Abstract
Eleven PhoP-PhoS homolog pairs were identified by searching the Enterococcus faecalis V583 genome sequence database at The Institute for Genomic Research with the Bacillus subtilis PhoP-PhoS sequences. Each pair appears to be a potential two-component system composed of a response regulator and a sensor kinase. Seven of the homologs were disrupted in E. faecalis strain OG1RF. TX10293, a mutant disrupted in one of these genes (etaR, the first gene of the gene pair designated etaRS), showed delayed killing and a higher 50% lethal dose in a mouse peritonitis model. The predicted EtaR protein sequence showed greatest similarity to LisR of Listeria monocytogenes (77%) and CsrR of Streptococcus pyogenes (70%); EtaS is 53% similar to LisK and 54% similar to CsrS. When grown in vitro, the TX10293 mutant was more sensitive to low pH (pH 3.4) and more resistant to high temperature (55°C) than wild-type OG1RF. In conclusion, many potential two-component systems are identified for E. faecalis, one of which, EtaRS, was shown to be involved in stress response and virulence.
Enterococci are normal flora of the human intestine, found in the feces of most healthy adults. These organisms are also one of the leading causes of nosocomial infections, producing various diseases including bacteremia, urinary tract infections, endocarditis, intra-abdominal and pelvic infections, neonatal infections, and central nervous system infections, among others (15). Seventy to ninety percent of clinical enterococcal isolates are Enterococcus faecalis, with Enterococcus faecium playing an increasingly important role in the past decade.
Bacteria often use two-component systems to control expression of virulence factors in response to environmental changes throughout the various stages of infection. These two-component systems are generally composed of a sensor kinase that recognizes one or more specific environmental signals and a response regulator protein that mediates cell response by regulating expression of specific operons or modulating protein functions (9). The genes encoding the sensor and regulator are often cotranscribed as a single transcript (9). An example is the CsrRS (also called CovRS) system of Streptococcus pyogenes. The CsrRS system is a two-component system that was first identified as a negative regulator of hyaluronic acid capsule synthesis (8, 12). In an attempt to identify potential two-component systems in S. pyogenes, PhoP-PhoS, a global regulatory system in Bacillus subtilis, was used as query, and CsrRS was identified as a PhoPS homolog (6) that negatively regulates the transcription of several virulence factors, including ska (encoding streptokinase), sagA (encoding streptolysin S), speMF (encoding mitogenic factor), and hasA, the first gene in the operon for capsule synthesis (6). The phosphorylated CsrR was then found to be able to bind to the promoters of ska, sagA, speMF, and hasA with different affinities, consistent with its function as a response regulator and its differential regulation of these virulence genes (14). Two-component systems in enterococci have not been well studied, except for the VanRS and VanRBSB systems, which regulate resistance of enterococci to vancomycin (1).
To identify potential two-component regulatory gene pairs, the E. faecalis V583 genome sequence at The Institute for Genomic Research (TIGR) was searched using the sequences of B. subtilis PhoP-PhoS (10). Here we report that disruption of one set of phoPS homologs, etaRS (enterococcal two-component system a), affects the virulence of E. faecalis in a mouse peritonitis model. In vitro, the disruption mutant also demonstrated a rate of survival at high temperature (55°C) and low pH (3.4) that was different from that of wild-type OG1RF.
MATERIALS AND METHODS
Bacterial strains and media.
The wild-type E. faecalis strain used in this study, OG1RF, has been described previously (16). Escherichia coli was grown in Luria-Bertani broth or agar with appropriate antibiotics at 37°C. Brain heart infusion (BHI) medium (Difco Laboratories, Detroit, Mich.) was used for growth of E. faecalis. SR medium was used for electroporation of E. faecalis. The concentration of kanamycin used for selection was 25 μg/ml for E. coli and 2,000 μg/ml for E. faecalis.
DNA manipulations.
DNA amplification (PCR), cloning, and Southern blotting were performed using standard methods (19) unless otherwise described. Transformation of E. coli and electroporation of E. faecalis were as described previously (2, 13).
Construction and analysis of disruption mutants.
An internal fragment of the first gene of each of the potential two-component gene pairs was amplified from OG1RF genomic DNA (oligonucleotides used are listed in Table 1) and cloned into the TOPO TA cloning vector (Invitrogen, Carlsbad, Calif.). The fragments were then released from the vector by EcoRI and cloned into pTEX4577 (17), which is a derivative of pBluescript KS(−) and contains a kanamycin resistance gene of gram-positive origin. The resulting constructs were electroporated into E. faecalis OG1RF followed by selection on SR agar plates with 2,000 μg of kanamycin per ml. The correct insertions were confirmed by PCR and Southern blot analysis. Growth curves of OG1RF and mutants and stability of single-crossover disruptions in mutants were determined as described previously (22).
TABLE 1.
The PhoP-PhoS homologs identified in E. faecalis V583 and outcome of disruption in E. faecalis OG1RF
| Locus name | E value (R/S) | Directiona | Oligonucleotidesb | Disruption outcomec | Best hit (R/S)d |
|---|---|---|---|---|---|
| 10276 | 5e-75/4e-05 | R→S | 5′ TTGGAAAAAGAAGGCTATC | Viable | Response regulator of Listeria innocua/sensor kinase of Listeria monocytogenes; NP_471974/NP_466023 |
| 5′ ACGTAAATGTCTAATGTGGAC | |||||
| 10299 | 3e-68/2e-26 | R→S | 5′ AAAATGGATGGCTTAGAA | Not recovered | VicR/VicK of Enterococcus faecalis; CAB64972/CAB64973 |
| 5′ AAATACGTTGGATGACTTG | |||||
| 10275 | 9e-49/3e-19 | R→S | 5′ ATGATGATAAAGAAATTGTAGAAC | Viable | Response regulator/histidine kinase of Lactobacillus sakei; AAD10265/AAD10266 |
| 5′ CACACGATTTGGATGACT | |||||
| 10292 | 2e-46/1e-20 | R→S | 5′ TAAAGATGATGGTAAAGAAGG | Viable | Response regulator/histidine kinase of Lactobacillus sakei; AAD10267/AAD10268 |
| 5′ ATATGGGCATCAACTGTC | |||||
| 10293 | 1e-41/2e-82 | R→S | 5′ AGATGAAAAGAACTTAGCGAGATT | Viable | LisR of Listeria monocytogenes/sensor kinase of Lactococcus lactis; AAF03932/NP_267749 |
| 5′ CGTTCACGTTTTCCATTAGC | |||||
| 10321e | 5e-41/1e-11 | R→S | NA | NA | VanRB/VanSB of Enterococcus faecalis; Q47744/Q47745 |
| 10303 | 3e-38/2e-25 | S→R | 5′ TGCCCAAAAAGAAAATCCAT | Viable | Response regulator/histidine kinase of Clostridium acetobutylicum; NP_346962/NP_346958 |
| 5′ AAGCGTTCGAATAAATAAGGTAAT | |||||
| 10288f | 2e-37/6e-10 | S→R | 5′ TTAATAAAACTGCTGAACCA | NA | Response regulator/sensor kinase of Listeria innocua; NP_472154/NP_472155 |
| 5′ TCCATTTTACCATTGTGC | |||||
| 37200 | 4e-30/8e-25 | S→R | 5′ AAATATGAAGCTAATCGGAAAGAA | Viable | Response regulator/histidine kinase of Clostridium acetobutylicum; NP_346962/NP_346958 |
| 5′ AAGCGTTCGAATAAATAAGGTAAT | |||||
| 10298 | 6e-27/5e-07 | R→S | 5′ CGAGAATTAATTAGCGAGGAA | Viable | Response regulator/histidine kinase of Lactobacillus sakei; AAD10258/AAD10259 |
| 5′ CCGTCAGCGTATTGTCATC | |||||
| 10285f | 2e-26/3e-06 | R→S | Could not amplify | NA | Response regulator VncR/sensor kinase VncS of Streptococcus pneumoniae; NP_345115/NP_345116 |
Direction of transcription. In all cases, the gene on the left was disrupted. R, response regulator; S, sensor kinase.
Oligonucleotides used to amplify the gene on the left. NA, not attempted.
The outcome of gene disruption.
The GenBank accession numbers are given.
The vancomycin resistance regulatory system VanRBSB.
PCR product not obtained from OG1RF.
Mouse peritonitis model.
The mouse peritonitis model and the 50% lethal dose (LD50) were described previously (18, 20, 22) and applied in this study with minor modifications: 25% sterile rat fecal extract was used. Determination of Kaplan-Meier survival curves and log rank analysis were performed using StatView (Abacus Concepts, Inc., Berkeley, Calif.).
Acid and heat sensitivity assay.
Overnight cultures were inoculated into 10 ml of fresh BHI, and log phase cells (35 Klett units; Klett-Summerson photoelectric colorimeter, Klett MFG Co., Inc., New York, N.Y.) were collected, washed, and resuspended in 1 ml of 10 mM MgSO4, which was kept at 4°C for up to 5 days. On the day of experiment, these cells (in 10 mM MgSO4) were diluted 1:50 into BHI and incubated at 37°C. When log phase was reached (35 Klett units), cells were diluted 1:50 into BHI that had been adjusted to pH 3.4 with 3 M lactic acid and then incubated at 37°C for 40 min (for acid sensitivity assay). For heat sensitivity assay, cells were diluted 1:50 into BHI and incubated at 55°C for 40 min. The starting numbers of cells added to the tubes prepared for acid or heat sensitivity assays were determined by plating serial dilutions on BHI agar plates and incubating overnight. After 40 min, the number of survivors was determined as described above. The percentage of cells surviving was calculated by comparing the starting and the 40-min cell counts. The experiments were performed twice, duplicates were used in each experiment, and the significance of the difference between wild-type OG1RF and the mutant was determined by a t test (for two-group comparisons).
Ethanol and high-salt sensitivity assay.
Overnight cultures of OG1RF, TX10293, and TX37200 were diluted 1:50 into BHI with 5% ethanol or 6.5% NaCl and incubated at 37°C. Klett units were measured every 2 h up to 8 h and then at 24 h.
RESULTS
Identification of PhoP-PhoS homologs in E. faecalis.
The E. faecalis V583 genome sequence (obtained from TIGR) was searched for potential two-component regulatory gene pairs using the B. subtilis PhoP and PhoS polypeptide sequences to perform BLAST searches. Eleven gene pairs, to which we gave the numerical designations listed in Table 1, were identified. Each gene pair is composed of a gene encoding a potential response regulator and a gene encoding a potential sensor kinase. Sequence analysis also suggested that each gene pair forms a potential operon, although the gene order varies (Table 1). One of the gene pairs encodes the known VanRBSB system which is involved in regulation of VanB-type vancomycin resistance gene expression in E. faecalis (5) and was not studied further.
Inactivation of PhoP-PhoS homologs in E. faecalis OG1RF.
V583 has multiple resistances to antibiotic markers of commonly used vectors; thus, OG1RF, a strain commonly used in the laboratory for genetic manipulations, was used to construct disruption mutants. Based on the sequences identified in V583, oligonucleotides (listed in Table 1) were designed to amplify from OG1RF the internal part of the first (upstream) gene of each of the 10 gene pairs studied. We could not amplify the sequence at locus 10285 from either OG1RF or V583, so this gene pair was not studied further. For the sequence at locus 10288, we did not get a PCR product from OG1RF genomic DNA with the designed primers, while amplification from V583 genomic DNA was successful under the same conditions, suggesting that the gene may not be present in OG1RF or may have a different sequence in OG1RF. The PCR products for the other eight genes were obtained and used to make disruption mutants in OG1RF. We isolated seven mutants: TX10276, TX10275, TX10292, TX10293, TX10303, TX37200, and TX10298. Disruption mutation at locus 10299 was not obtained, suggesting the possibility that this gene pair may be essential for bacterial growth.
Growth rates and stability of the mutants.
The growth rates of OG1RF and the seven mutants in BHI broth at 37°C were examined. The results showed that all the mutants had similar doubling times in log phase and similar densities at stationary phase. After 24 h, the cell counts of these cultures were comparable for OG1RF and the seven mutants. The colony counts on BHI and BHI-kanamycin were comparable for all the mutants, suggesting that the disruption mutations were stable under this growth condition.
Examination of the two-component system mutants in a mouse peritonitis model.
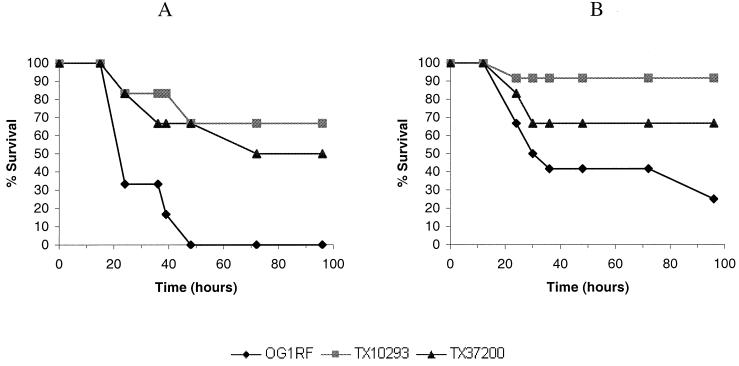
To test if disruption of these potential two-component systems had an effect on the virulence of OG1RF, the seven mutants were examined in a mouse peritonitis model, along with wild-type OG1RF. In the mouse peritonitis model, initial experiments showed that two mutants, TX10293 and TX37200, had higher survival rates than wild-type OG1RF when the number of cells inoculated was the same or slightly greater than that for the wild type (P = 0.009 for TX10293 versus OG1RF and P = 0.022 for TX37200 versus OG1RF; the inocula were 5.0 × 107, 5.0 × 107, and 8.0 × 107 CFU for OG1RF, TX10293, and TX37200) (Fig. 1A). The two sets of genes disrupted in TX10293 and TX37200, respectively, were named etaRS and etbRS (enterococcal two-component systems a and b). The other mutants did not show a significant difference in lethality from that of OG1RF (data not shown). To confirm these results, the mutants TX10293 and TX37200 were further examined in a larger-scale experiment. A group of 12 mice was used for each of the three inocula (based on the optical density at 600 nm and confirmed by cell counts). The etaR mutant, TX10293, again showed a higher percentage of surviving mice and delayed killing compared with that of OG1RF, while the etbS mutant, TX37200, showed a similar trend, but the difference between TX37200 and the wild type, OG1RF, was not significant (P = 0.0013 for TX10293 versus OG1RF, and P = 0.092 for TX37200 versus OG1RF; the inocula were 2.7 × 107, 3.0 × 107, and 3.2 × 107 CFU for OG1RF, TX10293, and TX37200) (Fig. 1B). The LD50s for OG1RF, TX10293, and TX37200 were 1.6 × 107, 7.3 × 107, and 3.9 × 107, respectively. The results suggest that the two-component system EtaRS contributes to virulence of E. faecalis in the mouse peritonitis model. Because of the less consistent results for the etbS mutant, the role of the EtbRS system in E. faecalis virulence is not clear.
FIG. 1.
Percentages of surviving mice injected with OG1RF, TX10293, or TX37200 over time. (A) The inoculum size and the number of mice used for each strain were as follows: OG1RF, 5.0 × 107 CFU (six mice); TX10293, 5.0 × 107 CFU (six mice); and TX37200, 8.0 × 107 CFU (six mice). (B) The inoculum size and the number of mice used for each strain were as follows: OG1RF, 2.7 × 107 (12 mice); TX10293, 3.0 × 107 CFU (12 mice); TX37200, 3.2 × 107 CFU (12 mice). The LD50 values were calculated based on this experiment (see the text).
The numbers of colonies recovered from the spleens of dead mice receiving the mutants were comparable on BHI agar and on BHI-kanamycin agar plates, indicating that the disruptions were at least relatively stable in vivo. It was observed that TX10293, the etaR mutant, was mucoid on the primary isolation plates when recovered from the spleens, suggesting that the EtaRS system may negatively regulate capsule synthesis of E. faecalis in vivo. The recovered strains were frozen immediately. However, when restreaked, the etaR mutant was not mucoid, suggesting that in vivo conditions were also important for this phenotype or the mucoidy was disadvantageous in vitro.
Sequence analysis of etaRS genes and their encoded proteins.
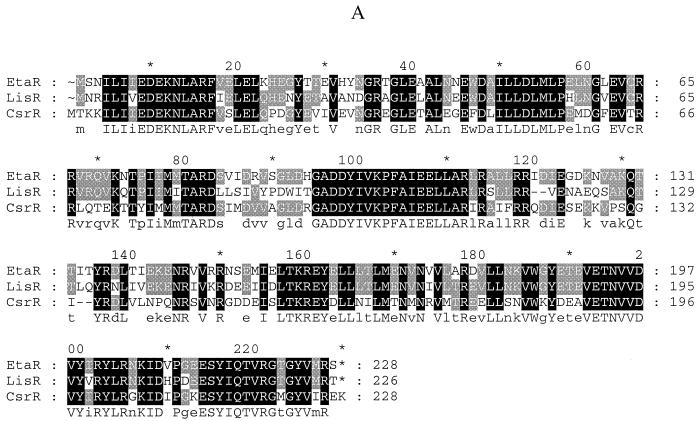
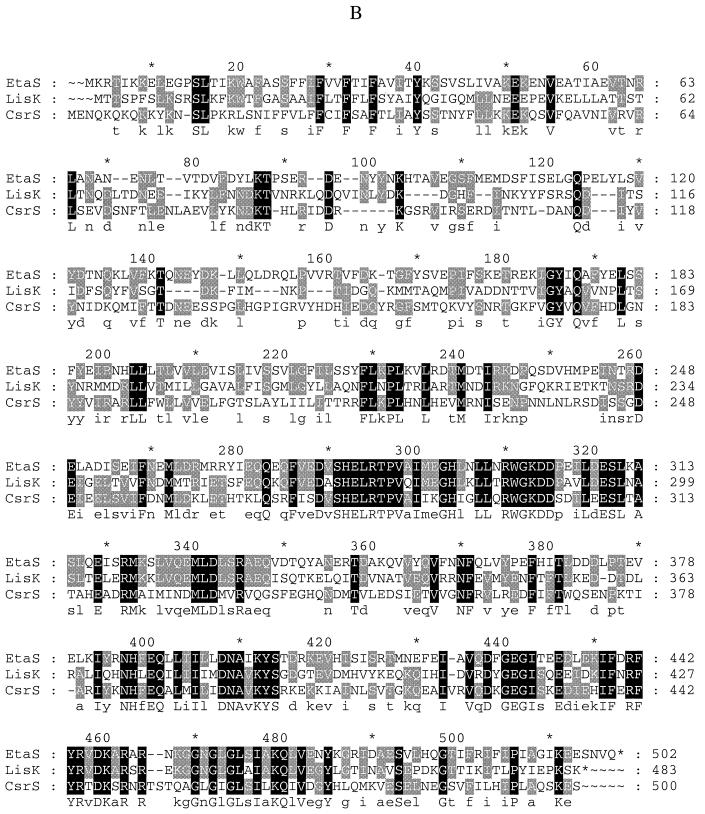
Sequence analysis of the 10293 locus showed that the sensor kinase homolog (etaS) follows the response regulator homolog (etaR), and there are 11 bp between the two open reading frames and no second promoter; thus, they are likely to be cotranscribed. BLAST searches were performed using predicted EtaRS polypeptide sequences, and two regulatory systems, LisRK of Listeria monocytogenes (3) and CsrRS of S. pyogenes (6, 8, 12), showed the highest sequence similarity of all hits to the EtaRS system (Fig. 2). The response regulator LisR showed 68% identity and 77% similarity to EtaR, while CsrR showed 60% identity and 70% similarity to EtaR. The sensor kinase LisK showed 37% identity and 53% similarity to EtaS, while CsrS showed 33% identity and 54% similarity to EtaS. Upstream of etaR, an open reading frame was identified with 76% protein sequence identity and 88% similarity to the 6-phosphogluconate dehydrogenase gene of Lactococcus lactis (SwissPROT accession no. P96789). This open reading frame is transcribed in the same orientation as etaR. Interestingly, upstream of lisR there is also an open reading frame encoding a 6-phosphogluconate homolog, suggesting that lisRK and etaRS have similar gene organizations at their loci.
FIG. 2.
(A) Alignment of EtaR, LisR, and CsrR polypeptide sequences. (B) Alignment of EtaS, LisK, and CsrS polypeptide sequences. The Pileup program in the GCG software package (Genetics Computer Group, Madison, Wis.) was used to make the alignment, and the GeneDoc software was used for editing and shading (the bottom line shows the consensus sequence).
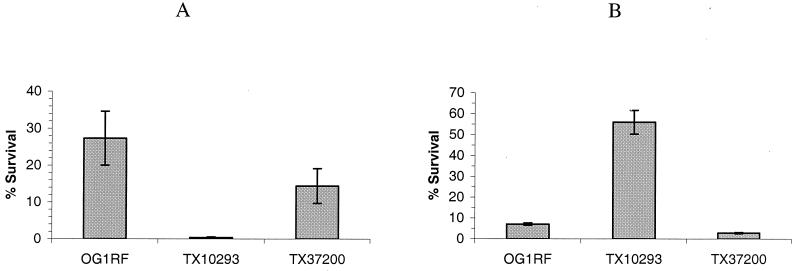
Survival of mutants TX10293 and TX37200 in low pH and high temperature.
To study the environmental cues that the EtaRS and EtbRS might respond to, different conditions were used in vitro to examine the phenotypes of TX10293 and TX37200. No significant difference was detected for growth of OG1RF, TX10293, and TX37200 in BHI with 5% ethanol or 6.5% NaCl (data not shown). When exposed to a low pH (pH 3.4), TX37200 showed a survival rate similar to that of wild-type OG1RF, and the survival rate of TX10293 was approximately 100-fold lower than that of wild-type OG1RF (P = 0.034) (Fig. 3A). When exposed to high temperature (55°C), TX10293 showed about 10-fold greater survival than wild-type OG1RF (P = 0.006), and TX37200 had two- to threefold less survival than OG1RF (P = 0.01) (Fig. 3B). The experiments were performed twice with reproducible results (P = 0.035, 0.005, and 0.035, respectively).
FIG. 3.
(A) Survival of OG1RF, TX10293, and TX37200 in BHI, pH 3.4, adjusted with lactic acid. (B) Survival of OG1RF, TX10293, and TX37200 in BHI at 55°C. The percent surviving 40 min from time zero is shown (see Materials and Methods for details).
DISCUSSION
Enterococci are reported as being able to grow in harsh environments, such as 6.5% NaCl, pH 9.6, and a wide range of temperature (10 to 45°C, with survival at 60°C for 30 min) (15); indeed, in our study, OG1RF was found to be able to survive in pH 3.4 for at least 40 min. Bacteria commonly use two-component regulatory systems to adapt to environmental challenge, and adaptation is also felt to be essential for bacterial survival and growth in mammalian hosts (9). Since expression of virulence factors can also be regulated by two-component systems during the infection, we felt that a study of the two-component systems in enterococci might provide information on their pathogenesis. In this study, genes representing seven PhoP-PhoS homologs were disrupted in E. faecalis OG1RF, and one of the mutants, TX10293 (etaR mutant), was attenuated in the mouse peritonitis model, suggesting that the EtaRS system is involved in virulence of E. faecalis.
The EtaRS system shows a high level of similarity to the two-component system LisRK of L. monocytogenes (3), and the etaRS and lisRK loci have similar gene organization. The etaR disruption mutant TX10293 was shown to be significantly more sensitive to low pH than the parent strain, OG1RF, during logarithmic growth, similar to the phenotype of the lisK deletion mutant (3). The etaR mutant (TX10293) also showed a tolerance for high temperature different from that of OG1RF. While the lisK deletion mutant showed a higher tolerance for a high ethanol concentration (5%) than the parent strain, growth of the etaR mutant (TX10293) in BHI with 5% ethanol was not significantly different from that of wild-type OG1RF (data not shown). These results suggest that the EtaRS system is involved in stress response, similar to the LisRK system in L. monocytogenes, although these systems may respond differently to different environmental cues. TX10293 showed lower resistance to low pH but higher resistance to high temperature than wild-type OG1RF, suggesting that the EtaRS system may regulate (negatively or positively) expression of different operon(s) in response to different environmental cues, or regulation of a specific operon may produce different phenotypes under different conditions. EtaRS also showed high similarity to the CsrRS system of S. pyogenes (6, 8, 12, 14). CsrRS has been shown to be a negative regulator of several virulence factors in S. pyogenes, and inactivation of CsrRS causes the strain to produce mucoid colonies. Interestingly, the E. faecalis etaR mutant (TX10293), when recovered from the mouse spleen, was mucoid on plates, while the wild-type OG1RF was nonmucoid, suggesting that the EtaRS system may negatively regulate capsule synthesis, similar to the CsrRS system. This phenotype was only observed once and disappeared quickly in vitro, suggesting that some specific condition(s) in vivo may trigger the phenotype or this phenotype may be disadvantageous in vitro.
We also found that the etaRS mutant of OG1RF was attenuated in a mouse peritonitis model, similar to a lisRK mutant of L. monocytogenes, which was attenuated in a mouse peritonitis model (3). A transposon disruption mutation in csrR reduced S. pyogenes internalization (11), and a spontaneous csrRS mutant of S. pyogenes showed enhanced virulence in a murine model of skin and soft tissue infection (4). Differences in these results may be due to different animal and tissue culture models, and/or the operons regulated by the EtaRS and CsrRS systems may not be the same. In addition, the csrRS mutant of S. pyogenes was generated spontaneously (4); thus, it was not clear whether genes other than csrRS were affected in these mutants.
In considering a possible link between the in vitro and in vivo phenotypes of the mutants, it is useful to consider some possible events in the mouse peritonitis model. It has been shown with L. monocytogenes that after bacteria are injected into the mouse peritoneum, the host immune response ensues with the arrival of neutrophils and macrophages (7). To survive this response, bacteria need to develop the ability to resist phagocytosis and/or to survive in the phagosome (21). At this stage, the sensing of and response to the in vivo environment (such as the stress conditions in the phagosome, including acidification and a wide range of lethal enzymes) are important (21), and such sensing and response could be mediated by two-component systems involved in stress response. One such example is the LisRK system of L. monocytogenes, which was initially identified as a two-component system involved in stress response (including response to low pH and high ethanol concentrations) and later was shown to be important for the virulence of L. monocytogenes. In this study, the potential two-component system EtaRS was also shown to be involved in stress response in vitro (affecting survival of E. faecalis at low pH and high temperature) and important for virulence of E. faecalis in vivo, which adds to the increasing evidence that stress responses mediated by two-component systems may regulate virulence of pathogens in vivo.
In summary, a number of potential two-component systems were identified for E. faecalis, one of which, EtaRS, was shown to be involved in response to heat and acidification in vitro and important for virulence of E. faecalis in a mouse peritonitis model. It will be of interest in future studies to look for gene(s) that are regulated by these two-component systems.
Acknowledgments
This work was supported by NIH grant AI47923 from the Division of Microbiology and Infectious Diseases to Barbara E. Murray.
We are grateful to The Institute for Genomic Research (TIGR) for providing the E. faecalis V583 genome sequence database.
Editor: E. I. Tuomanen
REFERENCES
- 1.Arthur, M., F. Depardieu, and P. Courvalin. 1999. Regulated interactions between partner and non-partner sensors and response regulators that control glycopeptide resistance gene expression in enterococci. Microbiology 145:1849-1858. [DOI] [PubMed] [Google Scholar]
- 2.Chung, C. T., S. Z. Niemela, and R. H. Miller. 1989. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. USA 86:2172-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotter, P. D., N. Emerson, C. G. M. Gahan, and C. Hill. 1999. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J. Bacteriol. 181:6840-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engleberg, N. C., A. Heath, A. Miller, C. Rivera, and V. J. DiRita. 2001. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J. Infect. Dis. 183:1043-1054. [DOI] [PubMed] [Google Scholar]
- 5.Evers, S., and Courvalin, P. 1996. Regulation of VanB-type vancomycin resistance gene expression by the VanS(B)-VanR(B) two component regulatory system in Enterococcus faecalis V583. J. Bacteriol. 178:1302-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Federle, M. J., K. S. Mciver, and J. R. Scott. 1999. A response regulator that repress transcription of several virulence operons in the group A streptococcus. J. Bacteriol. 181:3649-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gahan, C. G. M., and J. K. Collins. 1995. Non-dystrophic 129 REJ mice are susceptible to i.p. infection with Listeria monocytogenes despite an ability to recruit inflammatory neutrophils to the peritoneal cavity. Microb. Pathog. 18:355-364. [DOI] [PubMed] [Google Scholar]
- 8.Heath, A., V. J. DiRita, N. L. Barg, and N. C. Engleberg. 1999. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors: hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect. Immun. 67:5298-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoch, J. A., and T. J. Silhavy. 1995. Two-component signal transduction. ASM Press, Washington, D.C.
- 10.Hulett, F. M. 1996. The signal-transduction network for Pho regulation in Bacillus subtilis. Mol. Microbiol. 19:933-939. [DOI] [PubMed] [Google Scholar]
- 11.Jadoun, J., and S. Sela. 2000. Mutation in csrR global regulator reduces Streptococcus pyogenes internalization. Microb. Pathog. 29:311-317. [DOI] [PubMed] [Google Scholar]
- 12.Levin, J. C., and M. R. Wessels. 1998. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A streptococcus. Mol. Microbiol. 30:209-219. [DOI] [PubMed] [Google Scholar]
- 13.Li, X., G. M. Weinstock, and B. E. Murray. 1995. Generation of auxotrophic mutants of Enterococcus faecalis. J. Bacteriol. 177:6866-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller, A. A., N. C. Engleberg, and V. J. DiRita. 2001. Repression of virulence genes by phosphorylation-dependent oligomerization of CsrR at target promoters in S. pyogenes. Mol. Microbiol. 40:976-990. [DOI] [PubMed] [Google Scholar]
- 15.Murray, B. E. 1990. The life and times of the enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray, B. E., K. V. Singh, R P. Ross, J. D. Heath, G. M. Dunny, and G. M. Weinstock. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin, X., K. V. Singh, Y. Xu, G. M. Weinstock, and B. E. Murray. 1998. Effect of disruption of a gene encoding an autolysin of Enterococcus faecalis OG1RF. Antimicrob. Agents Chemother. 42:2882-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 19.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Singh, K. V., X. Qin, G. M. Weinstock, and B. E. Murray. 1998. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J. Infect. Dis. 178:1416-1420. [DOI] [PubMed] [Google Scholar]
- 21.Small, P. L. C., L. Ramakrishanan, and S. Falkow. 1994. Remodeling schemes of intracellular pathogens. Science 263:637-639. [DOI] [PubMed] [Google Scholar]
- 22.Xu, Y., K. V. Singh, X. Qin, B. E. Murray, and G. M. Weinstock. 2000. Analysis of a gene cluster of Enterococcus faecalis involved in polysaccharide biosynthesis. Infect. Immun. 68:815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]