Abstract
Infection of C57BL/6 mice with the third-stage larvae of Brugia pahangi results in a rapid expansion of NK1.1+ T cells in the spleen and draining lymph nodes. NK T cells produced interleukin-4 in the spleen within 24 h of infection, and these cells were CD4−.
NK T cells are an unusual population of T cells which coexpress receptors of the NK lineage (14, 21) and which are known to promptly produce large amounts of cytokines, in particular interleukin 4 (IL-4), upon primary stimulation. Most NK T cells are either CD4+ or CD4− CD8− αβ T cells (double negative [DN]). The majority of αβ NK1.1+ T cells are positively selected on class I or class I-related molecules and have restricted T-cell receptor usage (14). Their restricted T-cell receptor repertoire (Vα14-Jα281) has led some authors to propose that they may have evolved to recognize particular antigens. In both mouse and human systems, NK T cells recognize lipid and/or glycolipid antigens, often in the context of CD1 (1-3, 11, 19). For example, the recognition by Vα14+ NK T cells of the synthetic glycolipid antigen α-galactosylceramide (α-GalCer) in a CD1-restricted manner has proved to be a very useful model for characterizing these cells (5). Upon primary stimulation with α-GalCer, Vα14+ NK T cells can secrete either IL-4 or gamma interferon, depending upon the antigen-presenting cell (APC) (5); however, subsequent exposure to α-GalCer or immunization with α-GalCer predisposes to the development of Th2 cells (6, 20). The ability of NK T cells to secrete large quantities of cytokines following primary stimulation suggests that they may have a role in polarizing the subsequent adaptive T-helper-cell response.
Nematode parasites are among the most potent stimulators of Th2 responses (10), and it has been postulated that prototypic Th2 agents, for example, allergens and nematode parasites, may have the ability to activate a sufficient number of NK1.1+ T cells to result in rapid cytokine production, which can subsequently promote the development of the acquired immune response (22). In a previous study we demonstrated that infection of BALB/c mice with the third-stage larvae (L3) of Brugia pahangi elicits a significant burst of IL-4 transcription from a double-negative (CD4− CD8−) αβ T cell within 24 h of infection (15). In this work we extend these observations to demonstrate that NK T cells are indeed the source of early IL-4 at 24 h postinfection (hpi) and that this response is dependent upon the presence of live L3.
Male C57BL/6 mice, 6 weeks old, were purchased from Harlan Olac (Bicester, United Kingdom) and were maintained in filter-topped cages. B. pahangi L3 were harvested from infected Aedes aegypti mosquitoes by standard methods (8), washed in sterile Hanks balanced salt solution (HBSS) (Gibco/BRL), and counted. C57BL/6 mice were injected with 50 L3 in HBSS intravenously (i.v.) or injected with 30 L3 per hind footpad.
At 24 hpi, spleen cells (from mice with i.v. infections) were processed from five individual mice. Popliteal lymph node cells (from mice with footpad infections) were pooled from five animals per group. Cell suspensions were incubated for 2 h with brefeldin A (10 μg/ml; Sigma) in RPMI medium at 37°C with 5% CO2. The cells were washed and resuspended in blocking buffer (Fc block, the supernatant from rat anti-mouse CD16/CD32 monoclonal antibody [MAb] 24G2, 10% normal mouse serum, 0.2% sodium azide in phosphate-buffered saline [PBS]). For cell surface phenotypic analysis, the appropriate MAb or isotype-matched control MAb (all from Pharmingen) was added at 1 μg per 106 cells and incubated on ice for 15 min. Antibodies used were Cy-Chrome-labeled anti-mouse CD3 (145-2c11), APC-labeled anti-mouse CD4 (RM4-5), and fluorescein isothiocyanate-labeled anti-NK1.1 (PK136). Isotype controls were Cy-Chrome-labeled hamster immunoglobulin G (IgG) (A19-3), APC-labeled rat IgG2a (R35-95), phycoerythrin-labeled rat IgG2b (R35-38), and fluorescein isothiocyanate-labeled mouse IgG2a (G155-178). Cells were washed with PBS and fixed with 4% formaldehyde in PBS at room temperature for 20 min. Following one wash with ice-cold PBS and two washes with permeabilization buffer (0.2% sodium azide, 0.1% saponin [pH 7.4 to 7.6]), the cells were resuspended in permeabilization buffer and stained with 2 μg of phycoerythrin-labeled anti-mouse IL-4 (BVD4-1D11) per 106 cells. The cells were washed twice and analyzed on a Becton Dickinson FACSCalibur using CELLQUEST software (Becton Dickinson). Viable lymphocytes were gated by forward and side light scatter, and the gates for positive staining for CD3 and NK1.1 were set by comparison with isotype control MAb. The percentage of lymphocytes coexpressing CD3 and NK1.1+ was determined from 100,000 total events per sample. For analysis of intracellular IL-4, the acquisition gate was set on CD3+ NK1.1+ cells, and the number of cells coexpressing IL-4 was determined. Differences between groups were compared using the t test, with P values below 0.05 being considered significant.
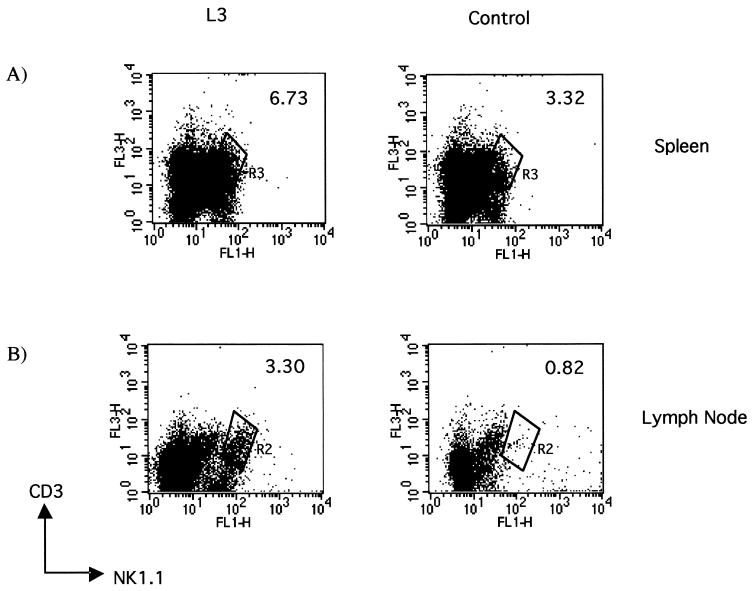
Infection of C57BL/6 mice with L3 of B. pahangi by the i.v. route resulted in a significant increase in the percentage of NK1.1+ T cells in L3-infected mice at 24 hpi, from 3.3% in control animals given HBSS i.v. to 6.7% in L3-infected animals (Fig. 1A). In six separate experiments there was a significant twofold increase in the number of lymphocytes that were CD3+ NK1.1+ in the spleen within 24 h of infection with L3 compared to that in uninfected controls given HBSS (P < 0.015). At this time point there was no difference between the total number of lymphocytes in the spleens of L3-infected mice and that in control mice.
FIG. 1.
NK T cells expand in response to infection with L3 of B. pahangi. Shown are representative scatter plots of CD3+ NK1.1+ lymphocytes in the spleens of C57BL/6 mice infected with 50 L3 of B. pahangi or control animals (A) or in the draining popliteal lymph nodes of mice injected with approximately 30 L3 or HBSS into the footpad (B) at 24 hpi. The cells were surface stained for CD3 and NK1.1. The data acquired were gated on viable lymphocytes as determined by forward and side scatter, and the percentage of CD3+ NK1.1+ cells was expressed. Spleen data presented are from one of six representative experiments, with each experiment containing five mice per group analyzed individually. Lymph node data are from one of four replicate experiments in which pooled cells from five mice per group were analyzed.
A greater expansion of NK T cells was observed in the draining popliteal lymph node following injection of L3 into the footpad. In the experiment shown in Fig. 1B, the percentage of NK T cells increased from 0.82% in the draining popliteal lymph nodes of mice given HBSS to 3.3% in those of mice given L3. In four separate experiments the fold increase in NK T cells in the draining popliteal lymph nodes of L3-infected mice averaged 4.1 ± 1.02 (mean ± standard deviation) (P < 0.028). The mean data from several different experiments are presented in Table 1. The popliteal lymph nodes increase significantly in size within 24 h of infection with L3, and when these results are expressed as the absolute numbers of NK1.1+ T cells in the lymph nodes, there is a disproportional increase (∼25-fold) in the numbers of NK1.1+ T cells, from a mean of 1.92 × 104 in uninfected mice to 5.12 × 105 in L3-infected mice (P < 0.002) (Table 2).
TABLE 1.
Mean numbers of NK T cells (CD3+ NK1.1+) per 105 cellsa
| Source of cells | NK T cells (mean ± SD) in mouse group
|
P | |
|---|---|---|---|
| L3 | Control | ||
| Spleen | 1,945 ± 718.9 | 1,003.3 ± 412.5 | <0.015 |
| Lymph node | 1,706.7 ± 395 | 426.7 ± 94.5 | <0.006 |
Mean number of NK T cells per 105 cells. The mean number of NK T cells per 105 cells was calculated from the percentage data acquired from spleen cells and lymph node cells at 24 hpi. Spleen cell data were collected from six individual experiments. Spleen cells from L3-infected mice contained significantly more NK T cells than those from control mice at 24 hpi (P < 0.015). Lymph node data are from one of four replicate experiments and represent the mean number of NK T cells per 105 cells present in lymph nodes pooled from five mice per group (P < 0.006 [L3-infected versus control mice]).
TABLE 2.
Mean absolute numbers of NK T cells (CD3+ NK 1.1+)a
| Source of cells | NK T cells (mean ± SD) in mouse group
|
P | |
|---|---|---|---|
| L3 | Control | ||
| Spleen | 7.78 × 105 ± 2.9 × 105 | 4.01 × 105 ± 1.65 × 105 | <0.015 |
| Lymph node | 5.12 × 105 ± 1.1.8 × 105 | 1.92 × 104 ± 0.43 × 104 | <0.002 |
Absolute numbers of NK T cells in the spleen and lymph node at 24 hpi. The mean absolute number of NK T cells was calculated from the percentage data acquired from spleen cells and lymph node cells at 24 hpi. Spleen cell data were collected from six individual experiments. Spleen cells from L3-infected mice contained significantly more NK T cells than those from control mice at 24 hpi (P < 0.015). Lymph node data are from one of four replicate experiments and represent the mean absolute number of NK T cells present in lymph nodes pooled from five mice per group (P < 0.002 [L3-infected versus control mice]).
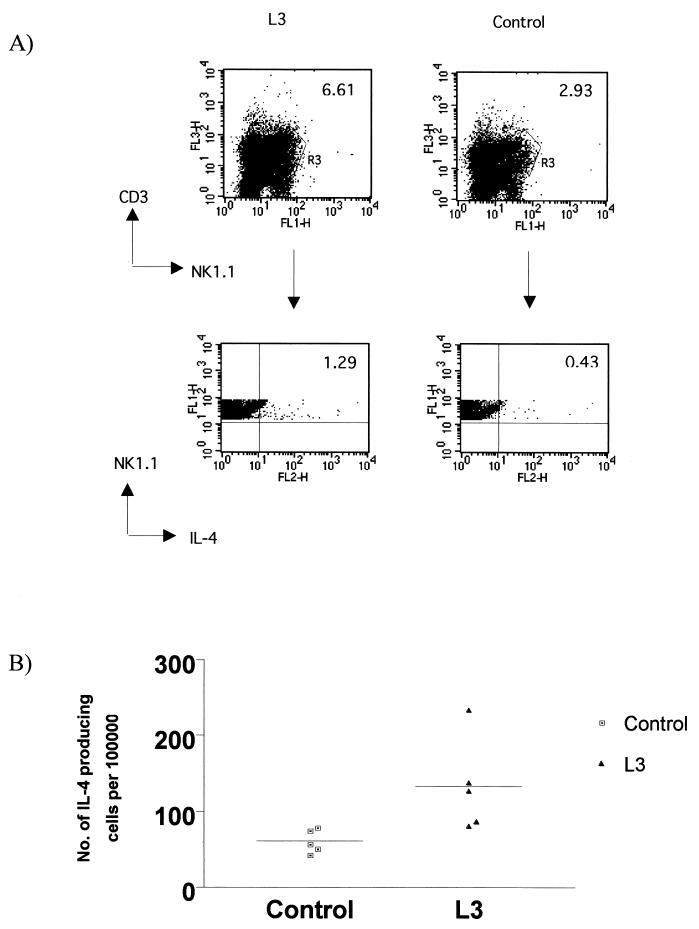
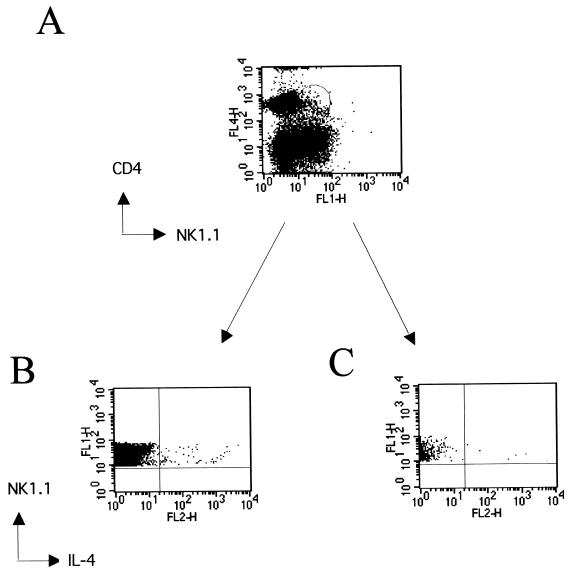
To determine if NK T cells contributed to the early IL-4 response to L3 infection, cells were harvested from the spleen (i.v. infection) at 24 hpi, labeled with the appropriate cell surface markers, permeabilized, and stained with anti-IL-4. For the flow cytometric analysis, the acquisition gate was set on CD3+ NK1.1+ cells, and the number of IL-4+ NK T cells was expressed. At 24 hpi, IL-4 production by NK T cells was observed in the spleen of L3-infected mice (Fig. 2A). In the experiment shown there was a significant threefold increase in the number of NK T cells producing IL-4 at 24 hpi in L3-infected mice compared to uninfected controls (P < 0.04) (Fig. 2B). No IL-4 was observed in CD3+ NK1.1− cells or CD3− NK1.1− cells, indicating that NK T cells are the major cellular source of the early burst of IL-4 in response to L3 infection. Further analysis revealed that essentially all the IL-4-producing cells in the spleen were CD4− (Fig. 3B). When the number of CD4− NK T cells that produced IL-4 was compared to CD4+ NK T cells, the figures were 108 ± 9 cells per 100,000 for CD4− cells and 4 ± 1.5 for CD4+ cells (means ± standard deviations). Further experiments demonstrated that live L3 were necessary for the induction of IL-4 from NK T cells (data not shown).
FIG. 2.
NK T cells produce IL-4 in response to L3 infection at 24 hpi. (A) Spleen cells were surface stained for CD3+ and NK1.1+, washed, fixed, and permeabilized to allow intracellular staining for IL-4. Viable lymphocytes were expressed on a dot plot of CD3 versus NK1.1 for spleen cells from L3-infected mice and control mice at 24 hpi. NK T cells (CD3+ NK1.1+) were selected, and the number of IL-4+ NK T cells was expressed. Data presented are from one of seven replicate experiments. (B) Scatter plot of the number of IL-4+ NK T cells per 105 total spleen cells. Data presented are from one representative experiment of five mice per group analyzed individually. The number of IL-4-producing NK T cells was significantly greater in L3-infected mice than in control mice (P < 0.0079).
FIG. 3.
IL-4-producing NK T cells are CD4−. Spleen cells were surface stained with CD4 and NK1.1 and then washed, fixed, and permeabilized to allow intracellular staining for IL-4. (A) Gates were set on CD4+ NK1.1+ and CD4− NK1.1− populations. CD4− NK1.1+ (B) and CD4+ NK1.1+ cells (C) were analyzed as shown in the lower panels.
These results demonstrate that infection of mice with L3 of B. pahangi induces the rapid production of IL-4 by NK1.1+ T cells. Consistent with previous observations in BALB/c mice, the IL-4-producing NK T cells were CD4−. The production of IL-4 by NK T cells was a relatively short-lived response, as by day 5 postinfection, no significant IL-4 production was observed by these cells in the spleen or lymph nodes of L3-infected mice (data not shown). Because of their capacity for rapid cytokine production, NK T cells may provide a link between innate and adaptive immune responses. Many NK T cells from both humans (16) and mice (3) are CD1 restricted and recognize lipid and glycolipid antigens such as mycolic acid from Mycobacterium tuberculosis and glycosylphosphatidylinositol (GPI)-anchored surface antigens of Plasmodium and Trypanosoma species (1, 16, 18). Whether the cells identified in the present study respond to lipid antigens derived from L3 remains unclear at present. In both mice and humans, many CD1-restricted T cells are auto-reactive, leading to the proposition that early production of IL-4 by CD1-restricted T cells may act to down-regulate proinflammatory responses (2).
The requirement of NK T cells for the development of a Th2 response remains controversial. Initially, NK T cells were proposed as a possible source of IL-4 that was essential for priming naïve CD4+ T cells to differentiate into Th2 effector cells (22). However, it was subsequently shown using β2-microglobulin knockout mice, which have greatly reduced numbers of NK T cells, that normal IL-4-dominated Th2 responses can develop in the absence of NK T cells (7, 23). Furthermore, in different models of infectious disease, additional sources of IL-4 at the start of an immune response have been identified, including eosinophils (17) and non-NK CD4+ T cells (13). Presumably, the cellular source of IL-4 varies with both the antigen and the site of infection, and as with many other facets of the immune response, it is likely that other cell types can compensate in the absence of NK T cells.
The prompt production of cytokines by NK T cells in response to L3 infection will affect the differentiation of naive CD4+ T cells by influencing the cytokine microenvironment in which they are stimulated (4). The immune response in human filariasis is characterized by a Th2 bias in which even apparently uninfected individuals exposed to the bites of infected mosquitoes express elevated levels of IL-4 (reviewed in reference 9). Moreover, recent studies in humans infected with filaria have shown that under conditions of intense L3 transmission, T-cell responses are profoundly affected while levels of IL-4 in plasma are elevated (12). Antigens derived from the L3 preferentially stimulated IL-4 release from basophils compared to antigen extracts from other life cycle stages (12). Thus, exposure to the L3 of filarial worms may be an important determinant of the eventual immune response in filariasis. Defining the nature of the L3-specific antigens that elicit the early IL-4 response will be a considerable challenge for future studies.
Acknowledgments
We are grateful to Paul Garside for help with the FACS analysis; Julie Strang, Isla Wheatley, and Victoria Gillan for technical assistance; and Richard O'Connor and Fiona Thompson for input into the project and critical reading of the manuscript. We thank F. Y. Liew, Department of Immunology, University of Glasgow, for access to the flow cytometer.
This project was funded by a grant from the MRC.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Beckman, E. M., S. A. Porcelli, C. T. Morita, S. M. Behar, S. T. Furlong, and M. B. Brenner. 1994. Recognition of lipid antigen by CD1-restricted αβ+ T cells. Nature 372:691-694. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac, A. 1995. CD1: presenting unusual antigens to unusual T lymphocytes. Science 269:185-186. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac, A., O. Lantz, M. E. Quimby, J. W. Yewdell, J. R. Bennick, and R. R. Brutkiewicz. 1995. CD1 recognition by mouse NK1+ T lymphocytes. Science 268:863-865. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Sasson, S. Z., K. Makedonski, J. Hu-Li., and W. E. Paul. 2000. Survival and cytokine polarization of naïve CD4+ T cells in vitro is largely dependent on exogenous cytokines. Eur. J. Immunol. 30:1308-1317. [DOI] [PubMed] [Google Scholar]
- 5.Burdin, N., L. Brossay, Y. Koezuka, S. T. Smiley, M. J. Grusby, M. Gui, M. Taniguchi, K. Hayakawa, and M. J. Kronenberg. 1998. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J. Immunol. 161:3271-3281. [PubMed] [Google Scholar]
- 6.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29:2014-2025. [DOI] [PubMed] [Google Scholar]
- 7.Brown, D. R., D. J. Fowell, D. B. Corry, T. A. Wynn, N. H. Moskowitz, A. W. Cheever, R. M. Locksley, and S. L. Reiner. 1997. β2-microglobulin-dependent NK1.1+ T cells are not essential for T helper cell 2 immune responses. J. Exp. Med. 184:1295-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devaney, E., and R. M. Jecock. 1991. The expression of the Mr 30,000 antigen in the third stage larvae of Brugia pahangi. Parasite Immunol. 13:75-87. [DOI] [PubMed] [Google Scholar]
- 9.Devaney, E., and J. Osborne. 2000. The L3 of Brugia: its role in immune modulation and protective immunity. Microbes Infect. 2:1363-1371. [DOI] [PubMed] [Google Scholar]
- 10.Holland, M. J., Y. M. Harcus, P. L. Riches, and R. M. Maizels. 2000. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur. J. Immunol. 30:1977-1987. [DOI] [PubMed] [Google Scholar]
- 11.Joyce, S., A. S. Woods, J. W. Yewdell, J. R. Bennick, A. D. De Silva, A. Boesteanu, S. P. Balk, R. J. Cotter, and R. R. Brutkiewicz. 1998. Natural ligand of mouse CD1d1: cellular glycosylphoshatidylinositol. Science 279:1541-1544. [DOI] [PubMed] [Google Scholar]
- 12.King, C. L. 2001. Transmission intensity and human immune responses to lymphatic filariasis. Parasite Immunol. 23:363-371. [DOI] [PubMed] [Google Scholar]
- 13.Launois, P., I. Mallard, S. Pingel, K. G. Swihart, I. Xenarios, H. Acha-Orbea, H. Digglemann, R. M. Locksley, H. Robson MacDonald, and J. A. Louis. 1997. IL-4 rapidly produced by Vβ4 Vα8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 6:541-549. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald, H. R. 1995. NK1.1+ T cell receptor αβ+ cells: new clues to their origin, specificity and function. J. Exp. Med. 182:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborne, J., and E. Devaney. 1998. The L3 of Brugia induces a Th2-polarized response following activation of an IL-4-producing CD4−CD8− αβ T cell population. Int. Immunol. 10:1583-1590. [DOI] [PubMed] [Google Scholar]
- 16.Porcelli, S., C. T. Morita, and M. B. Brenner. 1992. CD1b restricts the response of human CD4−CD8− T lymphocytes to a microbial antigen. Nature 360:593-597 [DOI] [PubMed] [Google Scholar]
- 17.Sabin, E. A., M. A., Kopf, and E. J. Pearce. 1996. Schistosoma mansoni egg induced early IL-4 production is dependent upon IL-5 and eosinophils. J. Exp. Med. 184:1871-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schofield, L., M. J. McConville, D. Hansen, A. S. Campbell, B. Fraser-Reid, M. J. Grusby, and S. D. Tachado. 1999. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NK T cells. Science 283:225-229. [DOI] [PubMed] [Google Scholar]
- 19.Seiling, P. A., D. Chatterjee, S. A. Porcelli, T. I. Prigozy, R. J. Mazzaccaro, T. Soriano, B. R. Bloom, M. B. Brenner, M. Kronenburg, P. J. Brennan, and R. L. Modlin. 1995. CD-1 restricted T cell recognition of microbial lipoglycan antigens. Science 269:227-230. [DOI] [PubMed] [Google Scholar]
- 20.Singh, N., S. Hong, D. C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163:2373-2377. [PubMed] [Google Scholar]
- 21.Vicari, A., and A. Zlotnik. 1996. Mouse NK1.1+ T cells: a new family of T cells. Immunol. Today 17:71-76. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimoto, T., and W. E. Paul. 1994. CD4+, NK1.1+ T cells promptly produce interleukin-4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 174:1285-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, Y., K. H. Rogers, and D. B. Lewis. 1996. β2-microglobulin-dependent T cells are dispensable for allergen-induced T helper 2 responses. J. Exp. Med. 184:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]