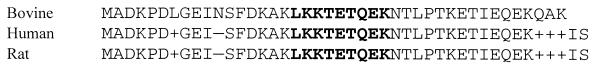Abstract
Bovine macrophages underwent apoptosis as a result of infection with a Mycobacterium bovis field strain. Macrophages infected with a multiplicity of infection (MOI) of 25:1 developed chromatin condensation and DNA fragmentation at 4 h and 8 h, respectively, whereas changes in chromatin condensation induced by MOIs of 10:1 and 1:1 required more time and had a reduced number of apoptotic cells. Not only infected macrophages underwent apoptosis, but also uninfected bystander macrophages became apoptotic. Increased differential expression of thymosin β-10 was identified in M. bovis-infected bovine macrophages by differential display reverse transcriptase PCR. Phagocytosis of latex beads had no effect on the expression of thymosin β-10, whereas bacterial suspensions upregulated thymosin β-10 expression, suggesting that M. bovis or mycobacterial products are essential in the process. Heat-inactivated M. bovis induced a slight increase in thymosin β-10 mRNA, whereas live virulent and attenuated M. bovis organisms increased the gene expression almost twofold. A mouse macrophage cell line (RAW 264.7) overexpressing the bovine thymosin β-10 transgene had spontaneous apoptosis at a higher rate (66.5%) than parental cells (4.7%) or RAW cells harboring the empty vector (22.8%). The apoptotic rates of the overexpressing cells were significantly higher when compared with both the empty vector transfected (P < 0.01) and parental cells (P < 0.001). Our evidence suggests that upregulation of thymosin β-10 in M. bovis-infected macrophages is linked with increased cell death due to apoptosis.
Thymosin β-10 is a G-actin binding protein that prevents globular actin monomers from polymerizing spontaneously. Although thymosin β-10 sequesters actin monomers in different cell types, it does not act simply as an actin monomer buffering protein (24, 28). In addition to regulating the rate of actin polymerization, thymosins are reported to regulate cell growth and differentiation (9, 14, 28). Upregulation of the thymosin β-10 protein predisposes cells to undergo apoptosis, whereas knocking out the gene endows cells with a robust F-actin (noncovalent helical filaments) network and increases resistance to both spontaneous and drug-induced cell death, as well as an enhanced ability to proliferate. Differential expression of thymosin β-10 influences cell proliferation, cell morphology, and expression of the anti-apoptotic protein bcl-2. Cells overexpressing thymosin β-10 have a reduced growth rate, grow in a disorganized fashion, and have increased bcl-2 expression (11).
It is well known that mycobacterial species induce macrophage apoptosis (1, 7, 13, 16, 22). However, most of the studies have focused on the human and murine models. In this study, an increase in the rate of chromatin condensation and DNA fragmentation in Mycobacterium bovis-infected bovine monocyte-derived macrophages demonstrated for the first time that M. bovis infection induces macrophage apoptosis in the preferred host bovine model. At the same time, a differential display screening of the infected cells detected upregulation of thymosin β-10 mRNA as another consequence of the infection. In an attempt to link both findings, the apoptotic rate of murine macrophages overexpressing the bovine thymosin β-10 mRNA was measured. Our results suggest that macrophage apoptosis may be linked to the overexpression of thymosin β-10.
MATERIALS AND METHODS
Microorganisms.
Two M. bovis strains were used, M. bovis BCG Montreal strain 9003 (kindly provided by Danuta Radzioch, McGill University, Montreal, Canada) and M. bovis “El Paso” strain, which is a wild-type strain isolated from the tuberculous lesions of a tuberculin skin test-positive cow from a 600-head El Paso, Tex., dairy herd which had a 50% rate of infection. The inoculum was prepared in Middlebrook 7H9 broth (Becton Dickinson, Cockeysville, Md.) supplemented with 0.05% Tween 80 and oleic acid-albumin-dextrose-catalase enrichment (Difco, Detroit, Mich.). Bacteria were grown at 37°C under shaking conditions during 8 days (middle logarithmic phase). The bacterial suspension was passed twice through a 26-gauge needle and sonicated for 30 s in order to disrupt the clumps. Aliquots of 1 ml were stored at −80°C. The inoculum was titrated by plating serial dilutions on 7H11 medium (Difco).
Infection of bovine macrophages.
Citrated bovine peripheral venous blood was obtained from healthy donors, processed by isopycnic centrifugation using a Percoll gradient (Pharmacia, Uppsala, Sweden), and cultured as previously described (3, 4). Macrophage monolayers in Teflon flasks were infected with M. bovis using different multiplicities of infection (MOIs), centrifuged at 200 × g for 10 min, and incubated at 37°C for 4 h. After the time allowed for phagocytosis, the cells were washed five times with 5 ml of fresh RPMI 1640 (Life Technologies, Gaithersburg, Md.) with l-glutamine, amino acids, sodium pyruvate, sodium bicarbonate (complete RPMI [CRPMI]), and 10% heat-inactivated fetal calf serum to remove the extracellular bacteria and incubated again at 37°C. This was considered time zero. Noninfected macrophages were treated in the same way to maintain valid comparisons.
Chromatin condensation detection.
A time course study every 2 h during the first 12 h and at 3-h intervals for the next 12 h was performed using three M. bovis MOIs (1:1, 10:1, 25:1). An aliquot of 1 × 105 to 5 × 105 bovine macrophages in CRPMI supplemented with 12.5% autologous serum was prepared and infected on coverslips in 35-mm petri dishes (Falcon, Lincoln Park, N.J.). After incubation, the cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, Pa.) during 40 min at 4°C. Three phosphate-buffered saline washes were performed, and propidium iodide (5 μg/ml with 100 μg of RNase) (Sigma, St. Louis, Mo.) was added. Chromatin condensation was analyzed by counting at least 100 cells per sample, using a fluorescence microscope.
DNA fragmentation analysis.
DNA fragmentation was identified using the terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay. M. bovis-infected macrophages were analyzed using two MOIs (10:1, 25:1) at 4 and 8 h postinfection and compared to noninfected cells. The APO-BRDU kit (Pharmingen, San Diego, Calif.) was used according to the manufacturer's instructions. All the samples were stained in the presence and in the absence of the TdT enzyme as a negative control. Flow cytometry analysis was carried out using a FACScalibur apparatus (Becton Dickinson). At least 10,000 cells were analyzed per sample (6).
Total RNA extraction.
Total RNA was isolated from noninfected and infected bovine macrophages (MOI, 10:1) after 0, 12, and 24 h postinoculation using TRI reagent (Molecular Research Center Inc., Cincinnati, Ohio) according to the manufacturer's instructions. At the end of the procedure, the RNA pellet was air dried, dissolved in water treated with diethyl pyrocarbonate to remove RNases, and stored at −80°C (5).
Differential gene expression analysis.
The RNA Image Kit (GenHunter Corporation, Nashville, Tenn.) was used according to the manufacturer's instructions. Reverse transcription reactions each with one of the three different anchored oligo(dT) primers were performed for each RNA sample. Reverse transcription was done as follows: 65°C for 5 min, 37°C for 60 min, 75°C for 5 min, and 4°C. At the end of the reaction, tubes were kept on ice for PCR or stored at −80°C for later use. PCR was performed as follows: 94°C for 30 s, 40°C for 2 min, and 72°C for 30 s for 40 cycles, 72°C for 5 min, and 4°C. The PCR products were electrophoresed through a 6% denaturing polyacrylamide gel for 3.5 h at 60 W. The differential expression of the candidate bands was confirmed in two independent experiments. The bands that consistently had differential expression were located and excised with a clean razor blade. The DNA was extracted from the gel and used for reamplification. The pCR-TRAP cloning system (GenHunter Corporation) was used to clone the reamplified cDNA probes. The presence of inserts was determined using the colony-PCR method (18, 19, 20).
Reverse Northern blot analysis.
The Reverse Prime cDNA Labeling Kit (GenHunter Corporation) was used to screen the candidates by reverse Northern blotting. Four reverse transcription reactions corresponding to each of the RNAs were compared according to the protocol provided by the manufacturer. The candidate probes were blotted onto quadruplicate Hybond-N nylon membranes (Amersham Life Sciences, Arlington Heights, Ill.) by using a dot blot microfiltration system. The membranes were UV cross-linked and rinsed in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) before hybridization. The membranes were prehybridized overnight in separate containers using 50% formamide base hybridization solution at 42°C. The counts-per-minute-normalized radioactively labeled cDNAs were denatured and added to the corresponding membrane. Membranes were hybridized overnight and washed twice for 15 min in 1× SSC-0.1% sodium dodecyl sulfate (SDS) at room temperature followed by washing at 60°C for 15 min in 0.25× SSC-0.1% SDS. The filters were dried between two pieces of 3M paper and processed for autoradiography.
RPA.
MAXIscript in vitro transcription and RNase protection assay (RPA) kits (Ambion, Austin, Tex.) were used according to the manufacturer's instructions (2). The transcription reactions were done to produce the labeled riboprobe corresponding to the differential display reverse transcriptase PCR candidate. For each experimental tube (noninfected and infected macrophages), the same amount of labeled probe (2 × 104 to 8 × 104 cpm) and 10 μg of total RNA were added, and two tubes with yeast RNA for positive and negative controls were included. The tubes were co-ethanol precipitated by adjusting the concentration of NH4OAc to 0.5 M and 2.5 volumes of ethanol. The pellets were resuspended in 20 μl of hybridization buffer and incubated at 42°C overnight. After hybridization, RNase digestion of the samples was performed during 30 min at 37°C. An aliquot of RNase inactivation-precipitation mixture was added to recover the protected fragments. The supernatant was removed and the pellet was resuspended in 10 μl of gel loading buffer. The samples were denatured and loaded on a 6% polyacrylamide gel and run at 250 V for 1 h in 1× Tris-borate-EDTA. The gel was transferred to filter paper, covered with plastic wrap, and exposed to X-ray film at −80°C with an intensifying screen. Quantification of mRNA expression was performed by densitometry using NIH 1.6 image software. The final value was obtained as a ratio of the size and density of the protected fragment representing the differentially expressed gene to the size and density of the protected fragment of the internal control (ribosomal 18S). The value of noninfected cells was considered baseline (1.0), and values for infected cells represented the variation of expression compared to the noninfected value.
Thymosin β-10 mRNA transcription.
Bovine monocyte-derived macrophages were inoculated with the M. bovis field strain, compared to cells exposed to a heat-inactivated M. bovis field strain, M. bovis BCG, or latex beads (MOI, 10:1), and incubated at 37°C for 0, 12, and 24 h. Total RNA was extracted and used to perform an RPA.
cDNA library screening.
A bovine macrophage cDNA library prepared in our laboratory carrying an average insert of 1.5 kb in the cloning vector pSPORT1 (Gibco BRL) was screened using a bovine thymosin β-10-radiolabeled probe obtained from macrophages to identify the full-length cDNA sequence of the gene (10). Filter membranes bearing colonies were incubated with prehybridization solution in hybridization tubes at 42°C for 12 h. The tubes were removed from the incubator, and 2 × 106 cpm of the labeled probe/ml was added. Tubes were reincubated for 24 h at 42°C. The filters were washed twice during 15 min in 100 ml of low-stringency buffer at room temperature. After rinsing with buffer, a high-stringency wash was conducted using 100 ml of the high-stringency buffer at 60°C to remove most of the background radioactivity. Filters were placed on blotting paper to remove excess fluid, covered with plastic wrap, and exposed to X-ray film. The positive clones were identified, purified, and subjected to two more rounds of screening to obtain 100% positive colonies. Minipreparations of plasmid DNA from the positive colonies were performed using the Wizard Plus Minipreps DNA purification system (Promega, Madison, Wis.). Plasmid DNA was analyzed by restriction digestion with MluI (Promega) to identify the size of the insert. Plasmids containing the insert with the expected size were sequenced using an ABI Prism 377 DNA sequencer (Perkin-Elmer).
Cell line.
RAW 264.7 cells (American Type Culture Collection, Rockville, Md.) were selected because they are well characterized for efficient transfection (24). The mouse cells were grown in RPMI 1640 (Life Technologies) supplemented with 10% heat-inactivated fetal calf serum (HyClone Laboratories, Logan, Utah) in 25-cm2 culture flasks (Corning Costar Corporation, Cambridge, Mass.).
Plasmid construction and transformations.
Restriction enzymes were purchased from Promega or Boehringer Mannheim (Indianapolis, Ind.). Ligations were performed for 16 h at 16°C in a total volume of 10 μl containing the vector, insert, 10× ligation buffer, and 1 to 3 U of T4 ligase (Promega). The bovine thymosin β-10 full-length cDNA was subcloned into the expression vector pcDNA3.1 (Invitrogen, Carlsbad, Calif.) by a directional cloning approach. A 470-bp XbaI-EcoRI fragment was excised from pSPORT1 (Gibco BRL), gel purified, and subcloned into the XbaI-EcoRI-cut pcDNA3.1 to create the overexpression vector pJGcDNA3.1. Plasmids were transfected into TOP10 competent Escherichia coli cells (Invitrogen). Positive colonies were detected by growth on Luria-Bertani plates containing 50 μg of ampicillin/ml (Sigma Chemical Company). Mini- and maxipreparations of plasmid DNA were performed using the Wizard Plus DNA purification system (Promega). Gel purification of DNA was performed using the QIAEX II gel extraction kit (Qiagen, Chatsworth, Calif.). The size and orientation of the insert were analyzed by restriction digestion and sequencing, respectively.
Transfection of the RAW 264.7 mouse macrophage cell line with the bovine thymosin β-10 construct.
Twenty-four hours prior to electroporation, the RAW 264.7 cells were split 1:6 in fresh culture medium (23). Five million cells were added to 10 μg of PvuI- (Promega) linearized plasmid pJGcDNA3.1 in a total volume of 50 μl of phosphate-buffered saline and transferred to a 0.4-cm gap electroporation cuvette (BTX, San Diego, Calif.). The cuvettes were left to equilibrate at room temperature for 5 min before electroporation at 750 V/cm, 1,050 μF, and 129 Ω in an ECM 600 electroporation system (BTX). Dilutions of the transfected cells were seeded in 90-mm petri dishes and allowed to recover for 48 h when selection was applied with the antibiotic compound G418 (Life Technologies), which blocks protein synthesis in mammalian cells, at a concentration of 400 μg/ml. After 10 to 15 days, colonies were isolated using cloning rings (Bellco Glass Inc., Vineland, N.J.) and expanded. An RPA was conducted to confirm the expression of the transgene. The murine macrophages were transfected also with an empty vector as a negative control. The presence of the empty pcDNA3.1 in the murine macrophages was confirmed by PCR with a forward primer (5′-GGCACCAAAATCAACGGGACTTTC-3′) and reverse primer (5′-GCAAACAACAGATGGCTGGCAAC-3′).
Apoptosis analysis.
The G418-selected colonies were screened to identify the rate of spontaneous apoptosis in the transfected clones and compared with the parental cells using TUNEL at 2, 4, 6, and 7 days. Results are the average of three independent experiments. Statistical analysis was performed using Student's t test (8).
Nucleotide sequence accession number.
The bovine macrophage thymosin β-10 cDNA sequence was submitted to the GenBank database under the accession number AF294616.
RESULTS
Chromatin condensation and DNA fragmentation analysis.
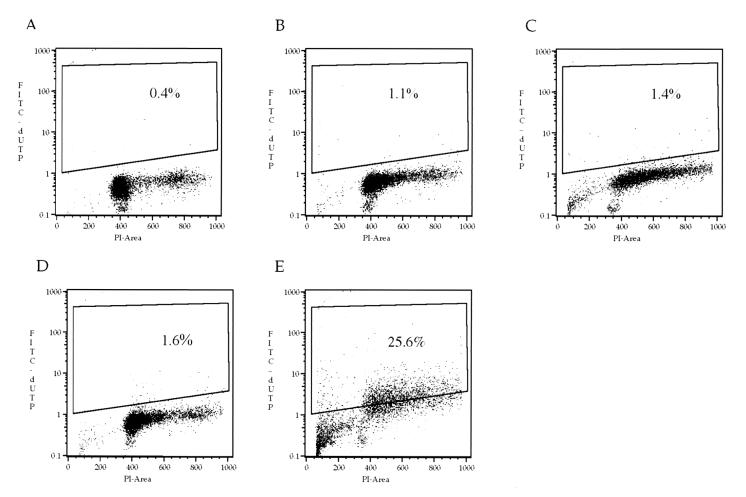
Chromatin condensation of M. bovis El Paso-infected macrophages was MOI and time dependent. Infected cells with MOIs of 1:1 and 10:1 developed chromatin condensation 12 h after infection, whereas an MOI of 25:1 induced the change as early as 4 h postinfection, with an increase over time (Fig. 1 and Table 1). The number of bacteria inoculated had a direct effect on the DNA fragmentation rate. Infection with 25 bacteria per macrophage required 8 h to increase the apoptosis level from 0.4% in noninfected cells to 25.6% (Fig. 2).
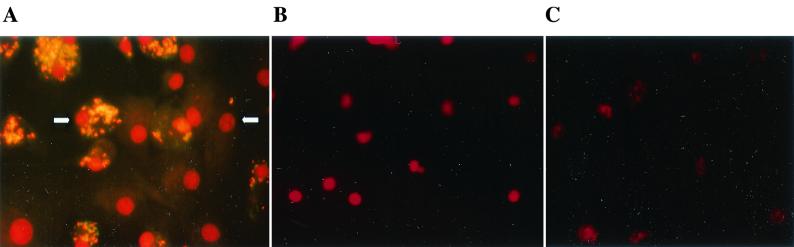
FIG. 1.
Propidium iodide-stained bovine macrophages. (A) M. bovis-infected macrophages (MOI, 25:1) 12 h postinoculation, demonstrating nuclei of M. bovis-containing (bright dots) and bystander (uninfected) macrophages with chromatin condensation (arrows). (B) Noninfected macrophages with normal nuclei. (C) Chromatin condensation of macrophages treated with camptothecin (10 μg/ml) for 72 h. Magnification, ×800.
TABLE 1.
Chromatin condensation of bovine macrophages either noninfected or infected with three different MOIs of M. bovis El Paso strain
| Time (h) | % Cells with chromatin condensation at MOI ofa:
|
|||
|---|---|---|---|---|
| C | 1:1 | 10:1 | 25:1 | |
| 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 9.5 ± 2.52 |
| 6 | 0 | 0 | 0 | 12.0 ± 2.91 |
| 8 | 0 | 0 | 0 | 19.0 ± 2.65 |
| 10 | 0 | 0 | 0 | 17.5 ± 2.52 |
| 12 | 0 | 8.0 ± 1.68 | 15.5 ± 1.88 | 33.5 ± 3.46 |
| 15 | 0 | 32.5 ± 2.70 | 47.5 ± 2.52 | 44.5 ± 2.22 |
| 18 | 0 | 55.5 ± 3.03 | 43.5 ± 2.22 | 60.5 ± 1.88 |
| 21 | 0 | 20.0 ± 5.31 | 72.0 ± 2.91 | 68.5 ± 2.22 |
| 24 | 0 | 18.0 ± 5.04 | 29.5 ± 3.46 | 77.0 ± 3.14 |
C, noninfected macrophages; values represent the mean ± standard error of the mean of two independent experiments.
FIG. 2.
DNA fragmentation analysis of M. bovis-infected bovine monocyte-derived macrophages. Bovine macrophages were infected using two MOIs (10:1, 25:1). Analysis of DNA fragmentation by the TUNEL method was conducted after 4 and 8 h postinfection. (A) Noninfected macrophages, 8 h of incubation; (B) infected macrophages (MOI, 10:1), 4 h of incubation; (C) infected macrophages (MOI, 10:1), 8 h of incubation; (D) infected macrophages (MOI, 25:1), 4 h of incubation; (E) infected macrophages (MOI, 25:1), 8 h of incubation. Results are representative of two replications.
Differential gene expression analysis.
A candidate gene differentially expressed after M. bovis infection was identified by differential display reverse transcriptase PCR in two independent experiments. The size of the candidate band was approximately 200 bp and increased shortly after infection (0 h). Band reamplification was performed with the same combination of primers used in the first screening and cloned into the pCRTrap vector. The differential expression of the candidate was confirmed by reverse Northern blotting and RPAs. The distribution and concentration of the signals were very similar to the band pattern observed in the differential display gels. The differentially expressed mRNA was found to have a 2-fold increase at 0 h and then decrease to 1.15- and 1.43-fold expression at 12 and 24 h postinfection, respectively (Fig. 3). A GenBank sequence database search identified a 90% sequence homology of the putative gene to the human thymosin β-10 mRNA (accession number M92381), rat thymosin β-10 mRNA (accession number M17698), and mouse thymosin β-10 mRNA (accession number Z48496).
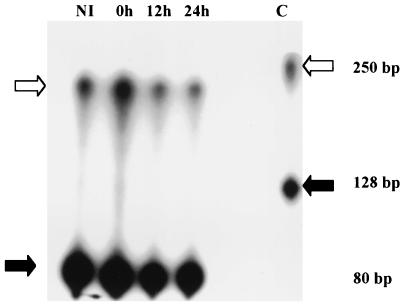
FIG. 3.
Results of RPA of bovine thymosin β-10. RNA from M. bovis-noninfected (NI) and -infected macrophages at 0, 12, and 24 h postinfection was hybridized to a thymosin β-10-radiolabeled probe, digested with RNase, and loaded into a 6% polyacrylamide gel. White arrows indicate the thymosin β-10-protected fragments on the right and the control probe (C) on the left, whereas the black arrows indicate the protected fragments of the ribosomal 18S internal control on the right and control probe on the left. Thymosin β-10 mRNA expression increased twofold after infection (0 h), while the level of expression in noninfected cells at 0 h was considered baseline. The fold increase was obtained as a ratio of the size and density of the protected fragment representing the differentially expressed gene to the size and density of the protected fragment of the internal control. Values are representative of two independent experiments.
Thymosin β-10 mRNA expression in bovine macrophages.
We demonstrated that M. bovis infection induces a twofold increase in the expression of thymosin β-10 mRNA in bovine monocyte-derived macrophages immediately after inoculation. To confirm the specificity of the result and to eliminate the phagocytic process as a potential nonspecific stimulus driving the upregulation of expression, bovine macrophages were inoculated with latex beads, heat-killed and live M. bovis El Paso, or M. bovis BCG. As expected, phagocytosis of latex beads did not change the expression of thymosin β-10 in macrophages at any time postinoculation. On the other hand, macrophages infected with M. bovis had an increased level of expression compared to noninoculated cells. The type of strain used had a direct effect on gene expression. Heat-killed M. bovis induced a slight increase in expression after 24 h of incubation, whereas live wild-type M. bovis and M. bovis BCG produced approximately twofold increases after 24 h of infection.
cDNA library screening.
A bovine macrophage cDNA library was screened to isolate the full-length cDNA sequence of thymosin β-10. The first screening identified an average of 10 positive colonies per filter containing 5,000 colonies. After three rounds of screening, 100% of the colonies were identified as positive by the thymosin β-10 probe. Restriction enzyme digestion of plasmid DNA from four of the positive selected colonies demonstrated the presence of an insert of about 500 bp in size in every single colony. Sequence analysis revealed two variants of the full-length cDNA sequence of the bovine thymosin β-10 gene. Two colonies of the four selected had a 451-bp fragment, whereas the other two had a 448-bp fragment with a 3-bp deletion at the beginning of the 5′ untranslated region (UTR). Sequence analysis identified the DNA fragment as the putative bovine thymosin β-10. The protein predicted from this sequence consists of 42 amino acids and contains the actin binding motif (Fig. 4).
FIG. 4.
Comparative amino acid sequence of bovine (accession number AF294616), human (accession number A27704), and rat (accession number M17698) thymosin β-10. The bovine sequence is 88% identical and 97% similar to the human and rat sequences. Amino acids in bold identify the actin binding motif.
Transformation experiments.
Several colonies were obtained after transformation of the competent E. coli with the pJGcDNA3.1 expression vector containing the bovine thymosin β-10 full-length cDNA. Seven transformants were analyzed by restriction digestion to verify the size of the insert. All the colonies selected contained an insert of about 500 bp, which is the expected size of the full-length cDNA corresponding to thymosin β-10. The orientation of the insert was investigated in three of the seven candidates by sequence analysis. All the inserts analyzed had the orientation required to be used in the overexpression studies, as expected from a directional cloning experiment.
Analysis of RAW 264.7 cells transfected with the bovine thymosin β-10 construct.
A total of 10 colonies transfected with the full-length cDNA of bovine thymosin β-10 was recovered from the cells surviving the selection pressure of G418 after 10 days. The expression of the transgene was investigated by RPA. Six of 10 selected clones had a protected fragment corresponding to the size of the bovine transgene; however, the expression of some transgenes was somewhat low. The clone with the highest mRNA expression was selected for further experiments. The presence of the empty pcDNA3.1 in the murine macrophages was confirmed by PCR.
Apoptosis analysis.
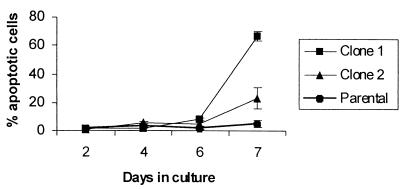
The apoptosis rates of one overexpressing clone and one clone transfected with the empty vector were analyzed by TUNEL at 2, 4, 6, and 7 days after splitting and compared with those of parental cells. Parental untransfected cells had an apoptotic rate ranging from 1.3 to 4.7% after 2 and 7 days in culture, whereas cells overexpressing thymosin β-10 increased the apoptotic rate from 1.1 to 66.5%, while cells harboring the empty vector increased the rate of apoptosis from 0.9 to 22.8% after 7 days in culture (Fig. 5). The apoptotic rates of the overexpressing cells were significantly different than rates in both empty vector transfected cells (P < 0.01) and parental cells (P < 0.001).
FIG. 5.
Percentage of spontaneous apoptosis of parental (untransfected) and bovine thymosin β-10-transfected RAW 264.7 cells. Clones 1 and 2 were transfected with the bovine thymosin β-10 full-length cDNA or the empty vector, respectively. Values represent the mean ± standard error of the mean of three independent experiments.
DISCUSSION
We report for the first time the induction of apoptosis in bovine macrophages infected with a virulent M. bovis field strain. Macrophage apoptosis was time and MOI dependent. Rates of apoptosis measured as chromatin condensation and DNA fragmentation progressed rapidly after infection and increased significantly postinfection. Also, the number of bacteria per macrophage had a direct effect on the apoptotic counts. Macrophages infected with an MOI of 25:1 developed chromatin condensation and DNA fragmentation at 4 and 8 h, respectively, whereas changes in chromatin condensation induced by MOIs of 10:1 and 1:1 required a longer time and resulted in fewer apoptotic cells. Mycobacterial species have been shown to induce macrophage apoptosis under different circumstances. Hayashi et al. (13) found that sonicated extracts of M. avium induced apoptosis in human monocytes and macrophages. Keane and colleagues (16) demonstrated that M. tuberculosis induced apoptosis of alveolar human macrophages; moreover, Placido and coworkers (22) identified the capacity of M. tuberculosis to induce apoptosis in bronchoalveolar lavage cells obtained from patients with reactive pulmonary tuberculosis. However, it also has been reported that infection with reduced numbers (MOI, 1:1) of viable M. tuberculosis organisms actually prevented apoptosis of monocytes (7). Results from this study suggest that in the bovine model, macrophage apoptosis is induced regardless of the MOI utilized. In addition, not only infected cells underwent apoptosis, but also uninfected bystander cells developed apoptosis (Fig. 1), suggesting a possible role of macrophage-secreted molecules in the induction of apoptosis in bystander cells. Apoptosis seems to play a role in the pathogenesis of tuberculosis, and whether the final outcome favors the host or the pathogen depends on the characteristics of the participants in the interaction. The presence of M. bovis in membrane-bound vesicles may help the host to clear the infection in other activated phagocytic cells; however, if the phagocytic cells are not competent, it may facilitate spread of the bacteria to new host cells.
We identified a twofold increase of the putative bovine thymosin β-10 mRNA in M. bovis-infected bovine macrophages shortly after infection. Gene upregulation after Mycobacterium infection of macrophages has been documented previously. Tchou-wong and colleagues (25) reported that M. tuberculosis induced the expression of interleukin-2 receptor (IL-2R) on human peripheral blood monocytes associated with the presence of NF-κB on the promoter site of IL-2R. In addition, Tomioka et al. (26) demonstrated that murine peritoneal macrophages infected with M. avium complex rapidly increased the expression of intercellular adhesion molecule-1 due to endogenous tumor necrosis factor alpha. Wang and coworkers (27) reported toll-like receptor 2 mRNA induction following infection of murine macrophages with M. avium.
To further investigate the specific role of M. bovis in the upregulation of bovine thymosin β-10, bovine macrophages were stimulated with live and heat-inactivated M. bovis, attenuated M. bovis BCG, and latex beads. Phagocytosis of latex beads did not affect the expression of thymosin β-10, whereas bacterial suspensions upregulated the gene expression, suggesting that M. bovis or mycobacterial products are active in the process. Heat-inactivated bacteria induced a slight increase in thymosin β-10 mRNA, whereas live virulent and attenuated M. bovis increased gene expression almost twofold. These results suggest that viability of mycobacteria contributes to thymosin β-10 upregulation within the 24-h incubation period. Upregulation of thymosin β-10 was observed after 24 h of incubation, which differs from the time of upregulation observed from the differential display results. The only difference between the two experiments was the origin of the macrophages used. The cells were obtained from two different donor cattle, which suggests an individual variation. In fact, the mRNA analysis of M. bovis-infected macrophages from another two donors confirmed an increase in the gene expression at 0 and 24 h postinfection, supporting the idea of individual variation in thymosin β-10 upregulation in response to M. bovis infection in macrophages (data not shown).
Two variants of the full-length sequence of the cDNA were obtained by screening the bovine macrophage cDNA library. The relevance of the 3-base deletion in the 5′ UTR of one of the variants is not known. However, the presence of protein variants has been documented previously. Lin et al. (21) reported a difference in 14 nucleotides at the 5′ UTR from the testis-specific thymosin β-10 and the ubiquitous thymosin in rats. The β thymosins are a series of homologous polypeptides that range in size between 41 and 45 amino acids (9, 12, 17). The protein predicted for the putative bovine thymosin β-10 consists of 42 amino acids and contains the G-actin binding motif. It also contains three of the four amino acids related to the tetrapeptide present in thymosin β-4 that exerts a high inhibitory activity on cell proliferation (9). Acceleration of apoptosis is one of the functions reported for thymosin β-10 (11). Upregulation of thymosin β-10 is associated with M. bovis infection in macrophages, suggesting a role of thymosin β-10 in macrophage death. We demonstrated that M. bovis infection induces bovine macrophages to undergo apoptosis. In an attempt to link the upregulation of thymosin β-10 in macrophages with the induction of apoptosis, overexpression analysis of a mouse macrophage cell line transfected with a construct carrying the bovine thymosin β-10 full-length cDNA was done. Cells transfected with the bovine thymosin β-10 cDNA construct survived the selection protocol, indicating the presence of the transgene. Although expression of thymosin β-10 was confirmed by RPA, the size and density of the protected fragments indicated a reduced level of expression for the overexpressing transfected cells. It is not surprising that there was a low level of expression in the selected clones carrying the full-length cDNA of thymosin β-10. One of the functions attributed to thymosin β-10 is the induction of apoptosis, a process that exerts a negative effect on the cells. Therefore, clones with high expression of the transgene are expected to die sooner, eliminating the possibility of identification during the selection process. It is likely that the only clones surviving the selection are those with a low level of gene expression. The thymosin β-10-transfected cells underwent spontaneous apoptosis at a higher rate than did cells transfected with the empty vector or untransfected cells. Cells transfected with the empty vector also exhibited increased apoptosis; however, the difference between those cells and untransfected cells was not significant. These observations suggest a role of the transgene in macrophage death. The molecular mechanism associated with this phenomenon is not known. A possible scenario should include the thymosin β-10 G-actin binding activity. The decrease in the concentration of free G-actin would impair the binding to DNase I by this protein, resulting in a higher rate of DNA degradation. A similar mechanism has been described for the IL-1β-converting enzyme. Cleavage of G-actin by IL-1β-converting enzyme results in a markedly decreased ability of G-actin both to inhibit the endonucleolytic activity of DNase I and to reduce the ability of G-actin to polymerize (15).
In summary, M. bovis infection induces both upregulation of thymosin β-10 and apoptosis in bovine macrophages. Viability of the microorganism was found to be required for optimal differential expression of the gene. Transfected clones overexpressing thymosin β-10 underwent apoptosis at a higher rate than the empty vector transfected and parental cells. Our evidence suggests that upregulation of thymosin β-10 in M. bovis-infected macrophages is linked with increased cell death due to apoptosis.
Acknowledgments
We gratefully acknowledge the critical discussions and suggestions provided by Jorge Piedrahita, Roger Smith III, and Robert Barthel and the technical assistance of Betty Rosenbaum and Renato de Lima Santos.
J. A. Gutiérrez-Pabello received financial support from the Universidad Nacional Autonoma de México through the Departamento de Superación Académica of the Dirección General de Asuntos del Personal Académico. This research was supported by the Texas Agricultural Experiment Station, project no. 8409.
Editor: R. N. Moore
REFERENCES
- 1.Balcewicz-Sablinska, M. K., J. Keane, H. Kornfeld, and H. G. Remold. 1998. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-α. J. Immunol. 161:2636-2641. [PubMed] [Google Scholar]
- 2.Calzone, F. J., R. S. Britten, and E. H. Davidson. 1987. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 152:611-632. [DOI] [PubMed] [Google Scholar]
- 3.Campbell, G. A., and L. G. Adams. 1992. The long-term culture of bovine monocyte-derived macrophages and their use in the study of intracellular proliferation of Brucella abortus. Vet. Immunol. Immunopathol. 34:291-305. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, G. A., L. G. Adams, and B. A. Sowa. 1994. Mechanisms of binding of Brucella abortus to mononuclear phagocytes from cows naturally resistant or susceptible to brucellosis. Vet. Immunol. Immunopathol. 41:295-306. [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski, P. 1993. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques 15:532-537. [PubMed] [Google Scholar]
- 6.Darzynkiewicz, Z., S. Bruno, G. Del Bino, W. Gorczyca, M. Hotz, P. Lassota, and F. Traganos. 1992. Features of apoptotic cells measured by flow cytometry. Cytometry 13:795-808. [DOI] [PubMed] [Google Scholar]
- 7.Durrbaum-Landmann, I., J. Gercken, H. D. Flad, and M. Ernst. 1996. Effect of in vitro infection of human monocytes with low numbers of Mycobacterium tuberculosis bacteria on monocyte apoptosis. Infect. Immun. 64:5384-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foil, L. D. 1990. GraphPad Instat software, version 1.15. Louisiana State University, Baton Rouge, La.
- 9.Grillon, C., K. Riegere, J. Bakala, D. Schott, J. Morgat, E. Hannapel, W. Voelter, and M. Lenfant. 1990. Involvement of thymosin β-4 and endoproteinase Asp-N in the biosynthesis of the tetrapeptide AcSerAspLysPro a regulator of the hematopoietic system. FEBS Lett. 274:30-34. [DOI] [PubMed] [Google Scholar]
- 10.Grunstein, M., and D. Hogness. 1975. Colony hybridization: a method for the isolating of cloned DNAs that contain a specific gene. Proc. Natl. Acad. Sci. USA 72:3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall, A. K. 1995. Thymosin β-10 accelerates apoptosis. Cell. Mol. Biol. Res. 41:167-180. [PubMed] [Google Scholar]
- 12.Hannappel, E., G.-J. Xu, J. Morgan, J. Hempstead, and B. L. Horecker. 1982. Thymosin β-4, a ubiquitous peptide in rat and mouse tissues. Proc. Natl. Acad. Sci. USA 79:2172-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi, T., A. Catanzaro, and S. P. Rao. 1997. Apoptosis of human monocytes and macrophages by Mycobacterium avium sonicate. Infect. Immun. 65:5262-5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janmey, P. A., and C. Chaponnier. 1995. Medical aspects of the actin cytoskeleton. Curr. Opin. Cell Biol. 7:111-117. [DOI] [PubMed] [Google Scholar]
- 15.Kalayar, C., T. Örd, M. P. Testa, L. Zhong, and D. E. Bredesen. 1996. Cleavage of actin by interleukin 1β-converting enzyme to reverse DNase I inhibition. Proc. Natl. Acad. Sci. USA 93:2234-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keane, J., M. K. Balcewicz-Sablinska, H. G. Remold, G. L. Chupp, B. B. Meek, M. J. Fenton, and H. Kornfeld. 1997. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 65:298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leondiadis, L., E. Livaniou, I. Vassiliadou, N. Ferderigos, D. S. Ithakissios, and G. P. Evangelatos. 1996. Development of specific anti-thymosin β-10 antipeptide antibodies for application in immunochemical techniques. Peptides 17:1091-1096. [DOI] [PubMed] [Google Scholar]
- 18.Liang, P., L. Averboukh, and A. B. Pardee. 1994. Method of differential display. Methods Mol. Genet. 5:3-16. [Google Scholar]
- 19.Liang, P., and A. B. Pardee. 1995. Recent advances in differential display. Curr. Opin. Immunol. 7:274-280. [DOI] [PubMed] [Google Scholar]
- 20.Liang, P., and A. B. Pardee. 1997. Differential display: a general protocol. Methods Mol. Biol. 85:3-11. [DOI] [PubMed] [Google Scholar]
- 21.Lin, S. C., and M. Morrison-Bogorad. 1991. Cloning and characterization of testis-specific thymosin β-10 cDNA. Expression in post-meiotic male germ cells. J. Biol. Chem. 266:23347-23353. [PubMed] [Google Scholar]
- 22.Placido, R., G. Mancino, A. Amendiola, F. Mariani, S. Vendetti, M. Piacentini, A. Sanduzzi, M. L. Bocchino, M. Zembala, and V. Colizzi. 1997. Apoptosis of human monocytes/macrophages in Mycobacterium tuberculosis infection. J. Pathol. 181:31-38. [DOI] [PubMed] [Google Scholar]
- 23.Stacey, K. J., I. L. Ross, and D. A. Hume. 1993. Electroporation and DNA-dependent cell death in murine macrophages. Immunol. Cell Biol. 71:75-85. [DOI] [PubMed] [Google Scholar]
- 24.Sun, H., K. Kwiatkowska, and H. L. Yin. 1996. β-thymosins are not simple actin monomer buffering proteins. J. Biol. Chem. 271:9223-9230. [PubMed] [Google Scholar]
- 25.Tchou-wong, K., O. Tanabe, C. Chi, T. Yie, and W. N. Rom. 1999. Activation of NF-κB in Mycobacterium tuberculosis-induced interleukin-2 receptor expression in mononuclear phagocytes. Am. J. Respir. Crit. Care Med. 159:1323-1329. [DOI] [PubMed] [Google Scholar]
- 26.Tomioka, H., T. Shimizu, W. W. Maw, and K. Ogasawara. 2000. Roles of tumor necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), and IL-10 in the modulation of intercellular adhesion molecule-1 (ICAM-1) expression by macrophages during mycobacterial infection. Clin. Exp. Immunol. 122:335-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, T., W. P. Lafuse, and B. S. Zwilling. 2000. Regulation of toll-like receptor 2 expression by macrophages following Mycobacterium avium infection. J. Immunol. 165:6308-6313. [DOI] [PubMed] [Google Scholar]
- 28.Yu, F., S. Lin, M. Morrison-Bogorad, and H. L. Yin. 1994. Effects of thymosin β-4 and thymosin β-10 on actin structures in living cells. Cell Motil. Cytoskel. 27:13-25. [DOI] [PubMed] [Google Scholar]