Abstract
The Pseudomonas aeruginosa protein ExoT is a bacterial GTPase-activating protein (GAP) that has in vitro activity toward Rho, Rac, and Cdc42 GTPases. Expression of ExoT both inhibits the internalization of strain PA103 by macrophages and epithelial cells and is associated with morphological changes (cell rounding and detachment) of infected cells. We find that expression of ExoT leads to the loss of GTP-bound RhoA, Rac1, and Cdc42 in transfected HeLa cells, demonstrating that ExoT has GAP activity in vivo toward all three GTPases. GAP activity is absolutely dependent on the presence of arginine at position 149 but is not affected by whether ExoT is expressed in the absence or presence of other P. aeruginosa type III secreted proteins. We also demonstrate that expression of ExoT in epithelial cells is sufficient to cause stress fiber disassembly by means of ExoT's GAP activity toward RhoA.
Several species of gram-negative bacterial pathogens, including Yersinia enterocolitica, Yersinia pseudotuberculosis, enteropathogenic Escherichia coli, and enterohemorrhagic E. coli, are able to block their internalization by professional phagocytes and epithelial cells (5, 13). This anti-internalization activity is mediated by bacterially synthesized proteins that are introduced into eukaryotic cells via the type III secretion systems of these pathogens. In the case of Yersinia, anti-internalization activity has been attributed to two proteins, YopE and YopH (27, 30); the identity of the enteropathogenic E. coli anti-internalization factor(s) is not yet known. Pseudomonas aeruginosa also blocks its internalization via type III secreted effector proteins (17); the bacterial effectors capable of inhibiting P. aeruginosa internalization by macrophages or epithelial cells are ExoS and ExoT (6, 7, 10). Both ExoS and ExoT have an arginine finger domain in their amino-terminal halves characteristic of GTPase-activating proteins (GAPs) (8). In the case of ExoT, mutation of the conserved Arg149 within this domain results in partial loss of anti-internalization activity (10). We have recently demonstrated that P. aeruginosa internalization by epithelial cells is accompanied by activation of the small GTPase RhoA and that expression of a constitutively active mutant of RhoA, RhoAV14, is sufficient to promote bacterial internalization (20). Neither Rac nor Cdc42 activation appears to promote Pseudomonas internalization by epithelial cells. Macrophage internalization of P. aeruginosa strain PA01, on the other hand, has been shown to be dependent upon Rac1 and Cdc42 activation (23). Purified ExoT acts as a GAP for Rho, Rac, and Cdc42 in vitro (21). In this paper we ask whether ExoT exhibits GAP activity toward these same GTPases in vivo and whether ExoT's GAP activity is altered by the presence of other host or bacterial proteins.
ExoT exhibits GAP activity toward Rho, Rac, and Cdc42 in vivo
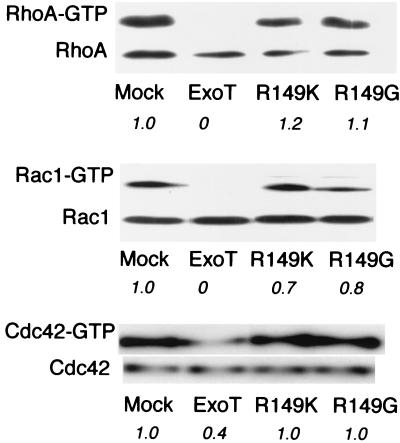
Strains and plasmids that we used are listed (Table 1). ExoT was PCR amplified from PA103 genomic DNA using primers BAKA18 (5′ GCGGGTACCATGGATATTCAATCATCTCAGCAG 3′) and BAKA19 (5′ GGCGGTACCTCAGGCCAGGTCGAGG 3′). The resulting fragment contains KpnI sites flanking the ExoT coding sequence, introduces an NcoI site at the ATG start codon, and changes the second amino acid of ExoT from aspartate to histidine. The PCR product was subcloned into pCruz Myc-B (Santa Cruz Biotechnology), creating a gene encoding a fusion protein with an amino-terminal Myc epitope tag under control of the cytomegalovirus (CMV) promoter (pMyc-T). Mammalian expression vectors expressing ExoT mutated at Arg149 to either glycine (R149G) or lysine (R149K) were constructed by an identical strategy, using pBK151 or pBK162 as template DNA for PCR amplifications (pMyc-T[R149G] and pMyc-T[R149K], respectively). The predicted sequences of these constructs were confirmed by nucleotide sequencing (University of California at San Francisco Biomolecular Resource Center sequencing facility). To assay the GAP activity of wild-type ExoT in the absence of other bacterial proteins, 5 × 105 HeLa cells (grown in DME-H21 [University of California at San Francisco Cell Culture Facility] supplemented with 10% fetal calf serum [Gibco] at 37°C in 5% CO2) were plated to 10-cm-diameter tissue culture dishes 24 h prior to transfection with 2 μg of pMyc-T in the presence of Effectene (Qiagen). Mock transfections were carried out in parallel. Cells were harvested 12 to 14 h after transfection, at which time Myc-tagged ExoT was detectable by indirect immunofluorescence in approximately 25% of cells (see below). The amount of RhoA-GTP present in mock- and ExoT-transfected cells was determined by selectively affinity precipitating GTP-bound RhoA with the rhotekin binding domain fused to glutathione reductase (GST-TRBD) and coupled to glutathione-Sepharose 4B (Amersham Pharmacia), a method originally described by Ren et al. (28). Activity of the coupled GST-TRBD reagent was assayed each time that reagent was prepared by demonstrating complete affinity precipitation of RhoA activated by cytotoxic necrotizing factor 1 treatment of Madin-Darby canine kidney (MDCK) cells, while specificity of the reagent for the GTP-bound form of RhoA was demonstrated by its inability to affinity precipitate the dominant negative allele of RhoA, RhoAN19, from MDCK cells induced to express this myc-tagged protein (20) (data not shown). Cell lysates were prepared as described previously (20), and total protein was determined (Pierce BCA Assay kit). Twenty microliters of each lysate was set aside (“total”); 500 μl was incubated with GST-TRBD coupled to glutathione-Sepharose 4B beads, which allowed selective precipitation of RhoA-GTP (“GTP-bound”). Total and GTP-bound samples were loaded in their entirety onto 13% polyacrylamide gels, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blotted to polyvinylidene difluoride (Millipore). Membranes were blocked with 5% (wt/vol) dry milk, incubated with anti-RhoA antibody (1:200) (Santa Cruz Biotechnology) followed by goat anti-mouse immunoglobulin G (IgG)-horseradish peroxidase conjugate (1:2,000) (Bio-Rad), developed using ECL Plus (Amersham), and exposed to Biomax ML film (Kodak). The amount of RhoA in each sample was approximated by scanning films and determining the density of scanned bands using the IPLabGel program. The amount of GTP-bound versus total RhoA was calculated and normalized to the amount present in mock-transfected cells. As seen in Fig. 1, ExoT-transfected cells showed no detectable RhoA-GTP in this assay, while approximately 5% of RhoA was present in the GTP-bound form in mock-transfected cells. GAP activity toward RhoA was absolutely dependent on the presence of Arg149, as cells transfected with 2 μg of pMyc-T(R149G) or pMyc-T(R149K) (expression constructs identical to pMyc-T except for a single amino acid change at position 149 of ExoT) showed amounts of Rho-GTP comparable to those observed in mock-transfected cells (Fig. 1).
TABLE 1.
Bacterial strains and plasmidsa
| Strain or plasmid | Description | Reference(s) or source |
|---|---|---|
| PA103 | Virulent lung isolate of P. aeruginosa | 2, 25 |
| PA103ΔU | PA103 with an in-frame deletion corresponding to amino acids 330 to 571 of exoU | 10 |
| XL2-Blue | E. coli strain used for cloning; recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac[F′ proAB lacIqZΔM15 Tn10(Tetr) Amy Chlr] | Stratagene |
| pCruz Myc-B | Mammalian expression vector containing multiple cloning site 3′ to Myc tag | Santa Cruz Biotechnology |
| pCruz Myc-LacZ | pCruz Myc carrying LacZ cloned in frame to amino-terminal Myc epitope tag | Santa Cruz Biotechnology |
| pCruz HA-B | Mammalian expression vector containing multiple cloning site 3′ to HA tag | Santa Cruz Biotechnology |
| pBK151 | ExoT(R149K) subcloned into pUCP20 | 10 |
| pBK162 | ExoT(R149G) subcloned into pUCP20 | 10 |
| pMyc-T | pCruz Myc-B carrying ExoT open reading frame cloned in frame to amino-terminal Myc epitope tag | This study |
| pMyc-T(R149K) | pCruz Myc-B carrying ExoT(R149K) open reading frame cloned in frame to amino-terminal Myc epitope tag | This study |
| pMyc-T(R149G) | pCruz Myc-B carrying ExoT(R149G) open reading frame cloned in frame to amino-terminal Myc epitope tag | This study |
| pHA-T | pCruz HA-B carrying ExoT open reading frame cloned in frame to amino-terminal HA epitope tag | This study |
| pMyc-RhoAV14 | Mammalian expression vector carrying Myc-RhoAV14 under expression of CMV promoter | Daniel Kalman (Emory University) |
| pMyc-RacV12 | Mammalian expression vector carrying Myc-Rac1V12 under expression of CMV promoter | Daniel Kalman (Emory University) |
| pGST-TRBD | Rhotekin binding domain subcloned into pGEX-2T | 28 |
| pGST-CRIB | CRIB domain of human Pak3 subcloned into pGEX-2T | 3 |
Abbreviations: Tetr, tetracycline resistant; Chlr, chloramphenicol resistant.
FIG. 1.
Cells transfected with wild-type ExoT but not with ExoT(R149G) or ExoT(R149K) show loss of GTP-bound RhoA, Rac1, and Cdc42. HeLa cells were transfected with pMycB-LacZ (Mock), pMycB-T, pMycB-T(R149K), or pMycB-T(R149G) 12 h prior to lysis and determination of total and GTP-bound RhoA, Rac1, and Cdc42 levels as described in the text. (Twenty microliters of lysate was analyzed for total RhoA, Rac1, or Cdc42, while 500 μl of lysate was used in the affinity precipitations.) The experiments shown are characteristic of three or four independent assays. Italicized numbers indicate the fraction of each GTPase present in the GTP-bound form as normalized to mock-transfected cells.
The absence of detectable RhoA-GTP in the ExoT-transfected sample is particularly striking, especially since ExoT expression cannot be detected in the majority of cells at this time point (see below). There is no evidence that ExoT liberated during cell lysis inactivates RhoA within the lysate in trans, as mixing lysate from ExoT-transfected cells with that from mock-transfected cells prior to incubation with GST-TRBD beads did not result in less GTP-RhoA being recovered from the mock-transfected cells (data not shown). We conclude, therefore, that many more cells are expressing ExoT, albeit at low levels, than can be detected by our staining procedure. This would be consistent with our observation that at 20 to 24 h posttransfection with ExoT, <10% of cells remain attached to coverslips; mock- or T(R149K)/T(R149G)-transfected cells do not show such cell loss, suggesting that it is related to the GAP activity of ExoT and not to nonspecific toxicity following transfection (data not shown).
Activation of RhoA is sufficient to increase P. aeruginosa internalization by epithelial cells; however, activation of neither Rac1 nor Cdc42 promotes P. aeruginosa internalization (20). We therefore asked whether the anti-internalization factor ExoT might exhibit specificity toward RhoA in vivo and show no significant activity toward Rac or Cdc42 when expressed in epithelial cells. Using the Cdc42/Rac interactive binding (CRIB) domain of hPak3 fused to GST as an affinity precipitation reagent selective for the GTP-bound forms of Rac1 and Cdc42 (3), we were able to precipitate Rac1-GTP and Cdc42-GTP from transfected cells in a method analogous to that described above for Rho-GTP determinations. The GST-CRIB reagent's activity and specificity were confirmed by demonstrating that this reagent selectively precipitated the constitutively active alleles of Rac1 (myc-Rac1V12) and Cdc42 (myc-Cdc42V12) from stably transfected MDCK cell lines expressing these proteins but was unable to pull down the dominant negative allele myc-RacN17 or myc-Cdc42N17 (20) (data not shown). As seen in Fig. 1, transfected cells exhibited diminished levels of both Rac-GTP and Cdc42-GTP compared to mock-transfected cells, demonstrating that all three GTPases serve as in vivo substrates for ExoT. The decreases in Rac-GTP and Cdc42-GTP levels were again dependent on the presence of arginine at position 149 in ExoT, as cells that were transfected with ExoT mutated at position 149 to glycine or lysine showed minimal changes in Rac1-GTP or Cdc42-GTP levels compared to mock-transfected cells (Fig. 1).
Decreased expression levels of transfected ExoT(R149G) or ExoT(R149K) relative to the wild-type protein could account for our inability to observe GAP activity following transfection of these proteins into HeLa cells. Indirect immunofluorescence, however, confirmed that both T(R149G) (96 of 319, 30% positive) and T(R149K) (52 of 208, 25% positive) were strongly expressed in the same proportion of HeLa cells as the wild-type protein (108 of 438, 25% positive) at 12 to 14 h posttransfection. As was shown previously, ExoT mutated at Arg149 to Gly or Lys is detected in mammalian cells following translocation without evidence of increased protein degradation or instability (10).
Bacterially translocated ExoT exhibits GAP activity toward Rho, Rac, and Cdc42 in vivo.
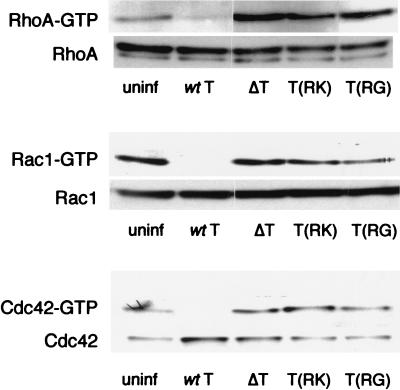
Expression of ExoT in transfected mammalian cells results in decreased levels of GTP-bound RhoA, Rac1, and, to a lesser extent, Cdc42. Thus, the specificity of ExoT's GAP activity in eukaryotic cells parallels that determined in vitro for purified ExoT and substrates (21). To determine whether other bacterial proteins may modify the GAP activity of ExoT, we assayed the in vivo GAP activity associated with infection by strains expressing wild-type ExoT. These experiments were carried out using PA103ΔU, a strain carrying an in-frame deletion within the exoU gene, so that cell necrosis caused by this type III secreted toxin would not interfere with these assays (10). HeLa cells (n = 106) were plated to 10-cm-diameter dishes 48 h prior to infection with PA103ΔU at a multiplicity of infection (MOI) of 25 to 50. Cells were lysed at 0, 1, and 2 h following bacterial infection, and GTP-bound RhoA, Rac1, and Cdc42 were detected as described above. GTP-bound levels of all three Rho family members decreased with time, demonstrating that ExoT had GAP activity toward all of these proteins when translocated into host cells in physiological amounts and in the context of other bacterial proteins (Fig. 2). As found in the transfected cells, the presence of arginine at position 149 was required for GAP activity, as infection with PA103ΔU/T(R149K) or PA103ΔU/T(R149G) did not decrease GTP-bound Rho, Rac, or Cdc42 levels (Fig. 3). Infected cells were examined by phase-contrast microscopy prior to lysis, allowing us to confirm that the characteristic changes in cell morphology associated with infection were present in most cells by 2 h postinfection (data not shown). Thus, as previously reported, PA103ΔU-infected cells showed cell body rounding; cells infected by bacteria carrying either Arg149 point mutation showed cytoplasmic retraction and cell body elongation; and cells infected with PA103ΔUΔT were indistinguishable from uninfected control cells (10).
FIG. 2.
Infection with ExoT expressing PA103ΔU results in loss of GTP-bound RhoA, Rac1, and Cdc42. HeLa cells were infected with PA103ΔU (MOI = 25 to 50) 0, 1, or 2 h prior to lysis, and determination of total and GTP-bound RhoA, Rac1, and Cdc42 levels was carried out as described in the text. Duplicate samples are shown for each time point. The experiments shown are representative of two or three independent assays.
FIG. 3.
Bacterially translocated ExoT(R149G) and ExoT(R149K) do not cause the loss of GTP-bound RhoA, Rac1, or Cdc42 in infected cells. HeLa cells were infected with PA103ΔU (wt T), PA103ΔUΔT (ΔT), PA103ΔU/T(R149K) [T(RK)], or PA103ΔU/T(R149G) [T(RG)] at an MOI of 25 to 50 for 2 h prior to cell lysis. Uninfected cells (uninf) were processed in parallel as a control. Total and GTP-bound RhoA, Rac1, and Cdc42 were detected as described in the text. The experiments shown are representative of two or three independent assays.
ExoT expression results in stress fiber disassembly and cell rounding.
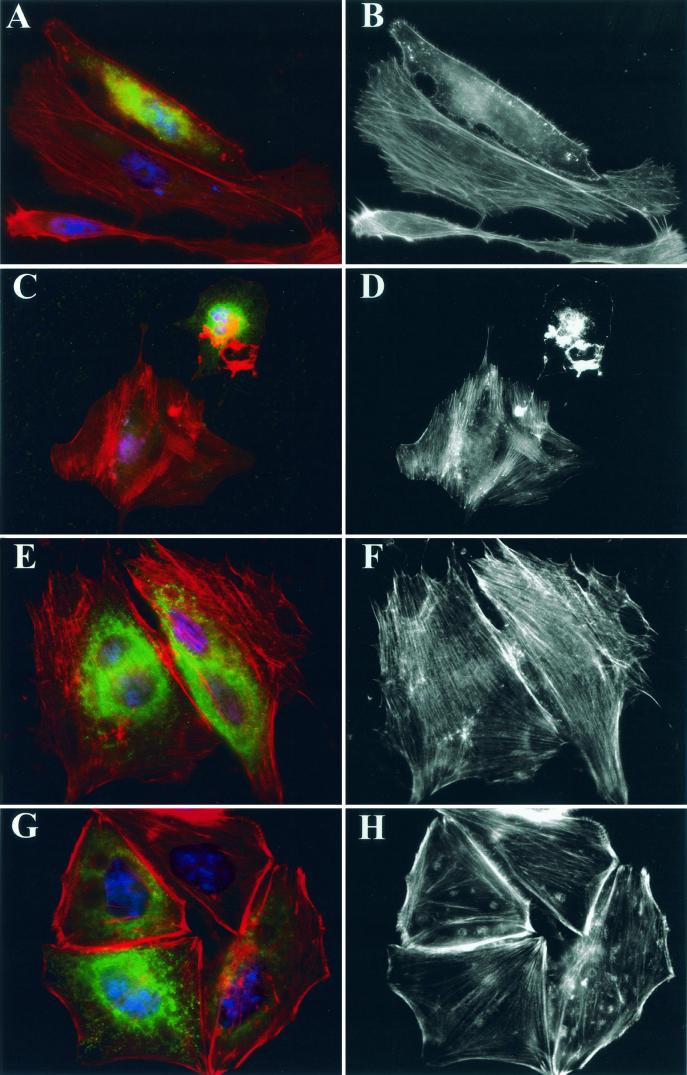
Infection of epithelial cells by bacteria expressing ExoT results in cell rounding and detachment (10, 11). We asked whether expression of ExoT in transfected cells was sufficient to cause these changes in cell morphology. HeLa cells (n = 5 × 104) were plated to glass coverslips and incubated for 24 h prior to transfection. Cells were transfected with 2 μg of pMyc-T, pMyc-T(R149K), or pMyc-T(R149G) 10 to 14 h prior to processing for indirect immunofluorescence. Coverslips were fixed in 4% paraformaldehyde and stained as described previously (11). Cells expressing wild-type or mutated Myc-tagged ExoT were detected by staining with monoclonal antibody (MAb) 9E10 (anti-Myc) diluted 1:250 (Santa Cruz Biotechnology) followed by Alexa488-conjugated anti-mouse MAb (Molecular Probes) diluted 1:500. Nuclei were stained with 4′,6′-diamidino-2-phenlyindole (DAPI) (1 μg/ml; Sigma), and the actin cytoskeleton was visualized with Texas red-phalloidin (0.03 μM; Molecular Probes). As seen in Fig. 4A to D, ExoT-transfected cells exhibited diffuse cytoplasmic staining with anti-Myc MAb 9E10; the intracellular distribution of bacterially translocated ExoT is not yet known. Actin stress fibers were absent in cells transfected with wild-type ExoT. Some cells still maintained a spread-out morphology despite the complete absence of stress fibers (Fig. 4A and B); however, the majority of transfected cells appeared rounded and showed a collapsed actin cytoskeleton by phalloidin staining (Fig. 4C and D). Transfection of cells with constructs expressing either the R149G (Fig. 4E and F) or R149K (Fig. 4G and H) point mutants of ExoT did not result in the loss of stress fibers or lead to cell rounding in HeLa cells, indicating that the GAP activity of ExoT is required for these changes in cell morphology.
FIG.4.
Cells transfected with wild-type ExoT but not ExoT(R149G) or ExoT(R149K) exhibit stress fiber loss and cell rounding. HeLa cells plated on coverslips were transfected with pMyc-T (A to D), pMyc-T(R149G) (E to F), or pMyc-T(R149K) (G to H) 10 to 14 h prior to fixation. All samples were stained with anti-Myc MAb 9E10 (green) to identify cells expressing ExoT, Texas red-phalloidin (red) to visualize the actin cytoskeleton, and DAPI (blue) to visualize nuclei. Samples were imaged on a Nikon Eclipse E800 microscope equipped with DAPI, fluorescein isothiocyanate, and rhodamine filter sets using a 100× objective, captured with a Spot charge-coupled device camera (Diagnostic Instruments, Inc.) using Spot Advanced 3.0.4 software, pseudocolored, and assembled into figures using Adobe Photoshop 5.0. Panels A, C, E, and G show triply stained cells; panels B, D, F, and H show phalloidin staining only. In panels A to D, cells expressing ExoT show clear loss of stress fibers; cells transfected with either point mutant (E to H) maintain normal stress fiber morphology.
Expression of constitutively active RhoAV14 blocks ExoT-dependent changes in the actin cytoskeleton.
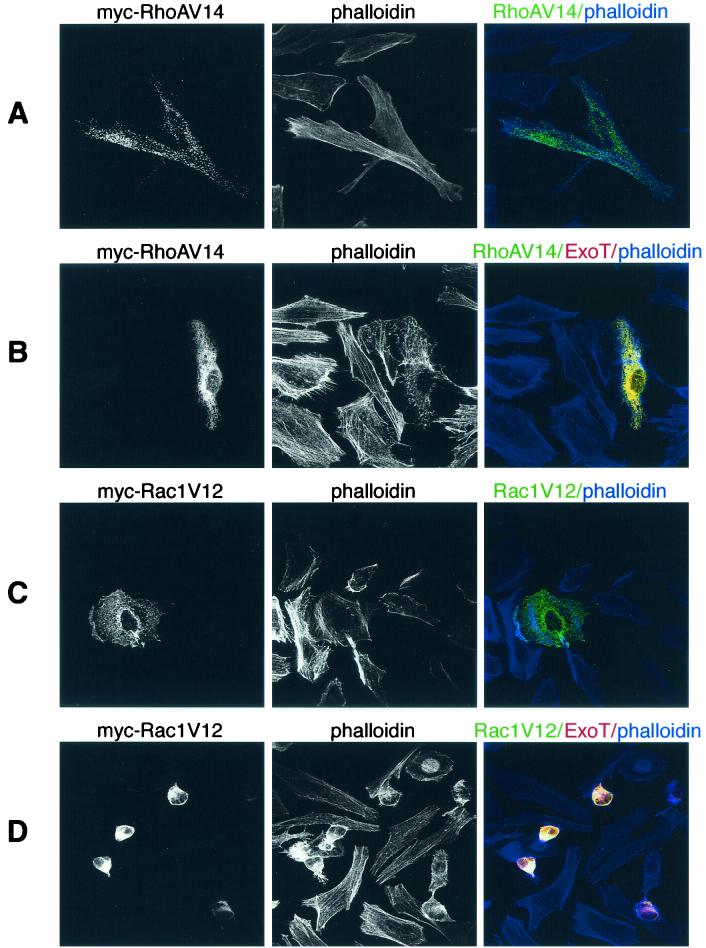
The formation and maintenance of stress fibers depend on RhoA activity (15); thus, ExoT could be causing stress fiber loss and cell rounding by inactivating RhoA. If this is the case, expression of a constitutively active allele of RhoA should prevent cell rounding caused by ExoT. In order to test this hypothesis, we constructed an expression vector, pHA-T, by subcloning the KpnI-KpnI fragment containing the ExoT coding sequence into pCruz HA-B (Santa Cruz Biotechnology). This plasmid expresses an amino-terminally hemagglutinin (HA)-tagged ExoT fusion protein from the CMV promoter. HeLa cells were plated to coverslips as described above and transfected with 1 μg of pHA-T plus 1 μg of either pRhoAv14 or pRac1V12, mammalian expression vectors encoding constitutively active alleles of RhoA or Rac1 from a CMV promoter, respectively. Doubly transfected cells were detected by staining with mouse anti-Myc (MAb 9E10) (1:250; Santa Cruz Biotechnology), which recognized RhoAV14- or Rac1V12-expressing cells, and rat anti-HA (MAb 3F10) (1:200; Boehringer Mannheim Biochemicals). We confirmed that the secondary antibodies employed in these studies (Alexa568-conjugated anti-rat IgG [Molecular Probes] and Alexa488-conjugated anti-mouse IgG [Molecular Probes]) reacted only against their cognate rat or mouse primary antibody at the concentration used for staining (1:500) (data not shown). Filamentous actin was visualized by staining with Bodipy 650/655-conjugated phalloidin (66 nM; Molecular Probes). As seen in Fig. 5, both Myc-RhoAV14 and Myc-Rac1V12 were easily detected in transfected cells. Myc-RhoAV14 is predominantly cytosolic, as expected, and transfected cells show many stress fibers (Fig. 5A), while Myc-Rac1V12-expressing cells show prominent lamellipodia to which this GTPase localizes (Fig. 5C). Cells cotransfected with pHA-T and pMyc-RhoAV14 (yellow) exhibit a spread morphology and possess some stress fibers (Fig. 5B). This is in marked contrast to cells coexpressing HA-ExoT and Myc-Rac1V12, which show stress fiber loss and cell rounding (Fig. 5D). Although it is possible that the differences in RhoAV14's versus Rac1V12's ability to prevent cell rounding may result from lower expression levels of the latter, we found no evidence to support this hypothesis. Although differing transfection efficiencies between samples precluded direct comparison of RhoAV14 versus Rac1V12 protein levels in cell lysates, anti-Myc staining detected strongly expressing cells in both RhoAV14 (Fig. 5A and B)- and Rac1V12 (Fig. 5C and D)-transfected samples. Nonetheless, no Rac1V12-transfected cell maintained stress fibers or showed a spread morphology. Thus, the visible effects of ExoT on the actin cytoskeleton in HeLa cells—i.e., stress fiber loss and cell rounding—appear to be a result of its GAP activity toward RhoA.
FIG. 5.
Expression of RhoAV14 but not of Rac1V12 prevents ExoT-mediated actin cytoskeleton disassembly and cell rounding. HeLa cells plated on coverslips were transfected with 1 μg of either pMyc-RhoAV14 (A and B) or pMyc-Rac1V12 (C and D) for 14 h prior to fixation; 1 μg of pHA-T was cotransfected in panels B and D. All samples were stained with anti-Myc MAb 9E10 (green) and anti-HA MAb 3F10 (red) to identify cells expressing RhoAV14 or Rac1V12 and ExoT, respectively. The actin cytoskeleton was visualized with Bodipy 650/655-conjugated phalloidin (blue). Cells were imaged with a Bio-Rad 1024 confocal microscope equipped with KrAg and HeNe lasers and fluorescein isothiocyanate, Texas red, and Cy5 filter sets using a 60× objective. Z sections were acquired sequentially and processed using NIH Image 1.62b software and were then merged and pseudocolored in Adobe Photoshop 5.0. Cells doubly transfected with ExoT and RhoAV14 (B), which appear yellow, maintained a spread-out shape and showed some stress fibers; cotransfection with Rac1V12, however, did not prevent cell rounding and complete stress fiber loss (yellow-stained cells, panel D).
Summary.
P. aeruginosa ExoT, like ExoS and Yersinia YopE, has been shown to have GAP activity toward purified RhoA, Rac1, and Cdc42 in vitro (21). In this study, we find that expression of ExoT in mammalian cells leads to decreases in GTP-bound RhoA, Rac1, and Cdc42, suggesting that this protein also exhibits GAP activity toward all three Rho family GTPases in vivo. The same result is obtained when ExoT is introduced into host cells during bacterial infection. This suggests that (i) no other bacterial or host protein acts to alter the specificity of ExoT toward these eukaryotic targets and that (ii) overexpression or mislocalization of ExoT in transfected cells is not responsible for its observed substrate specificity. Thus, the particular role played by RhoA in P. aeruginosa internalization by epithelial cells arises from the specific activation of this GTPase, and not of Rac1 or Cdc42, concomitant with bacterial internalization (B. I. Kazmierczak, K. Mostov, and J. N. Engel, unpublished data). On the other hand, the ability of ExoT to block P. aeruginosa phagocytosis by macrophages is likely attributable to the GAP activity of ExoT toward Rac1 and Cdc42 (23).
Recently, several groups have examined the in vitro and in vivo specificities of YopE. In vitro, YopE shows activity toward purified RhoA, Rac1, and Cdc42 (4, 30). In vivo activity toward RhoA and Rac1 has also been indirectly demonstrated, by showing that expression of a constitutively active allele of RhoA, RhoAV14, is sufficient to block actin stress fiber loss associated with YopE-expressing Y. pseudotuberculosis, while expression of constitutively active Rac1V12 inhibits YopE-dependent antiphagocytosis (4). These results are in apparent disagreement with a recent study by Andor et al. (1), where YopE was reported to inhibit ruffle formation following bradykinin stimulation of endothelial cells (the consequence of indirect Rac1 activation by Cdc42) but was unable to inhibit the direct activation of Cdc42, Rac1, or RhoA in response to bradykinin, sphingosine-1-phosphate, or thrombin stimulation, respectively, as assayed by microspike (Cdc42), ruffle (Rac1), or stress fiber formation (RhoA). The authors suggest that their findings indicate exquisite specificity of YopE toward Rac1 in vivo; however, it may also be that bacterially translocated YopE is present in the treated cells in too small an amount or too restricted a localization to overcome the stimulus presented by these pharmacologic activators of the Rho family GTPases.
P. aeruginosa ExoS has also undergone a recent examination of its in vivo activity toward Rho family GTPases. By assaying the distribution of GFP-Rac1 and GFP-Cdc42 fusion proteins, Krall et al. have demonstrated that the GAP domain of ExoS causes a relocalization of Rac1 and Cdc42 from the plasma membrane to the cytosol (22). As GTP-bound Rac1 and Cdc42 localize to the membrane, while GDP-bound Rac1 and Cdc42 are cytosolic, this suggests that the GAP domain of ExoS converts these two GTPases to the inactive form in vivo. RhoA also appeared to be a target of the ExoS GAP domain in vivo, as dominant active RhoA (RhoAQ63E) expression was required to prevent stress fiber loss in cells also transfected with the ExoS GAP domain (22). Thus, ExoS appears to exhibit GAP activity toward RhoA, Rac1, and Cdc42 in vivo as well as in vitro, much as we report here for ExoT.
Unlike YopE, ExoS, and ExoT, the Salmonella enterica serovar Typhimurium GAP SptP specifically activates GTP hydrolysis in vitro by Rac1 and Cdc42 and does not show appreciable GAP activity toward RhoA (8). This may reflect that the biological role of SptP in S. enterica serovar Typhimurium invasion is different from that proposed for the Yersinia and Pseudomonas GAPs. These latter organisms are thought to use their GAPs to block internalization by a wide variety of eukaryotic cells (professional and nonprofessional phagocytes). Conversely, S. enterica serovar Typhimurium possesses a type III secretion system (SPI 1) dedicated to promoting bacterial invasion of multiple cell types (9, 14) and translocates an effector, SopE, that activates Rac1 and Cdc42 and results in membrane ruffling and S. enterica serovar Typhimurium internalization (16). SptP, therefore, does not function to block invasion per se but rather returns the host cell cytoskeleton to baseline, presumably by inactivating Rac1 and Cdc42.
Though other bacterial toxins covalently modify their eukaryotic targets to activate (e.g., E. coli cytotoxic necrotizing factor 1) (24, 29) or inactivate them (e.g., Clostridium difficile toxin B) (19), a toxin such as ExoT can only shift the equilibrium between the active, or GTP-bound, form of a Rho family GTPase and the inactive, GDP-bound, form to favor the latter. Thus, the effect of such a protein is most visible on cells that are carrying out a task requiring continuous or repeated activation of a GTPase. The phenotypes that we have observed for ExoT fall into such a category. For example, the infection of epithelial cells with bacteria expressing wild-type ExoT (PA103ΔU) is sufficient to block cell migration in an in vitro wound closure model (11), a process that is known to require Rac1 and RhoA activation (26). Infection with mutants lacking wild-type ExoT—PA103ΔUΔT, ΔU/T(R149K), or ΔU/T(R149G)—does not affect wound closure. Likewise, tight junctions can be disrupted by infection with PA103ΔU but not by infection with PA103ΔUΔT (Kazmierczak and Engel, unpublished observations); the maintenance of tight junctions requires RhoA and Rac1 activation (18).
Lastly, it is clear that the enzymatic activity of ExoT toward the Rho family GTPases is absolutely dependent upon Arg149, as has been demonstrated for other bacterial GAPs, including P. aeruginosa ExoS (12, 22) and Y. enterocolitica YopE (4). The observation that bacterial strains carrying point mutations at Arg149 of ExoT are internalized to an intermediate extent, compared to strains with a deletion of ExoT versus strains expressing wild-type ExoT, suggests that bacterial GAP activity toward the Rho family GTPases accounts for only part of ExoT's anti-internalization activity (10). Studies to determine what roles other domains of ExoT play in this process are currently under way.
Acknowledgments
We thank Dan Kalman, Richard Cerione, Xiang-Dong Ren, and Martin Schwartz for generous gifts of plasmids; Dan Kalman, Melissa Woodrow, Lucy O'Brien, and Keith Mostov for helpful discussion and technical advice; and Rachel Kowal for excellent technical assistance.
This work was supported by NIH grants AI 01636 (B.I.K.) and AI 42806 (J.N.E.). J.N.E. is a Career Investigator of the American Lung Association.
Editor: D. L. Burns
REFERENCES
- 1.Andor, A., K. Trulzsch, M. Essler, A. Roggenkamp, A. Wiedemann, J. Heesemann, and M. Aepfelbacher. 2001. YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell. Microbiol. 3:301-310. [DOI] [PubMed] [Google Scholar]
- 2.Apodaca, G., M. Bomsel, R. Lindstedt, J. Engel, D. Frank, K. E. Mostov, and J. Wiener-Kronish. 1995. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation-defective host cells are resistant to bacterial killing. Infect. Immun. 63:1541-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagrodia, S., S. J. Taylor, K. A. Jordon, L. Van Aelst, and R. A. Cerione. 1998. A novel regulator of p21-activated kinases. J. Biol. Chem. 273:23633-23636. [DOI] [PubMed] [Google Scholar]
- 4.Black, D. B., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. [DOI] [PubMed] [Google Scholar]
- 5.Bliska, J. B. 2000. Yop effectors of Yersinia spp. and actin rearrangements. Trends Microbiol. 8:205-208. [DOI] [PubMed] [Google Scholar]
- 6.Cowell, B. A., D. Y. Chen, D. W. Frank, A. J. Vallis, and S. M. J. Fleiszig. 2000. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect. Immun. 68:403-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frithz-Lindsten, E., A. Holmström, L. Jacobsson, M. Soltani, J. Olsson, R. Rosqvist, and A. Forsberg. 1998. Functional conservation of the effector protein translocators PopB/YopB and PopD/YopD of Pseudomonas aeruginosa and Yersinia pseudotuberculosis. Mol. Microbiol. 29:1155-1165. [DOI] [PubMed] [Google Scholar]
- 8.Fu, Y., and J. E. Galan. 1999. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401:293-297. [DOI] [PubMed] [Google Scholar]
- 9.Galan, J. E. 1996. Molecular genetic bases of Salmonella entry into host cells. Mol. Microbiol. 20:263-271. [DOI] [PubMed] [Google Scholar]
- 10.Garrity-Ryan, L., B. Kazmierczak, R. Kowal, J. Commolli, A. Hauser, and J. Engel. 2000. The arginine finger domain of ExoT is required for actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 68:7100-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiser, T., B. Kazmierczak, L. Garrity, M. Matthay, and J. Engel. 2001. Pseudomonas aeruginosa ExoT inhibits in vitro lung epithelial wound repair. Cell. Microbiol. 3:223-236. [DOI] [PubMed] [Google Scholar]
- 12.Goehring, U. M., G. Schmidt, K. J. Pederson, K. Aktories, and J. T. Barbieri. 1999. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 274:36369-36372. [DOI] [PubMed] [Google Scholar]
- 13.Goosney, D. L., J. Celli, B. Kenny, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli inhibits phagocytosis. Infect. Immun. 67:490-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groisman, E. A., and H. Ochman. 1993. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 12:3779-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 16.Hardt, W.-D., L. M. Chen, K. E. Schubel, X. R. Bustelo, and J. E. Galan. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-822. [DOI] [PubMed] [Google Scholar]
- 17.Hauser, A. R., P. J. Kang, S. J. M. Fleiszig, K. Mostov, and J. Engel. 1998. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect. Immun. 66:1413-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jou, T.-S., E. Schneeberger, and W. J. Nelson. 1998. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 142:101-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Just, I., J. Selzer, M. Wilm, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375:500-503. [DOI] [PubMed] [Google Scholar]
- 20.Kazmierczak, B. I., T.-S. Jou, K. Mostov, and J. Engel. 2001. Rho-GTPase activity modulates Pseudomonas aeruginosa internalization by epithelial cells. Cell. Microbiol. 3:85-98. [DOI] [PubMed] [Google Scholar]
- 21.Krall, R., G. Schmidt, K. Aktories, and J. T. Barbieri. 2000. Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect. Immun. 68:6066-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krall, R., J. Sun, K. J. Pederson, and J. T. Barbieri. 2002. In vivo Rho GTPase-activating protein activity of Pseudomonas aeruginosa cytotoxin ExoS. Infect. Immun. 70:360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, D. J., D. Cox, J. Li, and S. Greenberg. 2000. Rac1 and Cdc42 are required for phagocytosis, but not NF-kappaB-dependent gene expression, in macrophages challenged with Pseudomonas aeruginosa. J. Biol. Chem. 275:141-146. [DOI] [PubMed] [Google Scholar]
- 24.Lerm, M., J. Selzer, A. Hoffmeyer, U. R. Rapp, K. Aktories, and G. Schmidt. 1999. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1: activation of c-Jun N-terminal kinase in HeLa cells. Infect. Immun. 67:496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, P. V. 1966. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis: identity of the lethal toxins produced in vitro and in vivo. J. Infect. Dis. 116:481-489. [DOI] [PubMed] [Google Scholar]
- 26.Nobes, C. D., and A. Hall. 1999. Rho GTPases control polarity, protrusion and adhesion during cell movement. J. Cell Biol. 144:1235-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persson, C., N. Carballeira, H. Wolf-Watz, and M. Fallman. 1997. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16:2307-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren, X.-D., W. B. Kiosses, and M. A. Schwartz. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18:578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387:725-729.9192900 [Google Scholar]
- 30.von Pawel-Rammingen, U. M., V. Telpnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737-748. [DOI] [PubMed] [Google Scholar]