Abstract
C3H and C57BL/6 mice are resistant to Leishmania major but develop chronic lesions with persistent parasite loads when they are infected with Leishmania amazonensis. These lesions develop in the absence of interleukin-4 (IL-4), indicating that susceptibility to this parasite is not a result of development of a Th2 response. Expression of the cytokine IL-10 during infection could account for the lack of IL-12 expression and poor cell-mediated immunity towards the parasite. Therefore, we tested the hypothesis that IL-10 plays a central role in downmodulating the Th1 response after L. amazonensis infection. Infection of C57BL/6 IL-10-deficient mice indicated that in the absence of IL-10 there was early enhancement of a Th1 response, which was downregulated during the more chronic stage of infection. In addition, although there were 1- to 2-log reductions in the parasite loads within the lesions, the parasites continued to persist, and they were associated with chronic lesions whose size was similar to that of the control lesions. These experiments indicated that L. amazonensis resistance to killing in vivo is only partially dependent on expression of host IL-10. However, IL-10-deficient mice had an enhanced delayed-type hypersensitivity response during the chronic phase of infection, indicating that there were Th1 type effector cells in vivo at this late stage of infection. These results indicate that although IL-10 plays a role in limiting the Th1 response during the acute infection phase, other immunomodulatory factors are responsible for limiting the Th1 response during the chronic phase.
Infection of mice with Leishmania spp. that cause cutaneous leishmaniasis has led to an understanding of many of the immunological events that are required for a successful host response towards these protozoan parasites. This experimental system has helped define factors necessary for the generation of the Th1 and Th2 types of immune responses in vivo (37, 42). Many studies have demonstrated that the host responses to different species or strains of Leishmania can vary in the same mouse strain. For example, BALB/c mice develop a fatal Th2 response after Leishmania major infection and yet are resistant to Leishmania braziliensis (9, 11). A more recent example is the finding that interleukin-4 (IL-4)-knockout (KO) and IL-4 receptor-KO mice have different susceptibilities to infection with different strains of L. major (27, 32, 33). Similarly, C3H and C57BL/6 mice are resistant to L. major but develop chronic lesions with persistent parasite loads when they are infected with L. amazonensis (2, 5, 7, 39, 45). These studies demonstrate that both host and parasite factors influence the outcome of an infection.
Recent work has shown that the in vivo T-cell response towards Leishmania amazonensis is different from that described for other Leishmania species. The chronic lesions characteristic of L. amazonensis infection in C57BL/6 and C57BL/10 mice are independent of expression of IL-4 and a corresponding Th2 response (2, 23). In addition, L. amazonensis infection in C3H mice results in low levels of production of IL-12 and gamma interferon (IFN-γ) by antigen-specific CD4+ T cells (23). However, lesion development and parasite burden have been shown to be exacerbated in the presence of CD4+ T cells, demonstrating that T cells are activated during L. amazonensis infection and that they contribute significantly to the immunopathology of the chronic disease (44). We have shown that chronic infection by L. amazonensis in C3H and C57BL/6 mice is persistent even after administration of exogenous IL-12 (23). The inability of IL-12 to drive an effective cell-mediated immune response during L. amazonensis infection suggests that the parasite can evoke a potent immunomodulatory mechanism to evade widespread parasite killing and promote a chronic infection. The importance of understanding the factors involved in maintenance of this chronic disease is highlighted by the fact that chronic cutaneous leishmaniasis in humans is often correlated with a poor T-cell-mediated immune response (43) or a mixed T-cell response (35).
IL-10 has been shown to be a potent immunomodulatory molecule (30). This cytokine has been shown to play a role in limiting IFN-γ production in T cells via downregulation of IL-12 production in both macrophages and dendritic cells and in downregulating antigen-presenting cell function by decreasing the expression of major histocompatibility complex class II and regulating the responsiveness and/or expression of costimulatory molecules in the antigen-presenting cell population (10, 13, 14-16, 26, 29, 36). In addition, IL-10 has been associated with decreased immune responses to cutaneous leishmaniasis in people (6, 40), and inhibition of IL-10 during the recall response increases IFN-γ levels (40). However, anti-IL-10 treatment did not alter the course of infection in L. major-susceptible BALB/c mice or L. major-resistant C57BL/6 mice (8). Recent reports have suggested that the primary role of IL-10 during Leishmania infection may be to limit macrophage activation since FcγR-mediated uptake of immunoglobulin-opsonized parasites by macrophages leads to IL-10-dependent inhibition of parasite killing (24). These studies also demonstrated that IL-10-deficient BALB/c mice infected with tissue-derived L. major amastigotes had significantly smaller lesions and fewer parasites associated with the lesions than wild-type (WT) control mice (24). In support of these data, a recent study demonstrated that mice deficient in either immunoglobulin production or FcγR expression were resistant to L. amazonensis infection, although the role of IL-10 in this infectious disease model is unknown (25).
Since C57BL/6 mice infected with L. amazonensis develop chronic lesions with a persistent parasite burden independent of a Th2 response, we tested the hypothesis that IL-10 is responsible for limiting the host immune response during such an infection. At 4 weeks postinfection, the tissue parasite load was decreased and there was an enhanced Th1 response in the absence of IL-10. However, at a more chronic stage of infection, parasites persisted within the lesions and there was no increase in the Th1 response in the draining lymph nodes (LN). In addition, lower numbers of IFN-γ-producing cells in the draining LN and lower levels of Leishmania-specific immunoglobulin G2a (IgG2a) antibody in the IL-10-deficient mice suggested that in vivo IFN-γ levels were not enhanced in the IL-10-deficient mice compared to those of WT mice. In contrast, the positive delayed-type hypersensitivity (DTH) response at 15 weeks postinfection demonstrated that potential Th1 immune effector cells were present in vivo. These results indicate that IL-10 does limit parasiticidal activity in the host but does not play a major role in determining the phenotype of the Th response during L. amazonensis infection.
MATERIALS AND METHODS
Parasites.
L. amazonensis parasites (MHOM/BR/00/LTB0016) were grown to the stationary phase in Grace's insect cell culture medium (Life Technologies, Grand Island, N.Y.) supplemented with 20% heat-inactivated fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Stationary-phase parasites were washed twice in phosphate-buffered saline (PBS) before they were used for infection. Freeze-thawed leishmanial antigen (FT-ag) was obtained from stationary-phase promastigotes that were washed twice in PBS, resuspended in PBS, and subjected to three freeze-thaw cycles consisting of −70 and 37°C. The protein concentration was determined by the bicinchoninic acid assay, the final concentration was adjusted to 2 mg/ml, and aliquots were stored at −70°C before use. The parasite burden was determined by performing a limiting dilution analysis as previously described (2).
Mice.
Female C57BL/6 and B6.129P2-Il10tm1Cgn mice were purchased from Jackson Laboratory (Bar Harbor, Maine). The mice were maintained in a specific-pathogen-free facility. The Committee on Animal Care at Iowa State University approved all protocols involving animals. Each mouse was inoculated with 5 × 106 stationary-phase promastigotes in 50 μl of PBS in a hind footpad. Lesion size was monitored with a dial micrometer (L. S. Starrett Co., Athol, Mass.), and the results were expressed as the difference between the footpad thickness for the uninfected foot and the footpad thickness for the infected foot.
DTH response.
At 15 weeks postinfection, the uninfected right hind foot of each mouse was measured, and 20-μg portions of FT-ag in 50 μl of PBS were injected into one-half of the infected mice; each of the other mice received an injection consisting of 50 μl of PBS in the subcutaneous tissue of the uninfected hind foot. At 24 h after antigen injection the right hind foot of each mouse was measured again. No increase in footpad thickness was detected at this time in the PBS-challenged mice (data not shown).
Recall response and ELISPOT analyses.
LN cells draining the infected feet were obtained 4 and 15 weeks postinfection as indicated below. The cells were disassociated with a Tenbrock tissue homogenizer and were resuspended in complete tissue culture medium (Dulbecco's minimal essential medium containing 4.5 mg of glucose/ml, 2 mM l-glutamine, 100 U of penicillin 6-potassium/ml, 100 mg of streptomycin/ml, 25 mM HEPES, and 50 μM 2-mercaptoethanol) at a density of 5 × 106 cells per ml in a final volume of 200 μl in U-bottom or flat-bottom 96-well plates. The cells were incubated in medium only (unstimulated), or they were stimulated with 50 μg of FT-ag per ml. Supernatants were harvested after 72 h, and IFN-γ and IL-4 levels were determined by enzyme-linked immunosorbent assays (ELISA); the sensitivity of both assays was ≥39 pg/ml. The numbers of IL-12p40- and IFN-γ-producing cells were determined ex vivo by using enzyme-linked immunospot (ELISPOT) assays with 4- and 12-h incubations, respectively, as described previously (41). The ELISA and IFN-γ and IL-12 ELISPOT assays were performed with commercially available antibodies (BD Pharmingen, San Diego, Calif.).
IgG2a antibody titers.
Leishmania-specific antibody contents were determined by using an ELISA based on a previously described assay (46). Briefly, polystyrene microtiter plates (Becton Dickinson, Franklin Lakes, N.J.) were coated with 1.0 μg of L. major FT-ag per ml or 1.0 μg of L. amazonensis FT-ag per ml in PBS (pH 9.4) overnight at 4°C. The plates were washed as described previously and blocked with 5% nonfat dry milk (Carnation) in PBS-0.05% Tween 20 for 2 h at 37°C. Serum samples were then serially diluted with 0.05% Tween 20 in PBS starting at a dilution of 1:100. The samples were incubated overnight at 4°C. The plates were washed in PBS-0.05% Tween 20 five times, and bound antibodies were detected with goat anti-mouse IgG2a directly conjugated to horseradish peroxidase (Southern Biotechnology Associates, Birmingham, Ala.) and diluted 1:5,000 in PBS containing 25% normal goat serum (pH 9.4). After 2 h of incubation at 37°C, the plates were washed with PBS-0.05% Tween 20, and the 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) was then added. The plates were read at A405 with a microplate reader (Molecular Devices, Sunnyvale, Calif.). The antibody titer was expressed as the optical density at 405 nm of the 1:300 dilution. All sera from uninfected animals were negative, as determined by optical densities at 405 nm of 1:300 dilutions (data not shown).
Immunohistochemistry.
At different times after infection, tissue from each mouse was fixed in 10% buffered formalin and routinely processed for paraffin-imbedded sectioning, and then 8-μm-thick sections were cut, applied to poly-l-lysine-coated slides, and stained with hematoxylin and eosin. Additional unstained sections were used for immunohistochemical detection of inducible nitric oxide synthase (iNOS) expression. Briefly, slides were incubated in a 1:10,000 dilution of a polyclonal rabbit IgG anti-mouse iNOS antibody (06-573; Upstate Biotechnology Incorporated, Lake Placid, N.Y.) in primary dilution buffer (Optimax; BioGenex, San Ramon, Calif.). Control sections were incubated with polyclonal rabbit IgG (12-370; Upstate Biotechnology). The primary antibody was detected by using adsorbed affinity-purified biotinylated goat anti-rabbit antibody (076-15-061; Kirkegaard and Perry) at a dilution of 1:200 in Optimax primary diluent. Super-sensitive-streptavidin-conjugated peroxidase was used for secondary antibody detection (BioGenex), and the signal was generated by using Nova Red (Vector Laboratories, Burlingame, Calif.). Tissue sections were then counterstained with Shandon's hematoxylin. Control sections were negative, as were footpad sections stained for iNOS from uninfected animals (data not shown).
Statistical procedures.
Statistical significance was determined by analysis of variance by using StatView (SAS Institute Inc.). Differences between means were considered significant if P was ≤0.05. Results are presented below as means ± standard errors, unless indicated otherwise.
RESULTS
L. amazonensis infection of IL-10-deficient mice.
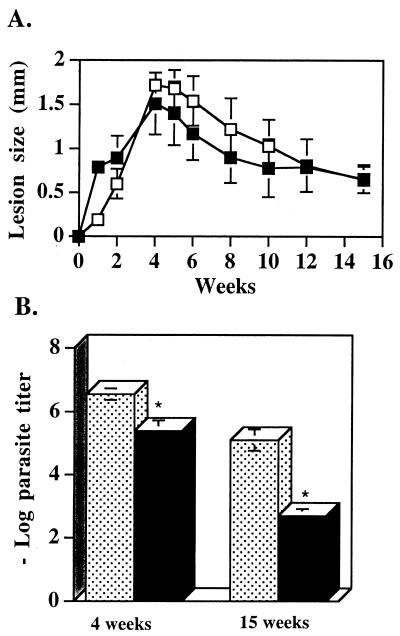
IL-10-deficient and C57BL/6 WT mice were infected with 5 × 106 stationary-phase L. amazonensis parasites. Lesion size was measured over time. Figure 1A shows that IL-10-deficient mice developed persistent lesions similar to those of the WT mice. Parasite numbers associated with the lesions were determined 4 and 15 weeks postinfection. The results indicate that although the lesion sizes were comparable in the two infections, there was a 1.1-log reduction in parasite load at 4 weeks postinfection and a 2.2-log reduction in parasite load at 15 weeks postinfection in the IL-10-deficient mice (Fig. 1B).
FIG. 1.
IL-10-deficient mice have chronic lesions with detectable parasites. (A) IL-10-deficient mice and WT controls were inoculated in the left hind footpad with 5 × 106 stationary-phase L. amazonensis promastigotes. Lesion size was monitored over time with a dial micrometer and was expressed as the difference between the footpad thickness for the infected foot and the footpad thickness for the uninfected foot. The results are representative of the results of three separate experiments performed with five mice per group. The values are the mean ± standard deviation lesion sizes for WT mice (□) and IL-10-deficient mice (▪). (B) Numbers of parasites in the infected feet at 4 and 15 weeks postinfection (see Materials and Methods). The stippled bars and the solid bars represent data obtained with WT and IL-10-deficient mice, respectively. The values are means ± standard errors based on three experiments performed with three to five mice per group. P was 0.005 and <0.001 for the 4- and 15-week postinfection values, respectively.
Histology and iNOS expression in tissue.
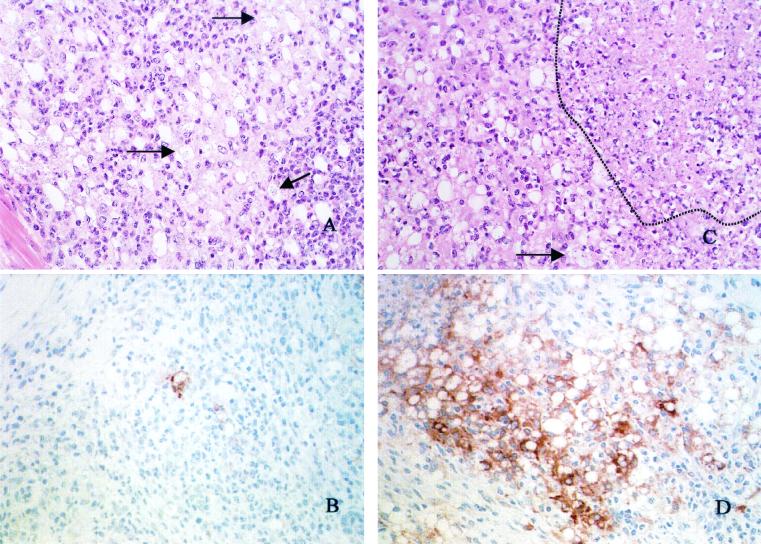
Hematoxylin- and eosin-stained sections from both IL-10-deficient mice and WT mice infected with L. amazonensis revealed similar lesion morphologies at 4 weeks postinfection. L. amazonensis-infected footpads in both IL-10-deficient mice and WT mice contained dense infiltrates of inflammatory cells that extended from the superficial dermis to the deep dermis. However, in the IL-10-deficient mice, within the inflammatory infiltrate were multifocal areas of necrosis involving up to 20% of the section. In the remaining areas there were dense infiltrates of macrophages, neutrophils, lymphocytes, and occasional plasma cells. Admixed with these leukocytes were moderate numbers of fibroblasts and fibrous connective tissue. Most macrophages had abundant cytoplasm with one to several variably sized vacuoles that were empty or contained one to several amastigotes. The numbers of amastigotes in the footpads of the WT mice were greater (Fig. 2A and C). An immunohistochemistry analysis in which we used antibodies that detected iNOS indicated that cells whose morphology was consistent with the morphology of macrophages and which were present in the lesions of both IL-10-deficient mice and WT mice upregulated this enzyme after infection. However, at 4 weeks postinfection, iNOS staining was more readily detectable and more intense in the lesions of the IL-10-deficient mice than in the lesions of the WT mice (Fig. 2B and D). Both iNOS-expressing cells and parasites were readily detectable at 15 weeks postinfection in WT and IL-10-deficient mice (data not shown).
FIG. 2.
L. amazonensis-infected IL-10-deficient mice have decreased parasite numbers and increased iNOS expression in vivo compared to WT mice. WT and IL-10-deficient mice were infected with L. amazonensis as described in the legend to Fig. 1, and the infected footpad of each mouse was harvested 4 weeks postinfection. (A) Histology of an L. amazonensis-infected footpad of a WT mouse. The arrows indicate parasites within macrophages. (B) Tissue section of an L. amazonensis-infected footpad of a WT mouse stained for iNOS (brown). (C) Histology of an L. amazonensis-infected footpad of an IL-10-deficient mouse. The arrow indicates parasites, and an area of necrosis is outlined. (D) Tissue section of an L. amazonensis-infected footpad of an IL-10-deficient mouse stained for iNOS (brown). The tissue sections are representative of tissue sections of footpads harvested from two individual WT or IL-10-deficient mice from three separate experiments. The tissue sections were processed for hematoxylin and eosin staining and iNOS expression as described in Materials and Methods. Magnification, ×20.
Ex vivo detection of IL-12p40-producing LN cells.
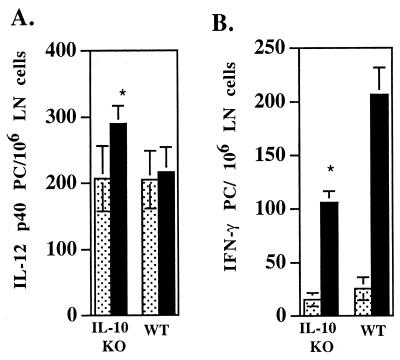
We have previously shown that in C3H mice the level of IL-12-producing cells in the LN draining the site of L. amazonensis infection remains near the level in uninfected mice. Since IL-10 has been shown to inhibit IL-12, we analyzed IL-12 production ex vivo by using the ELISPOT assay for IL-12p40-producing cells. IL-10-deficient mice infected with L. amazonensis as described above did have increased numbers of IL-12-producing cells in their draining LN at 4 weeks postinfection compared to the numbers of IL-12-producing cells in uninfected mice (Fig. 3A). Consistent with data obtained with C3H mice, the numbers of these cells in the WT mice were not greater than the numbers in uninfected mice.
FIG. 3.
IL-10-deficient mice have increased numbers of IL-12p40-producing cells and decreased numbers of IFN-γ-producing cells at 4 weeks postinfection. (A) IL-10-deficient (IL-10 KO) and WT mice were infected as described in the legend to Fig. 1. At 4 weeks postinfection the numbers of IL-12p40-producing cells in the draining LN were determined by an ELISPOT assay (see Materials and Methods). P was 0.04 for the infected IL-10-deficient mice compared to uninfected IL-10-deficient mice. (B) Numbers of IFN-γ-producing cells, as determined by an ELISPOT assay, from the same mice as described above for panel A. P was ≤0.001 for the infected IL-10-deficient mice compared to the infected WT mice. The stippled bars and the solid bars represent data for uninfected and infected mice, respectively. PC, producing cells. The values are means ± standard errors based on three separate experiments performed with three to five animals per group.
IFN-γ production.
An analysis of the numbers of IFN-γ-producing cells in the draining LN at 4 weeks postinfection demonstrated that the numbers of these cells were greater than the numbers in uninfected animals in both the IL-10-deficient mice and the WT mice. In contrast to the numbers of IL-12-producing cells, there were fewer cells producing IFN-γ in the IL-10-deficient mice than in the WT mice, although the background levels in uninfected animals were not statistically different (Fig. 3B). Therefore, the postinfection increase in the numbers of IFN-γ-producing cells compared to the levels in uninfected animals was greater for the WT mice (8.11-fold increase) than for the IL-10-deficient mice (6.88-fold increase).
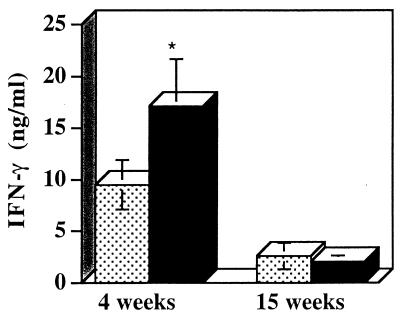
Despite the lower numbers of IFN-γ-producing cells detected ex vivo in the draining LN, the antigen-specific recall response at 4 weeks postinfection demonstrated that LN cells from infected IL-10-deficient mice did have the potential to produce more IFN-γ than LN cells from infected WT mice could produce. However, at 15 weeks postinfection, when there was still a persistent parasite load in the IL-10-deficient mice, these mice did not have increased IFN-γ in the recall response compared to the IFN-γ in the recall response in infected WT mice. In addition, the levels of IFN-γ produced in both groups of mice were less than the levels detected with the recall response at 4 weeks postinfection (Fig. 4). At 4 weeks postinfection IL-4 production was low in both the IL-10-deficient mice and the WT mice (mean production, 74 ± 80 and 73 ± 115.6 pg/ml, respectively). At 15 weeks postinfection there was 39.2 ± 97 pg of IL-4 per ml in the recall response of the IL-10-deficient mice and no IL-4 was detected in the WT mice.
FIG. 4.
Increased IFN-γ production from the recall response at 4 weeks postinfection but not at 15 weeks postinfection in L. amazonensis-infected IL-10-deficient mice. IL-10-deficient and WT mice were infected as described in the legend to Fig. 1. At the times indicated a 3-day antigen-specific recall response analysis was performed by using LN cells draining the site of infection. The levels of IFN-γ in the supernatants were determined by ELISA. The stippled bars and the solid bars represent data for WT and IL-10-deficient mice, respectively. The values are means ± standard errors based on three separate experiments performed with five mice per group. P was 0.0085 for the IL-10-deficient mice compared to the WT mice at 4 weeks postinfection.
Leishmania-specific IgG2a production.
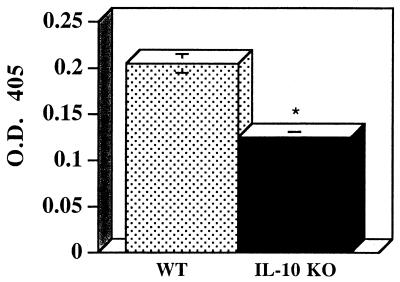
Given the dichotomy between the ELISPOT analysis results and the results of the recall response analysis, we measured the relative levels of Leishmania-specific IgG2a antibodies in order to assess in vivo IFN-γ production during the immune response. Figure 5 shows that at 15 weeks after L. amazonensis infection there was a significant decrease in the level of Leishmania-specific IgG2a isotype antibodies in the IL-10-deficient mice compared to the level in the WT control mice. These results are in agreement with the ELISPOT assay results which indicated that lower levels of IFN-γ were expressed in vivo in the IL-10-deficient mice.
FIG. 5.
L. amazonensis-specific IgG2a antibody levels are significantly decreased in IL-10-deficient mice. At 15 weeks postinfection serum was collected from IL-10-deficient mice (IL-10 KO) and WT mice, and the relative levels of Leishmania-specific IgG2a antibodies were determined as described in Materials and Methods. The values are means ± standard errors based on three separate experiments performed with five to seven animals per group. P = 0.027. O.D. 405, optical density at 405 nm.
DTH response.
To further assess the phenotype of the in vivo immune response, mice were tested for a DTH response by using the uninfected footpads. Half of the mice received an injection of antigen, and the other half received an injection of PBS as a control. After 24 h measurements of the injected feet were obtained, the mice were then sacrificed for the end point analysis. At 24 h after antigen challenge, the IL-10-deficient mice exhibited a significant increase in footpad thickness compared to the footpad thickness of the antigen-challenged WT mice (Fig. 6).
FIG. 6.
IL-10-deficient mice have an increased DTH response. At 15 weeks postinfection IL-10-deficient mice (IL-10 KO) and WT mice were each challenged with 20 μg of FT-ag in the uninfected footpad. Swelling was measured 24 h after the challenge. The values are means ± standard errors based on three separate experiments performed with three or four animals per group challenged with antigen (see Materials and Methods). P < 0.001.
DISCUSSION
To determine the role of IL-10 in limiting cell-mediated immunity during experimental L. amazonensis infection, we used IL-10-deficient mice with a C57BL/6 background. L. amazonensis infection of IL-10-deficient mice and WT mice resulted in the development of chronic lesions over time and readily detectable numbers of parasites associated with the lesions (Fig. 1 and 2). The lesions in both the IL-10-deficient mice and the controls contained parasites and a lymphocytic infiltrate at the infection site 4 weeks postinfection (Fig. 2). There were, however, increased areas of necrosis associated with the infection in the IL-10-deficient mice (Fig. 2). These results are consistent with reports that have described increased necrosis of the liver after systemic infection of IL-10-deficient mice with Trypanosoma cruzi, Schistosoma mansoni, and Toxoplasma gondii (17, 22, 49). Peroral infection of IL-10-deficient mice with T. gondii resulted in necrosis of substantial areas of the intestinal epithelium (48). The role that necrosis plays in limiting parasite persistence during Leishmania infection is unknown, but it is possible that increased necrosis of infected tissue could contribute to increased parasiticidal activity in the IL-10-deficient mice. Regardless of the increases in tissue necrosis, our study indicates that IL-10 expression does not influence the resolution of the chronic lesions that are characteristic of L. amazonensis infection in these mice. However, IL-10 does promote parasite survival, since in the absence of IL-10 1- to 2-log reductions in parasite load were detected at both 4 and 15 weeks postinfection (Fig. 1B). The reduced parasite load is similar to that reported for IL-10-deficient mice with a BALB/c background when they were infected with L. major. However, the lesions in the BALB/c mice were reduced compared to those in the control mice (24). The difference in these results is that after L. major infection, BALB/c mice develop progressive lesions with a concomitant Th2 response, whereas L. amazonensis infection in C57BL/6 mice results in chronic lesions in the absence of a Th2 response.
In our analysis of the IL-10-deficient mice after L. amazonensis infection, we detected an increase in the amount of IFN-γ in the recall response of the draining LN at 4 weeks after infection (Fig. 4). Although we did not determine the source of IFN-γ, the increase did correlate with a small but significant increase in the number of IL-12-producing cells in the draining LN and an enhanced DTH response. These results are consistent with an increase in a T-cell-mediated response and suggest that there is some biological significance to the increase in IL-12. The modest increase in the Th1 response after L. amazonensis infection is in contrast to the immune response of IL-10-deficient mice infected with T. gondii or T. cruzi. Systemic infections with these protozoans caused an immune hyperactivity that resulted in a lethal immune response mediated by IFN-γ from CD4+ T cells (17, 22, 31). In addition, in our experiments, the ratio of IFN-γ-producing cells in the LN of infected mice to IFN-γ-producing cells in the LN of uninfected mice was not greater for the IL-10-deficient mice than for the WT mice (Fig. 3B). In fact, the decrease in the number of IFN-γ-producing cells in the L. amazonensis-infected IL-10-deficient mice suggests that in the absence of IL-10, significant immunomodulatory mechanisms that limit IFN-γ production in vivo are upregulated. This conclusion is supported by the fact that the titers of IFN-γ-dependent Leishmania-specific IgG2a isotype antibodies were lower in the IL-10-deficient mice than in the WT mice (Fig. 5). Together, the data clearly indicate that any enhancement of the Th1 response due to the absence of host IL-10 is insufficient for an effective cell-mediated immune response to L. amazonensis and resolution of the infection.
There have been several studies demonstrating the association of IL-10 production with leishmanial infection in human and experimental murine infections. In these studies neutralization of IL-10 during in vitro analysis indicated that IL-10 plays a role in limiting IFN-γ production from CD4+ T cells and NK cells during Leishmania infection (40, 41). However, recent studies have demonstrated that during cutaneous leishmaniasis, IL-10 promotes parasite survival by influencing macrophage activation and that the role of IL-10 in determining the phenotype of the T-cell response is less important (18, 19, 24). In our studies, the observation that there is a low level of in vivo IFN-γ production but a high level of iNOS expression (Fig. 2D) with a decreased parasite load is consistent with the hypothesis that IL-10 plays a primary role in limiting macrophage activation during cutaneous leishmaniasis. The iNOS expression detected at 15 weeks postinfection (data not shown) suggests that there is persistent macrophage activation during later stages of the disease. These results are consistent with previous studies demonstrating the importance of NO in limiting parasite numbers in mice chronically infected with L. major (47). However, the persistence of L. amazonensis parasites in the IL-10-deficient mice indicates that the presence of these parasites during chronic infection is only partially dependent on expression of host IL-10.
After 15 weeks, localized footpad infection by L. amazonensis becomes relatively quiescent, and there is no increase in IL-12-producing cells in the draining LN, even in the presence of readily detectable parasites (data not shown). In addition, there is no detectable increase in the antigen-specific Th1 response in the draining LN of IL-10-deficient mice at this time (Fig. 4). Given the absence of a sustained increase in these parameters for the Th1 type of immunity in the draining LN, these results suggest that regulation of the Th1 response is independent of IL-10. These results are similar to those described for infection of IL-10-deficient mice with S. mansoni, which resulted in an increase in Th1 cytokines and a mixed Th1/Th2 immune phenotype during the acute immune response. As the disease progressed to a chronic stage, there was downregulation of Th1-associated cytokines. This regulation of the enhanced Th1 response was subsequently shown to be dependent on endogenous IL-4 (20, 49). Previous work with L. amazonensis has demonstrated that IL-4 does not play a role in limiting the immune response during this infection (2, 23). In agreement with the previous studies, very little IL-4 was detected in the recall responses of L. amazonensis-infected mice, whether they were IL-10-deficient mice or control mice. Therefore, we believe that downregulation of the Th1 response in L. amazonensis-infected IL-10-deficient mice is independent of IL-4.
The enhanced DTH response of L. amazonensis-infected IL-10-deficient mice does demonstrate that Th1 effector cells are present in vivo (Fig. 6). Previous studies have shown that IL-10 can inhibit the DTH response, at least in part, by limiting vascular permeability and swelling during the DTH response (28). Increases in these parameters of footpad swelling in IL-10-deficient mice during infection could account for the finding that the lesion size was equivalent to that in WT mice, even though the parasite load in the tissue was 1 to 2 logs lower in the IL-10-deficient mice. The dichotomy between the enhanced DTH response and the low IFN-γ levels detected in the recall response of the draining LN could be reconciled if the antigen interaction with the antigen-presenting cell-T-cell stimulation pathway in vivo was limited. This scenario could suggest that there are more antigen-specific memory T cells in the peripheral tissue and fewer antigen-specific Th1 effector, IFN-γ-producing cells in the draining LN. An increase in the peripheral activated memory cells has been described previously for IL-10-deficient mice, and it has recently been shown that few Th1 effector cells occur in secondary lymphoid organs in mice after an antigen challenge (3, 38). Regulation of antigen presentation in vivo would be consistent with in vitro data for the same organism that demonstrated that this parasite has several methods that prevent antigen stimulation of T cells (1, 4, 12, 34). Alternatively, increased iNOS expression is a possible mechanism of Th1 suppression through increased nitric oxide levels. This could account for local regulation of the immune response at the draining LN and is consistent with the role of nitric oxide in limiting T-cell responses during other intracellular protozoan infections, including infections of IL-10-deficient mice with Toxoplasma and infections of C57BL/6 mice with L. major (21, 31). Work is under way to determine what IL-10-independent mechanisms regulate the IL-12-producing cells and the Th1 cells that are detected in the recall response at 4 weeks postinfection and in the DTH response at 15 weeks postinfection.
In conclusion, the data presented here demonstrate that in the mouse model of cutaneous leishmaniasis, susceptibility to L. amazonensis is dependent not only on expression of IL-10. L. amazonensis parasites persist in IL-10-deficient mice, even in the presence of an enhanced Th1 response during the early stages of infection and in the presence of antigen-specific cells primed for Th1 effector function during the chronic phase. These persistent parasites are associated with chronic nonhealing lesions. Our results are consistent with a model proposing that IL-10 expression during Leishmania infection is important more for determining the level of macrophage activation and subsequent leishmanicidal activity than for influencing the T-cell response. Our results also demonstrate that L. amazonensis can evoke IL-10-independent mechanisms to limit in vivo IFN-γ production. Understanding these mechanisms at the systemic and cellular levels is necessary for us to propose adequate immunomodulatory strategies for preventive or curative procedures.
Acknowledgments
We thank Dennis Byrne and Jack Gallup for technical help.
This work was supported by National Institutes of Health grants AI48357, AI35914, and AI42334. Additional funds were provided by the Office of Biotechnology and the College of Veterinary Medicine at Iowa State University.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aebischer, T., D. Harbecke, and T. Ilg. 1999. Proteophosphoglycan, a major secreted product of intracellular Leishmania mexicana amastigotes, is a poor B-cell antigen and does not elicit a specific conventional CD4+ T-cell response. Infect. Immun. 67:5379-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonso, L. C., and P. Scott. 1993. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect. Immun. 61:2952-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annacker, O., R. Pimenta-Araujo, O. Burlen-Defranoux, T. C. Barbosa, A. Cumano, and A. Bandeira. 2001. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 166:3008-3018. [DOI] [PubMed] [Google Scholar]
- 4.Antoine, J. C., T. Lang, E. Prina, N. Courret, and R. Hellio. 1999. H-2M molecules, like MHC class II molecules, are targeted to parasitophorous vacuoles of Leishmania-infected macrophages and internalized by amastigotes of L. amazonensis and L. mexicana. J. Cell Sci. 112:2559-2570. [DOI] [PubMed] [Google Scholar]
- 5.Barral, A., E. A. Petersen, D. L. Sacks, and F. A. Neva. 1983. Late metastatic leishmaniasis in the mouse. A model for mucocutaneous disease. Am. J. Trop. Med. Hyg. 32:277-285. [DOI] [PubMed] [Google Scholar]
- 6.Bomfim, G., C. Nascimento, J. Costa, E. M. Carvalho, M. Barral-Netto, and A. Barral. 1996. Variation of cytokine patterns related to therapeutic response in diffuse cutaneous leishmaniasis. Exp. Parasitol. 84:188-194. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese, K. D. S., and S. C. da Costa. 1992. Enhancement of Leishmania amazonensis infection in BCG non-responder mice by BCG-antigen specific vaccine. Mem. Inst. Oswaldo Cruz 1:49-56. [DOI] [PubMed] [Google Scholar]
- 8.Chatelain, R., S. Mauze, and R. L. Coffman. 1999. Experimental Leishmania major infection in mice: role of IL-10. Parasite Immunol. 21:211-218. [DOI] [PubMed] [Google Scholar]
- 9.Childs, G. E., L. K. Lightner, L. McKinney, M. G. Groves, E. E. Price, and L. D. Hendricks. 1984. Inbred mice as model hosts for cutaneous leishmaniasis. I. Resistance and susceptibility to infection with Leishmania braziliensis. L. mexicana, and L. aethiopica. Ann. Trop. Med. Parasitol. 78:25-34. [DOI] [PubMed] [Google Scholar]
- 10.D'Andrea, A., M. Aste-Amezaga, N. M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178:1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeKrey, G. K., H. C. Lima, and R. G. Titus. 1998. Analysis of the immune responses of mice to infection with Leishmania braziliensis. Infect. Immun. 66:827-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Souza Leao, S., T. Lang, E. Prina, R. Hellio, and J. C. Antoine. 1995. Intracellular Leishmania amazonensis amastigotes internalize and degrade MHC class II molecules of their host cells. J. Cell Sci. 108:3219-3231. [DOI] [PubMed] [Google Scholar]
- 13.de Waal Malefyt, R., J. Haanen, H. Spits, M. G. Roncarolo, A. te Velde, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J. E. de Vries. 1991. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding, L., P. S. Linsley, L. Y. Huang, R. N. Germain, and E. M. Shevach. 1993. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J. Immunol. 151:1224-1234. [PubMed] [Google Scholar]
- 15.Fiorentino, D. F., A. Zlotnik, T. R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815-3822. [PubMed] [Google Scholar]
- 16.Fiorentino, D. F., A. Zlotnik, P. Vieira, T. R. Mosmann, M. Howard, K. W. Moore, and A. O'Garra. 1991. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146:3444-3451. [PubMed] [Google Scholar]
- 17.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 18.Groux, H., F. Cottrez, M. Rouleau, S. Mauze, S. Antonenko, S. Hurst, T. McNeil, M. Bigler, M. G. Roncarolo, and R. L. Coffman. 1999. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 162:1723-1729. [PubMed] [Google Scholar]
- 19.Hagenbaugh, A., S. Sharma, S. M. Dubinett, S. H. Wei, R. Aranda, H. Cheroutre, D. J. Fowell, S. Binder, B. Tsao, R. M. Locksley, K. W. Moore, and M. Kronenberg. 1997. Altered immune responses in interleukin 10 transgenic mice. J. Exp. Med. 185:2101-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann, K. F., A. W. Cheever, and T. A. Wynn. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164:6406-6416. [DOI] [PubMed] [Google Scholar]
- 21.Huang, F. P., W. Niedbala, X. Q. Wei, D. Xu, G. J. Feng, J. H. Robinson, C. Lam, and F. Y. Liew. 1998. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur. J. Immunol. 28:4062-4070. [DOI] [PubMed] [Google Scholar]
- 22.Hunter, C. A., L. A. Ellis-Neyes, T. Slifer, S. Kanaly, G. Grunig, M. Fort, D. Rennick, and F. G. Araujo. 1997. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J. Immunol. 158:3311-3316. [PubMed] [Google Scholar]
- 23.Jones, D. E., L. U. Buxbaum, and P. Scott. 2000. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J. Immunol. 165:364-372. [DOI] [PubMed] [Google Scholar]
- 24.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 25.Kima, P. E., S. L. Constant, L. Hannum, M. Colmenares, K. S. Lee, A. M. Haberman, M. J. Shlomchik, and D. McMahon-Pratt. 2000. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J. Exp. Med. 191:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopf, M., F. Brombacher, G. Kohler, G. Kienzle, K. H. Widmann, K. Lefrang, C. Humborg, B. Ledermann, and W. Solbach. 1996. IL-4-deficient Balb/c mice resist infection with Leishmania major. J. Exp. Med. 184:1127-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, L., J. F. Elliott, and T. R. Mosmann. 1994. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. J. Immunol. 153:3967-3978. [PubMed] [Google Scholar]
- 29.Macatonia, S. E., T. M. Doherty, S. C. Knight, and A. O'Garra. 1993. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J. Immunol. 150:3755-3765. [PubMed] [Google Scholar]
- 30.Moore, K. W., A. O'Garra, R. de Waal Malefyt, P. Vieira, and T. R. Mosmann. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165-190. [DOI] [PubMed] [Google Scholar]
- 31.Neyer, L. E., G. Grunig, M. Fort, J. S. Remington, D. Rennick, and C. A. Hunter. 1997. Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infect. Immun. 65:1675-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noben-Trauth, N., P. Kropf, and I. Muller. 1996. Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science 271:987-990. [DOI] [PubMed] [Google Scholar]
- 33.Noben-Trauth, N., W. E. Paul, and D. L. Sacks. 1999. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J. Immunol. 162:6132-6140. [PubMed] [Google Scholar]
- 34.Peters, C., M. Kawakami, M. Kaul, T. Ilg, P. Overath, and T. Aebischer. 1997. Secreted proteophosphoglycan of Leishmania mexicana amastigotes activates complement by triggering the mannan binding lectin pathway. Eur. J. Immunol. 27:2666-2672. [DOI] [PubMed] [Google Scholar]
- 35.Pirmez, C., M. Yamamura, K. Uyemura, M. Paes-Oliveira, F. Conceicao-Silva, and R. L. Modlin. 1993. Cytokine patterns in the pathogenesis of human leishmaniasis. J. Clin. Investig. 91:1390-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poe, J. C., D. H. Wagner, R. W. Miller, R. D. Stout, and J. Suttles. 1997. IL-4 and IL-10 modulation of CD40-mediated signaling of monocyte IL-1beta synthesis and rescue from apoptosis. J. Immunol. 159:846-852. [PubMed] [Google Scholar]
- 37.Reiner, S. L., and R. A. Seder. 1995. T helper cell differentiation in immune response. Curr. Opin. Immunol. 7:360-366. [DOI] [PubMed] [Google Scholar]
- 38.Reinhardt, R. L., A. Khoruts, R. Merica, T. Zell, and M. K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410:101-105. [DOI] [PubMed] [Google Scholar]
- 39.Roberts, M., J. Alexander, and J. M. Blackwell. 1990. Genetic analysis of Leishmania mexicana infection in mice: single gene (Scl-2) controlled predisposition to cutaneous lesion development. J. Immunogen. 17:89-100. [DOI] [PubMed] [Google Scholar]
- 40.Rocha, P. N., R. P. Almeida, O. Bacellar, A. R. de Jesus, D. C. Filho, A. C. Filho, A. Barral, R. L. Coffman, and E. M. Carvalho. 1999. Down-regulation of Th1 type of response in early human American cutaneous leishmaniasis. J. Infect. Dis. 180:1731-1734. [DOI] [PubMed] [Google Scholar]
- 41.Scharton-Kersten, T., L. C. Afonso, M. Wysocka, G. Trinchieri, and P. Scott. 1995. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 154:5320-5330. [PubMed] [Google Scholar]
- 42.Sher, A., and R. L. Coffman. 1992. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 10:385-409. [DOI] [PubMed] [Google Scholar]
- 43.Silveira, F. T., R. Lainson, J. J. Shaw, A. A. De Souza, E. A. Ishikawa, and R. R. Braga. 1991. Cutaneous leishmaniasis due to Leishmania (Leishmania) amazonensis in Amazonian Brazil, and the significance of a negative Montenegro skin-test in human infections. Trans. R. Soc. Trop. Med. Hyg. 85:735-738. [DOI] [PubMed] [Google Scholar]
- 44.Soong, L., C. H. Chang, J. Sun, B. J. Longley, Jr., N. H. Ruddle, R. A. Flavell, and D. McMahon-Pratt. 1997. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J. Immunol. 158:5374-5383. [PubMed] [Google Scholar]
- 45.Soong, L., J. C. Xu, I. S. Grewal, P. Kima, J. Sun, B. J. Longley, Jr., N. H. Ruddle, D. McMahon-Pratt, and R. A. Flavell. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263-273. [DOI] [PubMed] [Google Scholar]
- 46.Stamm, L. M., A. Raisanen-Sokolowski, M. Okano, M. E. Russell, J. R. David, and A. R. Satoskar. 1998. Mice with STAT6-targeted gene disruption develop a Th1 response and control cutaneous leishmaniasis. J. Immunol. 161:6180-6188. [PubMed] [Google Scholar]
- 47.Stenger, S., N. Donhauser, H. Thuring, M. Rollinghoff, and C. Bogdan. 1996. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 183:1501-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki, Y., A. Sher, G. Yap, D. Park, L. E. Neyer, O. Liesenfeld, M. Fort, H. Kang, and E. Gufwoli. 2000. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 164:5375-5382. [DOI] [PubMed] [Google Scholar]
- 49.Wynn, T. A., A. W. Cheever, M. E. Williams, S. Hieny, P. Caspar, R. Kuhn, W. Muller, and A. Sher. 1998. IL-10 regulates liver pathology in acute murine Schistosomiasis mansoni but is not required for immune down-modulation of chronic disease. J. Immunol. 160:4473-4480. [PubMed] [Google Scholar]