NF-κB is suggested by the presence of the NF-κB consensus motif in the promoter (12, 16, 17, 37). Moreover, McCole et al. reported that C. parvum infection of HCT-8 cells induces NF-κB activation (24), and a recent study extended these findings by demonstrating that this activation occurred exclusively in infected cells and not in bystander uninfected cells (11). These results are in accordance with our data showing that the mediators released from in vitro infected cells are not responsible for increased chemokine expression and that a direct infection is necessary. Altogether, these results clearly establish that IEC are a primary source of chemokines in the early host response to C. parvum.
The initial chemokine production in the mucosa may be amplified by recruitment of inflammatory cells producing their own set of proinflammatory cytokines and chemokines. As early as 4 days p.i. C. parvum infection of wild-type neonate mice resulted in strong overexpression of the mRNAs of IFN-γ-inducible chemokines (IP-10, MIG, and i-TAC) in the mucosa. In a previous study performed under similar conditions (21), we showed that IFN-γ mRNA levels were up-regulated 250-fold in infected neonate mucosa at day 4 p.i. compared to uninfected animals. As recently described by Dwinell et al., who used both an in vitro model and an in vivo human intestinal xenograft model, IFN-γ stimulates the production of non-ELR chemokines by human IEC (14). Our data obtained with the ICcl2 murine epithelial cell line confirm these data, and we therefore hypothesized that the increased expression of IP-10, MIG, and i-TAC mRNAs could be due to stimulation of IEC by the presence of high levels of IFN-γ in the infected mucosa at day 4 p.i. Moreover, as shown by immunofluorescence staining, macrophages were recruited during infection, and once activated by IFN-γ, these macrophages may also be an important source of the IP-10, MIG, and i-TAC released in the mucosa. As these chemokines interact with the C-X-CR3 receptor present on activated T cells, they probably participate in the substantial recruitment of T cells at sites of inflammation with high levels of IFN-γ production. Up-regulation of MIP-1β and RANTES mRNAs increased gradually in the neonate infected mucosa, reaching 81- and 107-fold, respectively, at day 15 p.i. RANTES and MIP-1β are probably initially produced by IEC in response to infection by the parasite, and the kinetics of RANTES and MIP-1β production can be related to the different waves of recruitment of T cells and macrophages. Once present in the infected mucosa, these cells may in turn produce MIP-1β and RANTES and consequently amplify this chemokine response.
It has been clearly established that IFN-γ plays a key role in protection against C. parvum. In this study, we explored the importance of IFN-γ in regulation of the local immune response by investigating the chemokine response during infection in wild-type and GKO mice. We found that following infection, LIX, KC, and MIP-2α mRNAs were overexpressed more in the mucosa of C. parvum-infected GKO mice than in the mucosa of control mice. Similar observations have been described for GKO mice during T. gondii and Trypanosoma cruzi infections (4, 5). In C. parvum-infected GKO adult mice, although the overexpression of ELR C-X-C chemokines could have been due to the higher level of infection, this overexpression was correlated with the increased recruitment of neutrophils observed in the lamina propria. In GKO adult mice the infection was chronic, and it therefore appears that the neutrophils may not play a beneficial role in the resolution of cryptosporidiosis or at least are not able to compensate for the diminished recruitment of other cell populations. CD3+ cells and macrophages were already present at significant levels in the mucosa of uninfected adults compared to the levels in uninfected neonates, which most probably reflected differences in the development stages of the mice. As adult wild-type C57BL/6J mice do not develop an infection with C. parvum, we could not verify that GKO mice were not able to resolve the infection because of altered recruitment of CD3+ cells in the mucosa and/or a defect in the capacity of resident CD3+ cells to produce IFN-γ. However, several arguments support the hypothesis that there is altered recruitment of T cells in GKO mice. (i) In C57BL/6J adult nude mice, which develop a transient C. parvum infection, there is a significant increase in the number of intraepithelial lymphocytes prior to and following C. parvum inoculation compared to the number of these cells in uninoculated controls (1). (ii) Following infection of GKO mice with another protozoan, T. cruzi, diminished recruitment of T cells is observed in the myocardium compared to the recruitment of T cells in wild-type mice (3). (iii) Since antigen-specific T-cell activation is intact in GKO mice (31, 33), the absence of overexpression of the three non-ELR C-X-C chemokines and the weak overexpression of the CC chemokines MIP-1β and RANTES suggested that there is altered recruitment of T cells rather than defective T-cell activation.
Several studies of neonate mice have shown that T lymphocytes are crucial in the development of a protective immune response to C. parvum. In C. parvum-infected newborn BALB/c mice treated with an anti-CD4 monoclonal antibody alone or combined with an anti-CD8 monoclonal antibody, the infection cannot be cured unless the antibody treatment is stopped (34). Moreover, major histocompatibility complex class II-deficient neonate mice remained infected 8 weeks after exposure, while in wild-type neonates the infection was eliminated in less than 3 weeks (2). These data highlight the importance of the recruitment of T cells in the infected tissue and consequently suggest that the chemokines that attract these cells play a crucial role. In infected wild-type neonates, we observed rapid and high overexpression of IP-10, MIG, and i-TAC, which was not observed in GKO neonates, and also strong overexpression of RANTES and MIP-1β associated with the recruitment of T cells and macrophages in the mucosa. By neutralizing the strong production of IP-10 in T. gondii-infected mice, Khan et al. inhibited the massive influx of T cells into tissues, which resulted in a more-than-1,000-fold increase in tissue parasite burden and a marked increase in mortality compared to the effects in control antibody-treated mice (20).
In conclusion, our results suggest that one of the mechanisms by which IFN-γ may confer protection against C. parvum is by recruitment of effector T cells and macrophages to sites of infection via the production of the non-ELR C-X-C chemokines and the MIP-1β and RANTES C-C chemokines. Further studies are needed to determine the precise role that these chemokines play in the susceptibility to and outcome of C. parvum infection in neonate mice.
Acknowledgments
We thank Geneviève Fort for technical help with oocyst preparation, Sébastien Lavilatte for mouse breeding, and Michèle Peloille for performing the sequencing during construction of the RT-PCR plasmids. We are also grateful to Alain Vandevalle (INSERM U478, Paris, France) and Dominique Kaiserlian (INSERM U404, Lyon, France) for providing ICcl2 cells and Mode-K cells, respectively, and to David Ojcius (Institut Pasteur, Paris, France) for a critical review of the manuscript and for helpful discussions and encouragement.
Editor: J. M. Mansfield
REFERENCES
- 1.Adjei, A. A., J. T. Jones, and F. J. Enriquez. 2000. Differential intraepithelial lymphocyte phenotypes following Cryptosporidium parvum challenge in susceptible and resistant athymic strains of mice. Parasitol. Int. 49:119-129. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre, S. A., P. H. Mason, and L. E. Perryman. 1994. Susceptibility of major histocompatibility complex (MHC) class I- and MHC class II-deficient mice to Cryptosporidium parvum infection. Infect. Immun. 62:697-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aliberti, J. C., F. S. Machado, J. T. Souto, A. P. Campanelli, M. M. Teixeira, R. T. Gazzinelli, and J. S. Silva. 1999. β-Chemokines enhance parasite uptake and promote nitric oxide-dependent microbiostatic activity in murine inflammatory macrophages infected with Trypanosoma cruzi. Infect. Immun. 67:4819-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliberti, J. C., J. T. Souto, A. P. Marino, J. Lannes-Vieira, M. M. Teixeira, J. Farber, R. T. Gazzinelli, and J. S. Silva. 2001. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am. J. Pathol. 158:1433-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amichay, D., R. T. Gazzinelli, G. Karupiah, T. R. Moench, A. Sher, and J. M. Farber. 1996. Genes for chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-gamma with patterns of tissue expression that suggest nonredundant roles in vivo. J. Immunol. 157:4511-4520. [PubMed] [Google Scholar]
- 6.Arnaud-Battandier, F., M. Naciri, A. Fisher, C. Ricour, C. Griscelli, and P. Yvore. 1982. Intestinal cryptosporidiosis: a new cause of human diarrhoea. Gastroenterol. Clin. Biol. 6:1045-1046. (In French.) [PubMed] [Google Scholar]
- 7.Bens, M., A. Bogdanova, F. Cluzeaud, L. Miquerol, S. Kerneis, J. P. Kraehenbuhl, A. Kahn, E. Pringault, and A. Vandewalle. 1996. Transimmortalized mouse intestinal cells (m-ICc12) that maintain a crypt phenotype. Am. J. Physiol. 270:C1666-C1674. [DOI] [PubMed] [Google Scholar]
- 8.Buzoni-Gatel, D., H. Debbabi, F. J. Mennechet, V. Martin, A. C. Lepage, J. D. Schwartzman, and L. H. Kasper. 2001. Murine ileitis after intracellular parasite infection is controlled by TGF-beta-producing intraepithelial lymphocytes. Gastroenterology 120:914-924. [DOI] [PubMed] [Google Scholar]
- 9.Casola, A., M. K. Estes, S. E. Crawford, P. L. Ogra, P. B. Ernst, R. P. Garofalo, and S. E. Crowe. 1998. Rotavirus infection of cultured intestinal epithelial cells induces secretion of CXC and CC chemokines. Gastroenterology 114:947-955. [DOI] [PubMed] [Google Scholar]
- 10.Chen, W. X., J. A. Harp, and A. G. Harmsen. 1993. Requirements for CD4+ cells and gamma interferon in resolution of established Cryptosporidium parvum infection in mice. Infect. Immun. 61:3928-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, X. M., S. A. Levine, P. L. Splinter, P. S. Tietz, A. L. Ganong, C. Jobin, G. J. Gores, C. V. Paya, and R. N. La. 2001. Cryptosporidium parvum activates nuclear factor kappaB in biliary epithelia preventing epithelial cell apoptosis. Gastroenterology 120:1774-1783. [DOI] [PubMed] [Google Scholar]
- 12.Danoff, T. M., P. A. Lalley, Y. S. Chang, P. S. Heeger, and E. G. Neilson. 1994. Cloning, genomic organization, and chromosomal localization of the Scya5 gene encoding the murine chemokine RANTES. J. Immunol. 152:1182-1189. [PubMed] [Google Scholar]
- 13.DuPont, H. L., C. L. Chappell, C. R. Sterling, P. C. Okhuysen, J. B. Rose, and W. Jakubowski. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332:855-859. [DOI] [PubMed] [Google Scholar]
- 14.Dwinell, M. B., N. Lugering, L. Eckmann, and M. F. Kagnoff. 2001. Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology 120:49-59. [DOI] [PubMed] [Google Scholar]
- 15.Eckmann, L., H. C. Jung, C. Schurer-Maly, A. Panja, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1993. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology 105:1689-1697. [DOI] [PubMed] [Google Scholar]
- 16.Freter, R. R., J. A. Alberta, G. Y. Hwang, A. L. Wrentmore, and C. D. Stiles. 1996. Platelet-derived growth factor induction of the immediate-early gene MCP-1 is mediated by NF-kappaB and a 90-kDa phosphoprotein coactivator. J. Biol. Chem. 271:17417-17424. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 18.Godwin, T. A. 1991. Cryptosporidiosis in the acquired immunodeficiency syndrome: a study of 15 autopsy cases. Hum. Pathol. 22:1215-1224. [DOI] [PubMed] [Google Scholar]
- 19.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan, I. A., J. A. MacLean, F. S. Lee, L. Casciotti, E. DeHaan, J. D. Schwartzman, and A. D. Luster. 2000. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity 12:483-494. [DOI] [PubMed] [Google Scholar]
- 21.Lacroix, S., R. Mancassola, M. Naciri, and F. Laurent. 2001. Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and gamma interferon-deficient mice: role of tumor necrosis factor alpha in protection. Infect. Immun. 69:1635-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laurent, F., L. Eckmann, T. C. Savidge, G. Morgan, C. Theodos, M. Naciri, and M. F. Kagnoff. 1997. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect. Immun. 65:5067-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurent, F., R. Mancassola, S. Lacroix, R. Menezes, and M. Naciri. 2001. Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect. Immun. 69:2527-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCole, D. F., L. Eckmann, F. Laurent, and M. F. Kagnoff. 2000. Intestinal epithelial cell apoptosis following Cryptosporidium parvum infection. Infect. Immun. 68:1710-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald, V., H. A. Robinson, J. P. Kelly, and G. J. Bancroft. 1996. Immunity to Cryptosporidium muris infection in mice is expressed through gut CD4+ intraepithelial lymphocytes. Infect. Immun. 64:2556-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mead, J. R., and X. You. 1998. Susceptibility differences to Cryptosporidium parvum infection in two strains of gamma interferon knockout mice. J. Parasitol. 84:1045-1048. [PubMed] [Google Scholar]
- 27.Ojcius, D. M., J. L. Perfettini, A. Bonnin, and F. Laurent. 1999. Caspase-dependent apoptosis during infection with Cryptosporidium parvum. Microbes Infect. 1:1163-1168. [DOI] [PubMed] [Google Scholar]
- 28.Panja, A., S. Goldberg, L. Eckmann, P. Krishen, and L. Mayer. 1998. The regulation and functional consequence of proinflammatory cytokine binding on human intestinal epithelial cells. J. Immunol. 161:3675-3684. [PubMed] [Google Scholar]
- 29.Rollo, E. E., K. P. Kumar, N. C. Reich, J. Cohen, J. Angel, H. B. Greenberg, R. Sheth, J. Anderson, B. Oh, S. J. Hempson, E. R. Mackow, and R. D. Shaw. 1999. The epithelial cell response to rotavirus infection. J. Immunol. 163:4442-4452. [PubMed] [Google Scholar]
- 30.Sangari, F. J., M. Petrofsky, and L. E. Bermudez. 1999. Mycobacterium avium infection of epithelial cells results in inhibition or delay in the release of interleukin-8 and RANTES. Infect. Immun. 67:5069-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith, L. M., M. T. Bonafonte, and J. R. Mead. 2000. Cytokine expression and specific lymphocyte proliferation in two strains of Cryptosporidium parvum-infected gamma-interferon knockout mice. J. Parasitol. 86:300-307. [DOI] [PubMed] [Google Scholar]
- 32.Song, F., K. Ito, T. L. Denning, D. Kuninger, J. Papaconstantinou, W. Gourley, G. Klimpel, E. Balish, J. Hokanson, and P. B. Ernst. 1999. Expression of the neutrophil chemokine KC in the colon of mice with enterocolitis and by intestinal epithelial cell lines: effects of flora and proinflammatory cytokines. J. Immunol. 162:2275-2280. [PubMed] [Google Scholar]
- 33.Theodos, C. M., K. L. Sullivan, J. K. Griffiths, and S. Tzipori. 1997. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection. Infect. Immun. 65:4761-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungar, B. L. P., J. A. Burris, C. A. Quinn, and F. D. Finkelman. 1990. New mouse models for chronic Cryptosporidium infection in immunodeficient hosts. Infect. Immun. 58:961-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Upton, S. J., M. Tilley, and D. B. Brillhart. 1995. Effects of select medium supplements on in vitro development of Cryptosporidium parvum in HCT-8 cells. J. Clin. Microbiol. 33:371-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vidal, K., I. Grosjean, J. P. Evillard, C. Gespach, and D. Kaiserlian. 1993. Immortalization of mouse intestinal epithelial cells by the SV40-large T gene. Phenotypic and immune characterization of the MODE-K cell line. J. Immunol. Methods 166:63-73. [DOI] [PubMed] [Google Scholar]
- 37.Widmer, U., K. R. Manogue, A. Cerami, and B. Sherry. 1993. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J. Immunol. 150:4996-5012. [PubMed] [Google Scholar]