Abstract
Cortactin and the translocated intimin receptor, Tir, interacted with each other in pedestal formation in HeLa cells infected with enteropathogenic Escherichia coli (EPEC). Cortactin is shown to be necessary for organizing actin pedestals in response to EPEC, based on the expression of green fluorescent protein-fused cortactin derivatives in HeLa cells.
Infection with enteropathogenic Escherichia coli (EPEC) is recognized as one of the most important causes of infantile diarrhea in developing countries (10). EPEC can trigger the rearrangement of the host cellular actin cytoskeleton, a common mode of action among several enteropathogenic bacteria (3). The dramatic EPEC-induced alteration of the host cell membrane culminates with the formation of a pedestal-like structure (2, 4). This process, also known as attaching and effacing, initiates when the bacteria bind to and inject several virulence factors (effectors) inside the host cell (2). The translocated intimin receptor (Tir) is one of the injected proteins. It is inserted into the host cell plasma membrane via a type III secretion system (2), and it functions as a receptor for another EPEC-produced protein, intimin (9). Several host cell proteins are then translocated or activated, resulting in the formation of F-actin-rich structures, also called pedestals (2). Actin polymerization by EPEC has been reported to depend on Wiskott-Aldrich Syndrome protein (WASP) or on neural-WASP (N-WASP) and actin-related protein (Arp2/3) complex activation (7). Recently, we reported that cortactin, an actin-binding protein, is recruited to the EPEC adherence site (1). To study this in more detail, we first tried to clarify whether or not cortactin interacts with Tir.
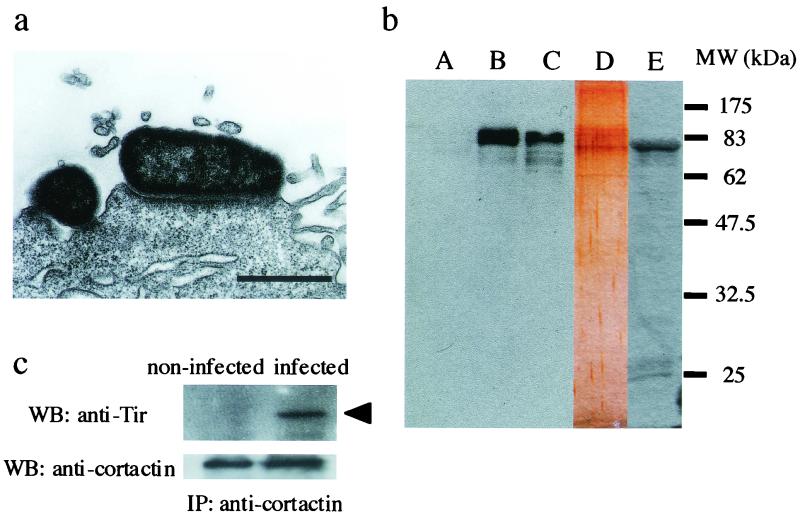
The EPEC strain serotype O111:H2 (RIMD0509829; provided by the Research Institute for Microbial Diseases, Osaka University, Osaka, Japan) was used to infect HeLa cell monolayers as described elsewhere (1). Infection time was set to 4 h at 37°C because distinct pedestals are produced by the EPEC strain used in this study during this period, as demonstrated by transmission electron microscopy (Fig. 1a). For the expression of recombinant whole Tir, the whole DNA fragment of tir was amplified by PCR with two specific primers (tir-1 [5′-GGGAGCTCATGCCTATTGGTAATCTTGGCCAC-3′] and tir-6 [5′-GGAAGCTTTTAAACGAAACGTGCGGG-3′]) linked with two restriction enzyme digestion sites (SacI and HindIII, respectively). The amplified PCR fragment was cloned into SacI and HindIII in pET28a(+) (Novagen). Purification of recombinant Tir fused to the His-T7 tag (His-T7-Tir) was performed with His-Bind resin (Novagen) according to the manufacturer's instructions. Anti-Tir polyclonal antibody was obtained by using purified recombinant Tir as described by Hartland et al. (6).
FIG. 1.
(a) Transmission electron micrograph showing EPEC attached to the top of a fully developed pedestal-like structure. Bar, 1 μm. (b) Immunoblots of eluates from His-T7-Tir affinity chromatography. HeLa cell lysates were applied and eluted after extensive washing. After electrophoresis, Western blotting with anti-cortactin antibody was performed. Lane A, eluate from the column with His-CPE, which specifically binds to claudin, used as a negative control for nonspecific protein binding. Lane B, eluate from the column with His-T7-Tir. Lane C, total HeLa cell lysate. Lane D, silver-stained gel electrophoresed eluate from the column with His-T7-Tir. Lane E, eluate from the column with His-T7-Tir detected by anti-T7 antibody. Molecular markers are indicated on the right. (c) Immunoprecipitation assay with anti-cortactin antibody on noninfected HeLa cell lysates (left ) or EPEC-infected HeLa cell lysates (right). Precipitated samples were detected with anti-Tir antibody (upper panel), and an arrowhead indicates Tir. Equal amounts of cortactin were included in both samples (lower panel).
To identify interactions between cortactin and Tir, affinity chromatography was performed as described elsewhere (5), by using His-T7-Tir (∼100 μg) or His-Clostridium perfringens enterotoxin (CPE), which binds to claudin (8) as a negative control, bound to Ni2+-charged resin. HeLa cell lysates (∼10 mg) were lysed with cold radioimmunoprecipitation assay (RIPA) buffer (1% Triton X-100; 50 mM Tris-HCl, pH 7.6; 400 μM NaVO4; 100 μg of phenylmethylsulfonyl fluoride/ml; 10 mM leupeptin) and diluted 10 times in binding buffer (5 mM imidazole; 500 mM NaCl; 20 mM Tris-HCl, pH 7.9). This lysate was loaded into the column and washed with binding buffer several times. Bound proteins were finally eluted with elution buffer (1 M imidazole; 500 mM NaCl; 20 mM Tris-HCl, pH 7.9). After the eluate had been dialyzed, it was concentrated 10 times by Centriplus (Amicon), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore). The membrane-bound protein was probed with the anti-cortactin antibody (Transduction Laboratories) at a dilution of 1:10,000, and it was developed by using horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG) antibody (Zymed) at a dilution of 1:10,000 and an ECL Western blotting detection kit (Amersham Pharmacia). The eluate from the His-T7-Tir column contained cortactin (80 to 85 kDa; Fig. 1b, lane B) similar to HeLa cell lysates (Fig. 1b, lane C). The eluate from the control column that retained His-CPE did not contain cortactin (Fig. 1b, lane A), indicating that cortactin specifically interacted with Tir (Fig. 1b, lane B). The protein profile of the eluate from the His-T7-Tir column was developed by silver staining with the two-dimensional silver stain DAIICHI kit (Daiichi Pure Chemicals, Tokyo, Japan) (Fig. 1b, lane D). Bands of ca. 83 kDa were detected as probable cortactin and recombinant Tir. His-T7-Tir was also confirmed by using anti-T7 antibody (Novagen) at a dilution of 1:10,000 (Fig. 1b, lane E).
We also used immunoprecipitation assays with an anti-cortactin antibody with EPEC-infected HeLa cells. After HeLa cells had been infected with EPEC, whole cells were washed with phosphate-buffered saline and lysed with RIPA buffer at 4°C. The samples were added to protein A/G plus agarose (Santa Cruz) to avoid nonspecific binding before the addition of the antibodies. After centrifugation to remove the protein A/G plus agarose, anti-cortactin antibody was added to the supernatant. To precipitate the immunocomplex, protein A/G plus agarose was added to the supernatant, and the mixture was centrifuged. The resulting precipitate was separated by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. Detection of Tir was performed with an anti-Tir antibody by Western blotting as described above. As shown in Fig. 1c, cortactin coimmunoprecipitated with Tir (Fig. 1c, right), and no signal appeared on membranes when noninfected HeLa cells were used (Fig. 1c, left). These results indicate that Tir interacts with cortactin during EPEC infection to HeLa cells.
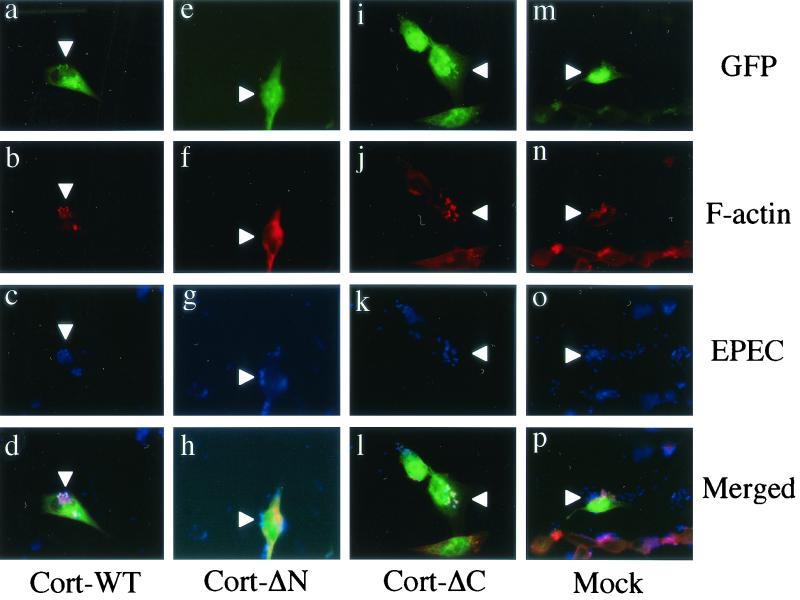
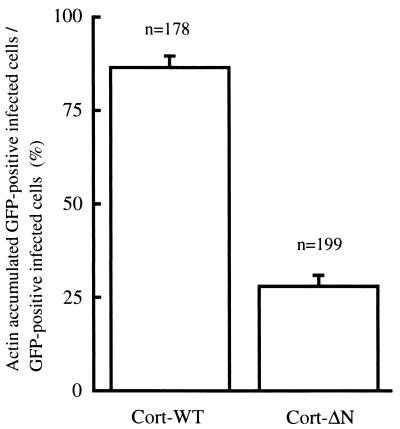
We further tried to determine the function of cortactin during pedestal formation by the expression of cortactin derivatives in HeLa cells. DNA fragments of wild-type cortactin (Cort-WT; 1 to 1,653 bp) and its deletion mutants (Cort-ΔN [904 to 1,653 bp], Cort-ΔC [1 to 964 bp]) were obtained by PCR amplification. Primer sets were designed as follows: Cort-WT, cort-1 (5′-GTCTAGAATGTGGAAAGCTTCAGCAGG-3′) and cort-2 (5′-GTCTAGACTACTGCCGCAGCTCCACAT-3′); Cort-ΔN, cort-3 (5′-GGTCGACTACTCCAA AGGATTCGGCGG-3′) and cort-4 (5′-GGGATCCCTACTGCCGCAGCTCCACAT-3′); and Cort-ΔC, cort-5 ( 5′-CGAATTCATGTGGAAAGCTTCAGCAGG-3′) and cort-6 (5′-GGTCGACACGCATTCTTATCCATCCGA-3′). Each PCR fragment, linked with a restriction enzyme digestion site (XbaI, XbaI, SalI, BamHI, EcoRI, and SalI, respectively), was cloned into the 3′ terminus of a green fluorescent protein (GFP) coding sequence in pEGFP-C1 (Clontech). After confirmation by DNA sequencing, the expression vectors were used to transfect HeLa cells by using Lipofectamine Plus reagent (Life Technologies) 2 days before infection. After the transfected HeLa cells were infected with EPEC, samples were subjected to immunofluorescence microscopy. F-actin was stained with rhodamine-phalloidin (Molecular Probes), and bacterial cells were visualized by using an anti-lipopolysaccharide rabbit antibody (anti-O111 antigen; Denkaseiken, Tokyo, Japan) and a secondary antibody, Alexa 350-labeled anti-rabbit IgG antibody (Molecular Probes). Cortactin derivatives were visualized by UV excitation of enhanced GFP. Immunofluorescent micrographs were acquired by using a fluorescence microscope (BX-50; Olympus) equipped with a cooled charge-coupled device camera. All images were processed in exactly the same manner by using Adobe Photoshop version 5.5 software. GFP-fused wild-type cortactin (Cort-WT) was observed to distribute diffusely into the cytoplasm of transfected HeLa cells. It behaved just like the endogenous cortactin, accumulating at the bacterial adherence site in HeLa cells exposed to EPEC (Fig. 2a to d). Pedestal formation was not affected in Cort-WT-expressing HeLa cells, as evaluated by the presence of the characteristic actin-rich structure (Fig. 2b) in the vicinity of the adhered bacteria (Fig. 2c). We then expressed various cortactin domains fused to GFP. Expression of the cortactin-deleted N terminus (Cort-ΔN), which contains the DDW (Asp-Asp-Trp) motif and tandem repeats for F-actin binding (11, 12, 13, 14), blocked F-actin accumulation in a dominant-negative manner (Fig. 2e to h). In infected HeLa cells expressing Cort-ΔN, few actin filaments were observed diffusely distributed in the area around the attached bacterium (Fig. 2e to h). Deletion of the C terminus of cortactin (Cort-ΔC), which contains a proline-rich domain and an src homology 3 (SH3) domain (14), was prepared, and the expression of Cort-ΔC in HeLa cells resulted in the localization of Cort-ΔC close to the bacterial adherence site (Fig. 2i to l). Inhibition of F-actin accumulation was not observed in HeLa cells expressing Cort-ΔC. HeLa cells expressing GFP alone by a mock vector were unable to recruit GFP to the sites that colocalized with bacteria and F-actin accumulation and demonstrated no inhibitory effects on F-actin accumulation (Fig. 2m to p). The inhibitory effect of actin accumulation caused by Cort-ΔN was analyzed by comparing the ratio of GFP-positive actin-nucleated infected cells per GFP-positive cells infected with EPEC by microscopic image analysis. As shown in Fig. 3, Cort-ΔN is shown to decrease the F-actin accumulation activity compared to that of Cort-WT (P < 0.005).
FIG. 2.
Immunofluorescence micrographs of EPEC-infected HeLa cells expressing cortactin derivatives. Expressed GFP-fused cortactin derivatives and GFP itself were visualized as green fluorescence (panels a, e, i, and m). F-actin was stained with rhodamine-phalloidin (red; panels b, f, j, and n), and bacterial cells were stained with anti-lipopolysaccharide and secondary antibody and Alexa 350-labeled anti-rabbit IgG antibody (blue; panels c, g, k, and o). Merged images are shown in panels d, h, l, and p. Arrowheads indicate the sites of bacteria attached to HeLa cells. Magnification, ×880.
FIG. 3.
Inhibitory effect of Cort-ΔN on actin accumulation in HeLa cells induced by EPEC infection. The inhibitory effect is indicated as a percentage based on the number of F-actin-accumulated GFP-positive infected HeLa cells compared to the total number of GFP-positive infected HeLa cells. The results are shown as means ± the standard deviations.
Cortactin is able to bind to F-actin through multiple sequences of an internal tandem repeat located at the N-terminal half of the molecule (14) and is thought to link the microfilaments to the cell membrane through proline-rich and SH3 domains present in the C-terminus portion of the molecule. A recent report demonstrated that the DDW motif, potentially conserved in WASP/Scar-1 family and Listeria ActA, in the N-terminal acidic region of cortactin binds to Arp2/3 complex (11, 12, 13) and that this interaction enhances the activity of actin polymerization of Arp2/3 complex and the developing branched actin filaments (11, 12). Our observations are consistent with the conclusions of these reports. First, only faint and vague F-actin accumulation could be observed beneath the attached bacteria in HeLa cells expressing Cort-ΔN, suggesting that the N-terminal region of cortactin may have a role in stimulating the actin nucleation activity of Arp2/3. Second, the inability of Cort-ΔN to stabilize F-actin clearly affects the formation of the compact actin-rich structure induced by EPEC. It is likely that Cort-ΔN defects to bind to the Arp2/3 complex, resulting in the inhibition of actin accumulation caused by EPEC. In a recent report (7), WASP (or N-WASP) was also reported as being necessary to form the actin pedestals induced by EPEC. However, this does not contradict our results, because binding of cortactin and WASP to Arp2/3 enhances Arp2/3 activity to polymerize F-actin in cooperation with each other (11, 12). In contrast, Cort-ΔC did not affect the F-actin accumulation, and this derivative was recruited to the site of adhered bacteria in HeLa cells (Fig. 2i to l). This result implied that the N terminus of cortactin is also necessary for the recruitment of cortactin itself; however, it is generally known that proline-rich and SH3 domains in the C terminus of cortactin have a role as the adapter to interact with another molecule. Accordingly, further experiments are indispensable and are now in progress to clarify these complex issues.
In summary, the data presented here demonstrate that interaction between cortactin and Tir plays an important role in the linkage and organization of F-actin in the EPEC-induced pedestals. Our results show that cortactin, especially its N terminus, plays an important role during EPEC-induced pedestal formation by binding the nascent actin filaments, drawing them close to the attached bacteria and forming the typical actin-rich structures that characterize the EPEC-induced pedestals.
Acknowledgments
This study was supported by a Grant-in-Aid from the Research for the Future Program of the Japan Society for the Promotion of Science (JSPS-RFTF 97L00704).
We thank K. Sano, T. Goto, and Y. Fujioka of the Osaka Medical College for helpful discussions and technical help. We also thank J. Katahira of the Research Institute for Microbial Diseases, Osaka University, for contributing the CPE. V.V.C. thanks the Laboratorio Weinmann for its support.
REFERENCES
- 1.Cantarelli, V. V., A. Takahashi, Y. Akeda, and T. Honda. 2000. Interaction of enteropathogenic or enterohemorrhagic Escherichia coli with HeLa cells results in translocation of cortactin to the bacterial adherence site. Infect. Immun. 68:382-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnenberg, M. S., J. B. Kaper, and B. B. Finlay. 1997. Interaction between enteropathogenic Escherichia coli and host epithelial cells. Trends. Microbiol. 5:109-114. [DOI] [PubMed] [Google Scholar]
- 3.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goosney, D. L., M. de Grado, and B. B. Finlay. 1999. Putting E. coli on a pedestal: a unique system to study signal transduction and the actin cytoskeleton. Trends. Cell Biol. 9:11-14. [DOI] [PubMed] [Google Scholar]
- 5.Goosney, D. L., R. DeVinney, R. A. Pfuetzner, E. A. Frey, N. C. Strynadka, and B. B. Finlay. 2000. Enteropathogenic E. coli translocated intimin receptor, Tir, interacts directly with alpha-actinin. Curr. Biol. 10:735-738. [DOI] [PubMed] [Google Scholar]
- 6.Hartland, E. L., M. Batchelor, R. M. Delahay, C. Hale, S. Matthews, G. Dougan, S. Knutton, I. Connerton, and G. Frankel. 1999. Binding of intimin from enteropathogenic Escherichia coli to Tir and host cells. Mol. Microbiol. 32:151-158. [DOI] [PubMed] [Google Scholar]
- 7.Kalman, D., O. D. Weiner, D. L. Goosney, J. W. Sedat, B. B. Finlay, A. Abo, and J. M. Bishop. 1999. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat. Cell Biol. 1:389-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katahira, J., N. Inoue, Y. Horiguchi, M. Matsuda, and N. Sugimoto. 1997. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J. Cell Biol. 136:1239-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 10.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, S. Scotman, and B. Rowe. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxin and are non-invasive. Lancet i:1119-1122. [DOI] [PubMed] [Google Scholar]
- 11.Uruno, T., J. Liu, P. Zhang., Y. Fan, C. Egile, R. Li, S. C. Mueller, and X. Zhan. 2001. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 3:259-266. [DOI] [PubMed] [Google Scholar]
- 12.Weaver, A. M., A. V. Karginov, A. W. Kinley, S. A. Weed, Y. Li, J. T. Parsons, and J. A. Cooper. 2001. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11:370-374. [DOI] [PubMed] [Google Scholar]
- 13.Weed, S. A., A. V. Karginov, D. A. Schafer, A. M. Weaver, A. W. Kinley, J. A. Cooper, and J. T. Parsons. 2000. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 151:29-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu, H., and J. T. Parsons. 1993. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J. Cell Biol. 120:1417-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]