Abstract
Perchlorate is a known environmental contaminant, largely due to widespread military use as a propellant. Perchlorate acts pharmacologically as a competitive inhibitor of thyroidal iodide uptake in mammals, but the impacts of perchlorate contamination in aquatic ecosystems and, in particular, the effects on fish are unclear. Our studies aimed to investigate the effects of concentrations of ammonium perchlorate that can occur in the environment (1, 10, and 100 mg/L) on the development of fathead minnows, Pimephales promelas. For these studies, exposures started with embryos of < 24-hr postfertilization and were terminated after 28 days. Serial sectioning of thyroid follicles showed thyroid hyperplasia with increased follicular epithelial cell height and reduced colloid in all groups of fish that had been exposed to perchlorate for 28 days, compared with control fish. Whole-body thyroxine (T4) content (a measure of total circulating T4) in fish exposed to 100 mg/L perchlorate was elevated compared with the T4 content of control fish, but 3,5,3′-triiodothyronine (T3) content was not significantly affected in any exposure group. Despite the apparent regulation of T3, after 28 days of exposure to ammonium perchlorate, fish exposed to the two higher levels (10 and 100 mg/L) were developmentally retarded, with a lack of scales and poor pigmentation, and significantly lower wet weight and standard length than were control fish. Our study indicates that environmental levels of ammonium perchlorate affect thyroid function in fish and that in the early life stages these effects may be associated with developmental retardation.
Keywords: development, endocrine disruption, fathead minnow, perchlorate, thyroid, thyroxine, triiodothyronine
In recent years there has been increasing concern about the presence of perchlorate in ground and surface waters and the percolation of perchlorate into drinking waters [Urbansky 1998; U.S. Environmental Protection Agency (EPA) 2002]. Ground and surface waters in several U.S. states have shown measurable concentrations of perchlorate at 8 μg/L to 3.7 g/L (Urbansky 1998). The major source of ground and surface water contamination is ammonium perchlorate, the primary ingredient of the solid propellant in rockets and missiles (Logan 2001; U.S. EPA 2002). Perchlorate salts are also used in smaller amounts as components of air bag inflators, road flares, and fireworks; in electroplating and in tanning and finishing leathers; and as mordants for fabrics and in producing paints and enamels (Logan 2001; U.S. EPA 2002. Discharge from rocket fuel manufacturing plants, demilitarization of weapons, and the washing out and refueling of rockets are responsible for most of the ammonium perchlorate released into the environment (Urbansky 1998; U.S. EPA 2002). Indeed, at the Longhorn Army Ammunition Plant in Texas (USA), perchlorate has been measured at 30–31 mg/L in a water treatment holding pond (Smith et al. 2001).
Perchlorate has several chemical properties that make environmental contamination difficult to resolve and decontamination difficult to achieve (Logan 2001). The perchlorate anion is persistent because of its tetrahedral structure (Wolff 1998). Perchlorate salts completely ionize in solution, and the perchlorate anion is highly mobile (Logan 2001). As a result of these properties, groundwater contamination inevitably presents a risk to drinking water quality, and perchlorate has been detected in many drinking water supplies. In Nevada, 4–24 μg/L was detected in drinking water (Xiao et al. 2001), and in California a number of drinking water wells showed peaks of 4–820 μg/L (California Department of Health Services 2004). As a result, the U.S. EPA has estimated that perchlorate affects the quality of drinking water for 15 million people in the United States (Logan 2001).
Based on U.S. EPA guidance, and assessment of toxicity data, several U.S. states have set advisory levels for perchlorate in drinking water that vary between 1 and 18 μg/L. The most recent reappraisal in California set a public heath goal for drinking water (maximum contaminant level) of 6 μg/L (Office of Environmental Health Hazard Assessment 2004).
There is a long history of clinical use of perchlorate as a pharmacologic inhibitor of thyroid hormone synthesis (Hobson 1961; Wolff 1998). Thyroid gland follicles trap iodide required for the iodination of tyrosine molecules. The resulting iodothyronines are then reversibly combined with the storage protein, thyroglobulin, within the lumen of each of the thyroid follicles (Leatherland 1988, 1993). Perchlorate competitively inhibits iodide uptake by the sodium/iodide symporter at the basolateral membrane of the follicles (Capen 1997; Wolff 1998) and induces iodide efflux from the follicles by an as yet unexplained mechanism (Wolff 1998). These pharmacologic actions might be predicted to reduce circulating levels of thyroid hormones, and several studies in mammals given drinking water containing perchlorate at target doses of 0.01–100 mg/kg/day support this idea (Siglin et al. 2000; York et al. 2001a). However, this has not been observed in all studies (York et al. 2001b).
Despite the known action of perchlorate on iodide uptake by the mammalian thyroid gland (Capen 1997; Wolff 1998) and evidence of perchlorate occurrence in aquatic ecosystems, remarkably few studies have investigated the effects of perchlorate on thyroid function of aquatic vertebrates. In the amphibian Xenopus laevis, 5 μg/L perchlorate inhibited forelimb emergence during thyroid-dependent metamorphosis (Goleman et al. 2002), and at 18 μg/L significantly fewer Xenopus completed tail resorption. Metamorphosis of larval sea lamprey Petromyzon marinus (an agnathan fish) is also affected by exposure to perchlorate (Manzon and Youson 1997, 2002; Manzon et al. 2001), but only two studies have investigated the impact of perchlorate on teleost fish that form key components of freshwater ecosystems. Potassium perchlorate (500 mg/L) was found to inhibit fin formation and skin pigment differentiation in early life stages of zebrafish, Danio rerio (Brown 1997), and in a study of adult zebrafish a high level of ammonium perchlorate (18 mg/L) resulted in thyroid hypertrophy, hyperplasia, and colloid depletion after 8 weeks of exposure (Patino et al. 2003). However, neither of these studies determined the changes in circulating thyroid hormones or whole-body thyroid hormone content. Our studies aimed to gain an integrated picture of the impacts of environmentally relevant concentrations of ammonium perchlorate on the thyroid axis of teleost fish by measuring whole-body thyroxine (T4) and 3,5,3′-triiodothyronine (T3) content, and examination of thyroid structure, together with investigation of changes in development and growth. For our studies we employed the fat-head minnow as a model cyprinid species. Although this species has been widely used in ecotoxicologic studies, knowledge of its thyroid function and the normal levels and fluctuations of T4 and T3 has only recently been obtained (Crane 2003; Crane et al. 2004). These data have shown a surge in thyroid hormones during the early stages of development and indicate that exposure to thyroid disruptors may have greatest effect during early development. Therefore, in investigating the effects of perchlorate, we focused on the first 28 days of development from < 24 hr postfertilization through the transition to juvenile fish.
Materials and Methods
Semistatic exposure system.
In a 28-day semi-static study, fathead minnow newly fertilized eggs were exposed to ammonium perchlorate in triplicate at 0 (control), 1, 10, and 100 mg/L (equivalent to 0.85, 8.47, and 84.7 mg/L perchlorate anion) with 0.15, 1.53, and 15.3 mg/L NH4+ in the experimental systems. The presence of added NH4+ would have resulted in 0.5 μg/L, 5.1 μg/L, and 51 μg/L unionized ammonia in the three experimental systems, but these levels of ammonia are well below the chronic-effects threshold concentration for unionized ammonia in fathead minnow larvae and adults (270 μg/L) based on a 2-year full-life cycle assessment of survival, growth, and reproductive success (Thurston et al. 1986).
From study day 0 to day 13, groups of fertilized eggs (n = 60) were exposed to perchlorate in 2-L Pyrex glass beakers (working volume, 1 L). Most embryos hatched on study day 5. On posthatch day 8 (study day 13), fish were transferred to 3-L Pyrex glass beakers (working volume, 2 L). Finally, on posthatch day 15 (study day 20) fish were transferred to 12-L glass tanks (working volume, 3 L) for the remainder of the study.
Preparation of stock concentrate and test solutions.
One day before the start of the study, ammonium perchlorate stock concentrate (50 g/L) was made up in dechlorinated local tap water (filtered to 10 μm, maintained at 25 ± 2°C). The stock was renewed at 11-day intervals. Typical water quality parameters were hardness, 44.7–49.3 mg/L; free chlorine, < 2.0 μg/L; calcium, 14.1 mg/L; sodium, 28.6 mg/L; potassium, 1.43 mg/L; aluminum, 4.73 μg/L; iron, < 3.0 μg/L; lead, < 2.0 μg/L. Test solutions (nominal concentrations of ammonium perchlorate, 0, 1, 10, and 100 mg/L) were made from the stock concentrate by dilution in dechlorinated water. Test and control solutions were renewed on study day 5 (principal hatch day) and then three times per week thereafter.
Embryo selection, maintenance, and exposure.
On day 0, fathead minnow embryos (n = 240) at blastula and morula developmental stages were gathered from mating tiles in the husbandry unit at Brixham Environmental Laboratory. Healthy embryos were selected, in batches of five, under a dissection microscope and randomly assigned to incubation cups, until each contained 10 embryos. Incubation cups were hung on oscillating incubation units over the fish tanks, maintaining the embryos constantly moving up and down through the test water at 2 cycles/min. There were two egg cups per tank, each containing a total of 10 embryos (20 embryos per tank), and a total of 60 embryos per exposure concentration, in triplicate. Embryos were inspected on a daily basis, and dead ones were removed. Most of the larvae hatched on study day 5 and were transferred to new test solution.
Maintenance of fry.
From posthatch day 0 to day 6, larvae were fed a suspension of rotifers (3,000 rotifers/mL; 2 mL three times per day on Monday–Friday and twice per day on weekends). On posthatch day 7, larvae were fed Artemia nauplii as well as rotifers. During posthatch days 8–15, fry were fed Artemia only, three times per day, and then from posthatch day 16 to the end of the study fry were fed Artemia twice per day and ground pellets (Ecostart 17; Biomar, Grangemouth, Scotland) once per day.
Sampling regime.
On study day 28, fish were sacrificed between 06:00 and 10:00 hr by immersion in a lethal dose of neutrally buffered ethyl 3-amino-benzoate methane sulfonate (MS222). Each fish was measured using digital calipers to determine standard length, weighed, and then snap frozen on dry ice. From each exposure concentration, 20 fish were sampled for whole-body thyroid hormone analyses, and a further 10 fish in each exposure group were fixed by immersion in 10% phosphate-buffered formalin for histologic examination of thyroid follicles, taking fish with an even distribution between the triplicate tanks. Fish for thyroid hormone assays were held at −80°C until analysis.
Histology.
Whole fish (n = 5) fixed in formalin were decalcified for 14 days in 5% formic acid in 5% formaldehyde. Fish were wax embedded and serially sectioned (6 μm) through all the thyroid follicles. Each follicle in each fish (5–13 follicles/fish) was traced through its entirety, and epithelial cell height was measured at the largest point.
Thyroid hormone extractions.
Thyroid hormones were extracted from fathead minnow larvae based on the technique described by Greenblatt et al. (1989). Larvae were placed in Teflon tubes on ice, and 2 mL 95% ethanol containing 1 mM 6-N-propyl-2-thiouracil (PTU) was added. Samples were homogenized (Ultra Turax T25; Janke and Kunkel, Staufen, Germany) and sonicated for 20 sec (Vibra-Cell, 50% output; Sonics and Materials, Meryin/Satigny, Switzerland). A further 2 mL of 95% ethanol with 1 mM PTU was added, and samples were vortexed. Samples were centrifuged for 10 min (10,000g, 4°C), the supernatant was decanted into clean Teflon tubes, and 2 mL 95% ethanol containing PTU was added to the pellets. Tubes were vortexed vigorously and recentrifuged for 10 min (10,000g, 4°C). Supernatants were pooled and evaporated to dryness under nitrogen, and desiccated samples were resuspended in 0.25 mL barbital buffer containing 2.5 mg/mL anilino naphthalene sulfonic acid (to disrupt the coupling between thyroid hormones and serum proteins, including lipoproteins), 0.25 mL ethanol, and 1 mL chloroform. Tubes were vigorously vortexed and then centrifuged for 10 min (1,500g, 4°C), producing two phases. The top ethanolic layer was removed using a glass pipette for radioimmunoassay (RIA) of thyroid hormones. The recovery of thyroid hormones was determined by addition of radioiodinated T4 or T3 after homogenization of whole larvae (n = 5). The recovery of 59.5 ± 3.25% T4 and 63.9 ± 3.27% T3 was comparable with those recoveries reported for larvae of other fish species (Greenblatt et al. 1989).
Radioimmunoassay of T3 and T4.
The RIAs used in these studies were developed and validated for use with fathead minnows (Crane 2003). For these assays, thyroid hormone standards (0.22–160 ng/mL T4; 0.06–10 ng/mL T3) were prepared in barbital buffer. Lyophilized polyclonal anti-T3 and anti-T4, raised in sheep (Diagnostics, Edinburgh, Scotland), were diluted in barbital buffer (1:7,000 for anti-T4, 1:10,000 for anti-T3). Radioiodinated T3 (I125-T3) and T4 (I125-T4; specific activities, 1,080–1,320 μCi/μg; New England Nuclear, Boston, MA, USA) were used at approximately 5,000 counts per minute (cpm) per tube for I125-T3 and 6,500 cpm per tube for I125-T4.
Extracted samples or standard solutions (30 μL) were incubated at 4°C overnight (in triplicate) with 100 μL antiserum and 100 μL radioiodinated solution, with additional “total counts” and “blank” tubes. The next morning, free and bound hormones were separated by addition of 100 μL Sac-Cel (Immunodiagnostic Systems Limited, Tyne and Wear, UK) and a solution of cellulose-coupled antibodies (anti-sheep/goat); tubes were centrifuged, and the pellet of bound radiolabeled hormone was counted (Cobra gamma counter; Packard, Boston, MA, USA). Thyroid hormone levels were estimated using RIA software (RIASMART; Packard).
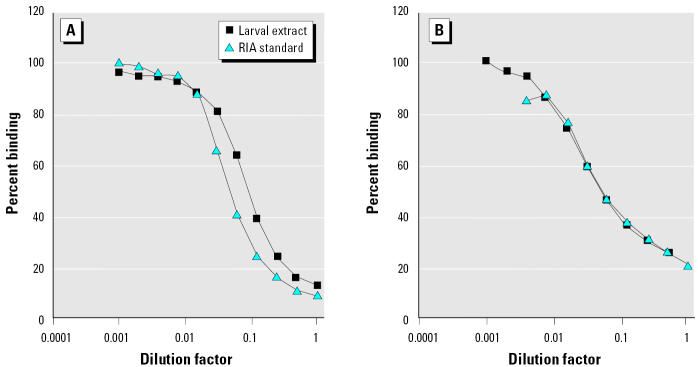
The thyroid hormone RIAs were validated for the estimation of thyroid hormones extracted from larvae, by running serial dilutions of an extracted pool of larvae in both T3 and T4 RIAs. These assays showed parallelism of larval extracts with the two standard curves (Figure 1). The cross-reaction of anti-T3 and anti-T4 with thyroid hormone metabolites [reverse triiodothyronine (rT3), diiodothyronine, monoiodotyrosine, and tyrosine] was determined by running serial dilutions in the two thyroid hormone RIAs. Anti-T4 showed 1.24% cross-reaction with T3 and 2.38% cross-reaction with rT3. Anti-T3 showed 5% cross-reaction with T4, 0.02% cross-reaction with rT3, and 3.4% cross-reaction with diiodothyronine. Other metabolites produced no displacement in the assay and thus exhibited negligible cross-reaction. The minimum detectable level of thyroid hormones, estimated as the mean plus two standard deviations of zero standards (n = 18 for T4, n = 9 for T3) was 2.04 pg/tube for T3 and 8.16 pg/tube for T4, which equates to whole-body contents of approximately 0.35 pg/mg T3 and 1.41 pg/mg T4. All RIA measurements were acquired in a single T3 assay and a single T4 assay. Intra-assay variation for the T3 RIA was 9.68% (n = 12), and for the T4, RIA was 5.33% (n = 15).
Figure 1. Parallelism between serially diluted larval extract and standards in the RIAs for (A) T4 and (B) T3.
Statistical analysis.
All data shown are mean ± SEM. Percentage survival and percentage hatch data were arcsine square root transformed before statistical analyses with modification where n = 0 or 1, as detailed by the U.S. EPA (1994), and analyzed by one-way analysis of variance (ANOVA) with post hoc Tukey honestly significantly different (Tukey HSD) tests. Wet weight, length, thyroid hormone content (adjusted for extraction efficiency), and follicular cell heights were analyzed using Kruskal-Wallis one-way ANOVA on ranks with Dunn’s test for multiple comparisons.
Results
Hatch and survival.
All test vessels showed hatching of at least 90% of the embryos (control, 98.3 ± 1.67%; ammonium perchlorate at 1 mg/L, 96.7 ± 1.67%; 10 mg/L, 98.3 ± 1.67%; 100 mg/L, 93.3 ± 1.67%). Thereafter, percentage survival was unaffected by exposure to ammonium perchlorate (control, 79.7 ± 10.3%; ammonium perchlorate at 1 mg/L, 70.3 ± 7.15%; 10 mg/L, 74.7 ± 11.30%; 100 mg/L, 82.0 ± 9.40%).
Development and growth.
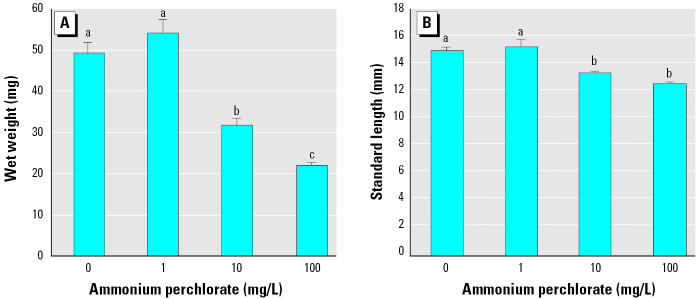
After 28 days (posthatch day 23), fish exposed to 10 mg/L and 100 mg/L ammonium perchlorate were visibly smaller than fish exposed to 1 mg/L ammonium perchlorate or controls. Wet weight (p < 0.05; Figure 2A) and standard length (p < 0.05; Figure 2B) were significantly lower in fish exposed to 10 mg/L and 100 mg/L ammonium perchlorate compared with fish exposed to 1 mg/L perchlorate and controls.
Figure 2. Wet weight (A) and standard length (B) of fathead minnows exposed to ammonium perchlorate from day 0 to day 28 (n = 30–36). Groups that are not significantly different are denoted by the same letter. Values shown are mean ± SEM.
Perchlorate exposure at the two higher concentrations resulted in delayed development. On study day 28, fish exposed to 10 and 100 mg/L had minimal appearance of scales, and the gut was still visible through the sides of the fish. In contrast, control fish had developed scales and pigmentation such that their viscera were no longer visible externally.
Histology.
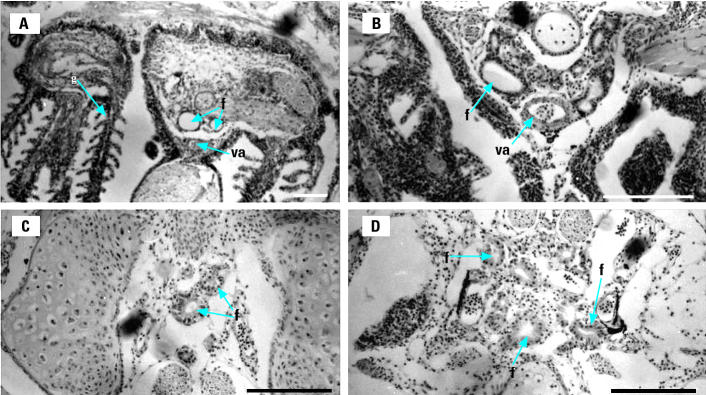
Fish exposed to all ammonium perchlorate concentrations exhibited significantly greater thyroid follicular epithelial cell height than did control fish (p < 0.05; Figure 3). Control fish had cuboidal follicular epithelial cells (Figure 4A), and the central colloid was full or showed only slight vacuolation. Individual fish exposed to 1 mg/L ammonium perchlorate showed a range of states of follicular colloid, from full colloid to visible vacuolation of the lightly stained central colloid (Figure 4B). In fish exposed to 10 mg/L, all follicles had more columnar epithelial cells (Figures 3 and 4C) and reduced follicular colloid (Figure 4C). Fish exposed to 100 mg/L ammonium perchlorate showed greatly enlarged epithelial cells (Figures 3 and 4D) and reduction or apparent absence of colloid (Figure 4D).
Figure 3. Follicular epithelial cell height of thyroid follicle of fathead minnows exposed to ammonium perchlorate from day 0 to day 28. These data are derived from five fish per treatment and between 5 and 13 follicles per fish. Groups that are not significantly different are denoted by the same letter. Values shown are mean ± SEM.
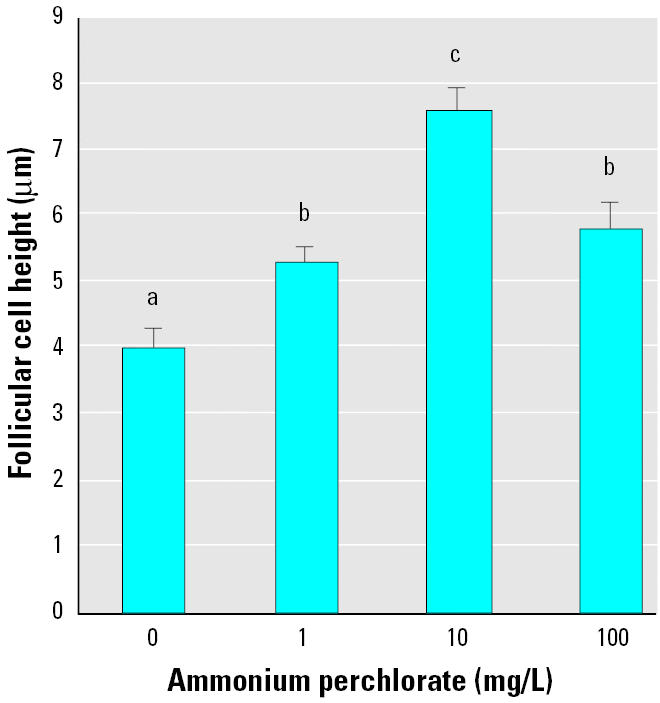
Figure 4. Thyroid follicles (f) in close proximity to the ventral aorta (va) and gills (g) of fathead minnows exposed to ammonium perchlorate from day 0 to day 28. (A) Control. (B) 1 mg/L ammonium perchlorate. (C) 10 mg/L ammonium perchlorate. (D) 100 mg/L ammonium perchlorate. Bars = 100 μm.
Whole-body thyroid hormone content.
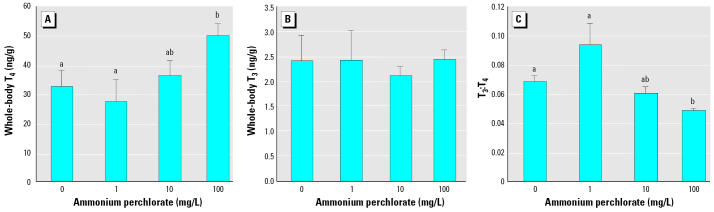
Whole-body T4 was significantly higher in fish exposed to 100 mg/L ammonium perchlorate than in either fish exposed to 1 mg/L or control fish (p < 0.05; Figure 5A). In contrast, there was no significant difference between the whole-body T3 content of con-trol fish and that of any group of fish exposed to ammonium perchlorate (Figure 5B). In line with these findings, thyroid hormone ratios (T3:T4) were significantly decreased in fish exposed to 100 mg/L perchlorate compared with control fish (p < 0.05; Figure 5C).
Figure 5. Whole-body T4 (A), T3 (B), and T3:T4 ratio (C) in fathead minnows exposed to ammonium perchlorate from embryo from day 0 to day 28 (n = 15–18). Groups.
Discussion
Exposure of fathead minnow embryos to ammonium perchlorate had no significant effect on their hatching, in agreement with a similar lack of effect of ammonium perchlorate on hatching by the amphibian Xenopus laevis at concentrations of < 1,000 mg/L (Goleman et al. 2002). Thereafter, however, exposure to ammonium perchlorate resulted in developmental retardation, both in X. laevis, where a reduced snout to vent length was observed after 16 days exposure of larvae to 425 mg/L ammonium perchlorate (Goleman et al. 2002), and in the present studies of fathead minnows exposed to 10 and 100 mg/L ammonium perchlorate.
The transition from larvae to juvenile fish in cyprinids such as the zebrafish and fathead minnow is characterized by the formation of scales, alongside other developmental changes (Brown 1997). In the present studies, development of scales and pigmentation was delayed in fathead minnows exposed to 10 mg/L and 100 mg/L ammonium perchlorate, indicating that the larval to juvenile transition in these fish had not been completed within the 28-day study period, whereas control fish successfully completed this transition. Impeded development and growth of fathead minnows held in perchlorate solutions was also indicated by the significantly lower wet weight and body length of fish exposed for 28 days to 10 mg/L and 100 mg/L ammonium perchlorate.
The reduced growth and inhibited development of fathead minnows exposed to the two higher concentrations of perchlorate is highly likely to reflect the cumulative impacts of ammonium perchlorate on thyroid status over the time course of the 28-day period of exposure, and further studies are now needed to define the effects of perchlorate on growth and development at earlier time points as well as their longer-term implications. Thyroid hormones are well known to play an important role in larval metamorphosis in flatfish (de Jesus et al. 1993; Inui et al. 1995) and in other species, such as the grouper, that undergo similar dramatic morphologic changes during metamorphosis from planktonic larvae to bottom dwellers (Trijuno et al. 2002), but the specific roles of thyroid hormones in regulating developmental processes in other teleostan fishes are less well defined. Our studies of fathead minnows have shown a peak in both T4 and T3 at 9 and 16 days posthatch, respectively (Crane 2003; Crane et al. 2004), suggesting roles in regulating particular developmental processes at this time, such as cartilage and gut formation (Liu and Chan 2002). Furthermore, thyroid hormones have been implicated in regulating the growth of both larval and adult fish, acting either directly or indirectly, via stimulation of growth hormone or insulin-like growth factors (Boeuf et al. 1989; Deane et al. 2003; Ebbesson et al. 1998; Marti-Palanca and Perez-Sanchez 1994; Perez-Sanchez and Le Bail 1999; Woo et al. 1991).
Pharmacologic inhibition of thyroidal uptake of iodide by perchlorate (Capen 1997; Wolff 1998) has been associated with reduced circulating levels of thyroid hormones in studies of mammals and birds given ammonium perchlorate in drinking water (McNabb et al. 2004; Siglin et al. 2000; York et al. 2001a, 2004). Similarly, low circulating concentrations of T4 and T3 have been reported in fish exposed to potassium perchlorate at 100–500 mg/L (Manzon and Youson 1997; Manzon et al. 1998). However, in our studies, despite the observed effects of ammonium perchlorate on growth and development of fathead minnows achieved over the 28-day experiment, there was no evidence of a significant depression in whole-body thyroid hormones after this period of exposure.
Thyroid function involves a series of sequential steps, beginning with the acquisition of iodide. Fish have a well-developed capacity to take up iodide from the environmental medium across their gills and, unlike mammals, do not have to rely on the dietary supply (Eales and Brown 1993). As a result, there is little evidence of natural thyroid deficiencies. Nevertheless, it is possible that the changing supply of dietary iodide in our studies may have had significance when set against the perchlorate inhibition of iodide uptake. In our studies, the developing fathead minnows were initially fed on rotifers and later weaned onto brine shrimp hatched in saline and a resultant increase in dietary iodide could have contributed to an elevated iodide:perchlorate ratio, aiding in the regulation of whole-body T4 despite perchlorate exposure.
A further major influence on circulating thyroid hormones during our study would have been the natural regulatory systems in fish that normally achieve stable circulating levels. In fish, circulating T3 is almost exclusively determined by peripheral deiodination (Eales and Brown 1993; Van Putte et al. 2001), whereas T4 levels are self-regulating via the pituitary–thyroid axis. T4 plays a major role is regulating pituitary release of thyroid-stimulating hormone (TSH), whereas in fish, in contrast to the situation in mammals, T3 has been found to exert no significant feedback regulation on TSH release (Eales and Brown 1993). Thus, the pituitary–thyroid axis responds to increases or decreases in plasma T4 concentrations, initiating compensatory changes in the thyroid activity and potential restoration of the T4 level. Therefore, the effects of perchlorate on the iodide uptake of thyroidal tissue in fathead minnows would be predicted to initially that are not significantly different are denoted by the same letter. Values shown are mean ± SEM. depress circulating levels of T4 resulting in stimulation of the pituitary release of TSH and activation of thyroid tissue to attempt to regulate thyroid hormone levels. Despite the apparent lack of a depression in either T4 or T3 content after 28 days of exposure to per-chlorate, our histologic results showed a marked hypertrophy of follicular epithelial cells. Therefore, the observed thyroid hyperplasia after 28 days of exposure to perchlorate is very likely to have resulted from a hypothyroidism that occurred during the 28-day study period and hence a reduced negative feedback on the pituitary, stimulating release of TSH. Similar thyroid hyperplasia has been observed after treatment of fish with goitrogens such as thiourea and thiouracil that inhibit coupling and formation of iodotyrosines in the thyroid. These studies have, furthermore, shown dose-dependent effects with maintenance of circulating T4 at low levels of goitrogen but depressed circulating T4, despite a more pronounced increase in epithelial cell height of thyroid tissue at higher doses (Eales and Brown 1993).
Thyroid gland hyperplasia during prolonged perchlorate exposure has also been reported in the amphibian Bufo arenarum exposed to potassium perchlorate at 340 mg/L for 5 months (Miranda et al. 1996). Our studies provide evidence of thyroid hyperplasia in fathead minnows at all concentrations of perchlorate investigated, and over a relatively short time frame. In fathead minnows, a 1-month period of exposure to as little as 1 mg/L initiated a significant increase in epithelial cell height. Exposure to 10 mg/L ammonium perchlorate increased the normally cuboidal epithelium with epithelial cell heights of 4 ± 0.27 μm to a columnar epithelium with cell height of 7.6 ± 0.35 μm. In contrast, B. arenarum exposed for 5 months to 340 mg/L potassium perchlorate showed a more pronounced stimulation of thyroid hypertrophy with epithelial cell height increased from 7 to 23 μm.
Despite the histologic evidence of hypothyroidism in all perchlorate-exposed fathead minnows, there was no evidence of a sustained depression in whole-body content of thyroid hormones, and by the end of the 28 days of exposure to 100 mg/L ammonium perchlorate, T4 was significantly elevated compared with that of the control fathead minnows.
The stimulation of the thyroid follicles by increased TSH (Eales and Brown 1993) may have subsequently compensated for the perchlorate inhibition of iodide uptake, restoring (1 or 10 mg/L ammonium perchlorate) or even elevating T4 (100 mg/L ammonium perchlorate). This regulation may have been aided by increased iodide gained from the diet of brine shrimp and a reduced perchlorate:iodide ratio. An increased release of stored thyroid hormone from the thyroid follicles (again stimulated by TSH) is also a possible contributory factor. Further studies using pronase digestions of thyroidal tissues could provide information of hormone stores bound to thyroglobulin (Kowalczyk and Sotowska-Brochocka 2000; Plohman et al. 2002) and the changes during perchlorate exposure. Our histologic studies showed a marked reduction in colloid within the follicles after 28 days of exposure to perchlorate, and in the longer term a more persistent depression in T4 may therefore occur.
Although our studies indicated elevated whole-body T4 content at the highest concentration of perchlorate (100 mg/L) after 28 days of exposure, T3, the hormone that exerts the principal physiologic effects (Eales and Brown 1993), was unchanged. As a result, T3:T4 ratios were significantly reduced. Regulation of plasma T3, despite depressed plasma T4, was reported in rabbits and rats given perchlorate in drinking water (York et al. 2001a), although time-related impacts of perchlorate in drinking water with longer- term depression of both circulating T4 and T3 may occur as regulation breaks down (Siglin et al. 2000). Our study of fathead minnows only extended for 28 days, and further studies over a longer time period are now warranted to gain a fuller picture of the potential impacts of this pervasive thyroid toxicant in the wild.
Conclusions
The results reported here indicate that environmentally relevant levels of ammonium perchlorate are likely to affect the thyroid axis of teleost fish. Growth and development of the early life stages of fathead minnows were significantly retarded after a 28-day exposure to 10 or 100 mg/L perchlorate, and we suggest that these changes are the result of hypothyroidism during the early stages of exposure. Our histologic studies showed that thyroid follicular epithelial cell height is a sensitive and appropriate biomarker for perchlorate exposure in aquatic vertebrates. However, after 28 days of exposure to perchlorate, fathead minnows achieved homeostasis of the major physiologically active hormone T3 and T4 levels were similar in control fish and all perchlorate-exposed fish except at the highest concentration (100 mg/L). Further studies are needed to investigate whether there is up-regulation of T4 production or increased release at this stage in exposure. It also remains to be determined how fathead minnows exposed to perchlorate would survive and function in the longer term and, given the impeded growth and development, whether these fish would reach sexual maturity.
References
- Boeuf G, Le Bail PY, Prunet P. Growth hormone and thyroid hormones during Atlantic salmon, Salmo salar, smelting, and after transfer to seawater. Aquaculture. 1989;82:257–268. [Google Scholar]
- Brown D. The role of thyroid hormones in zebrafish and axolotl development. Proc Natl Acad Sci USA. 1997;94:13011–13016. doi: 10.1073/pnas.94.24.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capen CC. Risk assessment: selected organ and compound-related issues. Mechanistic data and risk assessment of selected toxic end points of the thyroid gland. Toxicol Pathol. 1997;25:39–48. doi: 10.1177/019262339702500109. [DOI] [PubMed] [Google Scholar]
- Crane HM. 2003. Thyroid Function in the Fathead Minnow, Pimephales promelas and Its Disruption by Methimazole and Ammonium Perchlorate [PhD thesis]. Exeter, UK:University of Exeter.
- Crane HM, Pickford DB, Hutchinson THH, Brown JA. Developmental changes of thyroid hormones in the fathead minnow, Pimephales promelas. Gen Comp Endocrinol. 2004;139:55–60. doi: 10.1016/j.ygcen.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Deane E, Kelly S, Collins P, Woo N. Larval development of silver sea bream (Sparus auratus): ontogeny of RNA-DNA ratio, GH, IGF-1, and Na-K-ATPase. Mar Biotechnol. 2003;5:79–91. doi: 10.1007/s10126-002-0052-7. [DOI] [PubMed] [Google Scholar]
- De Jesus EG, Hirano T, Inui Y. Flounder metamorphosis: its regulation by various hormones. Fish Physiol Biochem. 1993;11:323–238. doi: 10.1007/BF00004581. [DOI] [PubMed] [Google Scholar]
- California Department of Health Services 2004. Perchlorate in California Drinking Water: Monitoring Update. Sacramento, CA:California Department of Health Services. Available: http://www.dhs.ca.gov/ps/ddwem/chemicals/perchl/moni-toringupdate.htm [accessed 11 May 2004].
- Eales JG, Brown SB. Measurement and regulation of thyroidal status in teleost fish. Rev Fish Biol Fish. 1993;3:299–347. [Google Scholar]
- Ebbesson LOE, Björnsson BT, Stefansson SO, Ekström P. Propylthiouracil-induced hypothyroidism in coho salmon, Oncorhynchus kisutch: effects on plasma total thyroxine, total triiodothyronine, free thyroxine and growth hormone. Fish Physiol Biochem. 1998;19:305–313. [Google Scholar]
- Goleman WL, Urquidi LJ, Anderson TA, Smith EE, Kendall RJ, Carr JA. Environmentally relevant concentrations of ammonium perchlorate inhibit development and metamorphosis in Xenopus laevis. Environ Toxicol Chem. 2002;21:424–430. [PubMed] [Google Scholar]
- Greenblatt M, Brown CL, Lee M, Dauder S, Bern HA. Changes in thyroid hormone levels in eggs and larvae and in iodide uptake by eggs of coho and chinook salmon, Oncorhynchus kisutch and O. tschawytscha. Fish Physiol Biochem. 1989;6:261–278. doi: 10.1007/BF01881680. [DOI] [PubMed] [Google Scholar]
- Hobson QJG. 1961. Aplastic anaemia due to treatment with potassium perchlorate. Br Med J (13 May):1368–1369. [DOI] [PMC free article] [PubMed]
- Inui Y, Yamano K, Miwa S. The role of thyroid hormone in tissue development in metamorphosing flounder. Aquaculture. 1995;135:87–98. [Google Scholar]
- Kowalczyk P, Sotowska-Brochocka J. Changes in thyroid hormones in the serum and the thyroid gland of hibernating frogs, Rana temporaria L. Gen Comp Endocrinol. 2000;119:172–180. doi: 10.1006/gcen.2000.7510. [DOI] [PubMed] [Google Scholar]
- Leatherland JF. Endocrine factors affecting thyroid economy of teleost fish. Am Zool. 1988;28:319–328. [Google Scholar]
- Leatherland JF. Reflections on the thyroidology of fishes: from molecules to humankind. Guelph Ichthyol Rev. 1993;2:1–67. [Google Scholar]
- Liu YW, Chan WK. Thyroid hormones are important for embryonic to juvenile transitory phase in zebrafish. Differentiation. 2002;70:36–45. doi: 10.1046/j.1432-0436.2002.700104.x. [DOI] [PubMed] [Google Scholar]
- Logan BA. Assessing the outlook for perchlorate remediation. Environ Sci Technol. 2001;35:482A–487A. doi: 10.1021/es012564o. [DOI] [PubMed] [Google Scholar]
- Manzon RG, Eales JG, Youson JH. Blocking of KClO4-induced metamorphosis in premetamorphic sea lampreys by exogenous thyroid hormones (TH); effects of KClO4 and TH on serum TH concentrations and intestinal thyroxine outer-ring deiodination. Gen Comp Endocrinol. 1998;112:54–62. doi: 10.1006/gcen.1998.7129. [DOI] [PubMed] [Google Scholar]
- Manzon RG, Holmes JA, Youson JH. Variable effects of goitrogens in inducing precocious metamorphosis in sea lampreys (Petromyzon marinus) J Exp Zool. 2001;289:290–303. [PubMed] [Google Scholar]
- Manzon RG, Youson JH. The effects of exogenous thyroxine (T4) or triiodothyronine (T3), in the presence and absence of potassium perchlorate, on the incidence of metamorphosis and on serum T4 and T3 concentrations in larval sea lampreys (Petromyzon marinus L.) Gen Comp Endocrinol. 1997;106:211–220. doi: 10.1006/gcen.1996.6867. [DOI] [PubMed] [Google Scholar]
- Manzon RG, Youson JH. KClO4 inhibits thyroidal activity in the larval lamprey endostyle in vitro. Gen Comp Endocrinol. 2002;128:214–223. doi: 10.1016/s0016-6480(02)00505-1. [DOI] [PubMed] [Google Scholar]
- Marti-Palanca H, Perez-Sanchez J. Developmental regulation of growth hormone binding in the gilthead sea bream, Sparus aurata. Growth Reg. 1994;4:14–19. [PubMed] [Google Scholar]
- McNabb FM, Jang DA, Larsen CT. Does thyroid function in developing birds adapt to sustained ammonium perchlorate exposure? Toxicol Sci. 2004;82:106–113. doi: 10.1093/toxsci/kfh247. [DOI] [PubMed] [Google Scholar]
- Miranda LA, Pisano A, Casco V. Ultrastructural study on the thyroid glands of Bufo arenarum larvae kept in potassium perchlorate solution. Biocell. 1996;20:147–153. [PubMed] [Google Scholar]
- Office of Environmental Health Hazard Assessment 2004. Public Health Goals for Perchlorate in Drinking Water. Sacramento, CA:Office of Environmental Health Hazard Assessment, California Environmental Protection Agency. Available: http://www.oehha.ca.gov/water/phg/pdf/finalperchlorate31204.pdf [accessed 11 February 2005].
- Patino O, Wainscott MR, Cruz-Li EI, Balakrishnan S, McMurray C, Blazer VS, et al. Effects of ammonium perchlorate on the reproductive performance and thyroid follicle histology of zebrafish. Environ Toxicol Chem. 2003;22:1115–1121. [PubMed] [Google Scholar]
- Perez-Sanchez J, Le Bail PY. Growth hormone axis as a marker of nutritional status and growth performance in fish. Aquaculture. 1999;177:117–128. [Google Scholar]
- Plohman JC, Dick TA, Eales JG. Thyroid of lake sturgeon, Acipenser fulvescens—I. Hormone levels in blood and tissues. Gen Comp Endocrinol. 2002;125:47–55. doi: 10.1006/gcen.2001.7733. [DOI] [PubMed] [Google Scholar]
- Siglin JC, Mattie DR, Dodd DE, Hildebrandt PK, Baker WH. A 90-day drinking water toxicity study in rats of the environmental contaminant ammonium perchlorate. Toxicol Sci. 2000;57:61–74. doi: 10.1093/toxsci/57.1.61. [DOI] [PubMed] [Google Scholar]
- Smith PN, Theodorakis CW, Anderson TA, Kendall RJ. Preliminary assessment of perchlorate in ecological receptors at the Longhorn Army Ammunition Plant (LHAPP), Karnack, Texas. Ecotoxicology. 2001;10:313. doi: 10.1023/a:1016715502717. [DOI] [PubMed] [Google Scholar]
- Thurston RV, Russo RC, Meyn EL, Zajdel RK, Smith CE. Chronic effects of ammonia to fathead minnows. Trans Am Fish Soc. 1986;115:196–207. [Google Scholar]
- Trijuno DD, Yoseda K, Hirokawa T, Tagawa M, Tanaka M. Effects of thyroxine and thiourea on the metamorphosis of coral trout grouper, Plectropomus leopardus. Fish Sci. 2002;68:282–289. [Google Scholar]
- Urbansky ET. Perchlorate chemistry: implications for analysis and remediation. Bioremed J. 1998;2:81–95. [Google Scholar]
- U.S. EPA 1994. Short Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Water to Freshwater Organisms. Washington, DC:U.S. Environmental Protection Agency.
- U.S. EPA 2002. Perchlorate Environmental Contamination: Toxicological Review and Risk Characterization (2002 External Review Draft). NCEA-1-050. Washington, DC:U.S. Environmental Protection Agency. Available: http://cfpub2.epa.gov/ncea/cfm/recordisplay.cfm?deid=72117 [accessed 2 February 2005].
- Van Putte CL, MacKenzie DS, Eales JG. Characterisation of hepatic low-K(m) outer ring deiodination in red drum (Scianops ocellatus) Comp Biochem Physiol B Biochem Mol Biol. 2001;128:413–423. doi: 10.1016/s1096-4959(00)00348-1. [DOI] [PubMed] [Google Scholar]
- Wolff J. Perchlorate and the thyroid gland. Pharmacol Rev. 1998;50:89–105. [PubMed] [Google Scholar]
- Woo NYS, Chung ASB, Ng TB. Influence of oral administration of 3,5,3′-triiodo-K-thyronine on growth, digestion, food conversion and metabolism in the underyearling red sea bream, Chrysophrys major (Temminck & Schlegel) J Fish Biol. 1991;39:459–468. doi: 10.1007/BF00004504. [DOI] [PubMed] [Google Scholar]
- Xiao Li F, Squartsoff L, Lamm SH. Prevalence of thyroid diseases in Nevada counties with respect to perchlorate in drinking water. J Occup Environ Med. 2001;43:630–634. doi: 10.1097/00043764-200107000-00010. [DOI] [PubMed] [Google Scholar]
- York RG, Barnett J, Brown WR, Garman RH, Mattie DR, Dodd D. A rat neurodevelopmental evaluation of offspring, including evaluation of adult and neonatal thyroid from mothers treated with ammonium perchlorate in drinking water. Int J Toxicol. 2004;23:191–214. doi: 10.1080/10915810490475835. [DOI] [PubMed] [Google Scholar]
- York RG, Brown WR, Girard MF, Dollarhide JS. Oral (drinking water) developmental toxicity study of ammonium perchlorate in New Zealand white rabbits. Int J Toxicol. 2001a;20:199–205. doi: 10.1080/109158101750408028. [DOI] [PubMed] [Google Scholar]
- York RG, Brown WR, Girard MF, Dollarhide JS. Two generation reproduction study of ammonium perchlorate in drinking water in rats evaluates thyroid toxicity. Int J Toxicol. 2001b;20:183–197. doi: 10.1080/109158101750408019. [DOI] [PubMed] [Google Scholar]