Abstract
We conducted a phase I trial with healthy adults to evaluate WRSS1, a live, oral ΔvirG Shigella sonnei vaccine candidate. In a double-blind, randomized, dose-escalating fashion, inpatient volunteers received a single dose of either placebo (n = 7) or vaccine (n = 27) at 3 × 103 CFU (group 1), 3 × 104 CFU (group 2), 3 × 105 CFU (group 3), or 3 × 106 CFU (group 4). The vaccine was generally well tolerated, although a low-grade fever or mild diarrhea occurred in six (22%) of the vaccine recipients. WRSS1 was recovered from the stools of 50 to 100% of the vaccinees in each group. The geometric mean peak anti-lipopolysaccharide responses in groups 1 to 4, respectively, were 99, 39, 278, and 233 for immunoglobulin (IgA) antibody-secreting cell counts; 401, 201, 533, and 284 for serum reciprocal IgG titers; and 25, 3, 489, and 1,092 for fecal IgA reciprocal titers. Postvaccination increases in gamma interferon production in response to Shigella antigens occurred in some volunteers. We conclude that WRSS1 vaccine is remarkably immunogenic in doses ranging from 103 to 106 CFU but elicits clinical reactions that must be assessed in further volunteer trials.
Shigella infection causes considerable morbidity and mortality, mostly among young children living in developing countries (14). Development of a safe and effective vaccine could provide a valuable tool for controlling this recalcitrant disease. To make an impact globally, a vaccine must protect against the three serogroups responsible for most of the disease. In developing countries, Shigella dysenteriae type 1 causes epidemic and pandemic dysentery and S. flexneri (15 serotypes) is the most common agent of endemic disease, with a small (∼10%) (14) but clinically important (9) contribution from S. sonnei. S. sonnei is the main serogroup found in industrialized countries, where it causes approximately 70% of both endemic and travel-related cases of shigellosis (14).
One strategy for developing a multivalent Shigella vaccine is to construct a series of strains in which the fundamental mutation is a deletion in plasmid-located virG (also known as icsA). The product of virG is required for intercellular spread of Shigella within the intestinal epithelium (1, 15). Sansonetti and colleagues attenuated S. flexneri 2a by creating deletions in virG and iuc (25) (a chromosomal gene encoding the siderophore aerobactin). In volunteers, a single oral 104-CFU dose of this strain, designated SC602, was generally safe (although mild fever or diarrhea developed in 5 to 8% of the subjects) and immunogenic and elicited significant protection against a wild-type challenge (4). In the rabbit ileal-loop assay, the deletion in iuc produced little additional attenuation over that provided by mutated virG alone (25), prompting construction of additional Shigella vaccine strains attenuated solely on the basis of ΔvirG. In pursuit of this strategy, we performed a phase I evaluation of WRSS1, a live oral S. sonnei vaccine candidate that Hartman and Venkatesan have attenuated by a deletion within virG (7).
MATERIALS AND METHODS
Vaccine.
WRSS1 was constructed from the Mosely strain of S. sonnei as previously described (7). In brief, a parent strain was selected that exhibited stability of the form I (smooth) colonial phenotype. This screening process represented an attempt to overcome the predilection of S. sonnei strains to spontaneously lose the virulence plasmid, which bears the genes associated with invasion of epithelial cells, as well as the genes encoding the O antigen. sacB suicide vector pCVD422 was used to replace the wild-type virG allele with virG possessing a 212-bp deletion. In preclinical experiments, WRSS1 exhibited >95% form I colonies after overnight growth, did not produce keratoconjunctivitis in guinea pigs (Sereny test), and did not form plaques on cultured epithelial cells (indicating absence of cell-to-cell spread) but retained its invasiveness phenotype. In the guinea pig Sereny challenge model, WRSS1 was both immunogenic and protective (7).
WRSS1 was manufactured at the Walter Reed Army Institute of Research Pilot Bioproduction Facility in Forest Glen, Md. The final composition (3.7 × 1010 CFU of WRSS1 per ml in phosphate buffer saline containing 7.5% dextran T10, 2% sucrose, and 1.5% glycerol as a cryopreservative) was lyophilized, dispensed into vials, and stored at −70°C until use.
Subjects.
Healthy community volunteers 18 to 45 years old were recruited as previously described (12). Informed consent was obtained in accordance with the guidelines of the University of Maryland, Baltimore Institutional Review Board, the Surgeon General's Human Subjects Research Review Board, and the Human Use Review Committee of the Walter Reed Army Institute of Research.
Study design.
Two randomized, double-blind, placebo-controlled trials were conducted in the Inpatient Research Isolation Ward of the Center for Vaccine Development. In the first trial, three vaccine inocula (3 × 103, 3 × 104, and 3 × 105 CFU) were evaluated sequentially in a placebo-controlled fashion. In the second trial, volunteers were randomly assigned to receive either the vaccine (3 × 105 or 3 × 106 CFU) or a placebo. The groups that received 3 × 105 CFU in the first and second trials were combined for analysis (Table 1). The vaccine and placebo were prepared by unblinded study personnel who did not participate in the clinical care or assessment of volunteers.
TABLE 1.
Immune responses to WRSS1 by inoculum size
| Response | Placebo (n = 7) | 3 × 103 CFU (group 1 [n = 7]) | 3 × 104 CFU (group 2 [n = 4]) | 3 × 105 CFU (group 3 [n = 10]) | 3 × 106 CFU (group 4 [n = 6]) |
|---|---|---|---|---|---|
| Anti-LPS | |||||
| ASCa | |||||
| IgA | 0.2, 3.5 | 0.4, 98.5 | 1.2, 38.6 | 0.1, 277.7 | 0.2, 233.4 |
| IgG | 0.0, 0.5 | 0.5, 30.4 | 0.0, 5.2 | 0.0, 27.7 | 0.2, 58.0 |
| Serum antibodyb | |||||
| IgA | 34 (14) | 245 (71) | 120 (50) | 703 (70) | 566 (100) |
| IgG | 56 (43) | 401 (86) | 201 (50) | 533 (70) | 284 (83) |
| Fecal IgA antibodyb | 10 (0) | 26 (17) | 3 (0) | 489 (75) | 1,092 (100) |
| Anti-Ipa | |||||
| ASCa | |||||
| IgA | 0.0, 3.2 | 0.1, 7.1 | 0.3, 27.8 | 0.1, 33.2 | 0.0, 10.6 |
| IgG | 0.0, 0.3 | 0.3, 4.0 | 0.0, 26.8 | 0.0, 5.1 | 0.0, 4.6 |
| Serum antibodyb | |||||
| IgA | 28 (0) | 37 (14) | 85 (50) | 58 (30) | 89 (17) |
| IgG | 489 (14) | 298 (0) | 951 (25) | 284 (40) | 226 (0) |
| Fecal IgA antibodyb | 246 (80) | 170 (83) | 3 (0) | 325 (38) | 1,092 (25) |
For ASC, the prevaccination and peak postvaccination geometric mean numbers per 106 PBMC are shown. Samples were collected on days 7 and 10 postvaccination; the greater value from each subject was used to calculate the peak postvaccination geometric mean for the dose group.
For antibodies the geometric mean peak postvaccination reciprocal titer (percentage of subjects with a fourfold or greater rise in titer postvaccination) is shown. Following vaccination, samples were collected on days 7, 14, 21, and 28 for serum antibody titer measurement and on days 3, 7, 10, and 14 for fecal antibody titer measurement. The highest postvaccination titer for each subject was used to calculate the peak postvaccination geometric mean for the dose group.
Inoculation and clinical evaluation.
Fasting volunteers ingested 2 g of sodium bicarbonate buffer dissolved in 150 ml of water, followed 1 min later by 30 ml of water containing the assigned vaccine dose or no vaccine (placebo). A physician queried each subject daily about the occurrence of anorexia, malaise, abdominal cramps, or headache. Volunteers graded the severity of their headaches and cramps as follows: 1, scarcely or easily noticed symptom but continued normal activity; 2, could not take mind off symptom but continued normal activity; 3, went to bed because of symptom but some activity possible; 4, symptom prevented any activity. Clinical events occurring within 6 days of vaccination are reported herein.
Nurses measured oral temperatures every 8 h. They graded the volunteers' stools for consistency by using previously published criteria (16), examined each for blood, and weighed all loose stools (grades 3 to 5) to estimate volume. Six days after inoculation, subjects received ciprofloxacin orally for 3 days, 500 mg every 12 h, to eradicate vaccine excretion.
The following clinical reactions were designated illness: diarrhea (two or more loose stools totaling at least 200 ml in 48 h or a single loose stool totaling at least 300 ml), dysentery (gross blood in a loose stool), or fever (an oral temperature of at least 100°F measured at two readings 5 min apart).
Bacteriology.
Vaccine excretion was detected by culturing all of the stools passed during inpatient isolation and once weekly for 3 weeks after discharge by using previously published methods (11). Excretion was quantified for 6 days after vaccination as previously described (11).
Immune responses.
Immunoglobulin A (IgA) antibody-secreting cells (ASC) circulating in the peripheral blood that recognize S. sonnei lipopolysaccharide (LPS) and a water-extractable antigen (21) presumed to contain the invasion plasmid antigens (Ipa) were enumerated (per 106 peripheral blood mononuclear cells [PBMC]) by ELISPOT before and on days 7 and 10 after vaccination as previously described (26). Antibodies to the same antigens (LPS and Ipa) were measured by enzyme-linked immunosorbent assay in serum (IgG and IgA) before vaccination and on days 7, 14, 21, and 28 postvaccination (2, 21) and in stool (IgA) before vaccination and on days 3, 7, 10, and 14 postvaccination (12). Results are expressed as reciprocal titers.
PBMC were isolated from blood drawn before and 28 days after inoculation and cryopreserved in liquid nitrogen. Proliferative and cytokine responses (gamma interferon [IFN-γ] and interleukin-10 [IL-10]) to Shigella antigens (purified recombinant IpaB, IpaC, and IpaD and a WRSS1 homogenate, each at 5 μg/ml, and a particulate WRSS1 preparation at 4 × 105 particles per well [17, 22, 24]) were measured as previously described (24). Bovine serum albumin (BSA, 5 μg/ml) and anti-CD3/anti-CD28 beads (3 μl/ml; Dynal Biotech ASA, Oslo, Norway) were used as negative and positive controls, respectively. Data are presented as net increases in cytokine production and were calculated by subtracting the preimmunization cytokine levels (in picograms per milliliter) from postimmunization values for each antigen and volunteer. Sufficient numbers of PBMC were available for the evaluation of cell-mediated immune responses in 5, 4, 10, and 4 subjects in groups 1, 2, 3, and 4, respectively, and for seven placebo recipients.
Fecal lactoferrin.
Fecal lactoferrin was measured prevaccination, 72 h postvaccination, and in the first diarrheal stool (if diarrhea occurred) by latex bead agglutination to assess whether vaccination elicited an intestinal inflammatory response (19).
Statistical analysis.
Proliferative and cytokine responses of individual subjects were defined as significant rises in geometric mean levels, as evaluated by one-sided, paired t tests. Group differences in geometric mean proliferative and cytokine responses were analyzed by using t tests evaluating two-sided hypotheses. Statistical significance was assessed at the 5% level throughout.
RESULTS
Clinical response.
Six volunteers met the definition of illness that was possibly vaccine related. One (14%) of the subjects in group 1 developed a fever of 101.2°F on day 3 postvaccination. He experienced 2 days of cramps (grade 2), headache (grade 3), anorexia, malaise, and myalgias. He passed two loose stools (totaling 72 ml). Four subjects in group 3 developed illnesses, of which three (30%) were considered to be vaccine related. One had a fever (100.7°F) on day 3, the second developed diarrhea (two loose stools totaling 229 ml) on day 4, and the third had a fever (100.8°F) on day 2 that was associated with anorexia, malaise, grade 2 headache, arthralgia, and chest pains. He passed two loose stools totaling 170 ml. A fourth volunteer developed a fever (100.4°F) on day 6 that was attributed to a dental abscess. Two (33%) of the volunteers in group 4 met the criteria for illness. One had asymptomatic diarrhea (three loose stools totaling 244 ml) for the first 2 days after vaccination. The second had three diarrheal stools (totaling 365 ml) and mild (grade 1) cramps for 4 days after vaccination. No volunteer had dysentery or a high fever (≥102°F).
Nine vaccinees (33%) reported abdominal cramps, and 8 (30%) had headaches. However, only two subjects reported that their symptom interfered with normal activity (both were grade 3 headaches) and no subject reported a grade 4 symptom. These reactions did not occur in placebo recipients and were not dose related.
Vaccine shedding.
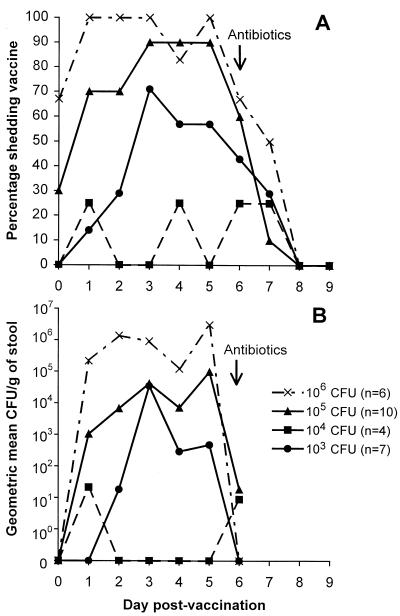
WRSS1 was recovered from the stools of 5 (71%), 2 (50%), 9 (90%), and 6 (100%) vaccinees in groups 1, 2, 3, and 4, respectively, but from none of the placebo recipients who were residing on the ward together with those excreting vaccine. The respective groupwise geometric mean excretion peaks were 5.5 × 104, 2.2 × 102, 1.3 × 106, and 7.5 × 106 CFU/g of stool. The percentage of subjects who excreted vaccine organisms (Fig. 1A) and the geometric mean CFU of vaccine in stool (Fig. 1B) remained relatively constant on each postvaccination day until antibiotics were administered.
FIG. 1.
Excretion of vaccine. Panel A shows the percentage of subjects shedding vaccine on each postinoculation day after receiving a single oral dose of either 103, 104, 105, or 106 CFU of strain WRSS1. Stools were inoculated in enrichment broth and plated on enteric media. Panel B shows the geometric mean number of CFU per gram of stool on the first 6 postinoculation days in each of the four groups of volunteers. To quantify excretion, stools were serially diluted and plated on MacConkey agar.
Fecal lactoferrin.
Fecal lactoferrin was detected postvaccination only in the volunteer who had developed a dental abscess.
Immune responses.
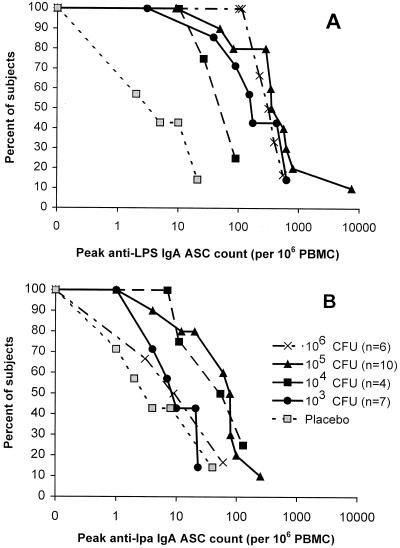
Vaccination elicited vigorous IgA ASC anti-LPS responses in all of the groups (Table 1). The counts exceeded 100 in most subjects, except for those in group 2 (Fig. 2A). ASC responses were less common and smaller in magnitude in the IgG anti-LPS assay (Table 1) and in both anti-Ipa assays (Fig. 2B; Table 1).
FIG. 2.
Reverse cumulative distribution curves (reference 23) by vaccine inoculum for ASC responses to S. sonnei LPS and Ipa. Each curve plots the proportion of vaccine or placebo recipients (ordinate) whose peak ASC count (per 106 PBMC), measured 7 to 10 days postinoculation, equals or exceeds the count shown on the abscissa.
Geometric mean peak postvaccination anti-LPS serum IgG and fecal IgA titers were also robust (Table 1). With the exception of those in group 2, most of the subjects exhibited a fourfold rise in serum and/or fecal anti-LPS antibody titers (Table 1). Whereas the anti-LPS IgA ASC and fecal antibody responses tended to increase with the dose, a similar trend was not apparent in serum antibody responses. In contrast, responses to Ipa were inconsistent, with many subjects exhibiting preexisting antibodies to this antigen (Table 1). Although no placebo recipient excreted WRSS1, several demonstrated immune responses, albeit of a smaller magnitude compared with those of vaccine recipients (Table 1; Fig. 2).
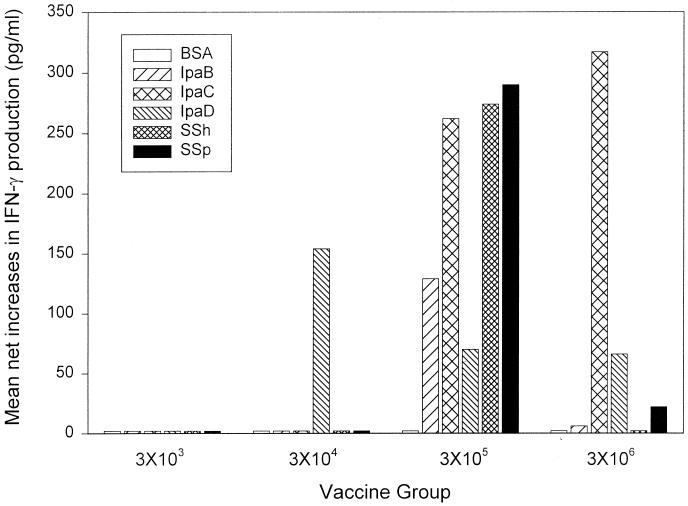
Mean net increases of >80 (range, 80 to 3,628) pg/ml in postvaccination IFN-γ production following PBMC exposure to two or more Shigella antigens were observed in 1 (20%) of 5, 1 (25%) of 4, 5 (50%)of 10, and 2 (50%) of 4 vaccinees in groups 1, 2, 3, and 4, respectively, and in 3 (43%) of 7 placebo recipients. These responses were most marked with the two larger vaccine doses (Fig. 3); however, there were no significant intergroup differences. No increases were observed in response to BSA. Postvaccination antigen-specific proliferative responses and increases in IL-10 production were not seen.
FIG. 3.
IFN-γ production by volunteers following oral immunization with S. sonnei. PBMC obtained from volunteers before immunization and at 28 days after immunization were evaluated for IFN-γ production in response to BSA (negative control) or Shigella antigens (IpaB, IpaC, IpaD, SSh [S. sonnei homogenate], and SSp [S. sonnei particulate]) as described in Materials and Methods. Results are expressed as mean net increases in IFN-γ production following immunization and include all of the evaluated volunteers in each group.
DISCUSSION
These findings demonstrate that WRSS1 is a remarkably immunogenic vaccine candidate. Although the precise immune responses that correlate with protection against shigellosis are not known, there is evidence that type-specific (anti-LPS) immunity is of critical importance. Vaccine-induced protective immunity that was serotype specific was demonstrated in field trials evaluating both streptomycin-dependent oral vaccines (18) and a parenteral S. sonnei O-antigen-specific polysaccharide conjugate vaccine (3). Similarly, homotypic immunity conferred by wild-type infection has been found in animal models (6), in epidemiologic studies (5), and in volunteer challenge experiments (8, 11). A response that has been correlated with protective efficacy in clinical trials is the anti-Shigella LPS IgA ASC count, which presumably reflects the degree of mucosal priming induced by an immunogen (10). In the field setting, preexisting IgA and IgG anti-LPS serum antibodies have been associated with protection against shigellosis following natural exposure (D. Cohen, M. S. Green, C. Block, R. Slepon, and Y. Lerman, Letter, J. Infect. Dis. 165:785-787, 1992). Thus, the observation that WRSS1 elicits vigorous anti-LPS IgA ASC and serum IgA and IgG antibody responses that are similar in magnitude to those elicited by other strains that prevented illness following experimental challenge (4) suggests that this vaccine may confer protection.
Furthermore, we have observed that immunization with WRSS1 elicits IFN-γ production by PBMC exposed to Shigella antigens, including highly purified preparations of IpaB, IpaC, and IpaD. Similar responses have been seen in volunteers who received other attenuated Shigella strains, including Shiga toxin-deficient S. dysenteriae 1 strain SC595 (24) and S. flexneri 2a strain CVD 1207 (13). However, this is the first time that an increase in IFN-γ production in response to IpaB was observed following immunization with attenuated Shigella strains. These results suggest that WRSS1 may be capable of eliciting a broad range of immune responses, including a type 1 cell-mediated immune response, which is likely to prevent invasion of eukaryotic cells and enhance the ability of macrophages to eliminate shigellae that gain access to the intracellular compartment (20, 24).
It is notable that some subjects exhibited immune responses to Shigella antigens following placebo inoculation, raising questions as to whether WRSS1 may have been transmitted while the subjects resided in the Isolation Ward. This and other trials have shown that exposure to very few Shigella organisms (≤103 CFU) elicits IFN-γ, serum antibody, and ASC responses (24). The lack of fecal vaccine excretion and clinical symptoms in placebo recipients renders the significance of these immunological observations uncertain. However, culture is a relatively insensitive technique for identifying WRSS1 in stool (103 CFU of WRSS1 per g of stool was the minimum count detected when stool was experimentally inoculated with known quantities of WRSS1 organisms [unpublished data]). The possibility of person-to-person transmission of WRSS1 should be carefully evaluated in future vaccine trials, for example, by attempting to detect shigellae in stools by molecular methods.
Decisions about further development of WRSS1 must take into account the objective clinical reactions (fever and diarrhea) observed in six vaccine recipients (22%). These reactions occurred across the dose range. Although symptoms were generally mild and brief, phase II studies must be designed to assess whether such reactions will be acceptable to subjects who are ambulatory and engaged in normal activities, particularly if WRSS1 is to be developed for use by travelers and military personnel. Large, placebo-controlled phase II studies will also gauge the relevance of the subjective complaints (headache, abdominal cramps) reported by vaccinees but not controls.
For reasons that could not be determined, recipients of 3 × 104 CFU of WRSS1 (group 2) seemed to have lower anti-LPS immune responses and diminished vaccine excretion compared with recipients of other doses. This could not be explained by an apparent loss of viability. There were no differences in the time interval from reconstitution to completion of vaccination or in the percentage of the inoculum that remained viable following vaccination of group 2 compared with that of the other groups (data not shown). Furthermore, one subject (a low responder) was inoculated on a different day than the other three. Given the small sample sizes, it is quite possible that, by chance alone, the occasional volunteers who did not respond to WRSS1 were clustered in group 2.
These data support the findings of previous studies with ΔvirG S. flexneri 2a strain SC602 that suggested that small doses (≤105 CFU) of a strain with a single mutation in virG may be sufficient to attenuate S. flexneri 2a for humans (4). By blocking the ability of shigellae to spread from cell to cell within the epithelium, the extent of infection is limited. However, when expanded clinical trials of SC602 were conducted among North American volunteers, it became apparent that larger doses (2 × 106 or 3 × 108 CFU) cause shigellosis (fever, diarrhea, and/or severe intestinal or constitutional symptoms) in the majority of subjects, illustrating the delicate balance between reactogenicity and immunogenicity that has challenged live, attenuated Shigella vaccine development (4). Most previous live Shigella vaccine constructs were developed by using an alternative strategy of creating multiple disabling mutations and then administering large doses of vaccine (ca. 108 to 109 CFU) (12, 13). While these highly attenuated constructs offer the potential for a greater margin of safety and further diminish the minimal risk of reversion by recombination, their immunogenicity has been lower compared with that of WRSS1.
We conclude that a single oral dose of WRSS1 in the range of 3 × 103 to 3 × 106 CFU is remarkably immunogenic, eliciting vigorous immune responses that generally appear to increase with vaccine inoculum size in assays that have been correlated with protection against shigellosis (anti-LPS IgA ASC and serum anti-LPS IgG). However, clinical reactions, including mild diarrhea, low-grade fever, headache, and abdominal cramps, occur in 22 to 33% of recipients. The clinical acceptability of this vaccine will be addressed in future trials among ambulatory subjects from areas where shigellosis is endemic and from areas where it is not endemic.
Acknowledgments
We thank the volunteers who participated in this study; Kathy Palmer for coordinating recruitment; Theresa Mowry, Ron Growchowski, and the nurses at the Center for Vaccine Development for providing clinical care; Sofie Livio and Mardi Reymann for technical assistance; Linda Rosendorf for regulatory support; and Dennis Lang for helpful suggestions.
This work was supported by contract N01-AI-45251 (to M.M.L.) and grant R21-AI-42802 (to M.B.S.) from the National Institute of Allergy and Infectious Diseases. Additional funds were provided by the Military Infectious Diseases Research Program, U.S. Army Medical Research and Materiel Command.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, the U.S. Department of the Army, or the U.S. Department of Defense, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Editor: J. D. Clements
REFERENCES
- 1.Bernardini, M. L., J. Mounier, H. D'Hauteville, M. Coquis-Rondon, and P. J. Sansonetti. 1989. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. USA 86:3867-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, R. E., M. M. Levine, M. L. Clements, G. Losonsky, D. Herrington, S. Berman, and S. B. Formal. 1987. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J. Infect. Dis. 155:1260-1265. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, D., S. Ashkenazi, M. S. Green, M. Gdalevich, G. Robin, R. Slepon, M. Yavzori, N. Orr, C. Block, I. Ashkenazi, J. Shemer, D. N. Taylor, T. L. Hale, J. C. Sadoff, D. Pavliakova, R. Schneerson, and J. B. Robbins. 1997. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 349:155-159. [DOI] [PubMed] [Google Scholar]
- 4.Coster, T. S., C. W. Hoge, L. L. Van de Verg, A. B. Hartman, E. V. Oaks, M. M. Venkatesan, D. Cohen, G. Robin, A. Fontaine-Thompson, P. J. Sansonetti, and T. L. Hale. 1999. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect. Immun. 67:3437-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreccio, C., V. Prado, A. Ojeda, M. Cayazzo, P. Abrego, L. Guers, and M. M. Levine. 1991. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in a poor periurban setting in Santiago, Chile. Am. J. Epidemiol. 134:614-627. [DOI] [PubMed] [Google Scholar]
- 6.Formal, S. B., E. V. Oaks, R. E. Olsen, M. Wingfield Eggleston, P. J. Snoy, and J. P. Cogan. 1991. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J. Infect. Dis. 164:533-537. [DOI] [PubMed] [Google Scholar]
- 7.Hartman, A. B., and M. M. Venkatesan. 1998. Construction of a stable attenuated Shigella sonnei ΔvirG vaccine strain, WRSS1, and protective efficacy and immunogenicity in the guinea pig keratoconjunctivitis model. Infect. Immun. 66:4572-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrington, D. A., L. Van de Verg, S. B. Formal, T. L. Hale, B. D. Tall, S. J. Cryz, E. C. Tramont, and M. M. Levine. 1990. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with a bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine 8:353-357. [DOI] [PubMed] [Google Scholar]
- 9.Khan, M. U., N. C. Roy, R. Islam, I. Huq, and B. Stoll. 1985. Fourteen years of shigellosis in Dhaka: an epidemiological analysis. Int. J. Epidemiol. 14:607-613. [DOI] [PubMed] [Google Scholar]
- 10.Kotloff, K. L., G. A. Losonsky, J. P. Nataro, S. S. Wasserman, T. L. Hale, D. N. Taylor, J. W. Newland, J. C. Sadoff, S. B. Formal, and M. M. Levine. 1995. Evaluation of the safety, immunogenicity and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli-Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine 13:495-502. [DOI] [PubMed] [Google Scholar]
- 11.Kotloff, K. L., J. P. Nataro, G. A. Losonsky, S. S. Wasserman, T. L. Hale, D. N. Taylor, J. C. Sadoff, and M. M. Levine. 1995. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine 13:1488-1494. [DOI] [PubMed] [Google Scholar]
- 12.Kotloff, K. L., F. Noriega, G. A. Losonsky, M. B. Sztein, S. S. Wasserman, J. P. Nataro, and M. M. Levine. 1996. Safety, immunogenicity, and transmissibility in humans of CVD 1203, a live oral Shigella flexneri 2a vaccine candidate attenuated by deletions in aroA and virG. Infect. Immun. 64:4542-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotloff, K. L., F. R. Noriega, T. Samandari, M. B. Sztein, G. A. Losonsky, J. P. Nataro, W. D. Picking, E. M. Barry, and M. M. Levine. 2000. Shigella flexneri 2a strain CVD 1207, with specific deletions in virG, sen, set, and guaBA, is highly attenuated in humans. Infect. Immun. 68:1034-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotloff, K. L., J. P. Winickoff, B. Ivanoff, J. D. Clemens, D. L. Swerdlow, P. J. Sansonetti, G. K. Adak, and M. M. Levine. 1999. Global burden of Shigella infections: implications for vaccine development and implementation. Bull. W. H. O. 77:651-656. [PMC free article] [PubMed] [Google Scholar]
- 15.Lett, M.-C., C. Sasakawa, N. Okada, T. Sakai, S. Makino, M. Yamada, K. Komatsu, and M. Yoshikawa. 1989. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J. Bacteriol. 171:353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine, M. M., R. E. Black, M. L. Clements, C. Lanata, S. Sears, T. Honda, C. R. Young, and R. A. Finkelstein. 1984. Evaluation in humans of attenuated Vibrio cholerae El Tor Ogawa strain Texas Star-SR as a live oral vaccine. Infect. Immun. 43:515-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marquart, M. E., W. L. Picking, and W. D. Picking. 1995. Structural analysis of invasion plasmid antigen D (IpaD) from Shigella flexneri. Biochem. Biophys. Res. Commun. 214:963-970. [DOI] [PubMed] [Google Scholar]
- 18.Mel, D. M., B. L. Arsic, B. D. Nikolic, and M. L. Radovanovic. 1968. Studies on vaccination against bacillary dysentery. 4. Oral immunization with live monotypic and combined vaccines. Bull. W. H. O. 39:375-380. [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, J. R., L. J. Barrett, K. Kotloff, and R. L. Guerrant. 1994. A rapid test for infectious and inflammatory enteritis. Arch. Intern. Med. 154:2660-2664. [DOI] [PubMed] [Google Scholar]
- 20.Niesel, D. W., C. B. Hess, Y. J. Cho, K. D. Klimpel, and G. R. Klimpel. 1986. Natural and recombinant interferons inhibit epithelial cell invasion by Shigella spp. Infect. Immun. 52:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oaks, E. V., T. L. Hale, and S. B. Formal. 1986. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect. Immun. 53:57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picking, W. L., J. A. Mertz, M. E. Marquart, and W. D. Picking. 1996. Cloning, expression, and affinity purification of recombinant Shigella flexneri invasion plasmid antigens IpaB and IpaC. Protein Expr. Purif. 8:401-408. [DOI] [PubMed] [Google Scholar]
- 23.Reed, G. F., B. D. Meade, and M. C. Steinhoff. 1995. The reverse cumulative distribution plot: a graphic method for exploratory analysis of antibody data. Pediatrics 96:600-603. [PubMed] [Google Scholar]
- 24.Samandari, T., K. L. Kotloff, G. A. Losonsky, W. D. Picking, P. J. Sansonetti, M. M. Levine, and M. B. Sztein. 2000. Production of IFN-gamma and IL-10 to Shigella invasins by mononuclear cells from volunteers orally inoculated with a Shiga toxin-deleted Shigella dysenteriae type 1 strain. J. Immunol. 164:2221-2232. [DOI] [PubMed] [Google Scholar]
- 25.Sansonetti, P. J., and J. Arondel. 1989. Construction and evaluation of a double mutant of Shigella flexneri as a candidate for oral vaccination against shigellosis. Vaccine 7:443-450. [DOI] [PubMed] [Google Scholar]
- 26.Van de Verg, L., D. A. Herrington, J. R. Murphy, S. S. Wasserman, S. B. Formal, and M. M. Levine. 1990. Specific immunoglobulin A-secreting cells in peripheral blood following oral immunization with bivalent Salmonella typhi-Shigella sonnei vaccine or infection with pathogenic S. sonnei in humans. Infect. Immun. 58:2002-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]