Abstract
Numerous pathogenic bacteria contain luxS, which is required for autoinducer-2 production. Here, we demonstrate that Neisseria meningitidis contains a functional copy of luxS that is necessary for full meningococcal virulence; strains with a luxS deletion are defective for bacteremia, a prerequisite of meningococcal pathogenesis.
Bacteria have evolved mechanisms to coordinate gene expression in response to population density. Known as quorum sensing, these mechanisms involve the production and detection of signaling molecules (called autoinducers or pheromones), which modulate critical functions including virulence factor production, plasmid conjugal transfer, activation of secretion systems, swarming, swimming and twitching motility, biofilm differentiation, and bioluminescence (for reviews, see references 2, 13, 15, 28, and 30). Thus, bacteria in populations display properties denied individual cells.
Gram-positive and gram-negative bacteria are known to utilize distinct signal molecules for quorum sensing. Gram-positive bacteria export and detect small peptides, while gram-negative bacteria produce membrane-permeative N-acyl homoserine lactones (13, 15). Recently, however, potential quorum sensing systems based on a signal molecule called autoinducer-2 (AI-2) were shown to be present in both gram-positive and gram-negative species (2, 23, 24). The luxS gene product is required for AI-2 production by Salmonella enterica serovar Typhimurium (24), Escherichia coli (19, 23), Shigella flexneri (6), Helicobacter pylori (9, 11), Porphyromonas gingivalis (4, 5), Streptococcus pyogenes (12), and Actinobacillus actinomycetemcomitans (8). The chemical structure of AI-2 appears to be conserved, possibly allowing interspecies communication (2, 3, 18).
Neisseria meningitidis colonizes the human nasopharynx and can cause meningitis and/or septicemia (16). Here, we demonstrate that N. meningitidis possesses a functional luxS necessary for AI-2 production and full meningococcal virulence.
Serogroup B N. meningitidis possesses a functional luxS gene.
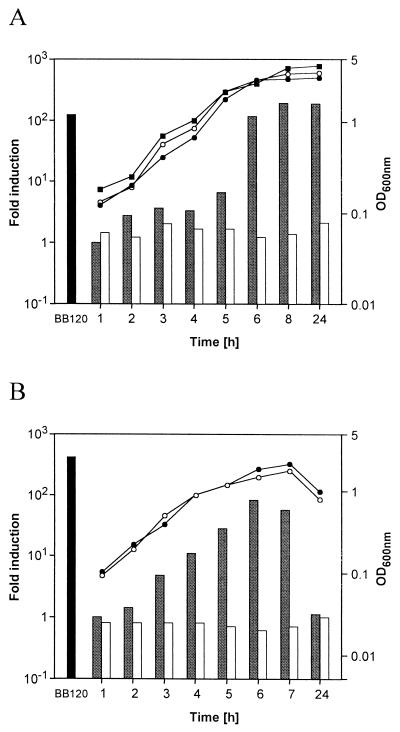
Investigation of the MC58 genome sequence (25) using LuxS from Vibrio harveyi (LuxSVh) led to the identification of NMB1981 (N. meningitidis serogroup B annotated sequence), a 504-bp open reading frame (ORF) with 80% identity to LuxSVh which is annotated as being of unknown function. To establish whether NMB1981 encodes a functional product, the gene was amplified with rTth DNA polymerase XL (Perkin-Elmer, Warrington, United Kingdom), ligated in both orientations into pGEM T-Easy (Promega, Madison, Wis.), and then transferred into Escherichia coli DH5α, which cannot produce AI-2 due to a mutation in luxS (24). Strains were grown at 37°C in Luria-Bertani medium, and AI-2 production was assayed using V. harveyi BB170 as the reporter (3). Exogenous AI-2 induces premature bioluminescence by this reporter strain. Only clones containing NMB1981 expressed through the vector-encoded promoter (pGEMluxS-F) produced culture supernatants with AI-2 activity (Fig. 1A); no activity was detected if the insert was in the opposite orientation (pGEMluxS-R). Identical results were obtained with the NMB1981 homologue from strain SD (data not shown). Thus, NMB1981 complements the defective luxS in DH5α and was designated luxSNm.
FIG. 1.
(A) Complementation of E. coli DH5α with luxSNm. The growth profiles and extracellular AI-2 activity of E. coli DH5α carrying either pGEM T-Easy or the same vector containing luxSNm in the forward or reverse orientation were measured. Growth profiles: empty vector, closed squares; luxSNm in forward orientation, closed circles; luxSNm in reverse orientation, open circles. AI-2 activity: V. harveyi BB120 (wild type, after 24 h), black bar; luxSNm in forward orientation, grey bars; luxSNm in reverse orientation, white bars. Activities are presented as the fold increase of bioluminescence in relation to E. coli DH5α carrying an empty vector at the respective time point. (B) Growth profiles and AI-2 activity of N. meningitidis. N. meningitidis strains MC58 (closed circles) and MC58ΔluxS (open circles) were grown, and the optical densities at 600 nm of cultures were measured. Culture supernatants of MC58 (grey bars), MC58ΔluxS (white bars), and V. harveyi BB120 (black bar) were assayed for AI-2 activity. Activities are presented as the fold increase of bioluminescence in relation to the control (sterile medium) at the respective time points. The results for MC58ΔluxSEct were identical to those for the wild type and are not shown. For panels A and B, single representative experiments are shown, although similar results were obtained on three occasions for both assays.
Expression of N. meningitidis AI-2 activity is dependent on luxSNm.
Next, we investigated whether N. meningitidis initiates luxSNm-dependent AI-2 production. A total of 432 bp of luxSNm (nucleotides 4 to 436 of the ORF) was deleted by inverse PCR and replaced with the kanamycin resistance gene from Tn903, yielding pGEMΔluxS-kan. The insert of pGEMΔluxS-kan was then cloned into pGIT5.3, a vector containing the Neisseria uptake sequence, and the resulting plasmid (pGIT5.3ΔluxS-kan) was introduced into N. meningitidis by transformation; Southern hybridization confirmed the integration of the deleted allele in MC58ΔluxS and B16/B6ΔluxS. A complemented strain, MC58ΔluxSEct, was constructed to enable the attribution of functions to luxSNm. luxSNm, under the control of an opa promoter, was introduced in the intergenic region between NMB102 and NMB103 (genes oriented in a tail-to-tail fashion), with ermC downstream of luxS. The construct (pYH204) was introduced into MC58ΔluxS by transformation, generating MC58ΔluxSEct; Southern analysis confirmed that integration into the NMB102/NMB103 intergenic region had occurred. N. meningitidis bacteria were grown at 37°C on brain heart infusion plates with Levinthal's supplement or in Mueller-Hinton medium for 24 h with shaking for AI-2 production. A list of the bacterial strains and plasmids used in this study is presented in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and/or details | Source or reference |
|---|---|---|
| Bacterial strains | ||
| E. coli DH5α | F′ endA1 supE44 thi-1 hsdR17(rK− mK+) recA1 gyrA relA1 Δ(lacIZYA-argF)U169 deoR [φ80d lacΔ(lacZ)M15] | Gibco-BRL |
| N. meningitidis | ||
| MC58 | Serogroup B; ET-5 | 25 |
| B16/B6 | Serogroup B; ET-37 | 17 |
| SD | Serogroup B: B:15:P1.16 | 1 |
| MC58ΔluxS | Deleted luxSNm replaced with kan | This study |
| MC58ΔluxSEct | Wild-type luxSNm located in the NMB102/103 intergenic region of MC58ΔluxS | This study |
| V. harveyi | ||
| BB120 | Wild type | 3 |
| BB170 | Sensor AI-1−, sensor AI-2+ | 3 |
| Plasmids | ||
| pGEM T-Easy | Cloning vector | Promega |
| pGEM luxS-F | 1.5-kb fragment encoding luxS of N. meningitidis MC58 and flanking region in pGEM-T Easy, forward orientation | This study |
| pGEM luxS-R | 1.5-kb fragment encoding luxS of N. meningitidis MC58 and flanking region in pGEM-T Easy, reverse orientation | This study |
| pGEMΔluxS-kan | luxSNm with internal deletion and Tn903 kanamycin resistance gene | This study |
| pGIT5.3 | Derived from pCRII; containing a chloramphenicol resistance cassette and Neisseria DNA uptake sequence | L. Barber and T. Baldwin, unpublished data |
| pGIT5.3ΔluxS-kan | pGIT5.3 derivative containing the insert of pGEMΔluxS-kan | This study |
| pYH204 | luxSNm in the NMB102/103 intergenic region with ermC | This study |
AI-2 activity was detected in MC58 culture supernatants from early logarithmic growth onward (Fig. 1B). While no AI-2 activity was observed in supernatants of MC58ΔluxS, the single ectopic luxS allele in MC58ΔluxSEct restored AI-2 activity to wild-type levels. Identical results were obtained with strain B16/B6 and its corresponding mutants (not shown).
N. meningitidis luxS mutants are attenuated for bacteremic infection.
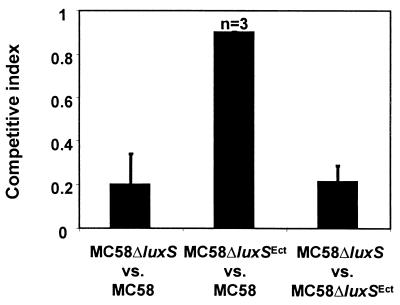
To investigate the influence of luxSNm on meningococcal pathogenesis, the ability of MC58ΔluxS to cause disseminated disease was compared directly against that of MC58. Bacteria were grown overnight on brain heart infusion agar and suspended in phosphate-buffered saline, and the number of CFU was determined. Bacteria (100 μl) in phosphate-buffered saline were injected intraperitoneally into five-day-old infant rats (Wistar; Harlan, Bicester, United Kingdom). The virulence of strains was assessed using groups of at least three animals in each of three experiments by determining their competitive index (CI). The CI is the proportion of mutant to wild-type bacteria recovered from the bloodstream following inoculation with a 1:1 ratio of the strains (26); results were compared by using a one-tailed Student's t test. MC58ΔluxS showed decreased survival in infected animals when compared with MC58 (P < 0.01; Fig. 2). Similar results were obtained with B16/B6 (not shown). Providing luxSNm in trans (MC58ΔluxSEct) restored the virulence to wild-type levels, demonstrating that the loss of luxS, not any polar effect, is responsible for attenuation.
FIG. 2.
MC58ΔluxS is defective for bacteremic infection compared with the parental strain, MC58. This defect is restored by supplying a single, intact copy of luxS in trans to MC58ΔluxS (MC58ΔluxSEct); the difference in the virulences of MC58ΔluxS and MC58ΔluxSEct is highly significant (P < 0.001). The CI is the proportion of the strains in the bloodstream 21 to 24 h following infection with a 1:1 ratio of the strains.
Complete genome sequences of bacterial pathogens provide an important resource for microbial research (21), though the annotations may miss biologically important homologies. While a luxS homologue was identified in the annotated Z2491 serogroup A strain sequence (14), the annotated MC58 genome (25) does not contain a luxS homologue. However, investigation of the MC58 database revealed an ORF closely related to luxSVh. We demonstrate that luxSNm complements DH5α and that N. meningitidis expresses AI-2 in a luxS-dependent fashion with maximal production during late exponential growth, consistent with a role in quorum sensing.
Studies with the infant rat model show that luxSNm contributes to the ability of the bacterium to cause bacteremia, a prerequisite for meningococcal pathogenicity. This model has been used to demonstrate the influence of other well-characterized virulence determinants on disseminated infection (20, 27). Although CIs provide a sensitive measure of a strain's virulence by eliminating host-to-host variation (22, 26), our findings may underestimate the impact of luxSNm on pathogenesis. AI-2 produced by the wild type could partially cross-feed a luxSNm mutant in mixed-inoculum experiments. However, the results are also consistent with a metabolic function of the LuxS protein, which is distinct from quorum sensing (18, 29).
Little is known about the regulatory networks governing the expression of N. meningitidis virulence factors apart from that of a LysR homologue, required for entry into host cells (7). Identification of the response elements and the genes controlled by AI-2-dependent signaling may help elucidate this aspect of pathogenesis and identify novel virulence determinants for this important human pathogen. Furthermore, the demonstration that luxSNm is required for full virulence suggests that interrupting this pathway may provide a means for preventing meningococcal disease (10).
Acknowledgments
We are grateful to Sharmila Bakshi, David Holden, Harry Smith, Nick West, and Paul Williams for valuable comments during preparation of the manuscript.
C.M.T. is an MRC Clinician Scientist, and A.G. was supported by a studentship from the University of Nottingham. Work in C.M.T.'s laboratory is supported by the Meningitis Research Fund. K.W. is supported by the MRC, and K.H. is a British Society for Antimicrobial Chemotherapy Fellow.
Editor: V. J. DiRita
REFERENCES
- 1.Ala'Aldeen, D. A., R. A. Wall, and S. P. Borriello. 1990. Immunogenicity and cross-reactivity of the 70-Kda iron-regulated protein of Neisseria meningitidis in man and animals. J. Med. Microbiol. 32:275-281. [DOI] [PubMed] [Google Scholar]
- 2.Bassler, B. L. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582-587. [DOI] [PubMed] [Google Scholar]
- 3.Bassler, B. L., E. P. Greenberg, and A. M. Stevens. 1997. Cross-species induction of luminescence in the quorum sensing bacterium Vibrio harveyi. J. Bacteriol. 179:4043-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess, N., D. Kirke, P. Williams, K. Winzer, K. R. Hardie, N. L. Meyer, J. Aduse-Opoku, M. A. Curtis, and M. Cámara. LuxS-dependent quorum sensing in Porphyromonas gingivalis modulates protease and haemagglutinin activities but is not essential for virulence. Microbiology, in press. [DOI] [PubMed]
- 5.Chung, W. O., Y. Park, R. J. Lamont, R. McNab, B. Barbieri, and D. R. Demuth. 2001. Signaling system in Porphyromonas gingivalis based on a LuxS protein. J. Bacteriol. 183:3903-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day, W. A., Jr., and A. T. Maurelli. 2001. Shigella flexneri LuxS quorum-sensing system modulates virB expression but is not essential for virulence. Infect. Immun. 69:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deghmane, A. E., S. Petit, A. Topilko, Y. Pereira, D. Giorgini, M Larribe, and M. K. Taha. 2000. Intimate adhesion of Neisseria meningitidis to human epithelial cells is under the control of the crgA gene, a novel LysR-type transcriptional regulator. EMBO J. 19:1068-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong, K., P., W. O. Chung, R. J. Lamont, and D. R. Demuth. 2001. Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS. Infect. Immun. 69:7625-7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsyth, M. H., and T. L. Cover. 2000. Intercellular communication in Helicobacter pylori: luxS is essential for the production of an extracellular signaling molecule. Infect. Immun. 68:3193-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartman, G., and R. Wise. 1998. Quorum sensing: potential means of treating gram-negative infections? Lancet 351:848-849. [DOI] [PubMed] [Google Scholar]
- 11.Joyce, E. A., B. L. Bassler, and A. Wright. 2000. Evidence for a signaling system in Helicobacter pylori: detection of a luxS-encoded autoinducer. J. Bacteriol. 182:3638-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyon, W. R., J. C. Madden, J. C. Levin, J. L. Stein, and M. G. Caparon. 2001. Mutation of luxS affects growth and virulence factor expression in Streptococcus pyogenes. Mol. Microbiol. 42:145-157. [DOI] [PubMed] [Google Scholar]
- 13.Novick, R. P., and T. W. Muir. 1999. Virulence gene regulation by peptides in staphylococci and other Gram-positive bacteria. Curr. Opin. Microbiol. 2:40-45. [DOI] [PubMed] [Google Scholar]
- 14.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M. A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 15.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in Gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsay, M., E. Kaczmarski, M. Rush, R. Mallard, P. Farrington, and J. White. 1997. Changing patterns of case ascertainment and trends in meningococcal disease in England and Wales. Commun. Dis. Rep. CDR Rev. 7:R49-R54. [PubMed] [Google Scholar]
- 17.Rokbi, B., G. Renauld-Mongenie, M. Mignon, B. Danve, D. Poncet, C. Chabanel, D. A. Caugant, and M.-J. Quentin-Millet. 2000. Allelic diversity of the two transferrin binding protein B gene isotypes among a collection of Neisseria meningitidis strains representative of serogroup B disease: implication for the composition of a recombinant TbpB-based vaccine. Infect. Immun. 68:4938-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schauder, S., K. Shokat, M. G. Surette, and B. L. Bassler. 2001. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 41:463-476. [DOI] [PubMed] [Google Scholar]
- 19.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stojiljkovic, I., V. Hwa, L. de Saint Martin, P. O'Gaora, X. Nassif, F. Heffron, and M. So. 1995. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol. Microbiol. 15:531-541. [DOI] [PubMed] [Google Scholar]
- 21.Strauss, E. J., and S. Falkow. 1997. Microbial pathogenesis: genomics and beyond. Science 276:707-712. [DOI] [PubMed] [Google Scholar]
- 22.Sun, Y. H., S. Bakshi, R. Chalmers, and C. M. Tang. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 6:1269-1273. [DOI] [PubMed] [Google Scholar]
- 23.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surette, M. G., M. B. Miller, and B. L. Bassler. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 96:1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 26.Unsworth, K. E., and D. W. Holden. 2000. Identification and analysis of bacterial virulence genes in vivo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogel, U., S. Hammerschmidt, and M. Frosch. 1996. Sialic acids of both the capsule and the sialylated lipooligosaccharide of Neisseria meningitidis serogroup B are prerequisites for virulence of meningococci in the infant rat. Med. Microbiol. Immunol. 185:81-87. [DOI] [PubMed] [Google Scholar]
- 28.Williams, P., M. Camara, A. Hardman, S. Swift, D. Milton, V. Hope, K. Winzer, B. Middleton, D. I. Pritchard, and B. W. Bycroft. 2000. Quorum sensing and the population dependent control of virulence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:667-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winzer, K., K. R. Hardie, N. Burgess, N. Doherty, D. Kirke, M. T. G. Holden, R. Linforth, K. A. Cornell, A. J. Taylor, P. J. Hill, and P. Williams. LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology, in press. [DOI] [PubMed]
- 30.Withers, H., S. Swift, and P. Williams. 2001. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr. Opin. Microbiol. 4:186-193. [DOI] [PubMed] [Google Scholar]