Abstract
Protection of cattle against bovine tuberculosis by vaccination could be an important control strategy in countries where there is persistent Mycobacterium bovis infection in wildlife and in developing countries where it is not economical to implement a tuberculin test and slaughter control program. The main aim of such a vaccination strategy would be to reduce transmission of infection by reducing the lung pathology caused by infection and preventing seeding of the organism to organs from which M. bovis could be excreted. Recent reports of successful DNA vaccination against Mycobacterium tuberculosis in small-animal models have suggested that DNA vaccines act by reducing lung pathology without sensitizing animals to tuberculin testing. We therefore evaluated the ability of vaccines consisting of DNA encoding the mycobacterial antigens MPB83 and 85A to reduce lung pathology and prevent hematogenous spread in guinea pigs challenged with a low dose of aerosolized M. bovis. Vaccination with MPB83 DNA reduced the severity of pulmonary lesions, as assessed by histopathology, and resembled M. bovis BCG vaccination in this respect. However, unlike BCG vaccination, MPB83 DNA vaccination did not protect challenged guinea pigs from hematogenous spread of organisms to the spleen. In contrast, vaccination with antigen 85A DNA, a promising DNA vaccine for human tuberculosis, had no measurable protective effect against infection with M. bovis.
It has been estimated that more than 50 million cattle are infected with Mycobacterium bovis worldwide and that the resulting economic losses are approximately $3 billion (40). In developed countries pasteurization of milk and control of bovine tuberculosis (TB) by test- and slaughter-based control measures have dramatically reduced the transmission of M. bovis infection from cattle to humans. In these countries M. bovis is now predominantly an occupational zoonosis with potential risk for workers on farms, in abattoirs, and in zoos (13, 19, 37). In contrast, human TB caused by M. bovis is still a major health issue in many developing countries (11, 12, 14, 20). A number of factors maintain the threat of bovine TB to human health, including the increase in the number of immunocompromised individuals (20), the emergence of strains of M. bovis resistant to known drugs (33), and, in some countries, an increasing prevalence of disease in cattle (25).
In countries with little or no wildlife reservoir, test and slaughter strategies have proved to be extremely successful in controlling bovine TB. However, this approach has been less successful in countries with significant reservoirs of M. bovis in their wildlife populations, and the cost of such a strategy precludes its use in many low-income countries. In these countries vaccination may be the only available option for the control of bovine TB. In Great Britain, a recent independent scientific review for the government of the recent sharp rise in bovine TB concluded that the best prospect for future control of the disease is to develop a vaccine to protect cattle against M. bovis infection (25). There are two main goals of vaccinating cattle against TB. The first is to prevent the establishment of infection in animals that are exposed to the organism, and the second is to prevent transmission of infection to other animals within a herd. In countries where tuberculin testing is used to control TB in cattle, either vaccination would need to be so effective that subsequent exposure to M. bovis did not sensitize animals to tuberculin or differential diagnostic tests would be required to distinguish vaccinated animals from animals infected with M. bovis (6, 44). In developing countries where tuberculin testing is not used to control M. bovis infection in cattle, the principal requirement of a vaccine would be to prevent transmission of the infection. While the goal of preventing establishment of infection may be difficult to achieve by vaccination, the goal of reducing transmission is less stringent and may be achievable by reducing lung pathology and preventing seeding of the organism to organs from which M. bovis could be excreted. In cattle, lesions are predominantly found in the lungs or lymph nodes of the thorax (30). A low-dose intratracheal challenge model has been established with cattle for testing vaccine efficacy; in this model the main readout is reduction in the number and severity of tuberculous lesions in the lungs and thoracic lymph nodes (6). Recent advances in DNA vaccination indicate that it may be possible to produce a vaccine that is active against M. bovis in cattle and reduces lung pathology without sensitizing the animals to the tuberculin skin test (2, 43). Such a vaccine would allow continued use of tuberculin-based strategies to remove animals that are not protected by vaccination.
Mycolyl transferase (antigen 85A [Ag85A]) (4) and MPB83, a lipoglycoprotein of unknown function (21), are major antigens of Mycobacterium tuberculosis and M. bovis during infection (21, 27, 31, 36). In a number of studies, vaccination of mice and guinea pigs with DNA encoding Ag85A resulted in strong cellular immune responses and protection against challenge with M. tuberculosis (2, 3, 22, 41, 42). Evaluation of vaccination with DNA encoding MPB83 has been more limited, but recent studies revealed immune responses in both mice and cattle following vaccination and protection of mice against challenge with M. bovis (7) and M. tuberculosis (29). Recently, it was shown that the prolonged survival of Ag85A DNA-vaccinated guinea pigs following aerogenic challenge with M. tuberculosis resulted from less severe pulmonary pathology rather than a reduction in the bacterial load in the lungs or spleen (2). The authors therefore suggested that assessments of the protective efficacy of vaccines other than M. bovis BCG should include assessments of long-term survival and pathology, as well as assessments of reductions in organ bacterial load.
In the present study, we evaluated vaccines consisting of DNA encoding Ag85A and MPB83 for their ability to protect guinea pigs challenged aerogenically with a low dose of M. bovis. Challenge with M. bovis caused extensive infiltration of lymphocytes into the lungs of unvaccinated guinea pigs. Like BCG vaccination, vaccination with either DNA construct provided significant protection against this lymphocytic infiltration. In addition, vaccination with DNA encoding MPB83 reduced the extent of granulomatous inflammation and caseation following aerogenic challenge with M. bovis, which also resembled an effect of BCG vaccination. However, unlike BCG vaccination, DNA vaccination did not reduce the load of acid-fast bacilli (AFB) in the lungs or protect challenged guinea pigs from hematogenous spread of organisms to the spleen.
MATERIALS AND METHODS
DNA constructs.
Plasmid pCMV-83 was made by inserting the mpt83 gene (which has a sequence identical to that of the mpb83 gene from M. bovis [21]) from M. tuberculosis H37Rv into the plasmid backbone pCMV4 as described previously (7). Plasmid pCMV4 is based on plasmid vector pCDNA3.1 from Invitrogen (Leek, The Netherlands), with addition at the HindIII restriction site of intron A from the human cytomegalovirus immediate-early gene (10). The control plasmid pCMV-link was made by removing mpt83 from pCMV-83 as described previously (7). Plasmid pVR1020-85A encoding Ag85A from M. tuberculosis and its plasmid vector, pVR1020, have been described previously (41). DNA used for immunization was prepared by using a QIAGEN-tip 10000 plasmid extraction kit and endotoxin-free buffers (QIAGEN Ltd., Dorking, United Kingdom) as recommended by the manufacturer. The DNA was resuspended in endotoxin-free phosphate-buffered saline (PBS) (Sigma, Poole, United Kingdom) prior to injection.
Bacteria and media.
Lyophilized M. bovis BCG Pasteur strain (obtained from the Statens Serum Institut, Copenhagen, Denmark) was cultured in 10 ml of M-ADC-TW broth (23) for 7 days and stored at −80°C in seed lots. The strain of M. bovis used in this study (AF2122/97) was isolated from a tuberculin test reactor cow in 1997 and was cultured at Veterinary Laboratories Agency Weybridge. For enumeration, BCG Pasteur was plated on Middlebrook 7H10 agar containing 0.2% (vol/vol) glycerol and 10% (vol/vol) Middlebrook oleic acid-albumin-dextrose-catalase (OADC) enrichment. M. bovis strain AF2122/97 was plated on Middlebrook 7H10 agar containing 4.16 mg of sodium pyruvate per ml and 10% (vol/vol) Middlebrook OADC enrichment. When necessary, serial dilutions of bacterial suspensions were prepared in water containing 0.05% (vol/vol) Tween 80 to maintain dispersion.
Inoculation of guinea pigs.
Outbred female Dunkin-Hartley guinea pigs that weighed between 350 and 450 g and were free of intercurrent infection were obtained from Charles River UK Ltd., Margate, United Kingdom. The guinea pigs (six animals per group) each received either 100 μl of PBS or 100 μg of a plasmid DNA vaccine in the biceps femoris muscle of both hind limbs on two occasions 3 weeks apart. For the BCG group, the concentration of the BCG vaccine was adjusted to 2 × 105 CFU/ml immediately prior to injection and the cells were dispersed by brief sonication by using a CV18 converter fitted with a 3-mm-diameter tip attached to a Vibracell control unit (Sonics & Materials Inc., Danbury, Conn.) set at 20% power. The guinea pigs were injected subcutaneously in the nape with 250 μl of the BCG preparation (representing an inoculum of approximately 5 × 104 CFU) on the day of the second DNA injection.
Aerosol infection of guinea pigs.
Vaccinated guinea pigs were challenged with M. bovis strain AF2122/97 via the aerosol route 8 weeks after the last inoculation by using a modified version of the mobile Henderson apparatus (15), as previously described (45). A suspension containing approximately 106 CFU/ml was used in order to obtain an inhaled retained dose in the lungs of approximately 10 organisms (9).
Postmortem examination of guinea pigs.
Infected animals were killed with peritoneal overdoses of sodium pentobarbitone 10 weeks after challenge or when an individual had lost 20% of its maximal body weight (the humane end point), whichever was sooner. Examination was carried out immediately after death. For each animal an external assessment of the condition of the body was followed by gross internal examination of the neck region and the thoracic and abdominal cavities. The lungs with the trachea, heart, and tracheobronchial lymph nodes attached were removed and placed in 10% Formol-buffered saline for subsequent examination. The whole spleen was removed aseptically and placed in 5 ml of sterile distilled water for bacteriological assessment.
Determination of pulmonary disease by using formalin-fixed tissue.
The trachea, bronchi, and heart were dissected away prior to detailed examination of the fixed lungs from each animal. The number of visible lesions on the dorsal surface of all lobes was recorded on a diagram of the lungs along with the positions and sizes of the lesions and information about whether necrosis or consolidation was present. Sections were then cut from the lungs of each animal and used for histopathological evaluation. A section was prepared from the base of the left apical lobe and from the right diaphragmatic lobe of each lung; we made sure that each lobe was sectioned at the same position for every animal. Duplicate sections were stained with hematoxylin and eosin stain and with van Giesson stain in order to aid visualization of fibrous tissue. The two sections were scored in a blinded fashion for the following features: area of the section occupied by granulomatous inflammation (granuloma area); fibrosis; calcification; and necrosis (coagulative or caseous). Necrosis was described as coagulative when the architecture of the tissue was preserved. In contrast, if all tissue architecture was lost, the necrosis was termed caseous (5). The extent of lymphocytic infiltration in each section was also assessed, along with the distribution of the lymphocytes (perivascular and/or peribronchiolar, peripheral to the granuloma, or inside granulomas). The extent or severity of each feature was assigned a score ranging from 0 to 4.
Bacterial enumeration.
Following Ziehl-Neelsen staining, approximate numbers of AFB were determined in the sections of lungs examined for histopathology. The following scoring system was used to describe the data: 0, no AFB observed in the entire section; 1, 1 to 15 AFB observed in the whole section; 2, AFB observed frequently and detected in most fields (at a magnification of ×40) with necrosis; 3, AFB very numerous and aggregates of AFB. Spleens were homogenized in 5 ml of sterile distilled water by using a rotating blade macerator system. Viable counts were determined by using serial dilutions of the macerate, and preparations were examined after 4 weeks of incubation at 37°C for growth of mycobacteria. The limit of detection was 25 CFU.
Statistical analyses.
Appropriate statistical tests were used, and all data were analyzed by using the InStat software package (version 3.00; GraphPad, San Diego, Calif.). The Mann-Whitney nonparametric test was used when the data were not sampled from Gaussian distributions (see Table 1 and Fig. 3). One-way analysis of variance was used for some of the data (see Fig. 1).
TABLE 1.
Semiquantitative assessment of the histopathology and bacterial load of guinea pig lungsa
| Group | Granuloma areab | Necrosisc | Caseation | Fibrosis | Calcification | Lymphocytesd | AFBe |
|---|---|---|---|---|---|---|---|
| PBS | 3.00 (0.74) | 1.92 (1.06) | 1.08 (1.17) | 1.00 (0.00) | 0.96 (1.05) | 1.89 (0.36) | 1.33 (0.49) |
| pVR1020 | 2.58 (0.67) | 1.50 (0.88) | 1.33 (1.16) | 1.21 (0.50) | 0.79 (1.01) | 1.42 (0.60) | 1.17 (0.39) |
| pVR1020-85A | 2.33 (0.98) | 1.96 (1.18) | 1.33 (1.30) | 1.08 (0.29) | 0.88 (0.98) | 1.28 (0.39)f | 1.33 (0.49) |
| pCMV-link | 2.00 (1.48) | 1.25 (1.12) | 1.25 (1.42) | 0.96 (0.62) | 0.96 (1.27) | 1.11 (0.78) | 0.92 (0.67) |
| pCMV-83 | 1.83 (1.34)g | 1.96 (0.81) | 0.71 (1.05) | 1.33 (0.49) | 0.88 (1.30) | 0.95 (0.63)h | 1.46 (0.84) |
| BCG | 1.25 (1.29)f | 0.88 (1.09)g | 0.25 (0.45) | 0.71 (0.75) | 0.08 (0.19)g | 0.92 (0.92)g | 0.50 (0.52)f |
The extent or severity of each parameter was assigned a score ranging from 0 to 4. The mean value for each group was derived from two sections for each animal. The values in parentheses are standard deviations.
Area of section occupied by granulomatous inflammation.
Coagulative necrosis. Caseous necrosis was scored separately.
Extent of lymphocytic infiltration, regardless of the distribution within the section.
AFB, as determined by Ziehl-Neelsen staining, were scored as follows: 0, no AFB observed in the entire section; 1, 1 to 15 AFB observed in the whole section; 2, AFB observed frequently and detected in most fields (at a magnification of ×40) with necrosis; 3, AFB very numerous and aggregates of AFB.
P < 0.005, as determined by the Mann-Whitney test.
P < 0.05, as determined by the Mann-Whitney test.
P < 0.0005, as determined by the Mann-Whitney test.
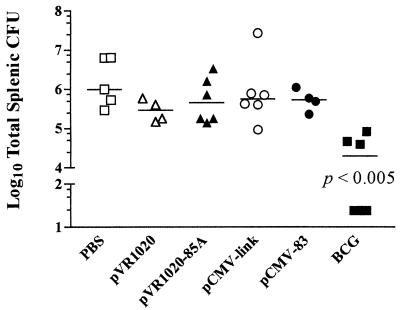
FIG. 3.
Ability of vaccines to protect guinea pigs from hematogenous spread following aerogenic challenge with M. bovis. Guinea pigs were vaccinated and then challenged 8 weeks later. The numbers of CFU recovered from the spleens of animals 10 weeks after challenge are shown (limit of detection, 25 CFU). The bars indicate the median values. Statistical significance was demonstrated by using the Mann-Whitney nonparametric test.
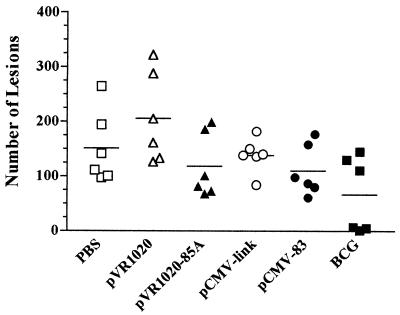
FIG. 1.
Number of gross lesions on the dorsal surfaces of fixed lungs 10 weeks after challenge with a low-dose aerosol of M. bovis strain AF2122/97. Guinea pigs were vaccinated and then challenged 8 weeks later. Each point represents an individual animal. The bar indicates the mean value for each group.
RESULTS
Influence of vaccination on the number of gross pulmonary lesions.
Animals were vaccinated with either pCMV-83 encoding MPB83, pVR1020-85A encoding Ag85A, or the control vector plasmids. Two additional groups of guinea pigs were vaccinated with BCG Pasteur and PBS. Eight weeks after the final vaccination all animals were exposed to a low-dose aerosol of M. bovis. One animal in the PBS group, two animals in the pVR1020-vaccinated group, and two animals in the pCMV-83-vaccinated group were killed at the humane end point before the end of the experiment. Bacteriological analysis was not performed on these animals. All remaining animals survived to 10 weeks after challenge and were then killed humanely and used to evaluate vaccine efficacy. Figure 1 shows the number of visible lesions on the dorsal surfaces of the fixed lungs. Although vaccination with pVR1020-85A, pCMV-83, and BCG reduced the mean numbers of lesions by 22, 27, and 56%, respectively, compared with that of the PBS control, these reductions were not statistically significant (as determined by analysis of variance).
Influence of vaccination on pulmonary histopathology.
To examine the pathology of the lungs in more detail, sections were cut from each animal at standardized positions, and a number of histopathological features were assessed in a blinded manner (Table 1). All test treatments reduced the extent of granulomatous inflammation compared with that of the PBS treatment, and the results obtained for the pCMV-83 and BCG groups were significantly different statistically. Representative examples of the histopathology observed in vaccinated animals and animals treated with PBS are shown in Fig. 2. Vaccination with both pCMV-83 and BCG also reduced the extent of caseation within granulomas. Only BCG vaccination reduced the load of AFB and the amounts of calcification, fibrosis, and necrosis apparent histologically, which supported the gross assessment made with lesions. There was a marked difference between groups in terms of the extent of lymphocytic infiltration. All three vaccinated groups had less-extensive lymphocytic infiltration per section than the controls (Fig. 2), and the differences were significant statistically, very significant in the case of pCMV-83 (P = 0.0003, as determined by the Mann-Whitney test). Although there were fewer lymphocytes, careful examination revealed that a greater proportion of the lymphocytes in the pCMV-83-vaccinated animals were located within granulomas rather than peripherally or perivascularly (data not shown). This was not observed for granulomas present in guinea pigs vaccinated with either of the other vaccines.
FIG. 2.
Representative micrographs of guinea pig lungs, showing the reduction in granulomatous inflammation in the BCG-vaccinated (A) and pCMV-83-vaccinated (B) groups compared with the pVR1020-85A-vaccinated group (C) and the PBS control (D). Vaccination with BCG or pCMV-83, but not vaccination with pVR1020-85A, reduced both the number and the size of the granulomas, as reflected in the pathology scores shown in Table 1. The sections were given the following scores for granuloma size: panel A, 1.00; panel B, 1.00; panel C, 2.00; and panel D, 2.00. These photographs also demonstrate the marked reductions in the numbers of lymphocytes observed in the three vaccinated groups compared with the control. The sections were given the following scores for lymphocytic infiltration: panel A, 1.00; panel B, 0.67; panel C, 1.00; and panel D, 1.67. Bar = 400 μm.
Vaccination with pCMV-83 did not reduce hematogenous spread of M. bovis.
The bacterial loads in the spleens of all animals that survived to the end of the experiment were determined (Fig. 3). Only vaccination with BCG reduced the number of bacteria in the spleens (P < 0.005, as determined by the Mann-Whitney test). The bacterial counts for three of the six animals vaccinated with BCG were below the limit of detection (25 CFU). These animals also had the fewest gross lung lesions (Fig. 1). The remaining three BCG-vaccinated animals had an average reduction in bacterial load compared with that of the PBS control of 1.7 log10. Neither vaccination with pVR1020-85A nor vaccination with pCMV-83 prevented hematogenous spread of bacteria from the lungs to the spleens.
DISCUSSION
DNA vaccines for TB, based on expression of selected mycobacterial antigens, have advantages over live vaccines in a number of respects. Such vaccines should be safer for immunocompromised individuals, quality control should be easier, and at the same time it should be possible to remove irrelevant or suppressive antigens. Another advantage is that studies to date have shown that unlike BCG vaccination, DNA vaccination does not sensitize guinea pigs or cattle to the tuberculin skin test (2, 43), which would allow continued use of tuberculin with vaccinated animals. Most importantly, a number of vaccines consisting of DNA encoding a single antigen or combined antigens have shown promising efficacy against TB in small-animal models (22, 24, 28, 29, 41, 42). In this study we evaluated two candidate DNA vaccines for bovine TB. The vaccine consisting of DNA encoding Ag85A was chosen because it has been shown to protect mice and guinea pigs against M. tuberculosis (2, 3, 22, 41). The vaccine consisting of DNA encoding MPB83 was chosen because MPB83 is an immunodominant antigen of M. bovis (21, 31, 36) and a vaccine consisting of DNA encoding this antigen has been shown to protect mice to some extent against challenge with M. bovis (7) and M. tuberculosis (29). This is the first report of evaluation of DNA vaccines for M. bovis with a guinea pig low-dose infection model.
Vaccination with DNA encoding MPB83 and Ag85A reduced the number of pulmonary lesions visible postmortem, although the reduction was not statistically significant for either vaccine. A more detailed study of the histopathology of the lungs of vaccinated and control guinea pigs revealed that vaccination with DNA encoding MPB83 reduced the extent of granulomatous inflammation and caseation to levels intermediate between the levels obtained with controls and the levels obtained with BCG vaccination, as shown in Fig. 2. Similar effects of vaccination on these parameters, as shown by histopathological assessment, have been reported by other workers (2, 39). Little or no reduction in the levels of these parameters was observed following vaccination with DNA encoding Ag85A.
In vaccination studies in which Ag85A and BCG were used to protect against M. tuberculosis aerosol challenge in guinea pigs, enhanced lymphocyte infiltration into tuberculous lesions was found to correlate with protection against M. tuberculosis (2). In contrast, we did not observe increased lymphocytic infiltration in the lungs of vaccinated animals following aerosol challenge with M. bovis. In fact, the opposite was observed. Since lymphocytes accumulate progressively in the developing tuberculous granulomas (1, 32), the observed difference may have been due to the fact that we examined tissue sections at least 5 weeks earlier than the tissue sections were examined in the M. tuberculosis study (2). However, although we assessed protection of the guinea pigs earlier in this study, the animals still developed the full spectrum of pulmonary pathology described for challenge with M. tuberculosis (2), including necrosis, caseation, fibrosis, and calcification. In fact, the protracted progression of M. tuberculosis pathology in guinea pigs appears to be temporally compressed in M. bovis infections (34, 35). The lower level of lymphocyte infiltration may also reflect differences in the immune responses to M. bovis and M. tuberculosis infections, as suggested by recent comparative studies (8, 45). The histopathology data support the view that the BCG and MPB83 vaccines retarded the progress of granuloma development, and this may also account for the reduction in lymphocytic infiltration (Fig. 2).
The ability to prevent hematogenous spread is a recognized feature of BCG vaccination (17, 26, 38). In this study, only BCG vaccination was able to prevent the dissemination of bacteria to the spleen and to reduce the load of AFB in the lungs; the latter finding was determined histologically. In previous studies, we demonstrated that vaccination with BCG confers a profound level of protection against colonization of the spleen by M. bovis 5 weeks after challenge and that the counts obtained with all spleens were below the lower limit of detection (8). In this study we found that one-half of the BCG-vaccinated guinea pigs still exhibited a high level of protection 10 weeks after challenge. Enumeration of the gross lung lesions (Fig. 1) suggested that in the other half pulmonary protection had waned, which allowed organisms to escape and colonize the spleen. The greater variability in the number of spleen CFU at 10 weeks following aerosol infection than in the number of spleen CFU at 5 weeks following aerosol infection probably resulted from the stochastic nature of the seeding of the spleen with bacteria from the lungs following the breakdown of local protective immunity.
The inability of Ag85A DNA vaccination to reduce the bacterial load in the lungs and spleens of guinea pigs challenged with M. tuberculosis has been reported previously (2). However, in the previous study the vaccine consisting of DNA encoding Ag85A mediated a protective effect through reduced pulmonary pathology, which led to enhanced survival of the animals. In the present study, Ag85A vaccination provided little or no protection against the pulmonary pathology caused by aerogenic challenge with M. bovis, although there are a number of differences between the two studies. We used M. bovis for challenge rather than M. tuberculosis, so it is possible that the greater virulence of M. bovis for guinea pigs (18) masked any protective effect of Ag85A vaccination. Another difference is that we used plasmid pVR1020-85A vaccine, whereas Baldwin and coworkers used pV1Jns-85A vaccine (2). This difference is unlikely to account for the difference in the efficacy of Ag85A DNA vaccination since both plasmids contain the same promoter, secretion signal, and drug resistance marker gene. Moreover, the two DNA vaccines have been found to be equally efficacious in mice (41, 42). A principal difference between the two studies is that we used half the concentration of vaccine DNA that Baldwin and colleagues used and we administered it on two occasions rather than three occasions. Also, expression of the Ag85 complex in M. tuberculosis may be different from expression of the Ag85 complex in M. bovis.
Vaccination with MPB83 DNA has been reported to protect mice against intravenous challenge with M. bovis (7) and aerogenic challenge with M. tuberculosis (29). The failure of the same vaccine to reduce the bacterial load in the lungs and spleens of guinea pigs could be due to the fact that guinea pigs are more susceptible to virulent mycobacteria than mice (18). Furthermore, the extents and natures of the immune response to MPB83 vaccination may be different in the two species. This has recently been reported for Ag85A: vaccination of cattle with DNA encoding this antigen failed to stimulate strong immune responses (43). We are currently performing vaccination and challenge studies with pCMV-83 in cattle. These studies should determine whether the mouse or the guinea pig is a better model for predicting the efficacy of candidate bovine TB vaccines in a natural host.
At present, the protection provided by DNA vaccines against TB has not exceeded the protection provided by BCG. However, there is cause for optimism. It is hoped that understanding the fundamental nature of vaccine protection in animal models that reflect the natural host will enable more efficacious DNA vaccines to be developed. The fact that the DNA vaccines tested to date do not compromise the tuberculin test in cattle (43) makes them particularly attractive candidates for control of bovine TB. Alternatively, given the ability of the MPB83 DNA vaccine to reduce lung pathology caused by M. bovis, a vaccination strategy consisting of DNA priming and BCG boosting might prove to be a more effective way to improve the protective efficacy of BCG against M. bovis challenge (16). However, since BCG sensitizes cattle to tuberculin, alternative diagnostic strategies would be required to implement this strategy. Recently, we and others have demonstrated that antigen or peptide cocktails based on ESAT-6 and CFP-10 used in a whole-blood gamma interferon assay can discriminate between M. bovis infection and BCG vaccination with a high degree of sensitivity and specificity (6, 44). Thus, the use of vaccination regimens consisting of DNA priming and BCG boosting may be feasible as long as the antigens used for diagnosis are not included in the vaccine. Finally, our observation that a DNA vaccine expressing Ag85A, which is a promising candidate vaccine for human TB, did not protect against challenge with M. bovis underlines the need for ongoing empirical testing of candidate vaccines for bovine TB.
Acknowledgments
This work was funded by the Department for Environment, Food and Rural Affairs (formerly Ministry of Agriculture, Fisheries and Food, Great Britain) and by grant G.0266.00 from the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (to K.H.).
We acknowledge the support of the animal services units at Veterinary Laboratories Agency Weybridge and CAMR.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abe, Y., K. Sugisaki, and A. M. Dannenberg, Jr. 1996. Rabbit vascular endothelial adhesion molecules: ELAM-1 is most elevated in acute inflammation, whereas VCAM-1 and ICAM-1 predominate in chronic inflammation. J. Leukoc. Biol. 60:692-703. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A. Frank, M. A. Lui, J. B. Ulmer, K. Huygen, D. M. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin, S. L., C. D. D'Souza, I. M. Orme, M. A. Liu, K. Huygen, O. Denis, A. Tang, L. Zhu, D. Montgomery, and J. B. Ulmer. 1999. Immunogenicity and protective efficacy of DNA vaccines encoding secreted and nonsecreted forms of Mycobacterium tuberculosis Ag85A. Tuber. Lung Dis. 79:251-259. [DOI] [PubMed] [Google Scholar]
- 4.Belisle, J. T., V. D. Vissa, T. Sievert, K. Takayama, P. J. Brennan, and G. S. Besra. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420-1422. [DOI] [PubMed] [Google Scholar]
- 5.Boyd, W. 1934. A text-book of pathology, 2nd ed. Henry Kimpton, London, United Kingdom.
- 6.Buddle, B. M. 2001. Vaccination of cattle against Mycobacterium bovis. Tuberculosis (Edinburgh) 81:125-132. [DOI] [PubMed] [Google Scholar]
- 7.Chambers, M. A., H. Vordermeier, A. Whelan, N. Commander, R. Tascon, D. Lowrie, and R. G. Hewinson. 2000. Vaccination of mice and cattle with plasmid DNA encoding the Mycobacterium bovis antigen MPB83. Clin. Infect. Dis. 30(Suppl. 3):S283-S287. [DOI] [PubMed] [Google Scholar]
- 8.Chambers, M. A., A. Williams, D. Gavier-Widen, A. Whelan, G. Hall, P. D. Marsh, B. R. Bloom, W. R. Jacobs, and R. G. Hewinson. 2000. Identification of a Mycobacterium bovis BCG auxotrophic mutant that protects guinea pigs against M. bovis and hematogenous spread of Mycobacterium tuberculosis without sensitization to tuberculin. Infect. Immun. 68:7094-7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers, M. A., A. Williams, D. Gavier-Widen, A. Whelan, C. Hughes, G. Hall, M. S. Lever, P. D. Marsh, and R. G. Hewinson. 2001. A guinea pig model of low-dose Mycobacterium bovis aerogenic infection. Vet. Microbiol. 80:213-226. [DOI] [PubMed] [Google Scholar]
- 10.Chapman, B., R. Thayer, K. Vincent, and N. Haigwood. 1991. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 19:3979-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosivi, O., J. M. Grange, C. J. Daborn, M. C. Raviglione, T. Fujikura, D. Cousins, R. A. Robinson, H. F. Huchzermeyer, I. de Kantor, and F. X. Meslin. 1998. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis. 4:59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daborn, C. J., J. M. Grange, and R. R. Kazwala. 1996. The bovine tuberculosis cycle--an African perspective. Soc. Appl. Bacteriol. Symp. Ser. 25:27S-32S. [DOI] [PubMed] [Google Scholar]
- 13.Dalovisio, J., M. Stetter, and S. Mikota-Wells. 1992. Rhinoceros' rhinorrhea: cause of an outbreak of infection due to airborne Mycobacterium bovis in zookeepers. Clin. Infect. Dis. 15:598-600. [DOI] [PubMed] [Google Scholar]
- 14.Dankner, W. M., N. J. Waecker, M. A. Essey, K. Moser, M. Thompson, and C. E. Davis. 1993. Mycobacterium bovis infections in San Diego: a clinicoepidemiologic study of 73 patients and a historical review of a forgotten pathogen. Medicine (Baltimore) 72:11-37. [PubMed] [Google Scholar]
- 15.Druett, H. A. 1969. A mobile form of the Henderson apparatus. J. Hyg. 67:437-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng, C. G., U. Palendira, C. Demangel, J. M. Spratt, A. S. Malin, and W. J. Britton. 2001. Priming by DNA immunization augments protective efficacy of Mycobacterium bovis Bacille Calmette-Guerin against tuberculosis. Infect. Immun. 69:4174-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fok, J. S., R. S. Ho, P. K. Arora, G. E. Harding, and D. W. Smith. 1976. Host-parasite relationships in experimental airborne tuberculosis. V. Lack of hematogenous dissemination of Mycobacterium tuberculosis to the lungs in animals vaccinated with Bacille Calmette-Guerin. J. Infect. Dis. 133:137-144. [DOI] [PubMed] [Google Scholar]
- 18.Francis, J. 1958. Tuberculosis in animals and man. Cassell & Co. Ltd., London, United Kingdom.
- 19.Georghiou, P., A. Patel, A. Konstantinos, J. Streeton, and P. Robinson. 1989. Mycobacterium bovis as an occupational hazard in abattoir workers. Aust. N. Z. J. Med. 19:409-410. [DOI] [PubMed] [Google Scholar]
- 20.Grange, J. M., C. Daborn, and O. Cosivi. 1994. HIV-related tuberculosis due to Mycobacterium bovis. Eur. Respir. J. 7:1564-1566. [DOI] [PubMed] [Google Scholar]
- 21.Hewinson, R., S. L. Michell, W. P. Russell, R. A. McAdam, and W. R. J. Jacobs. 1996. Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand. J. Immunol. 43:490-499. [DOI] [PubMed] [Google Scholar]
- 22.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J. P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs, W. R., Jr., G. V. Kalpana, J. D. Cirillo, L. Pascopella, S. B. Snapper, R. A. Udani, W. Jones, R. G. Barletta, and B. R. Bloom. 1991. Genetic systems for mycobacteria. Methods Enzymol. 204:537-555. [DOI] [PubMed] [Google Scholar]
- 24.Kamath, A. T., C. G. Feng, M. Macdonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs, J., R. Anderson, T. Clutton-Brock, I. Morrison, D. Young, and C. Donnelly. 1997. Bovine tuberculosis in cattle and badgers. Report by the Independent Scientific Review Group. Ministry of Agriculture, Fisheries and Food, London, United Kingdom.
- 26.Lagranderie, M., P. Ravisse, G. Marchal, M. Gheorghiu, V. Balasubramanian, E. H. Weigeshaus, and D. W. Smith. 1993. BCG-induced protection in guinea pigs vaccinated and challenged via the respiratory route. Tuber. Lung Dis. 74:38-46. [DOI] [PubMed] [Google Scholar]
- 27.Launois, P., R. DeLeys, M. N. Niang, A. Drowart, M. Andrien, P. Dierckx, J. L. Cartel, J. L. Sarthou, J. P. Van Vooren, and K. Huygen. 1994. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect. Immun. 62:3679-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowrie, D. B., C. L. Silva, M. J. Colston, S. Ragno, and R. E. Tascon. 1997. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine 15:834-838. [DOI] [PubMed] [Google Scholar]
- 29.Morris, S., C. Kelley, A. Howard, Z. Li, and F. Collins. 2000. The immunogenicity of single and combination DNA vaccines against tuberculosis. Vaccine 18:2155-2163. [DOI] [PubMed] [Google Scholar]
- 30.Neill, S. D., J. M. Pollock, D. B. Bryson, and J. Hanna. 1994. Pathogenesis of Mycobacterium bovis infection in cattle. Vet. Microbiol. 40:41-52. [DOI] [PubMed] [Google Scholar]
- 31.Newell, D. G., R. S. Clifton-Hadley, and C. L. Cheeseman. 1997. The kinetics of serum antibody responses to natural infections with Mycobacterium bovis in one badger social group. Epidemiol. Infect. 118:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orme, I. M., and A. M. Cooper. 1999. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol. Today. 20:307-312. [DOI] [PubMed] [Google Scholar]
- 33.Palenque, E., V. Villena, M. Rebollo, M. Jimenez, and S. Samper. 1998. Transmission of multidrug-resistant Mycobacterium bovis to an immunocompetent patient. Clin. Infect. Dis. 26:995-996. [DOI] [PubMed] [Google Scholar]
- 34.Ratcliffe, H. L. 1952. Tuberculosis induced by droplet nuclei infection: pulmonary tuberculosis of predetermined initial intensity in mammals. Am. J. Hyg. 55:36-48. [PubMed] [Google Scholar]
- 35.Ratcliffe, H. L., and V. S. Palladino. 1953. Tuberculosis induced by droplet nuclei infection: initial homogeneous response of small mammals (rats, mice, guinea pigs, and hamsters) to human and to bovine bacilli and the rate and pattern of tubercle development. J. Exp. Med. 97:61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhodes, S. G., D. Gavier-Widen, B. M. Buddle, A. O. Whelan, M. Singh, R. G. Hewinson, and H. M. Vordermeier. 2000. Antigen specificity in experimental bovine tuberculosis. Infect. Immun. 68:2573-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson, P., D. Morris, and R. Antic. 1988. Mycobacterium bovis as an occupational hazard in abattoir workers. Aust. N. Z. J. Med. 18:701-703. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues, L. C., V. K. Diwan, and J. G. Wheeler. 1993. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int. J. Epidemiol. 22:1154-1158. [DOI] [PubMed] [Google Scholar]
- 39.Smith, D. W., and E. H. Wiegeshaus. 1989. What animal models can teach us about the pathogenesis of tuberculosis in humans. Rev. Infect. Dis. 11(Suppl. 2):S385-S393. [DOI] [PubMed] [Google Scholar]
- 40.Steele, J. H. 1995. Regional and country status reports--introduction, p. 47-61. In Mycobacterium bovis infection in animals and humans. Iowa State University Press, Ames.
- 41.Tanghe, A., O. Denis, B. Lambrecht, V. Motte, T. van den Berg, and K. Huygen. 2000. Tuberculosis DNA vaccine encoding Ag85A is immunogenic and protective when administered by intramuscular needle injection but not by epidermal gene gun bombardment. Infect. Immun. 68:3854-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanghe, A., P. Lefevre, O. Denis, S. D'Souza, M. Braibant, E. Lozes, M. Singh, D. Montgomery, J. Content, and K. Huygen. 1999. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J. Immunol. 162:1113-1119. [PubMed] [Google Scholar]
- 43.Vordermeier, H. M., P. J. Cockle, A. O. Whelan, S. G. Rhodes, M. A. Chambers, D. Clifford, K. Huygen, R. Tascon, D. Lowrie, M. J. Colston, and R. G. Hewinson. 2001. Effective DNA vaccination of cattle with the mycobacterial antigens MPB83 and MPB70 does not compromise the specificity of the comparative intradermal tuberculin skin test. Vaccine 19:1246-1255. [DOI] [PubMed] [Google Scholar]
- 44.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams, A., A. Davies, P. D. Marsh, M. A. Chambers, and R. G. Hewinson. 2000. Comparison of the protective efficacy of Bacille Calmette-Guerin vaccination against aerosol challenge with Mycobacterium tuberculosis and Mycobacterium bovis. Clin. Infect. Dis. 30(Suppl. 3):S299-S301. [DOI] [PubMed] [Google Scholar]