Abstract
Transcutaneous immunization (TCI) is a new method for vaccine delivery that has been shown to induce immunity relevant to enteric disease vaccines. We evaluated the clinical safety and immunogenicity of a recombinant subunit vaccine against enterotoxigenic Escherichia coli (ETEC) delivered by TCI. Adult volunteers received patches containing the recombinant ETEC colonization factor CS6, either with heat-labile enterotoxin (LT) or patches containing CS6 alone. The vaccine was administered at 0, 1, and 3 months, and serum antibodies and antibody-secreting cells (ASCs) were assessed. Among the 26 volunteers that completed the trial, there were no responses to CS6 in the absence of LT. In the groups receiving both CS6 and LT, 68 and 53% were found to have serum anti-CS6 immunoglobulin G (IgG) and IgA, respectively; 37 and 42% had IgG and IgA anti-CS6 ASCs. All of the volunteers receiving LT had anti-LT IgG, and 90% had serum anti-LT IgA; 79 and 37% had anti-LT IgG and IgA ASCs. Delayed-type hypersensitivity (DTH), suggesting T-cell responses, was seen in 14 of 19 volunteers receiving LT and CS6; no DTH was seen in subjects receiving CS6 alone. This study demonstrated that protein antigens delivered by a simple patch could induce significant systemic immune responses but only in the presence of an adjuvant such as LT. The data suggest that an ETEC vaccine for travelers delivered by a patch may be a viable approach worthy of further evaluation.
An estimated 7.5 million cases of severe diarrhea and 400,000 deaths per year worldwide are caused by enterotoxigenic Escherichia coli (ETEC) among infants and children (8). ETEC is the most common cause of acute diarrhea among travelers to developing countries (3, 33, 44, 50), and ETEC is still a major problem for military personnel deployed in those countries. During the Persian Gulf War in 1990 to 1991, diarrhea from any cause was reported by 57% of the U.S. troops stationed in Saudi Arabia, and 20% reported lost duty time due to illness. ETEC and Shigella were the predominant causes of diarrhea among U.S. troops during this deployment (23, 56). ETEC is one of the main causes of food-borne disease in developing countries (51) and is an important cause of waterborne outbreaks of diarrheal disease (10, 22).
Measures to avoid traveler's diarrhea include hygienic measures that prevent the consumption of food or water contaminated with ETEC; however, these hygienic measures are difficult to maintain during travel to areas where ETEC-caused disease is endemic. Traveler's diarrhea is usually treated with oral antibiotics, rehydration, and intestinal antimotility agents (35). Antibiotic prophylaxis against ETEC traveler's diarrhea has been tested and shown to be effective (45); however, drug resistance of ETEC against multiple antibiotics has been documented since the early 1980s (39, 57) and continues to be an issue of growing concern (21, 27). ETEC infection induces an immune response that protects against disease on subsequent exposure (31). This suggests that a vaccine is a possible solution (30), yet presently there are no licensed ETEC vaccines.
To induce illness, ETEC first must adhere to intestinal epithelial cells through binding proteins called colonization factors (CF). CF interact with receptors on the host intestinal epithelial cells, allowing for adherence of ETEC to the intestinal mucosa (4, 5). After adhering to the mucosal cells, ETEC causes watery diarrhea by expressing heat-labile enterotoxin (LT), heat-stable enterotoxin (ST), or both toxins. Studies have shown CF to be immunogenic (29), and antibodies against CF produced by natural infection or by active immunization (25, 38, 49) or given through passive immunization (12, 48) can prevent diarrhea by blocking the adherence of ETEC to the intestinal mucosa. Three CF, CFA/I, CS3, and CS6, in addition to LT are present in the majority of ETEC isolates recovered from patients with ETEC diarrhea in 18 sites around the world (54). These CF and LTs have been targeted as important antigens in the development of an ETEC vaccine. CS6 is one of the most prevalent CF worldwide to be characterized (19, 20, 55).
Transcutaneous immunization (TCI) is a novel strategy for administering adjuvant and antigen to the skin surface. The adjuvant and antigen applied using TCI apparently target Langerhans cells in the skin, eliciting systemic antibodies, including antitoxin antibodies, as well as specific T-cell responses (16, 18, 32; R. Vassell, G. M. Glenn, M. C. Udey, T. Scharton-Kersten, and C. R. Alving, Fifth Natl. Symp. Basic Aspects Vaccines, abstr. A633, 1999). Although these toxins cannot readily be used orally and in their native forms in humans because of their enterotoxicity (30, 34), they have been shown to be safe in animal and human studies (13-16, 43). LT produced by ETEC is both a strong immunogen eliciting high titers of antitoxin antibodies and a potent adjuvant for immunizations (7). Although the contribution to protection against ETEC by antitoxin immunity in the human setting is debated (6, 52), animal studies strongly suggest that such immunity can be protective (14, 36, 37). In this study we demonstrate that LT acts as both an antigen and an adjuvant for a coadministered antigen, validating the observation that the adjuvant is critical for induction of strong systemic immune responses when delivering vaccines to the skin.
MATERIALS AND METHODS
Subjects and eligibility.
Healthy male and female volunteers, aged 18 to 45 years, were recruited from the Washington, D.C., metropolitan area. Exclusion criteria included travel to an area of ETEC diarrhea endemicity in the previous year; a recent history of traveler's diarrhea; pregnancy; infection with human immunodeficiency virus, hepatitis B virus, or hepatitis C virus; and allergy to antibiotics. Informed consent was obtained from all volunteers, and the human use guidelines of the U.S. Department of Defense were followed in the conduct of this trial. The protocol was approved by the Institutional Review Board of the Office of The Surgeon General, U.S. Army.
Vaccine composition.
The vaccine components consisted of CS6 antigen mixed with LT. CS6 was produced under present good manufacturing practices (GMP) at the Forest Glen Pilot BioProduction Facility of the Walter Reed Army Institute of Research, Silver Spring, Md. The bacterial strain used for the production of CS6 was constructed from a laboratory strain of E. coli (HB101), and a plasmid containing the four-gene operon necessary for CS6 expression was inserted by recombinant techniques. The CS6 genes were cloned from ETEC strain E8875 (55). The major steps in the production of CS6 included bacterial fermentation; purification of the CS6 from the fermentation broth by tangential flow filtration followed by ammonium sulfate precipitation; intermediate storage of the bulk CS6 protein in phosphate-buffered saline (PBS) solution at −80°C; thawing, stirring, and distribution into vials; and storage at −80°C. CS6 was formulated as purified protein in 2-ml serum vials with gray split rubber stoppers sealed with aluminum crimps. Each vial contained 0.9 ml of 1.3-mg/ml CS6 protein in PBS. Native LT of E. coli was produced under present GMP at the Swiss Serum and Vaccine Institute, Bern, Switzerland. The LT was produced from E. coli strain HE22 TP 235 Km. The LT was supplied as lyophilized powder. Each vial contained 500 μg of lyophilized LT and was reconstituted with 1 ml of sterile water. The doses of adjuvant (LT) and antigen (CS6) by vaccination group are shown in Table 1.
TABLE 1.
Number of volunteers by vaccine group (n = 26)
| Dose of antigen (μg) (CS6)a | No. of volunteers by dose of adjuvant (μg) (LT)a
|
|
|---|---|---|
| 500 | 0 | |
| 250 | 5 | 2 |
| 500 | 5 | 1 |
| 1,000 | 5 | 1 |
| 2,000 | 4 | 3 |
| Total | 19 | 7 |
Dose was split between two patches.
Vaccine administration.
The vaccine was administered in three doses. The first dose was administered on day 0, and the second and third immunizations on days 28 and 84, respectively, after the first immunization. The vaccine was administered transcutaneously using a semiocclusive patch consisting of a 2- by 2-in cotton gauze matrix (two-ply; no. 2556; Kendall) with a 2- by 2-in polyethylene (Saran Wrap) backing covered by a 4- by 4-in Tegaderm dressing (semiocclusive; catalog no. 1616; 3M).
At the time of vaccination the vaccine was applied in 500 μl of sterile saline and administered as a split dose on each upper arm. Each split dose contained the corresponding dose of CS6 (antigen) alone or in combination with 250 μg of LT (adjuvant). The upper arm was positioned in a half-extended manner on an examination table and was prepared by gently rubbing five times with an isopropyl alcohol (70%) swab. The cotton gauze was placed on each upper arm, and the immunization solution was applied to the gauze with a syringe. The polyethylene backing was then placed over the impregnated cotton gauze and covered with the Tegaderm dressing. Volunteers remained in the research clinic for 20 min following patch application for observation. Volunteers were instructed not to touch the patches or engage in strenuous physical activity during the time that the patches were worn. The patches were removed 6 h after application (range, 4 to 8 h). The immunization sites were then rinsed with 500 ml of water and patted dry. The volunteers were instructed to bathe or shower in the evening but to refrain from heavy scrubbing of the immunization site with soap to avoid unusual irritation of the skin. The volunteers were reimmunized at 28 and 84 days after the first immunization. Each volunteer received the same dose of vaccine on each immunization.
Postvaccination follow-up.
Volunteers were observed for 20 min after each dose for occurrence of immediate adverse effects. The volunteers were given a diary to record signs and symptoms observed after vaccination. Reported symptoms were graded as mild (noticeable), moderate (affecting normal daily activities), or severe (suspending normal daily activities). The volunteers were evaluated at 24 and 48 h for clinical assessment and evaluation of possible side effects. Volunteers who showed signs of vaccine skin reactions were instructed to return to the clinic at 72 h for additional clinical assessment. Volunteers were then followed as needed until side effects had completely resolved. One of the volunteers who developed a skin rash in the site of immunization was asked to undergo a skin biopsy. This biopsy was performed by a dermatologist, following standard procedure, and under a separate, written informed consent.
ASC immune responses.
The responses of antibody-secreting cells (ASCs) to the vaccine antigens were chosen as an immunological endpoint for this study, since previous studies have shown that ASC responses correlate with mucosal intestinal immune responses (53). Venous blood samples were obtained from the volunteers on day 0 before immunization and on days 7, 28, 35, 56, 84, 91, 98, and 112 after the first immunization. Blood specimens were collected using EDTA-treated tubes (Becton Dickinson Vacutainer Systems, Rutherford, N.J.). Peripheral blood mononuclear cells (PBMC) were isolated from the blood sample by gradient centrifugation on Ficoll-Hypaque (Sigma Chemical Co., St. Louis, Mo.) and were assayed for total and vaccine-specific numbers of IgA and IgG ASCs by the enzyme-linked immunospot (ELISpot) technique (9, 53). Individual wells of nitrocellulose-bottomed 96-well plates (Millititer HA; Millipore Corp., Bedford, Mass.) were coated with 0.1 ml of purified CS6 (20 μg/ml) or GM1 ganglioside (0.5 μg/ml) and were incubated overnight at 4°C. After a PBS wash, GM1-coated wells were exposed to LT (0.5 μg/ml) for 2 h at 37°C. After being washed with PBS, the plates were blocked with complete RPMI medium (Gibco BRL, Grand Island, N.Y.) supplemented with 5% fetal calf serum (Gibco BRL) and 50 μg of gentamicin (Gibco BRL)/ml. The PBMC were adjusted to 2 × 107 viable cells/ml in complete RPMI medium. A final 0.1-ml suspension of PBMC was added to each well (106 PBMC added per well), and plate contents were incubated for 4 h at 37°C in 5.0% CO2. Plates were washed. Their contents were incubated overnight at 4°C with a mixture of two affinity-purified goat anti-human immunoglobulin antibodies with distinct isotype specificities, one conjugated to alkaline phosphatase (immunoglobulin G [IgG]) and the other conjugated to horseradish peroxidase (IgA) (Southern Biotech Associates, Birmingham, Ala.), and were exposed to the appropriate chromogen-enzyme substrate (Sigma). Spots, corresponding to a zone of antibodies secreted by individual cells, were enumerated in triplicate wells under ×40 magnification, with data expressed as the number of spot-forming cells per 106 PBMC.
As previously described (1, 24, 25), we defined a positive ASC response as a ≥2-fold increase over baseline value of the ASCs per 106 PBMC, when the number of ASCs was ≥ 0.5 per 106 PBMC in the baseline sample. If the number of preimmune ASCs was less than 0.5 per 106 PBMC, a value of >1.0 per 106 PBMC after dosing was considered a positive response.
Serum antibody measurements.
Venous blood samples were obtained from the volunteers before immunization and on days 14 and 28 after each immunization for measurements of serum antibody titers. IgA and IgG antibody titers against LT were measured by the GM1-enzyme-linked immunosorbent assay (ELISA) method (24, 47), and those against the CS6 were determined by ELISA methods as previously described (17, 46). LT (provided by the Swiss Serum and Vaccine Institute) and CS6 (GMP lot 0695; provided by F. Cassels, Walter Reed Army Institute of Research, Silver Spring, Md.) were used as solid-phase antigens. The LT and CS6 used for the ELISAs were from the same lots used for the vaccine preparation. Individual microtiter wells (immunoplates; Nunc, Roskilde, Denmark) were coated with GM1 ganglioside (0.5 μg/ml) (Sigma) at room temperature overnight or with 0.1 ml of a 1.0-μg/ml preparation of CS6 at 37°C overnight. The GM1-coated wells were then washed with PBS and incubated with 0.1 ml of LT (0.5 μg/ml) for 2 h at 37°C. After being blocked with 0.1% bovine serum albumin (Sigma), the serum samples were threefold serially diluted (initial dilution 1:5) and were then incubated at room temperature for 90 min. Bound antibodies were demonstrated by addition of rabbit anti-human IgA or IgG conjugated with horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, Pa.) and were incubated at room temperature for 90 min, followed by addition of o-phenylenediamine-H2O2 (Sigma). The endpoint titers were assigned as the interpolated dilutions of the samples giving an optical density at 450 nm of 0.4 above background (absorbance at 450 nm). Titers were adjusted in relation to a reference specimen included in each test to compensate for day-to-day variation. For both antigens, pre- and postdosing serum samples from the same volunteer were always tested side by side. The antibody titer ascribed to each sample represented the geometric mean of duplicate determinations performed on different days. Reciprocal endpoint titers that were < 5 were assigned a value of 2.5 for computational purposes. Based on our calculations of the methodological error of each ELISA, previous to the study, we defined a significant response (seroconversion) as a ≥2-fold increase in endpoint titer between pre- and postimmunization specimens (26), with the added criterion that the postimmunization reciprocal titer be ≥ 10.
Statistical methods.
All volunteers receiving the three scheduled doses of vaccine were included in the postdosing safety and immunogenicity analyses. Proportions were compared using the 2- by n χ2 test for which α = 0.05 and power = 0.8. Fisher's exact test was used in 2- by 2 tables when the number contained in one of the cells was < 5 (40). The median number of ASCs and median plasma antibody titer fold increases were compared separately using the Wilcoxon rank test to assess the boosting effect of each consecutive dose of vaccination (11). All statistical tests were two-tailed.
RESULTS
Vaccine administration.
Thirty-three volunteers were enrolled and received at least one dose of the study vaccine. The volunteers were 21 to 44 years of age; by sex, 17 females and 16 males; and by race, 15 black, 16 white, and 2 Asian. Of the 33 volunteers, 7 did not complete the study for reasons unrelated to the study: conflict with their work schedule (4), moving from the D.C. metropolitan area (2), and admission to a local clinic for illegal drug use (1). Twenty-six volunteers received the three scheduled doses of the vaccine and completed all the postvaccination follow-up visits, and the data on these volunteers are shown. These volunteers were 21 to 44 years of age; by sex, 13 females and 13 males; and by race, 12 black, 12 white, and 2 Asian. The number of volunteers by vaccine dose is shown in Table 1.
Reactogenicity.
Of the volunteers that received a combination of LT/CS6, 74% (14 of 19) developed a maculopapular rash at the site of vaccination. No volunteers receiving CS6 alone developed a rash. The reaction was mild in 13 volunteers and moderate in 1 volunteer. White volunteers developed the rash significantly more frequently than black volunteers (11 of 11 versus 3 of 8; P < 0.005). Seven reactions occurred after the administration of the second dose and 14 occurred after the third dose; all seven volunteers with a second dose-related rash also developed the rash after the application of the third dose. The clinical diagnosis was contact dermatitis (delayed-type hypersensitivity [DTH]). One volunteer with the characteristic rash underwent a biopsy of the affected skin after receiving the third dose of LT/CS6. The biopsy showed mild dermal chronic inflammation (lymphocytic) with focal spongiosis. The pathological diagnosis was subacute spongiotic dermatitis, characteristic of DTH. The rash usually began within 24 h after patch application. Rash developing after the second dose lasted a median of 9 days (range, 1 to 14); the third dose rash lasted a median of 6 days (range, 1 to 11). Volunteers were offered 0.1% triamcinolone cream for relief of potential vaccine-related symptoms. None of the subjects used the cream after the first or second immunization. Eight patients with rashes that occurred after the third dose were treated with the triamcinolone cream. There were no apparent clinical differences regarding the appearance and severity of local symptoms (pruritus) or signs (erythema, papules) when compared by vaccination dose. There were no statistically significant differences in the magnitudes of the serological immune responses between the users of triamcinolone cream and the nonusers.
Immunogenicity.
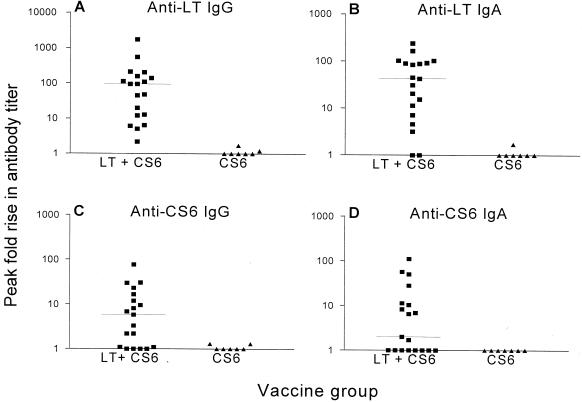
Immune responses were detected only in volunteers receiving both adjuvant and antigen (Fig. 1), although one volunteer who received only two doses of CS6 alone had a positive anti-CS6 IgA response (but no CS6 IgG) at a single time point. There were no significant differences in the frequency or the magnitude of the serum antibody or ASC responses to LT and CS6 between the four groups that received the adjuvant and antigen combination; therefore, data were pooled for further statistical analysis and presentation. All volunteers (100%) receiving LT demonstrated a serum anti-LT IgG response, and 90% produced anti-LT IgA. Anti-CS6 serum antibody responses rates were lower than the anti-LT response rate with 68 and 53% of volunteers showing a greater-than-twofold rise in anti-CS6 IgG and IgA titers, respectively. The individual peak fold rises in serum antibodies to LT and CS6 are depicted in Fig. 1. Robust responses to both LT and CS6 were observed, although there was a great deal of variability in the magnitude of the response. The mean anti-LT IgG response to LT exceeded the mean fold response previously described by nearly a log (16) and was greater than the response to CS6.
FIG. 1.
Individual IgG and IgA peak fold rise in antibody titer to LT (A and B) and CS6 (C and D) among volunteers immunized with adjuvant combined with antigen (LT + CS6) or with antigen alone (CS6). The transverse bar represents the median peak fold rise in antibody titer.
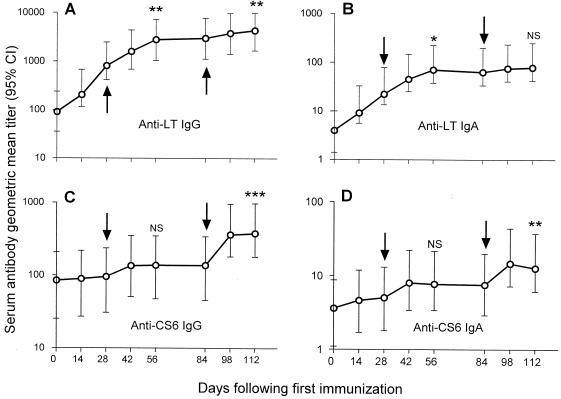
The kinetics of the serum antibody responses are depicted in Fig. 2. The postdose serum antibody titers for each group were combined and are presented as geometric mean titers with 95% confidence intervals. The kinetics of the immune responses to LT differ markedly to the kinetics of the response to CS6 in that strong priming and boosting responses to LT were seen, whereas the CS6 responses were primarily seen with boosting. Memory responses to CS6 appear to occur, as suggested by the significant difference in the pooled anti-CS6 IgG and IgA responses after both the second and third immunizations.
FIG. 2.
Kinetics of the anti-LT (A and B) and anti-CS6 (C and D) IgA and IgG antibody responses among volunteers immunized and boosted (arrows) using the transcutaneous route. The circles indicate the geometric mean titer by the day after the first immunization; the bars denote the corresponding 95% confidence intervals (CI). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001; NS, not statistically significant according to Wilcoxon signed rank test, comparing antibody titer responses between boosting immunizations.
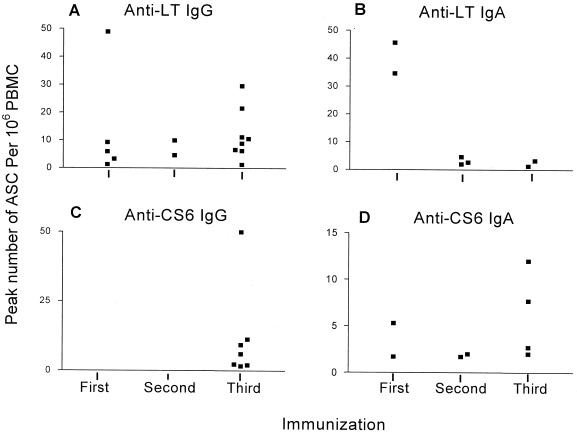
The percentage of ASC response rate and median number of antigen-specific ASCs per 106 PBMC are shown in Table 2. Both CS6 and LT-specific ASCs were detected. The time and magnitude of peak number of ASCs for each individual responder by specific ASC type are depicted in Fig. 3. In the majority of responders, peak ASCs were detected after the second or third immunization. All seven volunteers that demonstrated anti-CS6 IgG ASCs had their peak number of ASCs after the third immunization.
TABLE 2.
Vaccine antigen-specific ASC responses in volunteers receiving the LT/CS6 combination (n = 19)
| ASC type | No. of responders (%)a | Median no. of ASCs/106 PBMC (range)b |
|---|---|---|
| Anti-LT IgG | 15 (79) | 9 (1.3-49) |
| Anti-LT IgA | 7 (37) | 2.4 (1.3-46) |
| Anti-CS6 IgG | 7 (37) | 6 (1.7-77) |
| Anti-CS6 IgA | 8 (42) | 2.4 (1.7-12) |
A positive ASC response was defined as a ≥2-fold increase over baseline value of the ASCs per 106 PBMC, when the number of ASCs was ≥0.5 per 106 PBMC in the baseline sample. If the number of preimmune ASCs was less than 0.5 per 106 PBMC, a value of >1.0 per 106 PBMC after dosing was considered a positive response.
Only positive responses were included when calculating the median number of ASCs (range).
FIG. 3.
Individual peak number of anti-LT (A and B) and anti-CS6 (C and D) ASCs per 106 PBMC among responders to the immunization with adjuvant combined with antigen (LT + CS6), by the immunization after which the peak value was attained.
DISCUSSION
Our study is the first report of the use of recombinant CS6 as antigen in a human vaccine and the use of an antigen in combination with adjuvant applied using TCI in human volunteers. Robust immune responses to CS6 were seen only in volunteers receiving both adjuvant and antigen, validating the observation that the adjuvant is critical for induction of strong systemic immune responses as seen previously in animal studies (58). A previous study of natural disease in humans infected with ETEC strains expressing CS6 showed that CS6-specific immune responses developed following infection (20). Clinical trials using the live ETEC strain B7A in a human challenge model have shown the immune response to this CS6 expressing organism to be protective against developing diarrhea when the volunteers were rechallenged with the same strain, although the role of immunity to CS6 in protection was not evaluated (31). The immune responses to CS6 in the present trial are similar to responses after more recent challenge studies with the ETEC strain B7A, which expresses CS6 (M. K. Wolf, E. Hall, D. N. Taylor, T. Coster, F. Trespalacios, F. Cassels, A. DeLorimier, and C. McQueen, 35th U.S.-Japan Cholera Other Bacterial Enteric Infect. Joint Panel Meet., abstr. 37, 1999). Specifically, immunological analyses performed at our laboratory, using the same laboratory methods, showed that the IgA serum and ASC anti-CS6 responses elicited by TCI using LT in combination with CS6 were similar to responses elicited after ingestion of the same ETEC challenge strain (Wolf et al., 35th U.S.-Japan Cholera Other Bacterial Enteric Infect. Joint Panel Meet.). These results suggest that the use of TCI with antigen in combination with adjuvant can potentially elicit protective immunity similar to natural infection with ETEC. The results are particularly encouraging given the primitive nature of both the patch delivery system and formulation used in this trial.
The immune responses to CS6 and LT seen in humans using TCI are also similar to the response seen in preclinical animal studies using TCI (59). Both antitoxin and anti-CS6 immunity were seen, demonstrating that LT could act both as adjuvant and antigen for an ETEC vaccine. The preclinical studies clearly demonstrate that anti-LT antibodies generated using TCI can protect against enteric toxin challenge (58). The role of antitoxin immunity in human protection is debated; however, TCI may induce a more robust and durable immunity to LT than the B subunit immunity that has been shown to be protective in field studies (6, 17). Without an enteric animal challenge model for CS6, the relevance of the CS6 antibody responses can only be evaluated in clinical field or challenge studies. Preclinical studies also suggest that far more efficient use of antigen and adjuvant is possible, and the lack of significant differences in the CS6 response between dosing groups in the present trial suggests that lower doses may be effective. The immune kinetics demonstrate boosting of the antibody response (Fig. 2) and, in combination with the elicitation of DTH, suggest that T-cell-based memory is induced, consistent with preclinical observations (18). Previous studies demonstrated that anti-LT immunity is stable and long-lasting in concert with strong memory responses (16).
Clinical trials of an oral, inactivated, whole-cell ETEC vaccine have shown this vaccine to be relatively safe and immunogenic in adults (41) and children (42). However, these studies also showed that 13% of the preschool children receiving the vaccine failed to properly ingest the vaccine preparation and that 4 to 9% of the children vaccinated had gastrointestinal symptoms of reaction to either the vaccine or the buffer solution in the vaccine preparation. The results of this study suggest that TCI is a potential alternative method for effectively administering an ETEC vaccine that can avoid the difficulties of oral administration of a vaccine or the hazards of unsafe injections of other vaccines (28). The only adverse event related to the vaccine studied was a mild DTH developed by most of the volunteers who received the LT and CS6 combination. However, the DTH was self-limited and cleared without sequelae in all volunteers. The significantly less frequent DTH manifested in the black volunteers than in the white volunteers is consistent with previous findings showing that darker skin is less susceptible than lighter skin to diverse skin allergens (2). The use of triamcinolone cream by eight volunteers after the third dose decreased the symptom intensity and probably shortened the duration of the DTHs, apparently without interfering with the serological immune responses.
LT has been previously administered to humans using TCI, resulting in significant serological anti-LT IgA and IgG responses without eliciting DTH (16). This previous study using the same adjuvant and patch materials and the finding that DTH occurred only in the presence of adjuvant and CS6 implicate the specific CS6 response as the potential cause for the DTH, although the true cause is not known at this time. It is possible that a robust immunological response may be elicited by TCI without eliciting DTH by carefully selecting the patch materials, patch wear protocols, and the antigens and adjuvant to be used in the vaccine preparation.
The results of this study support the feasibility of developing a transcutaneous vaccine against ETEC. The study also shows that elicitation of immune responses to vaccine antigens using a simple patch applied to the skin requires the use of adjuvant. The combination of antitoxin immunity and anti-CS6 immunity against CS6-expressing strains, which largely produce ST toxin, may provide a combination of immunity against LT- and ST-mediated disease (56). Further studies on the transcutaneous administration of the other common ETEC CF CFA/I and CS3 in combination with CS6 and adjuvant should continue, followed by immunization and challenge studies in order to measure the functional and protective responses elicited by TCI against ETEC diarrhea.
Acknowledgments
We acknowledge the contributions of Moshe Shmuklarsky and the personnel of the Department of Clinical Trials at WRAIR for clinical and administrative support; the personnel of the WRAIR Pilot BioProduction Facility for CS6 manufacturing support; Henry Wong for performing the skin biopsy; the personnel of the Department of Membrane Biochemistry at WRAIR; and the IOMAI Corporation for their support of vaccine preparation. We gratefully acknowledge the cooperation of the study volunteers without whom this study would not be possible.
The work presented was supported and performed under a Cooperative Research and Development Agreement between Walter Reed Army Institute of Research and IOMAI Corporation, Gaithersburg, Md. Funding for the study was provided by the Military Infectious Disease Research Program, U.S. Army Medical Research and Material Command, Ft. Detrick, Md.
Editor: D. L. Burns
REFERENCES
- 1.Åhrén, C., M. Jertborn, and A.-M. Svennerholm. 1998. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect. Immun. 66:3311-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berardesca, E., and H. I. Maibach. 1988. Contact dermatitis in blacks. Dermatol. Clin. 6:363-368. [PubMed] [Google Scholar]
- 3.Caeiro, J. P., M. T. Estrada-Garcia, Z. D. Jiang, J. J. Mathewson, J. A. Adachi, R. Steffen, and H. L. DuPont. 1999. Improved detection of enterotoxigenic Escherichia coli among patients with travelers' diarrhea, by use of the polymerase chain reaction technique. J. Infect. Dis. 180:2053-2055. [DOI] [PubMed] [Google Scholar]
- 4.Cassels, F. J., and M. K. Wolf. 1995. Colonization factors of diarrheagenic E. coli and their intestinal receptors. J. Ind. Microbiol. 15:214-226. [DOI] [PubMed] [Google Scholar]
- 5.Cheney, C. P., P. A. Schad, S. B. Formal, and E. C. Boedeker. 1980. Species specificity of in vitro Escherichia coli adherence to host intestinal cell membranes and its correlation with in vivo colonization and infectivity. Infect. Immun. 28:1019-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens, J. D., D. A. Sack, J. R. Harris, J. Chakraborty, P. K. Neogy, B. Stanton, N. Huda, M. U. Khan, B. A. Kay, and M. R. Khan. 1988. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J. Infect. Dis. 158:372-377. [DOI] [PubMed] [Google Scholar]
- 7.Clements, J. D., N. M. Hartzog, and F. L. Lyon. 1988. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 6:269-277. [DOI] [PubMed] [Google Scholar]
- 8.Committee on Issues and Priorities for New Vaccine Development, Division of Health Promotion and Disease Prevention and Division of International Health, Institute of Medicine. 1986. The prospects for immunizing against Escherichia coli (ETEC), p. 178-185. In New vaccine development: establishing priorities, vol. 2. Diseases of importance in developing countries. National Academy Press, Washington, D.C.
- 9.Czerkinsky, C., Z. Moldoveanu, J. Mestecky, L. A. Nilsson, and O. Ouchterlony. 1988. A novel two colour ELISPOT assay. I. Simultaneous detection of distinct types of antibody-secreting cells. J. Immunol. Methods 115:31-37. [DOI] [PubMed] [Google Scholar]
- 10.Daniels, N. A., J. Neimann, A. Karpati, U. D. Parashar, K. D. Greene, J. G. Wells, A. Srivastava, R. V. Tauxe, E. D. Mintz, and R. Quick. 2000. Traveler's diarrhea at sea: three outbreaks of waterborne enterotoxigenic Escherichia coli on cruise ships. J. Infect. Dis. 181:1491-1495. [DOI] [PubMed] [Google Scholar]
- 11.Forrester, J. C., and H. K. Ury. 1969. The Signed-Rank (Wilcoxon) test in the rapid analysis of biological data. Lancet i:239-241. [DOI] [PubMed] [Google Scholar]
- 12.Freedman, D. J., C. O. Tacket, A. Delehanty, D. R. Maneval, J. Nataro, and J. H. Crabb. 1998. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J. Infect. Dis. 177:662-667. [DOI] [PubMed] [Google Scholar]
- 13.Glenn, G. M., M. Rao, G. R. Matyas, and C. R. Alving. 1998. Skin immunization made possible by cholera toxin. Nature 391:851. [DOI] [PubMed] [Google Scholar]
- 14.Glenn, G. M., T. Scharton-Kersten, R. Vassell, C. P. Mallett, T. L. Hale, and C. R. Alving. 1998. Transcutaneous immunization with cholera toxin protects mice against lethal mucosal toxin challenge. J. Immunol. 161:3211-3214. [PubMed] [Google Scholar]
- 15.Glenn, G. M., T. Scharton-Kersten, R. Vassell, G. R. Matyas, and C. R. Alving. 1999. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect. Immun. 67:1100-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glenn, G. M., D. N. Taylor, X. Li, S. Frankel, A. Montemarano, and C. R. Alving. 2000. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat. Med. 6:1403-1406. [DOI] [PubMed] [Google Scholar]
- 17.Hall, E. R., T. F. Wierzba, C. Ahren, M. R. Rao, S. Bassily, W. Francis, F. Y. Girgis, M. Safwat, Y. J. Lee, A. M. Svennerholm, J. D. Clemens, and S. J. Savarino. 2001. Induction of systemic antifimbria and antitoxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine. Infect. Immun. 69:2853-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammond, S. A., D. Walwender, C. R. Alving, and G. M. Glenn. 2001. Transcutaneous immunization: T cell responses and boosting of existing immunity. Vaccine 19:2701-2707. [DOI] [PubMed] [Google Scholar]
- 19.Helander, A., G. C. Hansson, and A. M. Svennerholm. 1997. Binding of enterotoxigenic Escherichia coli to isolated enterocytes and intestinal mucus. Microb. Pathog. 23:335-346. [DOI] [PubMed] [Google Scholar]
- 20.Helander, A., C. Wennerås, F. Qadri, and A.-M. Svennerholm. 1998. Antibody responses in humans against coli surface antigen 6 of enterotoxigenic Escherichia coli. Infect. Immun. 66:4507-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoge, C. W., J. M. Gambel, A. Srijan, C. Pitarangsi, and P. Echeverria. 1998. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin. Infect. Dis. 26:341-345. [DOI] [PubMed] [Google Scholar]
- 22.Huerta, M., I. Grotto, M. Gdalevich, D. Mimouni, B. Gavrieli, M. Yavzori, D. Cohen, and O. Shpilberg. 2000. A waterborne outbreak of gastroenteritis in the Golan Heights due to enterotoxigenic Escherichia coli. Infection 28:267-271. [DOI] [PubMed] [Google Scholar]
- 23.Hyams, K. C., A. L. Bourgeois, B. R. Merrell, P. Rozmajzl, J. Escamilla, S. A. Thornton, G. M. Wasserman, A. Burke, P. Echeverria, and K. Y. Green. 1991. Diarrheal disease during Operation Desert Shield. N. Engl. J. Med. 325:1423-1428. [DOI] [PubMed] [Google Scholar]
- 24.Jertborn, M., C. Ahren, J. Holmgren, and A. M. Svennerholm. 1998. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine 16:255-260. [DOI] [PubMed] [Google Scholar]
- 25.Jertborn, M., C. Åhrén, and A.-M. Svennerholm. 2001. Dose-dependent circulating immunoglobulin A antibody-secreting cell and serum antibody responses in Swedish volunteers to an oral inactivated enterotoxigenic Escherichia coli vaccine. Clin. Diagn. Lab. Immunol. 8:424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jertborn, M., A.-M. Svennerholm, and J. Holmgren. 1986. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J. Clin. Microbiol. 24:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang, Z. D., J. J. Mathewson, C. D. Ericsson, A. M. Svennerholm, C. Pulido, and H. L. DuPont. 2000. Characterization of enterotoxigenic Escherichia coli strains in patients with travelers' diarrhea acquired in Guadalajara, Mexico, 1992-1997. J. Infect. Dis. 181:779-782. [DOI] [PubMed] [Google Scholar]
- 28.Jodar, L., P. Duclos, J. B. Milstien, E. Griffiths, M. T. Aguado, and C. J. Clements. 2001. Ensuring vaccine safety in immunization programmes--a W. H. O. perspective. Vaccine 19:1594-1605. [DOI] [PubMed] [Google Scholar]
- 29.Levine, M. M. 1981. Adhesion of enterotoxigenic Escherichia coli in humans and animals. Ciba Found. Symp. 80:142-160. [DOI] [PubMed] [Google Scholar]
- 30.Levine, M. M. 1983. Travellers' diarrhoea: prospects for successful immunoprophylaxis. Scand. J. Gastroenterol. Suppl. 84:121-134. [PubMed] [Google Scholar]
- 31.Levine, M. M., D. R. Nalin, D. L. Hoover, E. J. Bergquist, R. B. Hornick, and C. R. Young. 1979. Immunity to enterotoxigenic Escherichia coli. Infect. Immun. 23:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lycke, N. 1997. The mechanism of cholera toxin adjuvanticity. Res. Immunol. 148:504-520. [DOI] [PubMed] [Google Scholar]
- 33.Mattila, L., A. Siitonen, H. Kyronseppa, I. Simula, P. Oksanen, M. Stenvik, P. Salo, H. Peltola, and the Finnish-Moroccan Study Group. 1992. Seasonal variation in etiology of travelers' diarrhea. J. Infect. Dis. 165:385-388. [DOI] [PubMed] [Google Scholar]
- 34.Michetti, P., C. Kreiss, K. L. Kotloff, N. Porta, J. L. Blanco, D. Bachmann, M. Herranz, P. F. Saldinger, I. Corthesy-Theulaz, G. Losonsky, R. Nichols, J. Simon, M. Stolte, S. Ackerman, T. P. Monath, and A. L. Blum. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804-812. [DOI] [PubMed] [Google Scholar]
- 35.Murphy, G. S., B. P. Petrucelli, H. Kollaritsch, and D. N. Taylor. 2001. Treatment of traveler's diarrhea, p. 165-176. In H. L. DuPont and R. Steffen (ed.), Textbook of travel medicine and health. B. C. Decker, Inc., Hamilton, Ontario, Canada.
- 36.Pierce, N. F., E. A. Kaniecki, and R. S. Northrup. 1972. Protection against experimental cholera by antitoxin. J. Infect. Dis. 126:606. [DOI] [PubMed] [Google Scholar]
- 37.Pierce, N. F., and H. Y. Reynolds. 1974. Immunity to experimental cholera. I. Protective effect of humoral IgG antitoxin demonstrated by passive immunization. J. Immunol. 113:1017-1023. [PubMed] [Google Scholar]
- 38.Qadri, F., C. Wenneras, F. Ahmed, M. Asaduzzaman, D. Saha, M. J. Albert, R. B. Sack, and A. Svennerholm. 2000. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi adults and children. Vaccine 18:2704-2712. [DOI] [PubMed] [Google Scholar]
- 39.Sack, R. B., M. Santosham, J. L. Froehlich, C. Medina, F. Orskov, and I. Orskov. 1984. Doxycycline prophylaxis of travelers' diarrhea in Honduras, an area where resistance to doxycycline is common among enterotoxigenic Escherichia coli. Am. J. Trop. Med. Hyg. 33:460-466. [DOI] [PubMed] [Google Scholar]
- 40.Sahai, H., and A. Khurshid. 1995. On analysis of epidemiological data involving a 2 x 2 contingency table: an overview of Fisher's exact test and Yates' correction for continuity. J. Biopharm. Stat. 5:43-70. [DOI] [PubMed] [Google Scholar]
- 41.Savarino, S. J., F. M. Brown, E. Hall, S. Bassily, F. Youssef, T. Wierzba, L. Peruski, N. A. El-Masry, M. Safwat, M. Rao, M. Jertborn, A. M. Svennerholm, Y. J. Lee, and J. D. Clemens. 1998. Safety and immunogenicity of an oral, killed enterotoxigenic Escherichia coli-cholera toxin B subunit vaccine in Egyptian adults. J. Infect. Dis. 177:796-799. [DOI] [PubMed] [Google Scholar]
- 42.Savarino, S. J., E. R. Hall, S. Bassily, F. M. Brown, F. Youssef, T. F. Wierzba, L. Peruski, N. A. El-Masry, M. Safwat, M. Rao, H. El Mohamady, R. Abu-Elyazeed, A. Naficy, A. M. Svennerholm, M. Jertborn, Y. J. Lee, J. D. Clemens, and the PRIDE Study Group. 1999. Oral, inactivated, whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine: results of the initial evaluation in children. J. Infect. Dis. 179:107-114. [DOI] [PubMed] [Google Scholar]
- 43.Scharton-Kersten, T., J. Yu, R. Vassell, D. O'Hagan, C. R. Alving, and G. M. Glenn. 2000. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins, subunits, and unrelated adjuvants. Infect. Immun. 68:5306-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultsz, C., J. van den Ende, F. Cobelens, T. Vervoort, A. van Gompel, J. C. F. M. Wetsteyn, and J. Dankert. 2000. Diarrheagenic Escherichia coli and acute and persistent diarrhea in returned travelers. J. Clin. Microbiol. 38:3550-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott, D. A., R. L. Haberberger, S. A. Thornton, and K. C. Hyams. 1990. Norfloxacin for the prophylaxis of travelers' diarrhea in U. S. military personnel. Am. J. Trop. Med. Hyg. 42:160-164. [DOI] [PubMed] [Google Scholar]
- 46.Stoll, B. J., A. M. Svennerholm, L. Gothefors, D. Barua, S. Huda, and J. Holmgren. 1986. Local and systemic antibody responses to naturally acquired enterotoxigenic Escherichia coli diarrhea in an endemic area. J. Infect. Dis. 153:527-534. [DOI] [PubMed] [Google Scholar]
- 47.Svennerholm, A. M., J. Holmgren, R. Black, M. Levine, and M. Merson. 1983. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J. Infect. Dis. 147:514-522. [DOI] [PubMed] [Google Scholar]
- 48.Tacket, C. O., G. Losonsky, H. Link, Y. Hoang, P. Guesry, H. Hilpert, and M. M. Levine. 1988. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N. Engl. J. Med. 318:1240-1243. [DOI] [PubMed] [Google Scholar]
- 49.Tacket, C. O., R. H. Reid, E. C. Boedeker, G. Losonsky, J. P. Nataro, H. Bhagat, and R. Edelman. 1994. Enteral immunization and challenge of volunteers given enterotoxigenic E. coli CFA/II encapsulated in biodegradable microspheres. Vaccine 12:1270-1274. [DOI] [PubMed] [Google Scholar]
- 50.Taylor, D. N., P. Echeverria, M. J. Blaser, C. Pitarangsi, N. Blacklow, J. Cross, and B. G. Weniger. 1985. Polymicrobial aetiology of travellers' diarrhoea. Lancet i:381-383. [DOI] [PubMed] [Google Scholar]
- 51.Todd, E. C. 1997. Epidemiology of foodborne diseases: a worldwide review. World Health Stat. Q. 50:30-50. [PubMed] [Google Scholar]
- 52.Trach, D. D., J. D. Clemens, N. T. Ke, H. T. Thuy, N. D. Son, D. G. Canh, P. V. Hang, and M. R. Rao. 1997. Field trial of a locally produced, killed, oral cholera vaccine in Vietnam. Lancet 349:231-235. [DOI] [PubMed] [Google Scholar]
- 53.Wennerås, C., A.-M. Svennerholm, C. Åhrén, and C. Czerkinsky. 1992. Antibody-secreting cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia coli vaccine. Infect. Immun. 60:2605-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolf, M. K. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin. Microbiol. Rev. 10:569-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolf, M. K., L. A. de Haan, F. J. Cassels, G. A. Willshaw, R. Warren, E. C. Boedeker, and W. Gaastra. 1997. The CS6 colonization factor of human enterotoxigenic Escherichia coli contains two heterologous major subunits. FEMS Microbiol. Lett. 148:35-42. [DOI] [PubMed] [Google Scholar]
- 56.Wolf, M. K., D. N. Taylor, E. C. Boedeker, K. C. Hyams, D. R. Maneval, M. M. Levine, K. Tamura, R. A. Wilson, and P. Echeverria. 1993. Characterization of enterotoxigenic Escherichia coli isolated from U.S. troops deployed to the Middle East. J. Clin. Microbiol. 31:851-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood, L. V., D. R. Morgan, and H. L. DuPont. 1983. Antimicrobial resistance of gram-negative bacteria isolated from foods in Mexico. J. Infect. Dis. 148:766. [DOI] [PubMed] [Google Scholar]
- 58.Yu, J., F. Cassels, T. Scharton-Kersten, S. A. Hammond, A. Hartman, E. Angov, B. Corthesy, C. Alving, and G. Glenn. 2002. Transcutaneous immunization using colonization factor and heat-labile enterotoxin induces correlates of protective immunity for enterotoxigenic Escherichia coli. Infect. Immun. 70:1056-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]