Abstract
Although Helicobacter pylori has generally been considered an extracellular pathogen, a number of in vitro infection experiments and biopsy examinations have shown that it is capable of occasionally entering mammalian host cells. Here, we characterized this entry process by using AGS cells as a host cell model. In gentamicin protection-invasion assays, the number of H. pylori colonies recovered was lower than that for Salmonella enterica serovar Typhimurium X22, Escherichia coli expressing InvA, and Yersinia enterocolitica YO:9 grown at 25°C but higher than that for Neisseria gonorrhoeae VP1 and Y. enterocolitica YO:9 grown at 37°C. At the ultrastructural level, the entry process was observed to occur via a zipper-like mechanism. Internalized H. pylori was bound in tight LAMP-1-containing vacuoles in close association with condensed filamentous actin and tyrosine phosphorylation signals. Wortmannin, a potent inhibitor of phosphatidylinositol 3-kinase, and calphostin C, an inhibitor of protein kinase C, both inhibited the entry of H. pylori in a sensitive and dose-dependent manner; however, the level of entry was enhanced by sodium vanadate, an inhibitor of tyrosine phosphatases and ATPases. Furthermore, the cytokine tumor necrosis factor alpha antagonized the entry of H. pylori into AGS cells. Collectively, these results demonstrate that the entry of H. pylori into AGS cells occurs via a zipper-like mechanism which involves various host signal transduction events.
Helicobacter pylori is a gram-negative microaerophilic rod first isolated from human gastric biopsy specimens (53). Once acquired, H. pylori can persist for life (11, 15, 16). Infections caused by H. pylori are associated with chronic superficial gastritis, characterized by inflammation in the gastric mucosa. At a later stage, the infection can lead to chronic active gastritis, which shows the typical feature of infiltration of polymorphonuclear cells (14). Decades of colonization by H. pylori may lead to gastric adenocarinoma and gastric mucosa-associated lymphoid tissue lymphoma (34, 60, 83, 84). At the cellular level, H. pylori infection has been shown to induce the secretion of proinflammatory cytokines, cell proliferation, and apoptosis of epithelial cells (7, 36, 59). An understanding of the strategies by which H. pylori persists in the gastric epithelium and the mechanisms underlying the host responses induced is thus of crucial importance to better understanding the pathogenesis of H. pylori infections.
The exact location in which H. pylori persists and induces host responses in the gastric epithelium has been a subject of debate. Early reports indicated that H. pylori was not present in the gastric mucosa but was present in the mucus layer overlying the gastric tissue (52, 53, 56). However, in recent years, a number of biospy studies (28, 30, 62) and cell culture infection models (19, 31, 50, 64, 73, 79, 85) have provided increasing evidence for the intracellular localization of H. pylori. Clearance of intracellular H. pylori has been demonstrated in vitro by using antibiotics with known intracellular activity (40). Furthermore, professional phagocytes appear to be unable to eradicate the pathogen during inflammation and polymorphonuclear cell infiltration (4, 47, 65), supporting the notion that H. pylori can evade host immune responses by intracellular penetration of the mucosal epithelium. It has been proposed that the intracellular environment can serve as a reservoir for H. pylori to repopulate the stomach and to cause persistent infections despite antimicrobial therapy (30, 40).
The molecular mechanisms involved in host cell entry vary widely from one pathogen to another (33). For example, initial engulfment of Yersinia, Neisseria, Listeria, and Streptococcus occurs via zipper-like receptor-mediated endocytosis (27, 32, 55), which is characterized by invagination of the host cell membrane at the site of bacterial attachment to the extent that the host cell membrane zips up around the entire surface of the bacterium (44). Enteropathogenic Escherichia coli (EPEC) is thought to be internalized by a similar mechanism subsequent to the formation of an adherence pedestal (6, 71). In contrast, engulfment of Salmonella and Shigella occurs by macropinocytosis, involving the formation of host cell membrane ruffles (32, 35). While Yersinia can utilize the host β1-integrin receptor as a receptor for induced uptake (43), EPEC translocates its own receptor into the host cell membrane for specific entry into epithelial cells (46). Furthermore, to promote their own uptake, pathogens often have developed a series of strategies to exploit host cell functions, including cytoskeletal rearrangement and signal transduction (37, 71). For example, invasion by Yersinia requires tyrosine kinase activities (70), invasion by Salmonella of epithelial cells is dependent on the activity of phospholipase C (33), and internalized Shigella is able to induce the assembly of actin tails to facilitate its subsequent cell-to-cell spread (13). Recently, it was demonstrated that Listeria facilitates its invasion into HeLa cells by activating host cell phosphatidylinositol 3-kinase (PI 3-kinase), a kinase shown to be important for phagocytosis, activation of the small GTPase Rac, and cytoskeletal rearrangement (41).
In contrast, the molecular mechanisms involved in in vitro host cell entry by H. pylori are still poorly understood. Previous findings indicated that H. pylori enters HEp-2 cells by receptor-mediated endocytosis requiring cytoskeletal rearrangement (31), whereas the entry of H. pylori into cultured gastric adenocarcinoma cells (AGS cells) has been shown to occur via the β1-integrin receptor in a tyrosine kinase-dependent manner (79). In this study, by using a variety of techniques, including the gentamicin protection assay, confocal laser scanning microscopy (CLSM), and electron microscopy, we further demonstrate that the entry of H. pylori into the model cell line AGS involves an intimate interaction between the pathogen and the host cell. The process occurs via a zipper-like mechanism reminiscent of that involved in the invasion of cultured cells by Yersinia. Internalized H. pylori was shown to be located in tight phagosomes and in close association with condensed actin filaments and localized tyrosine phosphorylation signals. Our data suggest that the entry of H. pylori into AGS cells requires protein kinase C (PKC) and PI 3-kinase but is inhibited by tumor necrosis factor alpha (TNF-α) as well as phosphatases and/or ATPases. Overall, our findings indicate that the entry of H. pylori into cultured gastric epithelial cells is a specific and multifactorial process reminiscent of the induced penetration of host cells observed with other invasive pathogens.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The type I H. pylori strains P1 and P12 are cagA+ clinical isolates from patients with nonulcer dyspepsia and duodenal ulcer, respectively (10, 67). These two strains are capable of translocating CagA and inducing the phosphorylation of CagA in the host cell (9, 10, 63). The previously entirely sequenced type I H. pylori strains 26695 (80) and J99 (2) were obtained from The Institute for Genomic Research and AstraZeneca, respectively. Although both 26695 and J99 are cagA+, they appear to lack the ability to induce the phosphorylation of CagA in the host cell (10, 63). P49 is a mouse-adapted strain which has been described previously (67). All Helicobacter strains were grown on agar plates supplemented with 10% fetal calf serum (FCS) (Biochrom KG, Berlin, Germany), vitamin mix, 1 μg of nystatin/ml, 5 μg of trimethoprim/ml, and 10 μg of vancomycin/ml under microaerobic conditions in the presence of 5% O2, 10% CO2, and 85% N2 at 37°C for 2 days. Neisseria gonorrhoeae VP1 (82) and Yersinia enterocolitica YO:9 (39) have been described elsewhere. E. coli H2356 (invA+) is an E. coli DH5α strain carrying a plasmid that expresses the Yersinia invasin InvA. Salmonella enterica serovar Typhimurium strain X22 is a Salmonella serovar Typhimurium Ty21A galE human-adapted strain provided by Paul Manning (University of Adelaide, Adelaide, South Australia, Australia). Strain X22 was grown on Luria-Bertani (LB) agar at 37°C in 5% CO2 overnight, whereas Y. enterocolitica YO:9 was grown on LB agar at either 37 or 25°C in 5% CO2 overnight. Y. enterocolitica YO:9 was grown at 37°C when the expression of the antiphagocytic Yop proteins was required for inhibiting host cell entry (5), whereas the bacterium was grown at 25°C when repression of the expression of the Yop proteins was required for promoting host cell entry. N. gonorrhoeae VP1 and E. coli H2356 (invA+) were grown on gonococci (GC) agar and LB agar supplemented with 30 μg of chloramphenicol/ml, respectively, at 37°C in 5% CO2 overnight.
Infection of host cells with H. pylori.
For infection assays, AGS cells (ATCC CRL 1739, human gastric adenocarcinoma epithelial cell line), HEp-2 cells (human laryngeal carcinoma cell line), or HeLa cells were seeded to give 3.2 × 105 cells in RPMI medium supplemented with 5% FCS (Gibco BRL, Eggenstein, Germany) per well in 24-well tissue culture plates. The plates were incubated at 37°C in 5% CO2 for 18 h. The culture medium was replaced with fresh RPMI medium without FCS 2 h before the inoculation of bacteria. Bacteria were resuspended in brain heart infusion medium, and 20-μl aliquots of the bacterial suspension (1.6 × 109 per ml) were added to host cells to achieve a multiplicity of infection (MOI) of 100. As a control, 20 μl of brain heart infusion medium was added to host cells. Infection was carried out at 37°C in 5% CO2 for 3 h unless otherwise stated.
TEM and SEM.
Coverslips containing infected cultures were fixed first in 700 μl of 3.8% paraformaldehyde (PFA) preequilibrated to 37°C for 15 min and subsequently in 700 μl of ice-cold 2% glutaraldehyde. For transmission electron microscopy (TEM), the glutaraldehyde-fixed samples were postfixed in 1% osmium tetroxide in phosphate buffered saline (PBS) for 1 h on ice, rinsed with double-distilled water, and then treated with 1% aqueous uranyl acetate for 1 h at 4°C. Samples were dehydrated through a graded series of ethanol and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and viewed in a Philips CM10 electron microscope. For scanning electron microscopy (SEM), glutaraldehyde-fixed cells were postfixed with 1% osmium tetroxide in PBS, dehydrated in ethanol, and dried at the critical point in CO2. The samples were sputter coated with 8-nm gold-palladium and examined at a 20-kV accelerating voltage in a Hitachi S-800 field emission scanning electron microscope.
Inhibitor assays.
For uptake inhibition studies, AGS cells were pretreated with 0 to 300 μM sodium ortho-vanadate (Calbiochem, Bad Soden, Germany), 0 to 600 nM calphostin C (Sigma, Deisenhofen, Germany), 0 to 3 μM wortmannin (Calbiochem), 0 to 100 μM Genistein (Calbiochem), or 0 to 20 ng of TNF-α (R&D Systems, Minneapolis, Minn.)/ml for 15 min at 37°C in 5% CO2. Subsequent infection with H. pylori was carried out as described above. After infection, AGS cells were washed three times with warm RPMI medium to remove nonadherent bacteria and then were fixed with 3.8% paraformaldehyde in PBS. The fixed cell samples were subjected to immunofluorescence (IF) staining for visualization of extracellular and intracellular H. pylori, filamentous actin, and lysosomal marker LAMP-1 by using the procedures described below.
IF labeling and CLSM.
IF labeling of infected host cells was performed at room temperature according to a protocol described elsewhere (25). Specimens were fixed in 2% paraformaldehyde at room temperature for at least 15 min. All antibodies were diluted in PBS-0.2% bovine serum albumin, and all incubations with antibodies were carried out for 1 h. H. pylori was labeled by using a rabbit polyclonal antibody against H. pylori (V4074; Sigma) diluted 1:20. Filamentous actin in the host cell was labeled with fluorescein isothiocyanate (FITC)-conjugated phalloidin (Sigma) diluted 1:100. Phagosomes were labeled by using a mouse antibody against the human lysosomal marker LAMP-1 (H4A3; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City) diluted 1:100. Tyrosine phosphorylation was detected by using a mouse antiphosphotyrosine antibody (PY99; Santa Cruz Biotechnology, Santa Cruz, Calif.) diluted 1:1,000.
For differential staining of intracellular and extracellular bacteria, fixed cells were treated as described in the following steps: (i) washed three times with PBS for 5 min each time, (ii) incubated with PBS-0.2% bovine serum albumin for 15 min to block nonspecific binding, (iii) incubated with antibody V4074, (iv) incubated with 1:100-diluted Texas red (TR)-conjugated goat anti-rabbit immunoglobulin (IgG) antibody, (v) incubated with 0.1% Triton X-100 for 15 min to permeabilize the mammalian cell membrane, (vi) incubated with antibody V4074, and (vii) incubated with 1:100-diluted Cy5-conjugated goat anti-rabbit IgG antibody mixed with FITC-conjugated phalloidin. For differential staining of intracellular bacteria, extracellular bacteria, and human LAMP-1, fixed cells were treated with similar procedures except that the specimens were incubated in step iv with FITC-conjugated goat anti-rabbit IgG antibody, in step vi with both V4074 and H4A3, and in step vii with both Cy5-conjugated goat anti-rabbit IgG antibody and TR-conjugated goat anti-mouse IgG antibody. For differential staining of intracellular bacteria, extracellular bacteria, and phosphotyrosine, the specimens were incubated with 0.1% Triton X-100, with V4074, and with Cy5-conjugated goat anti-rabbit IgG antibody. The specimens were then probed with antibody PY99 and incubated with both TR-conjugated goat anti-mouse IgG antibody and FITC-conjugated phalloidin. IF-labeled samples were analyzed by using a TCS NT confocal laser scanning microscope equipped with an argon-krypton mixed-gas laser source (Leica Lasertechnik, Heidelberg, Germany). In all experiments, a minimum of 90 cells were examined per sample.
Gentamicin protection assay.
Infection of AGS cells at a density of 3.2 × 105 AGS cells per well was carried out as described above. After infection, the AGS-bacterium coculture was washed three times with 1 ml of warm RPMI medium per well to remove nonadherent bacteria. To determine the CFU corresponding to intracellular bacteria, the AGS cell monolayers were treated with gentamicin (200 μg/ml; Sigma) at 37°C in 5% CO2 for 1 h, washed three times with warm RPMI medium, and then incubated with 1 ml of 0.1% saponin in PBS at 37°C for 15 min. The treated monolayers were resuspended thoroughly, diluted, and plated on serum agar (Helicobacter), LB agar (Yersinia and Salmonella), or GC agar (Neisseria). To determine the total CFU corresponding to host-associated bacteria, the infected monolayers were incubated with 1 ml of 0.1% saponin in PBS at 37°C for 15 min without prior treatment with gentamicin. The resulting suspensions were diluted and plated as described above. Both the CFU of intracellular bacteria and the total CFU of cell-associated bacteria are given as CFU per well of AGS cells.
RESULTS
H. pylori enters AGS cells at a level lower than that of Salmonella and Escherichia expressing InvA but higher than that of Neisseria.
Despite increasing evidence supporting the entry of H. pylori into epithelial cells both in vivo and in vitro, evidence on the relative invasiveness of H. pylori in comparison with that of other commonly known invasive pathogens is scarce. To address this question, AGS cells were infected in parallel with H. pylori, Salmonella serovar Typhimurium, Y. enterocolitica, N. gonorrhoeae, and E. coli H2356 expressing the Yersinia invasin InvA for 6 h at an MOI of 100. The extent to which the various pathogens entered AGS cells was assessed by quantitation of viable adherent and intracellular bacteria by using the gentamicin protection assay. The results shown in Table 1 demonstrate that the number of gentamicin-resistant CFU of H. pylori 26695 obtained was lower than that for Salmonella serovar Typhimurium X22, E. coli H2356, and Y. enterocolitica Y:O9 grown at 25°C but was higher than that for N. gonorrhoeae VP1 and Y. enterocolitica Y:O9 grown at 37°C. These results indicated that H. pylori 26695 exhibits an invasiveness higher than that of the invasive N. gonorrhoeae strain VP1 and Y. enterocolitica Y:O9 grown at 37°C but lower than that of the highly invasive Salmonella serovar Typhimurium X22, E. coli H2356, and Y. enterocolitica Y:O9 grown at 25°C. When the ratios of the percentages of internalized CFU and total cell-associated CFU were compared for the various pathogens, the percentage of internalization shown by H. pylori was found to be lower than that shown by E. coli H2356 but higher than that shown by Salmonella serovar Typhimurium X22, Y. enterocolitica YO:9, and N. gonorrhoeae VP1 (Table 1). These findings suggest that both the level and the efficiency with which H. pylori enters AGS cells are comparable to those exhibited by other invasive bacteria.
TABLE 1.
Comparison of extent of entry into AGS cells for H. pylori and other commonly known invasive pathogensa
| Organism | No. of CFU/well
|
% Internalizationb | |
|---|---|---|---|
| Total cell associated (106) | Gentamicin resistant (104) | ||
| H. pylori 26695 | 1.4 ± 0.4 | 3.5 ± 0.03 | 2.5 ± 0.8 |
| E. coli H2356 (invA+) | 43 ± 5.8 | 700 ± 120 | 16 ± 5 |
| S. typhimurium X22 | 7.0 ± 0.4 | 12 ± 3 | 1.7 ± 0.5 |
| Y. enterocolitica YO:9 (grown at 25°C) | 21 ± 4 | 15 ± 3.4 | 0.7 ± 0.3 |
| Y. enterocolitica YO:9 (grown at 37°C) | 19 ± 0.5 | 2.1 ± 0.2 | 0.1 ± 0.01 |
| N. gonorrhoeae VP1 | 3.1 ± 0.9 | 0.75 ± 0.2 | 0.24 ± 0.15 |
Bacteria were added to AGS cell monolayers at an MOI of 100 for 6 h at 37°C in a humidified atmosphere supplemented with 5% CO2. Data were obtained in triplicate from gentamicin protection assays in three independent experiments and are expressed as CFU per well of AGS cells (mean and standard deviation) (see Materials and Methods).
Ratio of the number of gentamicin-resistant CFU per well to the total number of cell-associated CFU per well (mean and standard deviation).
Entry of H. pylori is both strain and host cell dependent.
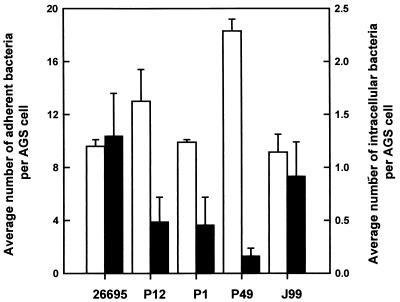
To further characterize the internalization of H. pylori by mammalian host cells, the abilities of different H. pylori strains to enter AGS cells were compared. Infection of AGS cells was carried out in parallel by using various H. pylori strains at an MOI of 100 for 3 h at 37°C. The levels of internalization were determined by differential IF staining in combination with CLSM, a technique that enables extracellular and intracellular H. pylori, both viable and nonviable, to be quantitated by microscopy and thus allows quantitation of the level of host cell entry in a manner independent of bacterial viability. The results shown in Fig. 1 indicate that H. pylori strains 26695 and J99 both adhered to and entered AGS cells to similar extents. In contrast, strains P1, P12, and P49 were internalized at a lower level despite a level of adherence similar to (strain P1) or even higher than (strains P12 and P49) that shown by strain 26695. This spectrum of invasiveness indicated that H. pylori entry into AGS cells is a strain-dependent process, suggesting that bacterial determinants are involved in modulating the level of uptake.
FIG. 1.
Strain-to-strain variation in the frequency of host cell entry. Confluent AGS cell monolayers were infected with the various strains of H. pylori at an MOI of 100 for 3 h at 37°C. Intracellular (black bars) and adherent (white bars) bacteria were detected by IF labeling. AGS cells were stained for F actin. Quantitation of intracellular and adherent H. pylori was performed by CLSM. Data were obtained in triplicate from at least two independent experiments. Error bars indicate standard deviations.
To investigate whether host cells also play a role in governing the invasiveness of H. pylori, three adherent epithelial cells lines, AGS, HeLa, and HEp-2, were infected in parallel with H. pylori 26695. The postinfection cultures were subjected to differential IF staining for the quantitation of intracellular and extracellular H. pylori. Table 2 shows that while H. pylori adhered equally well to all three cell lines, the level of entry was significantly higher with AGS cells than with HEp-2 or HeLa cells. These results clearly indicated that host cells also play a significant role in determining the level of H. pylori entry. Taken together, these findings suggest that the in vitro host cell entry of H. pylori is a multifactorial process requiring contributions from both the pathogen and the host cell.
TABLE 2.
Comparison of the level of internalization of H. pylori by AGS, HeLa, and HEp-2 cellsa
| Host cell line | No. of bacteria/host cell
|
|
|---|---|---|
| Adherent | Intracellular | |
| AGS | 18 ± 2 | 1.3 ± 0.55 |
| HeLa | 18 ± 1.5 | 0.05 ± 0.02 |
| HEp-2 | 18 ± 6.8 | 0.05 ± 0.05 |
Bacteria were added to cell monolayers at an MOI of 100 for 3 h at 37°C in a humidified atmosphere supplemented with 5% CO2. Quantitation of adherent and intracellular bacteria was performed in triplicate by CLSM; data are expressed as mean and standard deviation.
Ultrastructural analysis of attachment, engulfment, and internalization of H. pylori with AGS cells.
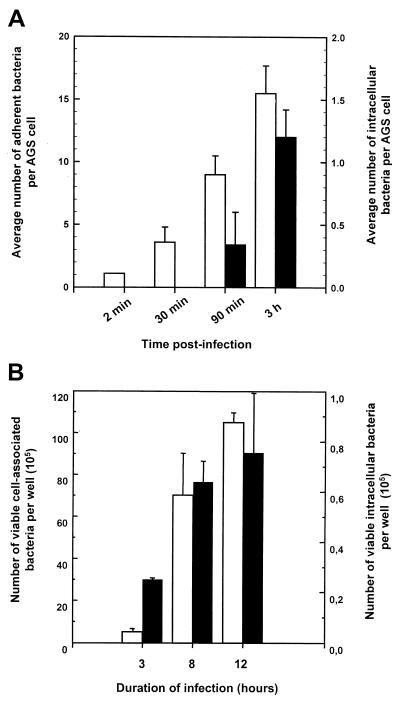
As a first step toward understanding the molecular mechanism of the interaction between H. pylori and AGS cells, samples obtained at various times after infection of AGS cultures were examined at the ultrastructural level by both SEM and TEM. The time course of this investigation was designed based on the kinetics of the internalization of H. pylori by AGS cells as characterized by both the gentamicin protection assay and CLSM (Fig. 2). The data obtained highlight two interesting features. First, the extent of adherence and internalization of H. pylori by AGS cells increased continuously for at least 12 h (Fig. 2B). Second, while adherence occurred rapidly after inoculation, no intracellular bacteria were detected at 30 min, whereas increasing numbers of intracellular bacteria were found at 90 min and later (Fig. 2A). Based on these observations, we decided to examine the ultrastructures of the infected AGS cell monolayers at 30 min, 90 min, 3 h, and 12 h postinfection.
FIG. 2.
Kinetics of entry of H. pylori into AGS cells. (A) Time course of infection during the first 3 h of infection of AGS cells with H. pylori 26695 at an MOI of 100. Intracellular (black bars) and adherent (white bars) H. pylori 26695 bacteria were quantitated as described in the legend to Fig. 1. (B) Recovery of viable intracellular bacteria during 24 h of infection. The number of CFU of viable cell-associated adherent (white bars) and intracellular (black bars) H. pylori at the various time points was determined by the gentamicin protection assay. Data were obtained in triplicate from at least three independent experiments and are presented as the number of viable cell-associated bacteria or the number of viable intracellular bacteria per well of AGS cells. Error bars indicate standard deviations.
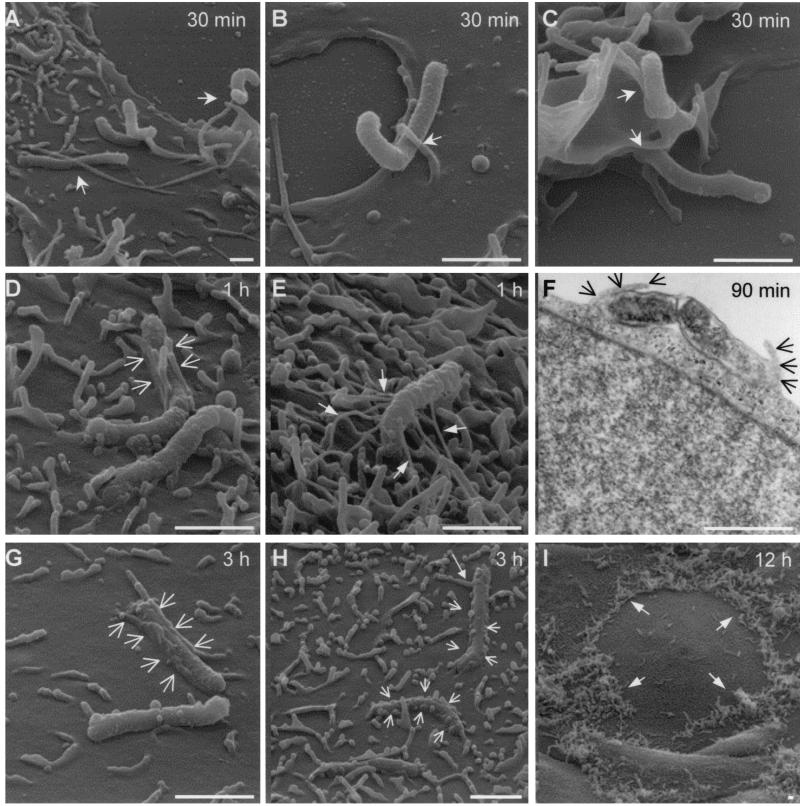
SEM showed that intimate contact between H. pylori and the host cell plasma membrane had been established 30 min postinfection, with the majority of adherent bacteria binding lengthwise to the host cell surface (Fig. 3A). Contact was seen mostly between the bacteria and the host cell microvilli (Fig. 3A and B), although an interaction of attached H. pylori with ruffle-like structures formed by the host cell membrane, particularly on the cell periphery, was also common (Fig. 3C). In addition, some microvilli in contact with H. pylori seemed to have extended beyond their normal length; it was not clear whether this effect was a consequence or a prerequisite of the bacterial contact (Fig. 3A).
FIG. 3.
SEM and TEM analyses of H. pylori 26695 adherence to and entry into AGS cells. AGS cells were infected with H. pylori 26695 at an MOI of 100 for 12 h at 37°C. Samples obtained at various times postinfection were analyzed by SEM and TEM. H. pylori adhered to AGS cells by intimate contact with the host cell microvilli (A and B, arrows) and pseudopods (C, arrows). Fibrillar structures connecting adherent H. pylori to AGS cells were occasionally seen (E, arrows). Features of zipper-like engulfment (D, F, G, and H, arrows) were observed at 1 to 3 h postinfection, whereas adherence to the host cell periphery was prominent by 12 h postinfection (I, arrows). Bars, 1.5 μm.
A more extensive interaction between H. pylori and the host cell was seen after 60 min of coculturing. The host cell microvilli or protrusions established close contact with the bacteria, and these host structures appeared to have wrapped around the rod-shaped H. pylori (Fig. 3D, arrows), reminiscent of bacterial phagocytosis occurring via a zipper-like mechanism. Supporting data obtained from TEM provided a cross-sectional view of the engulfment process (Fig. 3F). Intriguingly, fibrillar structures similar to those observed in biopsy samples described previously (28) were found in the vicinity of H. pylori (Fig. 3E). The nature and origin of these structures, however, remain unclear.
Engulfment of H. pylori in a zipper-like manner was even more frequently observed in the 3-h samples (Fig. 3G and H, short arrows), with the host cell membrane wrapping around almost the entire length of the H. pylori rod. Interestingly, some long-range interactions between H. pylori and host cell membrane protrusions were also detected (Fig. 3H, long arrow). Low-magnification micrographs revealed that by 12 h postinfection, a large number of H. pylori organisms had already adhered to AGS cells, predominantly at the cell periphery or near cell-cell junctions (Fig. 3I). In contrast to previous observations indicating that the bacteria turned from spiral to coccoid form within hours of attachment to AGS cells (73), our data showed that more than 80% of the attached H. pylori organisms remained spiral at 12 h postinfection.
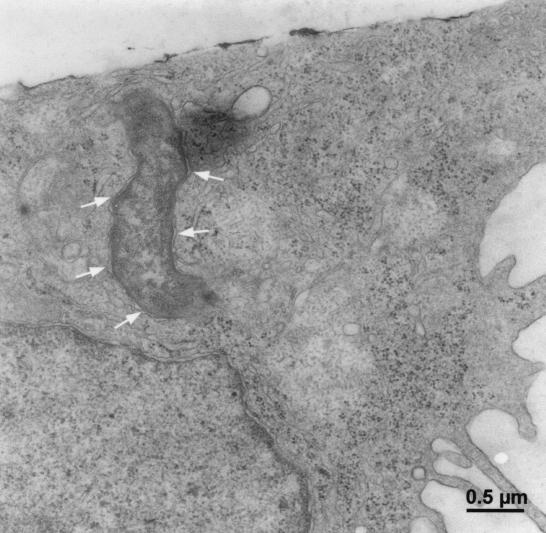
The internalization of H. pylori was confirmed by TEM, which showed the presence of intact H. pylori in the host cell cytoplasm (Fig. 4). While the internalized H. pylori organisms were often found in tight vacuoles, most of the internalized bacteria showed no obvious signs of degradation at up to 12 h postinfection. In contrast to some in vivo reports suggesting that the attachment of H. pylori is associated with pedestal formation (14, 73, 77), no classical adherence-induced pedestal formation (as known for EPEC) was observed in vitro in this study.
FIG. 4.
Transmission electron micrograph showing the intracellular location of H. pylori 26695 in AGS cells 3 h postinfection. AGS cells were infected with H. pylori 26695 at an MOI of 100 for 1.5 h at 37°C. Samples were fixed and subsequently analyzed by TEM. The phagosomal membrane is indicated by arrows.
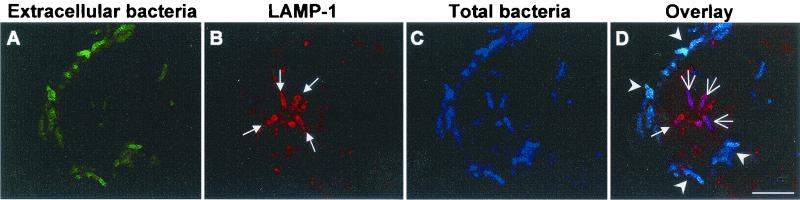
Internalized H. pylori was localized in late-endosome-like LAMP-1-containing vacuoles.
At 3 h postinfection, H. pylori was found in LAMP-1-containing vacuoles inside the host cytosol, as analyzed by CLSM (Fig. 5). LAMP-1 is a lysosomal marker protein, and this observation indicates that the initial H. pylori-containing vacuoles underwent fusion with lysosomes to form phagolysosomes. Phagolysosomes containing H. pylori could be readily detected in perinuclear positions even at 12 h postinfection (data not shown). While the intraphagosomal environment remains to be investigated in more detail, the phagosomal location of intracellular H. pylori is in line with the previous hypothesis that H. pylori uptake occurs via receptor-mediated endocytosis (31). Furthermore, these results reveal that internalized H. pylori neither escapes into the host cell cytoplasm nor prevents the fusion of phagolysosomes within at least the first 12 h of infection.
FIG. 5.
Confocal laser scanning micrograph showing the localization of H. pylori in LAMP-1-containing phagosomes 3 h postinfection. AGS cell monolayers were infected with H. pylori 26695 at an MOI of 100 for 3 h at 37°C. Samples were then fixed and subjected to triple IF labeling. Total H. pylori was stained blue (C). Extracellular H. pylori was stained green (A) and appeared light blue in the overlay (D, arrowheads). The human LAMP-1 protein was stained red (B and D, small arrows). Intracellular H. pylori which colocalized with the LAMP-1-containing phagosomes appeared magenta (D, large arrows). Bar, 10 μm.
Internalized H. pylori is associated with condensed actin filaments and protein tyrosine phosphorylation in host cells.
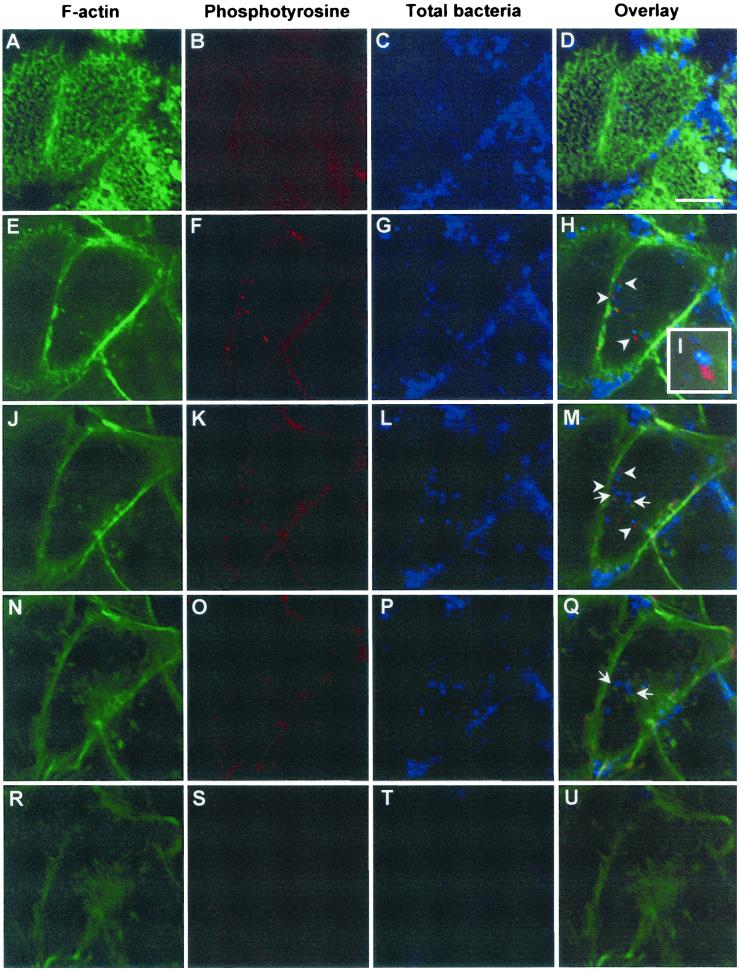
Consistent with the formation on the host cell membrane of pseudopods characteristic of significant cytoskeletal rearrangement during the engulfment of H. pylori, as revealed by TEM and SEM, IF staining with FITC-conjugated phalloidin, a dye which binds specifically to filamentious actin, showed condensation of actin filaments around and beneath the adherent bacteria (data not shown). This result is consistent with previous observations (73). However, in addition to the adherence-associated actin rearrangement, we observed that a significant proportion of intracellular H. pylori organisms was also closely associated with condensed patches of host filamentous actin (Fig. 6M, arrows). Very often, intracellular H. pylori colocalized with these condensed actin patches (Fig. 6Q, left arrow). This finding is, to our knowledge, the first report of actin condensation in close association with internalized H. pylori.
FIG. 6.
CLSM analysis of the spatial relationship among intracellular H. pylori, host actin cytoskeleton, and protein phosphotyrosine signals. AGS cells were infected with H. pylori 26695 at an MOI of 100 for 3 h at 37°C, washed, and fixed with PFA. Specimens were stained for F actin (A, E, J, N, and R), for phosphotyrosine (B, F, K, O, and S), and for H. pylori (C, G, L, P, and T). Images were obtained in various focal planes (panels in rows) by scanning from the apex to the basal side of the host cell (panels in columns, from top to bottom). The proximity between bacteria and phosphotyrosine signals is indicated by arrowheads in the overlay of the three channels (D, H, M, Q, and U) and in enlargement in inset I, whereas the close association of bacteria with F actin can be seen. The orange color indicates colocalization of F actin with phosphotyrosine (M and Q, arrows). Bar, 10 μm.
We subsequently examined whether this actin rearrangement is associated with host signal transduction processes such as tyrosine phosphorylation events. When infected AGS cells were examined by CLSM with an antiphosphotyrosine antibody, tyrosine phosphorylation patterns were found to be located not only at the typical focal adhesion sites at the end of the stress fibers on the edge of host cells but also in close proximity to intracellular H. pylori (Fig. 6H, I, and M, arrowheads). Tyrosine phosphorylation patterns often colocalized with the condensed actin patches near intracellular H. pylori. In a few instances, the intracellular bacteria appeared to be surrounded by a ring of tyrosine phosphorylation signal (data not shown). Since tyrosine-phosphorylated proteins were often detected in conjunction with the condensation of actin, we speculate that the phosphorylation event and the actin rearrangement observed may be functionally or structurally linked. These signals do not correspond to phosphorylated bacterial CagA proteins, because both wild-type H. pylori and an isogenic P1 ΔcagA mutant showed similar effects. Our findings clearly demonstrated that the internalization of H. pylori induces both signal transduction and cytoskeletal rearrangement in the host. It remains to be determined whether these host responses are consequences of the uptake per se or are cellular responses induced by intracellular H. pylori from within the phagolysosome.
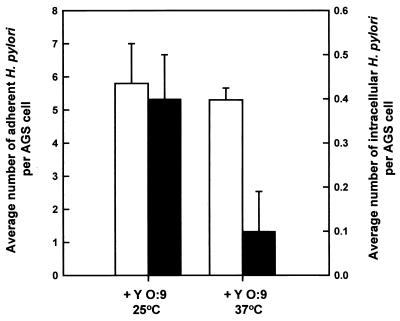
Entry of H. pylori can be blocked by Yersinia (grown at 37oC).
When grown at 37°C, Yersinia has been shown to express a series of Yop proteins which, upon contact with the host cell, translocate into the host cell cytoplasm and perturb host signal transduction; the outcome of this event is inhibition of phagocytosis by either phagocytic cells or nonphagocytic epithelial cells (5, 23, 78). We thus decided to use Yersinia as a tool to further test our hypothesis that H. pylori invasion involves host signal transduction. AGS cell monolayers were preinfected with either Y. enterocolitica YO:9 grown at 37°C or Y. enterocolitica YO:9 grown at 25°C at an MOI of 50 for 1 h. The step was followed by incubation with H. pylori at an MOI of 100 for an additional 3 h. Postinfection samples were fixed and then stained with H. pylori-specific antibodies for the quantitation of extracellular and intracellular H. pylori. Figure 7 shows that preinfection of AGS cells with Y. enterocolitica YO:9 grown at 37°C resulted in a reduced number of intracellular H. pylori organisms, whereas preinfection with Y. enterocolitica YO:9 grown at 25°C did not. The fact that we were still able to detect a significant level of invasion with Y. enterocolitica YO:9 grown at 25°C argues against the possibility that the reduction in H. pylori entry observed in the presence of Y. enterocolitica YO:9 grown at 37°C was due to sequestration of adherence sites. The above results indicate that the mechanism of H. pylori entry into AGS cells involves one or more of the host signaling steps targeted by the Yersinia Yop proteins.
FIG. 7.
Effects of preinfection of Y. enterocolitica YO:9 grown at either 37or 25°C on the level of H. pylori entry into AGS cells. AGS cells were first infected with Y. enterocolitica YO:9 grown at 37 or 25 oC for 1 h at an MOI of 50 and then infected with H. pylori 26695 at an MOI of 50 for 3 h. Intracellular (black bars) and adherent (white bars) bacteria were detected by IF labeling as described in the text. Intracellular and adherent H. pylori bacteria were quantitated by CLSM. Error bars indicate standard deviations.
Entry requires PI 3-kinase and PKC activities but is inhibited by tyrosine phosphatase(s).
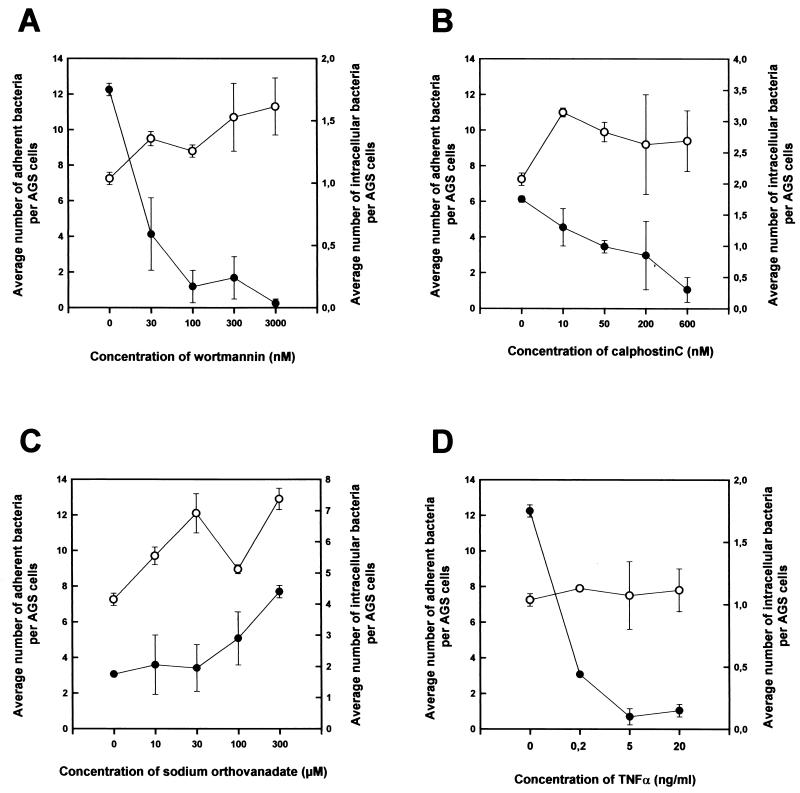
To pinpoint the host signaling components important for H. pylori entry, we examined the effects of a series of inhibitors of eukaryotic cellular components on the frequency of H. pylori internalization. AGS cells were treated with the inhibitor of interest for 15 min at 37°C. H. pylori was then added to the pretreated cells, after which infection was carried out for 3 h at 37°C. This step was followed by assessment of the internalization frequency by IF staining. The concentrations of the inhibitors used were confirmed by light microscopy and CLSM to exert no significant effect on either the morphology of the host cells or the level of H. pylori adherence (Fig. 8). The viability of the host cells was confirmed by trypan blue staining (data not shown).
FIG. 8.
Effect of wortmannin, calphostin C, sodium ortho-vanadate, or TNF-α on the level of H. pylori entry into AGS cells. Wortmannin (A), calphostin C (B), sodium ortho-vanadate (C), or TNF-α (D) was added to AGS cultures at the appropriate concentration, and the cultures were incubated at 37°C for 15 min. H. pylori 26695 was then added to the cell cultures at an MOI of 100. Infection was carried out for 3 h at 37°C in the presence of the inhibitor, after which samples were washed and fixed with PFA. Staining for intracellular and extracellular bacteria and host cells was performed as described in the text. Intracellular (•) and adherent (○) H. pylori 26695 bacteria were quantitated by CLSM. Data obtained were consistent among the staining strategies. Error bars indicate standard deviations.
Treatment with 100 μm Genistein, a broad-spectrum inhibitor of tyrosine kinases, resulted in a dramatic decrease in the level of H. pylori entry into AGS cells (a decrease from 1.7 ± 0.2 to 0.4 ± 0.1 bacteria per AGS cell), indicating that tyrosine kinases play a significant role in the uptake of H. pylori into AGS cells. This observation coincides with results described elsewhere (79). The other three inhibitors used included wortmannin, a potent specific inhibitor of PI 3-kinase (88); calphostin C, a PKC inhibitor (48); and sodium ortho-vanadate, which inhibits tyrosine phosphatases and ATPases (21, 51, 66). PI 3-kinase, a lipid kinase responsible for converting the membrane phospholipid phosphatidylinositol-4,5-bisphosphate into phosphatidylinositol-3,4,5-trisphosphate, plays an important role in signal transduction in a wide range of cellular functions, such as cytokine induction, cell proliferation, and cytoskeletal rearrangement (81). PKC is a protein kinase which can be activated by Ca2+ and diacylglycerol and is involved in the modulation of a wide range of metabolic processes, including cytoskeletal rearrangement and oxidative burst (61, 87). The activities of both kinases have been implicated to be important for bacterial uptake (41, 55). The results shown in Fig. 8 demonstrate that wortmannin and calpholstin C both inhibited the internalization of H. pylori in a dose-dependent manner without affecting the level of adherence. In contrast, sodium ortho-vanadate caused a dose-dependent enhancement of the level of uptake. These findings suggest that both PI 3-kinase and PKC are required for the internalization of H. pylori by AGS cells, whereas the action of one or more tyrosine phosphatases and/or ATPases is likely to antagonize the uptake process.
The attachment of H. pylori has been shown to induce host epithelial cells to secrete a series of cytokines, including interleukin 8 (IL-8), IL-1, TNF-α, and gamma interferon (59, 75, 76, 90). Given that TNF-α can up-regulate receptors on the host cell surface and may therefore influence bacterial uptake (58), we decided to examine its effect on the internalization of H. pylori. Surprisingly, TNF-α significantly inhibited H. pylori entry in a dose-dependent manner without affecting the level of bacterial adherence (Fig. 8). Our results suggest that TNF-α-mediated signaling interferes with the signaling events that mediate H. pylori entry.
DISCUSSION
For a very long time, H. pylori was considered an extracellular pathogen (52, 53, 56), but an increasing number of reports based on biospy examination and in vitro infection experiments have indicated that at least some host-associated H. pylori bacteria are located within host epithelial cells (19, 28, 31, 50, 62, 64, 73, 79, 85). In the present study, we used AGS cells as an infection model in order to demonstrate that the penetration of gastric epithelial cells is a multifactorial process resulting from an intricate interplay between H. pylori and host cells. The first question that we addressed concerned the invasiveness of H. pylori relative to that of other well-characterized, invasive, pathogenic bacteria. A direct comparison between the invasiveness of H. pylori and that of several other pathogens revealed that H. pylori entered AGS cells at a lower level than Salmonella serovar Typhimurium X22, E. coli H2356 expressing the Yersinia invasin InvA, and Y. enterocolitica YO:9 grown at 25°C but at a higher level than N. gonorrhoeae VP1 and Y. enterocolitica YO:9 grown at 37°C (and therefore expressing phagocytosis-inhibiting Yop proteins). Thus, the level of H. pylori entry into epithelial cells falls within the same range as that of other known invasive pathogens. The relatively low level of internalization observed with H. pylori is consistent with both previous in vitro findings and in vivo pathological observations that H. pylori infection is rarely associated with significant penetration of host epithelial cells or acute massive cell destruction (17). Taken together, these observations are consistent with the chronic nature of H. pylori infections.
Despite similar degrees of adherence, H. pylori invades AGS cells to a greater extent than it does HeLa or HEp-2 cells. This result indicates that adherence is by no means the single major determinant for host cell entry and supports the notion that adherent H. pylori induces host cell responses to promote its subsequent uptake. Furthermore, our kinetic data demonstrate a lag of at least 30 min between the adherence of H. pylori and its subsequent uptake; in addition, scanning electron micrographs showed significant cytoskeletal rearrangement, including the formation of pseudopod structures on the host plasma membrane, occurring at between 30 and 60 min postinfection. These pseudopod structures are apparently important for establishing intimate contact with the bacteria, since by 3 h postinfection, these structures appeared to have “zipped” along the entire length of H. pylori in a manner reminiscent of the zipper-like engulfment of Yersinia and Listeria (32, 44, 54). In addition, IF and CLSM analyses indicated that intracellular H. pylori bacteria are in close proximity to sites of host signaling. For example, both tyrosine phoshorylation and condensation of actin filaments were observed adjacent to intracellular H. pylori. These observations are all indicative of signal-induced uptake of H. pylori.
Direct evidence that H. pylori may activate host signaling factors to promote its uptake was obtained in assays with protein inhibitors. Our results indicate that both PKC and PI 3-kinase are important for efficient internalization of H. pylori, as inhibitors of these enzymes effectively reduced the level of uptake in a sensitive and dose-dependent manner. Our data further suggest that tyrosine kinases promote the entry of H. pylori into AGS cells, whereas phosphatases and/or ATPases antagonize uptake. Overall, these observations suggest that H. pylori, upon adherence to AGS cells, induces a variety of host cell responses, including cytoskeletal rearrangement and activation of specific kinases. We propose that the triggered host responses are some of the key factors involved in promoting the entry of H. pylori into AGS cells. More detailed investigations are under way to characterize the host signal transduction pathways involved in H. pylori uptake.
With the aid of CLSM and specific antibodies against various host cell factors, we were able to probe the molecular environment of intracellular H. pylori. First, intracellular H. pylori bacteria are closely associated with actin condensation in the host cell cytoplasm. These patches of condensed actin filaments appear to be different from the actin cup structures which typically circumscribe phagosomes (38). It is therefore unclear whether this actin rearrangement is a prerequisite for the internalization of H. pylori or is an effect caused by it. Given that internalized H. pylori bacteria are isolated inside phagosomes, H. pylori would need to trigger actin condensation from within these organelles according to the latter scenario. Recent findings have suggested that H. pylori may use a type IV secretion machinery to inject the CagA protein into the host cell cytoplasm (8, 9, 10, 63, 74, 75, 77); the same or a similar mechanism may be responsible for secreting CagA and/or other effector proteins across the phagosomal membrane to induce actin condensation and the other observed signal transduction events. Indeed, recent findings have suggested that the intraphagosomal survival of Legionella may be dependent on a type IV secretion system (49) and that type I strains of H. pylori can trigger megasome formation in macrophages (1).
We observed tyrosine phosphorylation signals at one end of intracellular H. pylori bacteria and in the vicinity of the condensed actin patches. This observation provided further evidence that intracellular H. pylori bacteria are closely associated with host signaling events. Since it has been shown that some phagolysomal proteins can be phosphorylated at their tyrosine residues (29), the phosphotyrosine signals that we observed may well have been of a phagolysosomal origin. However, these signals are probably not due to the tyrosine phosphorylation of CagA within the host (8, 9, 10, 63, 74, 75, 77), since we observed significant phosphotyrosine signals adjacent to an internalized CagA mutant. The invasion of several other pathogens has been shown to require the activity of tyrosine kinases (24, 70). For example, the integrin receptor-mediated invasion of Yersinia has been shown to require tyrosine phosphorylation of focal adhesion kinase (3). In addition, the involvement of β1-integrin has been implicated in the entry of H. pylori into AGS cells (79). Therefore, we propose that the observed tyrosine phosphorylation may well be part of the downstream events of β1-integrin-mediated signaling. Since β1-integrin plays a crucial role in focal adhesion (20), our observation that intracellular H. pylori bacteria were found associated with tyrosine phosphorylation at locations characteristic of focal adhesion plaques (e.g., the tips of stress fibers or regions of actin condensation) further supports this proposition. Recently, we observed that FCS resulted in a twofold increase in the level of H. pylori entry into AGS cells without affecting the level of adherence (unpublished results), while previous findings demonstrated the binding of vitronectin by H. pylori (69). Since pathogens such as N. gonorrhoeae are able to efficiently trigger αvβ5-integrin-mediated uptake by binding directly to vitronectin (26), it is quite possible that the binding of serum factors such as vitronectin by H. pylori may further stimulate entry into AGS cells via the β1-integrin-mediated signaling pathway.
The significant inhibition of H. pylori internalization by the potent inhibitor wortmannin suggested that PI 3-kinase is important for mediating the entry of H. pylori into AGS cells. The role of this lipid kinase in the induced uptake of other pathogens into nonphagocytic cells was recently demonstrated for invasion by Yersinia and Listeria (41, 72). One of the adhesins of Listeria, InlB, was shown to trigger the activation of PI 3-kinase and the association of the p85 regulatory subunit of PI 3-kinase with Gab1, Cbl, and Shc kinases (41, 42). These events are believed to result in the activation of Rho GTPases, which could subsequently induce actin cytoskeletal rearrangement and hence bacterial uptake (42). It is therefore possible that H. pylori uses a similar signal transduction pathway to induce its own uptake. Indeed, recent findings show that H. pylori is capable of activating Rho GTPases, an observation which argues strongly for the ability of H. pylori to activate PI 3-kinase (22). Interestingly, the involvement of PI 3-kinase has also been implicated in the NF-κB activation pathway (68). Since H. pylori is capable of activating NF-κB (45, 57, 76) and hence the induction of cytokines (18), it is possible that the activation of PI 3-kinase by H. pylori is part of the NF-κB signaling pathway that leads to the induction of several cytokines during H. pylori infection.
Our study showed that PKC is another host protein kinase important for the entry of H. pylori into AGS cells. While the activity of PKC correlates poorly with the invasion frequency of Yersinia (70), PKC has been shown to be involved in host cell invasion by Neisseria (55). It is believed that PKC induces bacterial uptake by nonphagocytic cells by activating proteins which regulate actin cytoskeletal rearrangement, for example, Rac (86). It remains to be determined whether H. pylori activates PKC by using the conventional pathway via the activation of phospholipase C and sphingomyelinase, subsequently causing increases in the amount of diacylglycerol and the intracellular Ca2+ level (61). Interestingly, previous data suggested that PKC is not required for the induction of IL-8 by H. pylori (12), implying that the signal transduction pathway triggered by H. pylori during the induction of IL-8 may be, at least to a certain extent, distinct from that involved in its uptake.
Enhancement of the level of entry of H. pylori into AGS cells by sodium vanadate implies that either phosphatases or ATPases or both are likely to be involved in inhibiting H. pylori entry. Inhibition of the H. pylori entry process by phosphatases would be consistent with the speculated involvement of β1-integrin and the subsequent tyrosine phosphorylation of focal adhesion kinase in the entry of H. pylori into AGS cells (79). An involvement of ATPases in inhibiting the entry of H. pylori into AGS cells would suggest that the uptake of H. pylori requires ATP hydrolysis. Interestingly, one of the putative gene products encoded by the cag pathogenicity island of H. pylori, VirB11, shows ATPase activity that is possibly involved in the opening and closing of the transport channel (89). Since the cag pathogenicity island has been suggested to play a role in the entry of H. pylori into AGS cells (64, 79), it will be of considerable interest to examine whether VirB11 is important for H. pylori invasion activity.
While few data are available to support a direct role of TNF-α in bacterial uptake, our study demonstrated an inhibitory effect of the cytokine TNF-α on the entry of H. pylori into AGS cells. An indirect role of TNF-α in the induced uptake of Neisseria into endothelial cells has been suggested (58). This cytokine was shown to promote neisserial uptake by up-regulating the expression of CEACAM1, a CD66 family receptor required for promoting the invasion of endothelial cells by Neisseria (58). However, instead of promoting H. pylori entry, TNF-α inhibited the uptake of H. pylori by AGS cells in a dose-dependent manner. Given that TNF-α is a potent mediator of apoptosis, one possible explanation for this inhibitory effect is that the apoptotic signaling induced by TNF-α directly interferes with the uptake of H. pylori. While this proposition remains to be proven, the finding itself has interesting implications. TNF-α has been shown to be induced during H. pylori infection in vivo (18). The inhibitory effect of TNF-α on the uptake of H. pylori by gastric epithelial cells may therefore explain why intracellular H. pylori bacteria have been found only rarely in biopsy samples. More importantly, this information suggests that the host immune response may play a role in determining the rate of H. pylori entry into host cells.
In summary, various strains of H. pylori are able to invade AGS cells, indicating that the invasion of AGS cells is a general characteristic of H. pylori. The multiple lines of evidence obtained to demonstrate that this entry is a specific multifactorial process include the following. (i) Different H. pylori strains show statistically significant differences in their levels of entry, indicating that uptake is determined at least partly by bacterial factors. (ii) AGS cells take up H. pylori to a greater extent than do HeLa or HEp-2 cells, indicating that uptake is dependent on host cell determinants. (iii) The engulfment of H. pylori occurs via a zipper-like mechanism, involving intimate contact with host cell microvilli and surface membrane pseudopod structures. (iv) Internalized H. pylori bacteria are found in specific host organelles (e.g., LAMP-1-containing phagosomes), indicating that H. pylori bacteria are internalized by a specific phagocytic mechanism. (v) Distinct host signaling responses are associated with internalized H. pylori, as tyrosine phosphorylation and cytoskeletal rearrangement were readily detected in the promixity of intracellular H. pylori. (vi) The uptake of H. pylori can be specifically blocked by inhibitors that inactivate host signaling factors, including PKC and PI 3-kinase, and enhancement of uptake occurs in the presence of vanadate, an inhibitor of phosphatases and ATPases. These results collectively indicate triggered bacterial uptake resulting from an intricate interplay between the pathogen and the host cells.
H. pylori was shown in recent studies to be capable of delaying and/or inhibiting phagocytosis in professional phagocytes (1, 67) while being able to invade epithelial cells. These two apparently contrasting activities of H. pylori could well be attributed to the vast differences between the respective host cell types. One plausible explanation would be that H. pylori interacts with professional phagocytes and epithelial cells via different receptors and hence different signal transduction pathways. The identification of the respective host and bacterial determinants involved should shed light on this intriguing duality.
The present study provides the basis for identifying host and bacterial factors mediating the entry of H. pylori into cultured gastric epithelial cells. The identification of H. pylori mutants defective in the ability to enter host cells, in combination with appropriate animal models, will be of crucial importance for evaluating the physiological significance of H. pylori invasion. It is quite possible that the specific entry of H. pylori into epithelial cells allows this organism to evade the host immune response. Our findings from a gentamicin protection assay and TEM showing that H. pylori organisms are likely to remain viable within epithelial cells for at least 12 h support this notion. Thus, invasion of epithelial cells could represent one of the many strategies used by H. pylori to survive and persist in the human stomach.
Acknowledgments
We are grateful to Christoph Dehio, Gaby Haas, Ralph Jack, Michael Naumann, and Antonia Costacurta for insightful discussions and critical reading of the manuscript. We also thank Ilka Müller and Bozena Pichler-Brand for excellent technical assistance.
This work was supported by a Max-Planck-Institute stipend granted to Terry Kwok.
Editor: E. I. Tuomanen
REFERENCES
- 1.Allen, L. A., L. S. Schlesinger, and B. Kang. 2000. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 191:115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm, R. A., and T. J. Trust. 1999. Analysis of the genetic diversity of Helicobacter pylori: the tale of two genomes. J. Mol. Med. 77:834-846. [DOI] [PubMed] [Google Scholar]
- 3.Alrutz, M., and R. Isberg. 1998. Involvement of focal adhesion kinase in invasin-mediated uptake. Proc. Natl. Acad. Sci. USA 95:13658-13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen, L. P., J. Blom, and H. Nielsen. 1993. Survival and ultrastructural changes of Helicobacter pylori after phagocytosis by human polymorphonuclear phagocytes and monocytes. APMIS 101:61-72. [PubMed] [Google Scholar]
- 5.Andersson, K., N. Carballeira, K. E. Magnusson, C. Persson, O. Stendahl, H. Wolf-Watz, and M. Fällman. 1996. YopH of Yersinia pseudotuberculosis interrupts early phospho-tyrosine signalling associated with phagocytosis. Mol. Microbiol. 20:1057-1069. [DOI] [PubMed] [Google Scholar]
- 6.Andrade, J. R., V. F. Da Veiga, M. R. De Santa Rosa, and I. Suassuna. 1989. An endocytic process in HEp-2 cells induced by enteropathogenic Escherichia coli. J. Med. Microbiol. 28:49-57. [DOI] [PubMed] [Google Scholar]
- 7.Anti, M., A. Armuzzi, A. Gasbarrini, and G. Gasbarrini. 1998. Importance of changes in epithelial cell turnover during Helicobacter pylori infection in gastric carcinogenesis. Gut 43(Suppl. 1):S27-S32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asahi, M., T. Azuma, S. Ito, Y. Ito, H. Suto, Y. Nagai, M. Tsubokawa, Y. Tohyama, S. Maeda, M. Omata, T. Suzuki, and C. Sasakawa. 2000. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J. Exp. Med. 191:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backert, S., S. Moese, M. Selbach, V. Brinkmann, and T. F. Meyer. 2001. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol. 42:631-644. [DOI] [PubMed] [Google Scholar]
- 10.Backert, S., E. Ziska, V. Brinkmann, U. Zimny-Arndt, A. Fauconnier, P. R. Jungblut, M. Naumann, and T. F. Meyer. 2000. Translocation and tyrosine phosphorylation of the Helicobacter pylori CagA protein in gastric epithelial cells. Cell. Microbiol. 2:155-164. [DOI] [PubMed] [Google Scholar]
- 11.Bayerdorffer, E., A. Neubauer, B. Rudolph, C. Thiede, N. Lehn, S. Eidt, and M. Stolte. 1995. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue after cure of Helicobacter pylori infection. Lancet 345:1591-1594. [DOI] [PubMed] [Google Scholar]
- 12.Beales, I. L., and J. Calam. 1997. Stimulation of IL-8 production in human gastric epithelial cells by Helicobacter pylori, IL-1 beta and TNF-alpha requires tyrosine kinase activity, but not protein kinase C. Cytokine 9:514-520. [DOI] [PubMed] [Google Scholar]
- 13.Bernardini, M. L., J. Mournier, H. d'Hauteville, M. Coquis-Rondon, and P. J. Sansonetti. 1989. Identification of icsA, a plasmid locus of Shigella flexnerii that governs bacterial intra- and inter-cellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. USA 86:3867-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaser, M. J. 1990. Helicobacter pylori and the pathogenesis of gastrointestinal inflammation. J. Infect. Dis. 161:626-633. [DOI] [PubMed] [Google Scholar]
- 15.Blaser, M. J. 1992. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology 102:720-727. [DOI] [PubMed] [Google Scholar]
- 16.Blaser, M. J. 1997. All Helicobacter pylori strains are not created equal: should all be eliminated? Lancet 349:1020-1022. [DOI] [PubMed] [Google Scholar]
- 17.Blaser, M. J., and J. Parsonnet. 1994. Parasitism by the ‘slow’ bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J. Clin. Investig. 94:4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodger, K., and J. E.Crabtree. 1998. Helicobacter pylori and gastric inflammation. Br. Med. Bull. 54:139-150. [DOI] [PubMed] [Google Scholar]
- 19.Bukholm, G., T. Tannæs, P. Nedenskov, Y. Esbensen, H. J. Grav, T. Hovig, S. Ariansen, and I. Guldvog. 1997. Colony variation of Helicobacter pylori: pathogenic potential is correlated to cell wall lipid composition. Scand. J. Gastroenterol. 32:445-454. [DOI] [PubMed] [Google Scholar]
- 20.Burridge, K., and M. Chrzanowska-Wodnicka. 1996. Focal adhesions, contractility and signaling. Annu. Rev. Cell Dev. Biol. 12:463-519. [DOI] [PubMed] [Google Scholar]
- 21.Cantley, L. C., Jr., L. Josephson, R. Warner, M. Yanagisawa, C. Lechene, and G. Guidotti. 1977. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J. Biol. Chem. 252:7421-7423. [PubMed] [Google Scholar]
- 22.Churin, T., K. Kardalinou, T. F. Meyer, and M. Naumann. 2001. Pathogenicity island-dependent activation of Rho GTPases Rac1 and Cdc42 in Helicobacter pylori infection. Mol. Microbiol. 40:815-823. [DOI] [PubMed] [Google Scholar]
- 23.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23:861-867. [DOI] [PubMed] [Google Scholar]
- 24.Crane, J. K., and J. S. Oh. 1997. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect. Immun. 65:3277-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehio, C., M. Meyer, J. Berger, H. Schwarz, and C. Lanz. 1997. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalization of the bacterial aggregate by a unique structure, the invasome. J. Cell Sci. 110:2141-2154. [DOI] [PubMed] [Google Scholar]
- 26.Dehio, M., O. G. Gómez-Duarte, C. Dehio, and T. F. Meyer. 1998. Vitronectin-dependent invasion of epithelial cells by Neisseria gonorrhoeae involves αv integrin receptors. FEBS Lett. 424:84-88. [DOI] [PubMed] [Google Scholar]
- 27.Dombek, P. E., D. E. Cue, J. Sedgewick, H. Lam, S. Ruschkowski, B. B. Finlay, and P. P. Cleary. 1999. High frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol. Microbiol. 31:859-870. [DOI] [PubMed] [Google Scholar]
- 28.El-Shoura, S. M. 1995. Helicobacter pylori. I. Ultrastructural sequences of adherence, attachment, and penetration into the gastric mucosa. Ultrastruct. Pathol. 19:323-333. [DOI] [PubMed] [Google Scholar]
- 29.Emans, N., N. N. Nzala, and M. Desjardins. 1996. Protein phosphorylation during phagosome maturation. FEBS Lett. 398:37-42. [DOI] [PubMed] [Google Scholar]
- 30.Engstrand, L., D. Y. Graham, A. Scheynius, R. M. Genta, and F. El-zaatari. 1997. Is the sanctuary where Helicobacter pylori avoids antibacterial treatment intracellular? Am. J. Clin. Pathol. 108:504-509. [DOI] [PubMed] [Google Scholar]
- 31.Evans, D. G., D. J. Evans, and D. Y. Graham. 1992. Adherence and internalization of Helicobacter pylori by HEp-2 cells. Gastroenterology 102:1557-1567. [DOI] [PubMed] [Google Scholar]
- 32.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276:718-725. [DOI] [PubMed] [Google Scholar]
- 33.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forman, D., D. G. Newell, F. Fullerton, J. W. G. Yarnell, A. R. Stacey, N. Wald, and F. Sitas. 1991. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. Br. Med. J. 302:1302-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francis, C. L., T. A. Ryan, B. D. Jones, S. J. Smith, and S. Falkow. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639-642. [DOI] [PubMed] [Google Scholar]
- 36.Fujiwara, Y., F. Wyle, T. Arakawa, M. J. Domek, T. Fukuda, K. Kobayashi, and A. Tarnawski. 1997. Helicobacter pylori culture supernatant inhibits binding and proliferative response of human gastric cells to epidermal growth factor: implications for H. pylori interference with ulcer healing? Digestion 58:299-303. [DOI] [PubMed] [Google Scholar]
- 37.Goosney, D. L., D. G. Knoechel, and B. B. Finlay. 1999. Enteropathogenic E. coli, Salmonella, and Shigella: masters of host cell cytoskeletal exploitation. Emerg. Infect. Dis. 5:216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenberg, S. 1995. Signal transduction in phagocytosis. Trends Cell. Biol. 5:93-99. [DOI] [PubMed] [Google Scholar]
- 39.Heesemann, J., U. Gross, N. Schmidt, and R. Laufs. 1986. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia sp. grown in calcium-deficient media. Infect. Immun. 54:561-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hulten, K., E. Hjelm, O. Cars, and L. Engstrand. 1996. In vitro activity of azithromycin against intracellular Helicobacter pylori. J. Antimicrob. Chemother. 37:483-489. [DOI] [PubMed] [Google Scholar]
- 41.Ireton, K., B. Payrastre, H. Chap, W. Ogawa, H. Sakaue, M. Kasuga, and P. Cossart. 1996. A role for phosphoinositide 3-kinase in bacterial invasion. Science 274:780-782. [DOI] [PubMed] [Google Scholar]
- 42.Ireton, K., B. Payrastre, and P. Cossart. 1999. The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositide 3-kinase. J. Biol. Chem. 274:17025-17032. [DOI] [PubMed] [Google Scholar]
- 43.Isberg, R. R., and J. M. Leong. 1990. Multiple beta 1 chain integrins are receptors for InvA, a protein that promotes bacterial penetration into mammalian cells. Cell 60:861-871. [DOI] [PubMed] [Google Scholar]
- 44.Isberg, R. R. 1991. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science 252:934-938. [DOI] [PubMed] [Google Scholar]
- 45.Keates, S., Y. S. Hitti, M. Upton, and C. P. Kelly. 1997. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology 113:1099-1109. [DOI] [PubMed] [Google Scholar]
- 46.Kenny, B., R. De Vinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-522. [DOI] [PubMed] [Google Scholar]
- 47.Kist, M., C. Spiegelhalder, T. Moriki, and H. E. Schaefer. 1993. Interaction of Helicobacter pylori (strain 151) and Campylobacter coli with human peripheral polymorphonuclear granulocytes. Int. J. Med. Microbiol. Virol. Parasitol. Infect. Dis. 280:58-72. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi, E., H. Nakano, M. Morimoto, and T. Tamaoki. 1989. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 159:548-553. [DOI] [PubMed] [Google Scholar]
- 49.Kwaik, Y. A. 1998. Fatal attaraction of mammalian cells to Legionella pneumophila. Mol. Microbiol. 30:689-695. [DOI] [PubMed] [Google Scholar]
- 50.Löfman, C., R. Rigo, M. Block, K. Hultén, H. Enroth, and L. Engstrand. 1997. Bacterium-host interactions monitored by time-lapse photography. Nat. Med. 3:930-931. [DOI] [PubMed] [Google Scholar]
- 51.Lopez, V., T. Stevens, and R. N. Lindquist. 1976. Vanadium ion inhibition of alkaline phosphatase-catalyzed phosphate ester hydrolysis. Arch. Biochem. Biophys. 175:31-38. [DOI] [PubMed] [Google Scholar]
- 52.Marshall, B. J. 1989. Histroy of the discovery of Campylobacter pylori, p. 7-24. In M. J. Blaser (ed.), Campylobacter pylori in gastritis and peptic ulcer disease. Igaku Shoin, New York, N.Y.
- 53.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 54.Mengaud, J., H. Ohayon, P. Gounon, R.-M. Mège, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 55.Meyer, T. F. 1998. Pathogenic Neisseriae—interplay between pro- and eukaryotic worlds. Folia Microbiol. 43:311-319. [DOI] [PubMed] [Google Scholar]
- 56.Molyneux, A. J., and M. D. Harris. 1993. Helicobacter pylori in gastric biopsies—should you trust the pathology report? J. R. Coll. Physicians 27:119-120. [PMC free article] [PubMed] [Google Scholar]
- 57.Münzenmaier, A., C. Lange, E. Glocker, A. Covacci, A. Moran, S. Bereswill, P. A. Baeuerle, M. Kist, and H. L. Pahl. 1997. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor kappa B. J. Immunol. 159:6140-6147. [PubMed] [Google Scholar]
- 58.Münzner, P., C. Dehio, T. Fujiwara, M. Achtman, T. F. Meyer, and S. D. Gray-Owen. 2000. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect. Immun. 68:3601-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakajima, N., H. Kuwayama, Y. Ito, A. Iwasaki, and Y. Arakawa. 1997. Helicobacter pylori, neutrophils, interleukins, and gastric epithelial proliferation. J. Clin. Gastroenterol. 25(Suppl. 1):S198-S202. [DOI] [PubMed] [Google Scholar]
- 60.Nedrud, J. G. 1999. Animal models for gastric Helicobacter immunology and vaccine studies. FEMS Immunol. Med. Microbiol. 24:243-250. [DOI] [PubMed] [Google Scholar]
- 61.Nishizuka, Y. 1986. Studies and perspectives of protein kinase C. Science 233:305-312. [DOI] [PubMed] [Google Scholar]
- 62.Noach, L. A., T. M. Rolf, and G. N. Tytgat. 1994. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J. Clin. Pathol. 47:699-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Odenbreit, S., J. Püls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 64.Petersen, A. M., J. Blom, L. P. Andersen, and K. A. Krogfelt. 2000. Role of strain type, AGS cells and fetal calf serum in Helicobacter pylori adhesion and invasion assays. FEMS Immunol. Med. Microbiol. 29:59-67. [DOI] [PubMed] [Google Scholar]
- 65.Pruul, H., P. C. Lee, C. S. Goodwin, and P. J. MacDonald. 1987. Interaction of Campylobacter pyloridis with human immune defense mechanisms. J. Med. Microbiol. 23:233-238. [DOI] [PubMed] [Google Scholar]
- 66.Pugazhenthi, S., F. Tanha, B. Dahl, and R. L. Khandelwal. 1996. Inhibition of a src homology 2 domain containing protein tyrosine phosphatase by vanadate in the primary culture of hepatocytes. Arch. Biochem. Biophys. 335:273-282. [DOI] [PubMed] [Google Scholar]
- 67.Ramarao, N., S. D. Gray-Owen, S. Backert, and T. F. Meyer. 2000. Helicobacter pylori inhibits phagocytosis by professional phagocytes involving type IV secretion components. Mol. Microbiol. 37:1389-1404. [DOI] [PubMed] [Google Scholar]
- 68.Reddy, S. A., J. H. Huang, and W. S. Liao. 1997. Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NFkappaB and AP-1 activation. J. Biol. Chem. 272:29167-29173. [DOI] [PubMed] [Google Scholar]
- 69.Ringner, M., K. H. Valkonen, and T. Wadstrom. 1994. Binding of vitronectin and plasminogen to Helicobacter pylori. FEMS Immunol. Med. Microbiol. 9:29-34. [DOI] [PubMed] [Google Scholar]
- 70.Rosenshine, I., V. Duronio, and B. B. Finlay. 1992. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect. Immun. 60:2211-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenshine, I., and B. B. Finlay. 1993. Exploitation of host signal transduction pathways and cytoskeletal functions by invasive bacteria. Bioessays 15:17-24. [DOI] [PubMed] [Google Scholar]
- 72.Schulte, R., R. Zumbihl, D. Kampik, A. Fauconnier, and I. B. Autenrieth. 1998. Wortmannin blocks Yersinia invasin-triggered internalization, but not interleukin-8 production by epithelial cells. Med. Microbiol. Immunol. (Berlin) 187:53-60. [DOI] [PubMed] [Google Scholar]
- 73.Segal, E. D., S. Falkow, and L. S. Tompkins. 1996. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc. Natl. Acad. Sci. USA 93:1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Selbach, M., S. Moese, T. F. Meyer, and S. Backert. 2002. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect. Immun. 70:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma, S. A., M. K. R. Tummuru, M. J. Blaser, and L. D. Kerr. 1997. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor kappa B in gastric epithelial cells. J. Immunol. 160:2401-2407. [PubMed] [Google Scholar]
- 77.Stein, M., R. Rappuoli, and A. Covacci. 2000. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. USA 3:1263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stuckey, J. A., H. L. Schubert, E. B. Faumann, Z. Y. Zhang, J. E. Dixon, and M. A. Saper. 1994. Crystal structure of Yersinia protein tyrosine phosphatase at 2.5 Å and the complex with tungstate. Nature 370:571-575. [DOI] [PubMed] [Google Scholar]
- 79.Su, B., S. Johansson, M. Fällman, M. Patarroyo, M. Granström, and S. Normark. 1999. Signal transduction-mediated adherence and entry of Helicobacter pylori into cultured cells. Gastroenterology 117:595-604. [DOI] [PubMed] [Google Scholar]
- 80.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 81.Vanhaesebroeck, B., S. J. Leevers, G. Panayotou, and M. D. Waterfield. 1997. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem. Sci. 22:267-272. [DOI] [PubMed] [Google Scholar]
- 82.van Putten, J. P. 1993. Phase conversion of lipopolysaccharide directs interconversion of invasive and immuno-resistant phenotypes of Neisseria gonorrhoeae. EMBO J. 12:4043-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walker, A. R. 1999. Helicobacter pylori and gastric cancer. Am. J. Med. 107:646-647. [DOI] [PubMed] [Google Scholar]
- 84.Watanabe, T., M. Tada, H. Nagai, S. Sasaki, and M. Nakao. 1998. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology 115:642-648. [DOI] [PubMed] [Google Scholar]
- 85.Wilkinson, S. M., J. R. Uhl, B. C. Kline, and F. R. Cockerill. 1998. Assessment of invasion frequencies of cultured HEp-2 cells by clinical isolates of Helicobacter pylori using an acridine orange assay. J. Clin. Pathol. 51:127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woods, A., and J. R. Couchman. 1992. Protein kinase C involvement in focal adhesion formation. J. Cell Sci. 101:277-290. [DOI] [PubMed] [Google Scholar]
- 87.Yamamori, T., O. Inanami, H. Nagahata, Y. Cui, and M. Kuwabara. 2000. Roles of p38 MAPK, PKC and PI3-K in the signaling pathways of NADPH oxidase activation and phagocytosis in bovine polymorphonuclear leukocytes. FEBS Lett. 467:253-258. [DOI] [PubMed] [Google Scholar]
- 88.Yano, H., S. Nakanishi, K. Kimura, N. Hanai, Y. Saitoh, Y. Fukui, Y. Nonomura, and Y. Matsuda. 1993. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J. Biol. Chem. 268:25846-25856. [PubMed] [Google Scholar]
- 89.Yeo, H. J., S. N. Savvides, A. B. Herr, E. Lanka, and G. Waksman. 2000. Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system. Mol. Cell 6:1461-1472. [DOI] [PubMed] [Google Scholar]
- 90.Zarrilli, R., V. Ricci, and M. Romano. 1999. Molecular response of gastric epithelial cells to Helicobacter pylori-induced cell damage. Cell. Microbiol. 1:93-99. [DOI] [PubMed] [Google Scholar]